Abstract
A series of novel benzo[b]thiophen-2-yl-3-(4-arylpiperazin-1-yl)-propan-1-one derivatives 6a–f, 7a–f and their corresponding alcohols 8a–f were synthesized and evaluated for their affinity towards 5-HT1A receptors. The influence of arylpiperazine moiety and benzo[b]thiophene ring substitutions on binding affinity was studied. The most promising analogue, 1-(benzo[b]thiophen-2-yl)-3-(4-(pyridin-2-yl)piperazin-1-yl)propan-1-one (7e) displayed micromolar affinity (Ki = 2.30 μM) toward 5-HT1A sites. Docking studies shed light on the relevant electrostatic interactions which could explain the observed affinity for this compound.
Keywords: arylpiperazines, benzo[b]thiophene, depression, 5-HT1A receptor, docking, microwave Michael addition
1. Introduction
Serotonin (5-hydroxytryptamine, 5-HT), is an important neurotransmitter that plays a role in regulating numerous physiological functions, including thermoregulation, vasoconstriction, sexual behavior, appetite and sleep [1,2,3]. Additionally, it has been consistently implicated in the pathophysiology of a number of psychiatric disorders such as depression and anxiety. The 5-HT selective reuptake inhibitors (SSRIs), are currently the first line therapy for depression. However, a serious drawback in the treatment by SSRIs is on one hand, the delay of therapeutic benefits believed to be caused by the 5-HT1A auto receptors’ inhibitory role, and on the other, the lack of selectivity of the obtained compounds. For these reasons efforts in developing newer antidepressants for a fast acting agent are still ongoing [4,5]. Among the various 5-HT receptors, the 5-HT1AR has been the most extensively studied, as a result, ligands acting as partial agonists on this subtype have demonstrated effectiveness in the treatment of anxiety and depression [6,7].
During the last twenty years several chemical scaffolds are known to bind 5-HT1AR sites, among these, arylpiperazine derivatives represent one of the most studied skeletons [8,9,10].
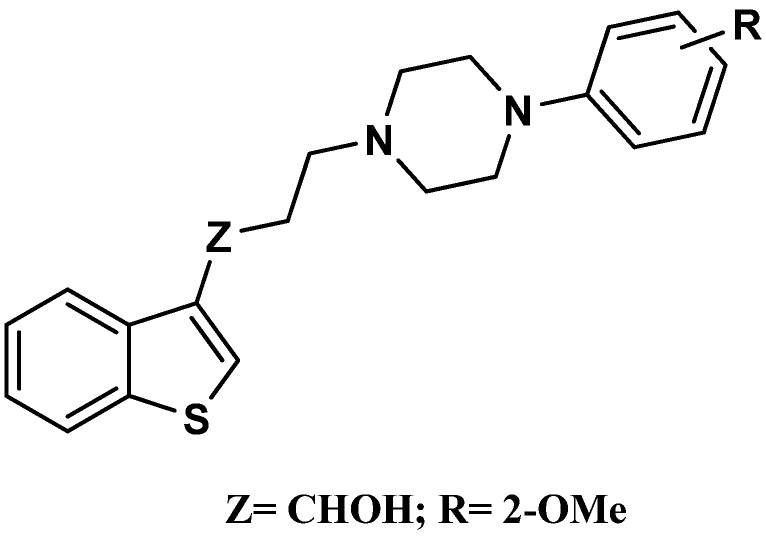
In the search for new antidepressants, Monge et al. reported the synthesis and biological evaluation of a series of unsubstituted benzothiophene derivatives connected at C-3 with subtituted aryl-piperazines by a polymethylenic chain (Figure 1). The best pharmacological activities were obtained with the benzothiophene hydroxylpropylarylpiperazine series, with the highest 5-HT1AR affinity (Ki = 20 nM) being observed for the 2-methoxyphenylpiperazine derivative [11,12].
Figure 1.
General structure of benzothiophene hydroxylpropylarylpiperazine series.
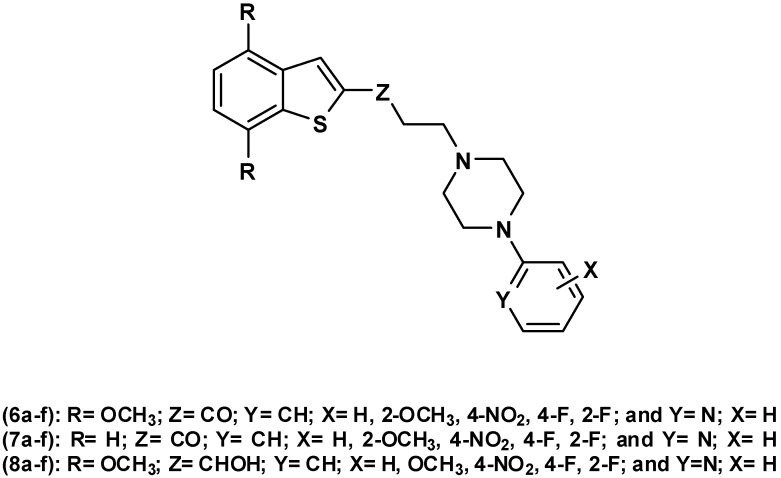
Recently, Perez-Silanes et al.[13] reported the synthesis and binding assays on SERT and 5-HT7R of a series of benzo[b]tiophen-2-yl-propenones connected to arylsulfonamide moieties, which displayed a rapid onset of action. Given our interest in this field, a new set of benzo[b]thiophenyl arylpiperazinyl propan-1-one derivatives was synthesized, in order to study the influence of a novel C-2 substitution pattern on 5-HT1AR binding affinity. It is expected that this new C-2 substitution pattern affects not only the electronic density on the benzo[b]thiophene moiety, but might provide compounds with improved 5-HT1AR affinities. The synthesized series are summarized in Figure 2.
In a previous work, we reported a versatile approach for obtaining novel benzothiophene bis-ligands using solvent-free microwave assisted aza-Michael addition, connecting functionalized arylpiperazines to benzo[b]tiophen-2-yl-propenones. This strategy is used as the base reaction in the present study [14,15].
Figure 2.
Series of benzo[b]thiophene compounds synthesized in this study.
With the aim of elucidating probable binding modes of these compounds upon binding to 5-HT1AR, molecular modeling and docking studies of substituted 2-[oxoalkyl](4-arylpiperazinyl)]benzo[b]-thiophene were carried out using an homology model obtained from the β2-adrenergic crystal structure deposited in the Protein Data Bank [16].
2. Results
2.1. Chemistry
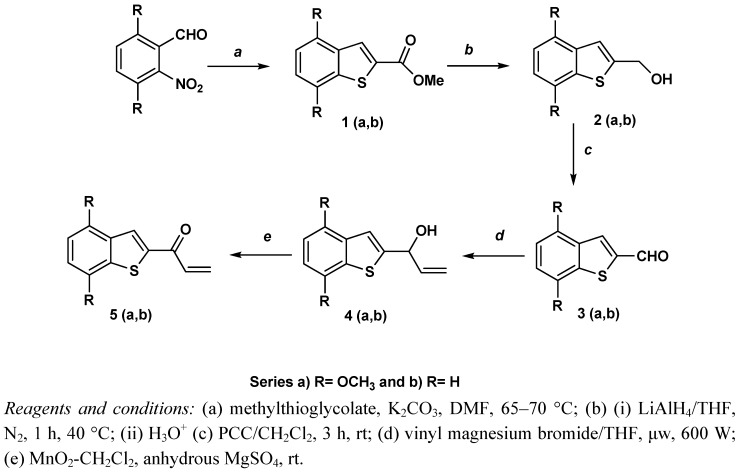
In an effort to obtain compounds with improved affinities, eighteen new benzothiophene arylpiperazines were synthesized and their bioactivities evaluated on 5-HT1AR. The presence of ketopropyl and hydroxypropyl spacers as connecting chains between the benzothiophene and arylpiperazine moieties, along with the introduction of substituted aromatic rings at the N-4 piperazine atom, were performed. The synthetic approach for the preparation of the target compounds, benzothiophene 2-propenones 5a,b, (series a: R = OCH3; series b: R = H) is outlined in Scheme 1. The general procedure is as follows: benzothiophene aldehydes 3a,b were prepared in three steps, starting from 2,5-dimethoxy-6-nitrobenzaldehyde and commercially available 2-nitrobenzaldehyde in presence of methylthioglycolate to provide esthers 1a and 1b in 72% and 75% yields, respectively. The esters were reduced with LiAlH4 to afford the alcohols 2a,b, with yields of 65% and 71%, respectively.
The synthesis of the key ketones 5a,b was carried out by a microwave supported Grignard reaction of the corresponding benzo[b]thiophene-2-carboxaldehydes 3a,b with vinyl magnesium bromide, followed by oxidation of the corresponding alcohols 4a,b with MnO2 to give ketones 5a,b in relatively good yields of 58% and 65%, respectively.
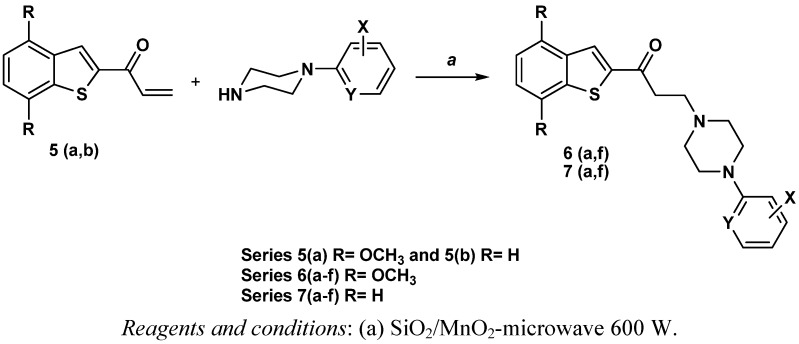
The ketoarylpiperazine derivatives 6a–f and 7a–f were prepared by Michael addition of ketones 5a,b with different arylpiperazines using free solvent microwave irradiation conditions according to Scheme 2. Melting points and reaction yields for these compounds are reported in Table 1.
Table 1.
Series (6a–f and 7a–f) obtained through Michael addition reaction between arylpiperazines and benzo[b]thiophenpropenone 5a,b.
| Entry | Y | X | R | Yield (%) | m.p (°C) | Entry | Y | X | R | Yield (%) | m.p (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6a | CH | H | OMe | 88 | 169–170 | 7a | CH | H | H | 59 | ….. a |
| 6b | CH | 2-F | OMe | 66 | 134–135 | 7b | CH | 2-F | H | 90 | ….. b |
| 6c | CH | 4-F | OMe | 60 | 131–132 | 7c | CH | 4-F | H | 38 | 117–118 |
| 6d | CH | 2-OMe | OMe | 67 | 128–129 | 7d | CH | 2-OMe | H | 50 | 45–47 |
| 6e | N | H | OMe | 60 | 174–175 | 7e | N | H | H | 71 | 56–58 |
| 6f | CH | 4-NO2 | OMe | 65 | 161–162 | 7f | CH | 4-NO2 | H | 69 | 138–141 |
a,b yellow oils.
Scheme 1.
Synthetic approach used to obtain 1-(benzo[b]thiophen-2-yl)prop-2-en-1-ones 5a–b.
Scheme 2.
Synthesis of series 6a–f and 7a–f under microwave conditions.
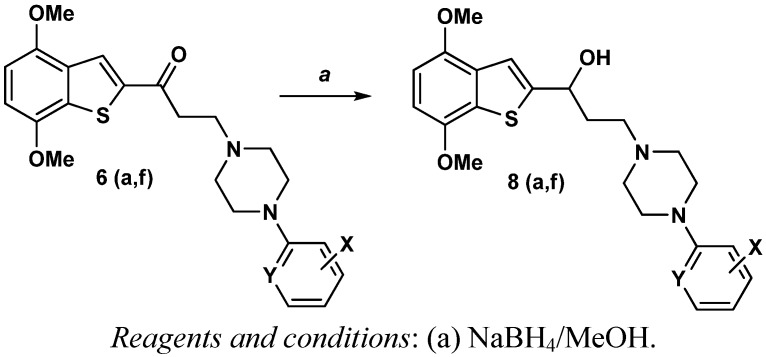
The corresponding hydroxyl derivatives 8a–f depicted in Scheme 3 were obtained in good yields (Table 2) upon treatment of the ketoarylpiperazine derivatives 7a–f with sodium borohydride in methanol.
Table 2.
Yields of the reduced derivatives 8a–f.
| Entry | Y | X | R | Yield (%) | m.p (°C) |
|---|---|---|---|---|---|
| 8a | CH | H | OMe | 68 | 148–149 |
| 8b | CH | 2-F | OMe | 63 | 128–129 |
| 8c | CH | 4-F | OMe | 80 | 55–56 |
| 8d | CH | 2-OMe | OMe | 93 | 55–56 |
| 8e | N | H | OMe | 77 | 157–158 |
| 8f | CH | 4-NO2 | OMe | 72 | 173–174 |
Scheme 3.
Synthesis of the reduced derivatives 8a–f.
2.2. Biological Properties
Target compounds 6a–f, 7a–f, and 8a–f were assessed for in vitro affinity on serotoninergic 5-HT1AR by radioligand binding assays, using [3H]-8-OH-DPAT in rat cerebral cortex membranes. All the compounds were DMSO soluble as bases. Due to practical considerations, in the experimental binding assays, these compounds were first tested at a fixed dose of 1 μM, and only for those that in the screening process showed a displacement of the radioligand of ≥55%, the dose-response curves were determined. The inhibition constant Ki was calculated from its IC50 value only for compound 7e, using the Cheng-Prusoff equation The results of these assays are summarized in Table 3. The 8-OH-DPAT displayed an inhibition percentage of 92.
Table 3.
Inhibition % for benzo[b]thiophene arylpiperazine derivatives.
| Entry | Y | X | R | Inhibition (%) | Entry | Y | X | R | Inhibition (%) |
|---|---|---|---|---|---|---|---|---|---|
| 6a | CH | H | OMe | 2 ± 2 | 7-d | CH | 2-OMe | H | 52 ± 7 |
| 6b | CH | 2-F | OMe | 14 ± 2 | 7-e | N | H | H | 60 ± 4 |
| 6c | CH | 4-F | OMe | 0 ± 3 | 7-f | CH | 4-NO2 | H | 0 ± 4 |
| 6d | CH | 2-OMe | OMe | 44 ± 2 | 8-a | CH | H | OMe | 24 ± 4 |
| 6e | N | H | OMe | 17 ± 5 | 8-b | CH | 2-F | OMe | 21 ± 1 |
| 6f | CH | 4-NO2 | OMe | 0 ± 3 | 8-c | CH | 4-F | OMe | 23 ± 3 |
| 7a | CH | H | H | …… a | 8-d | CH | 2-OMe | OMe | 38 ± 0 |
| 7b | CH | 2-F | H | 33 ± 3 | 8-e | N | H | OMe | 27 ± 3 |
| 7c | CH | 4-F | H | 31 ± 7 | 8-f | CH | 4-NO2 | OMe | 14 ± 2 |
a not measured.
All the synthesized compounds displayed inhibition with regards to [3H]-8-OH-DPAT binding, with percentages ranging from 0% (compounds 6c, 6f and 7f) to 60%, corresponding to compound 1-(benzothiophen-2-yl)-3-(4-(pyridin-2-yl)piperazin-1-yl)propan-1-one (7e). Consequently, a dose-response curve was plotted out using 7e, under the same pH conditions, temperature and time as stated in the Experimental section. Values for 7e concentrations encompassed six orders of magnitude (1 nM–1 mM), thus, its obtained IC50 and Ki values were 2.50 and 2.30 μM, respectively.
Focusing our aims toward to the exploration of new molecules endowed with 5-HT1A activity, binding values for compounds 6a–f, 7a–f and 8a–f were analyzed in order to examine the influence of functionalized arylpiperazines linked to benzo[b]thiophene moieties at C-2.
2.3. Docking Studies
2.3.1. Molecular Modeling Studies of 5-HT1AR in complex with Ligands 6d, 6f, 7f, 7e
In order to get a better understanding of the benzo[b]thiophene arylpiperazine receptor interactions, computational simulations were carried out using an homology model for the 3D structure of 5-HT1AR.
From the pharmacological results, the pairs 6d/6f and 7f/7e displayed the lowest and the highest binding affinities in these series, respectively. These results were interpreted according the interactions obtained in the docking studies. The results are summarized in Table 4.
Table 4.
Displayed interactions in the binding site.
| Residues 5-HT1AR | Ligands | |||
|---|---|---|---|---|
| 6d | 6f | 7f | 7e | |
| D116 | + | + | + | +, ¥ |
| F360 | Y | Y | Y | Ya |
| F361 | Y | - | - | NOI |
| L380 | NOI | - | - | + |
| N385 | NOI | |||
+: H-bonding; Y: Hydrophobic interactions; Ya: Edge-to-face interactions; -: Repulsive interactions; ¥: electrostatic interactions; NOI, no observed interactions.
The docking results were analyzed according to the structures with the best total energies, displaying the shortest H-bond between the aspartic residue D116 and the basic protonated nitrogen of the piperazine ring of the ligands. The electrostatic and hydrophobic interactions observed in the complexes were also considered.
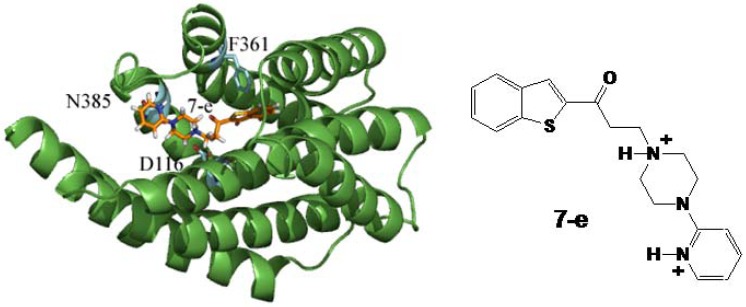
As mentioned in Section 2.2, compound 7e displayed the best binding profile in the radioligand studies with 60% of inhibition (Table 3) (Ki 2.30 μM). This result was interpreted according to the docking criteria described above and considering a diprotonated form of compound 7e as represented in Figure 3, which shows the molecular docking of compound 7e in its preferred conformation at the binding site of 5-HT1AR. The observed interactions were an H-bond between D116 and protonated N-1 at a distance of 2.83 Å, reinforced approximately by 10 kcal/mol product by the electrostatic interaction resulting between the aspartate moiety and the N-1 quaternary ammonia of the piperazine ring [17].
Figure 3.
Compound 7e docked to 5-HT1A molecular model receptor. Interacting residues with compound 7e are shown in bold.
Another H-bond was observed at 3.19 Å between the protonated nitrogen of the pyridine ring and the carbonyl amide group of the side chain of N385 reinforced by an ion-dipole at 2.95 Å. The stabilization of the latter interactions may vary energetically among 1–7 kcal/mol [16]. These interactions might explain the observed affinity of compound 7e obtained in the binding assays. This complex also displayed edge-to-face aromatic-interactions [18] between the F361 and the benzo[b]thiophene moiety of the ligand. These weak attractive interactions arise among aromatic groups that do not necessarily bear polar substituents; however, they play an important role in protein folding, ligand-binding, supramolecular complexes and drug-receptor interactions [18].
On the other hand, the model complex for 7f (displaying no activity), shows an H bond, at a distance of 3.01 Å between the N-1 and D116 along with repulsive interactions between the p-nitro group of the aromatic ring linked to piperazine with L380 at a distance of 2.79 Å, and the residue N385 at a distance of 2.89 Å. This suggests that groups bearing strong electron-withdrawing features decrease the affinity of the ligand for the binding site which can be attributed to two factors: one of electronic nature and other due to an unfavorable steric hindrance of the nitro group in that position. Hindrance effects have been also observed in the binding properties to the 5-HT1A receptors of structural related ligands such as coumarins [19]. Thus, it would be reasonable to think that these repulsive interactions are essential in the observed null activity for both compounds 6f/7f, confirming that voluminous groups at p-position of the aromatic ring linked to piperazine are detrimental for the proper affinity with the receptor.
Comparing these four ligand-receptor complexes, we conclude that several electrostatic or hydrophobic in nature stabilizing interactions are present. The molecular docking of series 6d, 6f, 7e and 7f showed that they bind to the 5-HT1AR in a similar fashion, being the common feature for these compounds the presence of a H-bond between the D116, and the protonated nitrogen of the piperazine ring with distances lower than 3.00 Å. However, the structural determinants for compounds showing measurable activity seem to be the absence of repulsive interactions, which can define in a greater extent the ability of the ligand to accomadate itself in the binding site.
Another compound that did not display any inhibitory activity was the p-fluoro compound 6c. This fact reinforces the idea that an electronegative substituent in p-position of the phenyl ring does not favor the activity. However, the decreased volume of fluorine atom compared to the nitro group indicates that this lack of activity could be attributed to other structural features such as the presence of methoxy groups on the benzo[b]thiophene ring. According to this, p-F derivatives 8c and 7c displayed certain level of activity, with inhibition percentages of 23% and 31%.
The evaluated compounds belonging to the 8a–f family showed better inhibition assays compared to their carbonylic analogues 6a–f, suggesting that hydroxyl groups might have some influence in the affinity for the 5-HT1AR. This may be due to the hydroxyl participation in H-bonding interactions, stabilizing the ligand-receptor complexes or changes in volume and orientation (in terms of bond length and angles) underwent by the carbon atom bearing the hydroxyl group, when the hybridization goes from sp2 to sp3. These results are in agreement with the report by Martinez [20] for a series of benzo[b]thiophene derivatives, where the carbonyl compouds were compared with their respective alcohol derivatives.
On the other hand, the carbonylic derivatives belonging to family 7a–f displayed an improved binding pattern compared to their structural analogues 6a–f, probably due to the lack of voluminous methoxyl groups on the benzothiophene ring, which might have a detrimental effect on the affinity displayed. In this sense, the existence or absence of these groups in such compounds probably represent the most important structural determinants for their displayed activity. The decreased activity, going from derivatives 6e (17%), 6b (14%) and 6c (0%) to their respective analogues without the methoxyl groups 7e (60%), 7b (33%) and 7c (31%), confirm this statement. A fact that confirms this observation is that two compounds with the best competition profiles do not bear methoxyl groups in their scaffolds, namely compounds 7d and 7e. Within compounds that displayed better inhibition profiles, three of them, namely 6d (44%); 8d (38%) and 7d (52%) bear a methoxy group at the ortho position of the aromatic ring linked to piperazine, strongly suggesting that the presence of this group represents a relevant and important characteristic for their increased activity. These results are in good agreement with previous studies, indicating that incorporation of o-methoxy groups lead to arylpiperazine compounds with higher affinities for 5-HT1A receptors [21,22,23,24,25,26] and 5-HT7[26].
3. Experimental
3.1. General
Melting points were determined on a hot-stage apparatus and are uncorrected. The IR spectra were recorded on a FT-IR Bruker IFS 55 spectrophotometer for KBr discs and wavenumbers are reported in cm−1. The 1H-NMR and 13C-NMR spectra were recorded on a Bruker DRX-300 spectrometer (at 300 and 75 MHz, respectively) in deuterochloroform, or DMSO-d6. Chemical shifts were recorded in ppm (δ) relative to TMS as an internal standard. J values are given in Hz. The following multiplicity abbreviations were utilized: s = singlet, bs = broad singlet; d = doublet; dd = doublet of doublet; td = triplet of doublet; m = multiplet. The microwave assisted procedures were carried out in a Milestone, Lavis 1000 Multiquant. High resolution mass spectrum were recorded on a Thermo Finnigan model MAT 95XP Mass Spectrometer. Silica gel Merck 60 (70–230 mesh) and DC-alufolien 60 F254 were used for column and TLC chromatography, respectively. Purification of each product was carried out by chromatography on a silica gel column and/or recrystallized from the appropriate solvent.
3.2. Synthesis
1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-2-propen-1-one (5a) [14,15]. To a solution of alcohol 4a (350 mg, 1.40 mmol) in CH2Cl2 (60 mL) was added MnO2 (365 mg, 4.20 mmol), and anhydrous MgSO4 (168 mg, 1.40 mmol), and the mixture stirred at room temperature for 3 h. The reaction mixture was filtered and the solvent removed under reduced pressure to give 250 mg, of crude enone 5a. The crude residue was purified by column chromatography on silica gel (CH2Cl2) and recrystallized from (EtOH/cyclohexane 2:1) to afford pure 5a (218 mg, 63%) as a yellow-orange solid. mp 99–100 °C; IRνmax (KBr): 3054 (C-H Ar), 2968 (C-H Aliph.), 1665 (C=O), 1556 (C=C) cm−1; 1H-NMR (CDCl3): 3.94 (s, 3H, Ar-OMe), 3.96 (s, 3H, Ar-OMe), 5.92 (dd, 1H, 3′-H, J = 10.4 Hz and J = 1.6 Hz), 6.55 (dd,1H, 3′-H, J = 17 Hz and J = 1.6 Hz), 6.67 (d, 1H, 5-H, J = 8.5 Hz), 6.80 (d, 1H, 6-H, J = 8.5 Hz), 7.22 (dd, 1H, 2′-H, J = 17 Hz and J = 10.4 Hz), 8.16 (s, 1H, 3-H); 13C-NMR (75 MHz, CDCl3): δ 55.8, 56.0, 104.6, 107.6, 127.0, 129.6, 131.4, 131.6, 133.7, 143.6, 148.6, 150.8, 183.8; HRMS (EI) calcd for C13H12O3S (M+): 248.05072, found: 248.05001.
3.2.1. General Procedure for the Synthesis of 1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-(4-arylpiperazin-1-yl)-1-propanone Derivatives 6a–f
1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-(4-phenylpiperazin-1-yl)-1-propanone (6a). To a solution of 1-(4,7-dimethoxybenzo[b]thiophen-2-yl)-2-propen-1-one (5a, 100 mg, 0.40 mmol) and 1-phenylpiperazine (93 mg, 0.57 mmol), in dichloromethane (20 mL) was added the supported inorganic reagent (MnO2–SiO2, 4:1, 1.0 g) and the suspension was vigorously stirred for 15 min at room temperature. The solvent was removed in vacuo and the solid was irradiated in a microwave oven at 900 W for 15 min. until TLC showed that the starting product had disappeared. The solid was thoroughly washed with AcOEt followed by remotion of the solvent to afford 7a as a crude in quantitative yield. The solid residue was purified by crystallization (EtOH/CH3CN 5:1), to give the title compound as pale yellow crystals (138 mg, 88% yield); mp 169–170 °C; IR (KBr): 3032 (C-H Ar), 2934 (C-H Aliph.), 1655 (C=O), 1600 (C=C) cm−1; 1H-NMR (CDCl3): δ 2.71 (t, 4H, 2′′-H and 6′′-H J = 5.0 Hz), 2.95 (t, 2H, 3′-H, J = 7.4 Hz), 3.22 (t, 2H, 3′′-H, 5′′-H, J = 5.0 Hz), 3.24 (t, 2H, 2′-H, J = 7.4 Hz,), 3.95 (s, 3H, Ar-OMe C-4 or C-7), 3.96 (s, 3H, Ar-OMe C-7 or C-4), 6.68 (d, 1H, 5-H, J = 8.4 Hz), 6.8 (d, 1H, 6-H, J = 8.4 Hz), 6.87 (t, 1H, 4′′´-H, J = 7.4 Hz), 6.94 (d, 2H, 2′′′-H, 6′′′-H, J = 8.5 Hz), 7.27 (t, 2H, 3′′′-H and 5′′′-H, J = 8.3 Hz), 8.15 (s, 1H, 3-H); 13C-NMR (75 MHz, CDCl3): δ 36.7, 49.1, 53.1, 53.3, 55.8, 56.1, 104.6, 107.4, 116.1, 119.73, 129.1, 126.5, 131.5, 133.4, 142.8, 148.6, 150.7, 151.3, 193.3; HRMS (EI) Calcd for C23H26N2O3S (M+): 410.16641, found: 410.16618.
3-[4-(2-Fluorophenyl)piperazin-1-yl]-1-(4,7-dimethoxybenzo[b]thiophen-2-yl)-1-propanone (6b).Prepared from 5a (50 mg, 0.20 mmol) and 1-(2-fluorophenyl)piperazine (55 mg, 0.31 mmol). Quantitative crude yield. Purified by recrystallization (EtOH/hexane 2:1), to give yellow crystals (53.6 mg, 65.7%); mp 134–135 °C; IRνmax (KBr): 3032 (C-H Ar), 2937 (C-H Aliph.), 1665 (C=O), 1500 (Ar C=C) cm−1; 1H-NMR (CDCl3): δ 2.72 (t, 4H, 2′′-H and 6′′-H, J = 4.8 Hz), 2.95 (t, 2H, 3′-H, J = 7.6 Hz), 3.12 (t, 4H, 3′′-H, 5′´-H, J = 4.8 Hz ), 3.23 (t, 2H, 2′-H, J = 7.6 Hz), 3.93 (s, 3H, Ar-OMe C-4 or C-7), 3.95 (s, 3H, Ar-OMe C-7 or C-4), 6.67 (d, 1H, 5-H, J = 8.5 Hz), 6.78 (d, 1H, 6-H, J = 8.5Hz), 6.90–7.09 (m, 4H, 3′′′-H, 4′′′-H, 5′′′-H, 6′′′-H), 8.14 (s, 1H, 3-H); 13C-NMR (CDCl3): δ 36.7, 50.5, 50.6, 53.2, 53.3, 55.8, 56.1, 104.6, 107.4, 116.1(d, 2J = 21 Hz), 118.9.0 (d, 3′J = 3.0 Hz), 122.5 (d, 2′J = 8.0 Hz), 124.5 (d, 4J = 3.6 Hz), 126.5, 131.5, 133.4, 140.1 (d, 3J = 8.6 Hz), 143.0, 148.6, 150.8, 155.8 (d, 1J = 245 Hz), 193.4; HRMS (EI) Calcd for (M+): C23H25FN2O3S 428.15699, found: 428.15686.
3-[4-(4-Fluorophenyl)piperazin-1-yl]-1-(4,7-dimethoxybenzo[b]thiophen-2-yl)-1-propanone (6c). Prepared from 5a (70 mg, 0.282 mmol) and 1-(4-fluorophenyl) piperazine (77 mg, 0.427 mmol). Crude yield 96%. Purified by recrystallization (EtOH) as yellow pale crystals (73 mg, 60%); mp 131–132 °C; IR νmax (KBr): 3032 (C-H Ar), 2940 (C-H Aliph.), 1655 (C=O), 1514 (Ar C=C) cm−1; 1H-NMR (CDCl3): δ 2.68 (t, 4H, 2′′-H and 6′′-H, J = 4.9 Hz), 2.92 (t, 2H, 3′-H, J = 7.5Hz), 3.11 (t, 4H, 3′′-H, 5′′-H, J = 4.9 Hz ), 3.21 (t, 2H, 2′-H, J = 7.5 Hz), 3.92 (s, 3H, Ar-OMe C-4 or C-7), 3.94 (s, 3H, Ar-OMe C-7 or C-4), 6.65 (d,1H, 5-H, J = 8.5 Hz), 6.77 (d, 1H, 6-H, J = 8.5 Hz), 6.82–6.98 (m, 4H, Ar-F (2′′′-H, 3′′′-H, 5′′′-H, 6′′′-H), 8.13 (s, 1H, 3-H); 13C-NMR (CDCl3): δ 36.8, 50.1, 53.2, 53.3, 55.8, 56.1, 104.6, 107.4, 115.5 (d, 2J = 22 Hz), 117.8.(d, 3J = 7.6 Hz),126.5, 131.5, 133.4, 140.1 (d, 3J = 8.6 Hz), 143.0, 148.0 (d, 4J = 2.2 Hz), 148.6, 150.8, 157.1 (d, 1J = 239 Hz), 193.3 (C=O); HRMS (EI) Calcd for C23H25FN2O3S (M+): 428.15699, found: 428.15584.
1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-[4-(2-methoxyphenyl)piperazin-1-yl]-1-propanone (6d). Prepared from 5a (53 mg, 0.21 mmol) and 1-(2-methoxyphenyl)piperazine (58 mg, 0.30 mmol). Crude yield 95.5%. Recrystallization from EtOH/hexane 5:1) gave pale white crystals (60 mg, 67% yield); mp 128–129 °C; IRνmax (KBr): 3031 (C-H Ar), 2940 (C-H Aliph.), 1663 (C=O), 1597 (C=C) cm−1; 1H-NMR (CDCl3): δ 2.75 (t, 4H, 2′′-H and 6′′-H, J = 4.4 Hz), 2.95 (t, 2H, 3′-H, J = 7.7 Hz), 3.31 (m, 2H, 3′′-H, 5′′-H), 3.27 (t, 2H, 2-H, J = 7.7 Hz), 3.87 (s, 3H, 2′′′-Ar-OMe), 3.94 (s, 3H, Ar-OMe C-4 or C-7), 3.95 (s, 3H, Ar-OMe C-7 or C-4), 6.67 (d, 1H, 5 -H, J = 8.5 Hz), 6.79 (d, 1H, 6-H, J = 8.5 Hz), 6.87–7.06 (m, 4H, 3′′′-H, 4′′′-H, 5′′′-H, and 6′′′-H Ar-OMe), 8.14 (s, 1H, 3-H); 13C-NMR (CDCl3): δ 36.8, 50.6, 53.4, 55.4, 55.8, 56.1, 104.6, 107.4, 111.2, 118.2, 120.0, 123.0, 126.5, 131.5, 133.4, 141.3, 143.0, 148.6, 150.8, 152.3, 193.5; HRMS (EI) Calcd for (M+): C24H28N2O4S 440.17698, found: 440.17541.
1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-(4-pyridin-2-yl)-piperazin-1-yl)-1-propanone (6e). Prepared from 5a (70 mg, 0.28 mmol) and 1-(pyridin-2-yl) piperazine (73 mg, 0.45 mmol). Quantitative crude yield. Purified by recrystallization (EtOH) as yellow pale crystals (66 mg, 60%); mp 174–175 °C; IRνmax (KBr): 3032 (C-H Ar), 2938 (C-H Aliph.), 1656 (C=O), 1594 (Ar, C=C) cm−1; 1H-NMR (CDCl3): δ 2.64 (t, 4H, 2′′-H and 6′′-H, J = 5.0 Hz), 2.92 (t, 2H, 3′-H, J = 7.5 Hz), 3.26 (t, 2H, 2′-H, J = 7.5 Hz), 3.55 (t, 4H, 3′′-H and 5′′-H; J = 5.0 Hz), 3.93 (s, 3H, Ar-OMe C-4 or C-7), 3.95 (s, 3H, Ar-OMe C-7 or C-4), 6.59-6.68 (m, 3H, 5-H, 4′′′-H, 6′′′-H), 6.79 (d, 1H, 6-H, J = 8.5 Hz), 7.5 (td, 1H, 5′′′-H, Jo = 7.6, Jm = 2.2 Hz), 8.14 (s, 1H, 3-H), 8.18 (d, 1H, 3′′′-H, J = 5.2 Hz); 13C-NMR (75 MHz, CDCl3): δ 36.8, 45.2, 53.1, 53.4, 55.8, 56.1, 104.7, 107.1, 107.4, 113.3, 126.5, 131.5, 133.4, 137.4, 142.9, 147.9, 148.6, 150.8, 159.5, 193.3; HRMS (EI) Calcd for (M+): C22H25N3O3S 411.16166, found: 411.15976.
1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-[4-(4-nitrophenyl)piperazin-1-yl]-1-propanone (6f). Prepared from 5a (72 mg, 0.29 mmol) and 1-(4-nitrophenyl) piperazine (88mg, 0.43 mmol). Crude yield quantitative. Purified by recrystallization (EtOH/CH3CN 5:1) as yellow crystals (82 mg, 65%); mp 161–162 °C; IRνmax (KBr): 3030 (C-H Ar), 2934 (C-H Aliph.), 1647 (C=O), 1597 (NO2), 1326 (NO2) cm−1; 1H-NMR (CDCl3): δ 2.66 (t, 4H, 2′′-H and 6′′-H, J = 5.0 Hz,), 2.95 (t, 2H, 3′-H, J = 7.1 Hz), 3.24 (t, 2H, 2′-H, J = 7.1 Hz), 3.41 (t, 4H, 3′′-H and 5′′-H, J = 5.0 Hz), 3.93 (s, 3H, Ar-OMe C-4 or C-7), 3.95 (s, 3H, Ar-OMe C-7 or C-4), 6.67 (d,1H, 5-H, J = 8.5 Hz), 6.79 (d, 3H, 6-H, and 2′′′-H, 6′′′-H Ar-NO2, J = 8.8 Hz), 8.1 (d, 2H, 3′′′-H and 5′′′-H, Ar-NO2, J = 9.3 Hz), 8.13 (s,1H, 3-H); 13C-NMR (CDCl3): δ 36.7, 47.0, 52.6, 53.2, 55.8, 56.1, 104.7, 107.5, 112.6, 125.9, 126.5, 131.4, 133.4, 138.4, 142.8, 148.6, 150.7, 154.8, 193.1; HRMS (EI) Calcd for (M+): C23H25N3O5S 455.15149, found: 455.15128.
3.2.2. Synthesis of 1-(Benzo[b]thiophen-2-yl)-3-(4-arylpiperazin-1-yl)-1-propanone Derivatives 7a–f
1-Benzo[b]thiophen-2-yl-2-propen-1-one (5b) [14]. To a solution of alcohol 4b (430 mg, 2.28 mmol) in CH2Cl2 (50 mL), was added MnO2 (991 mg, 11.4 mmol), anhydrous MgSO4 (275 mg, 2.28 mmol), and the mixture stirred, at r.t for 6 h. The crude residue was then filtered and concentrated in vacuo, to afford the crude product (321 mg), which was purified by column chromatography (CH2Cl2) to provide pure 5b (265 mg, 62%); mp 45–46 °C; IRνmax (KBr): 3032 (C-H Arom.), 1661 (C=O), 1601 (C=C); 1H-NMR (CDCl3): δ 5.92 (dd,1H, 3′-Hcys, Jcys = 10.4 Hz; Jgem = 1.5 Hz), 6.54 (dd, 1H, 3′-H trans, Jtrans = 17.0 Hz; Jgem = 1.5 Hz), 7.18 (dd, 1H, 2′-H, Jtrans = 17.0 Hz, Jcys = 10.4 Hz), 7.41 (td, 1H, 6-H or 5-H, Jo = 7.5 Hz; Jm = 1.25 Hz), 7.47 (td, 1H, 5-H or 6-H, Jo = 7.5 Hz; Jm = 1.25 Hz), 7.87 (t, 2H, 4-H and 7-H, Jo = 7.1 Hz), 8.0 (s, 1H, 3-H); 13C-NMR (CDCl3): δ 123.0, 125.3, 126.1, 127.6, 128.7, 128.8, 131.4, 139.2, 142.8, 144.2, 183.7; HRMS (EI) Calcd for C11H8OS (M+): 188.02959, found: 188.02904.
1-(Benzo[b]thiophen-2-yl)-3-(4-phenylpiperazin-1-yl)-1-propanone (7a). To a solution of 5b (131 mg, 0.71 mmol) and 1-phenylpiperazine (115 mg, 0.71 mmol), in dichloromethane (20 mL) was added the inorganic supported reagent SiO2–MnO2 (4:1) (2.0 g) and the suspension was vigorously stirred for 15 min at room temperature. The solvent was removed in vacuo and the solid was irradiated at 900 W for 15 min, until TLC showed that the starting product had disappeared. The solid was thoroughly washed with AcOEt followed by removal of the solvent to afford 7a as a crude in quantitative yield. The solid residue was purified by column chromatography (AcOEt) to give a pale yellow oil (145 mg, 59%); IRνmax. (KBr): 2917 (C-H Arom.), 2849 (C-H aliphatic), 1655 (C=O), 1599 (C=C) cm−1; 1H-NMR (CDCl3): δ 2.77 (t, 4H, 2′′-H and 6′′-H, J = 4.8 Hz); 3.01 (t, 2H, 3′-H, J = 7.2 Hz); 3.27 (t, 4H, 3′′-H and 5′′-H, J = 4.8 Hz); 3.32 (t, 2H, 2′-H, J = 7.2 Hz), 6.88 (t, 1H, 4′′′-H, J = 9.2 Hz); 6.97 (d, 2H, 2′′′-H and 6′′′-H, J = 8.0 Hz); 7.30 (t, 2H, 3′′′-H and 5′′′-H, J = 9.6 Hz); 7.46 (t, 2H, 6-H or 5-H, J = 6.8 Hz); 7.53 (t, 2H, 5-H or 6-H, J = 6.8 Hz); 7.94 (t, 2H, 4-H and 7-H, J = 8.4 Hz); 8.05 (s, 1H, 3-H); 13C-NMR (CDCl3) δ: 32.5; 49.1 (2C); 53.7 (2C); 56.5; 113.2 (2C); 116.1; 119.8; 123.0; 125.1; 127.5; 129.1 (2C); 138.4; 193.8; HRMS (EI) Calcd for (M+): 350.14528, found: 350.14605.
3-[4-(2-Fluorophenyl)piperazin-1-yl]-1-(benzo[b]thiophen-2-yl)-1-propanone (7b). To a solution of 5b (59 mg, 0.32 mmol) and 1-(2-fluorophenyl)piperazine (57 mg, 0.32 mmol), in dichloromethane (20 mL) was added the inorganic supported reagent SiO2–MnO2 (4:1, 2.0 g). After solvent removal the solid mixture was irradiated at 900 W for 10 min. Purification using a chromatographic column afforded 105 mg, (90%) of pure 7b as a yellow oil. IRνmax (KBr): 3040 (C-H arom.), 2822 (C-H aliph.), 1661 (C=O), 1501 (C=C) cm−1; 1H-NMR (CDCl3) δ: 2.69 (m, 4H, 2′′-H and 6′′-H); 2.92 (t, 2H, 3′-H, J = 6.8 Hz), 3.06 (m, 4H, 3′′-H and 5′′-H), 3.20 (t, 2H, 2′-H, J = 6.8 Hz), 6.85–7.02 (m, 4H, 3′′′-H and 6′′′-H), 7.35 (t, 1H, 5-H or 6-H, J = 7.2 Hz), 7.41 (t, 1H, 6-H or 5-H, J = 7.2 Hz); 7.81 (d, 1H, 7-H or 4-H, J = 8.0Hz); 7.85 (d, 1H, 4-H or 7-H, J = 8.0Hz); 7.98 (s, 1H, 3-H); 13C-NMR (CDCl3) δ: 36.8; 52.2 (2C); 53.1 (2C); 53.6; 113.7 (d, 2J = 49 Hz); 118.4 (d, 3′J = 2.8 Hz); 122.9 (d, 2′J = 7.0 Hz); 124.4, 125.5 (d, 4J = 3,5 Hz);128.6 (d, 3J = 66 Hz); 130.8; 132.9, 136.2, 138.0, 138.1, 142.2, 147.5, 158.0 (d, 1J = 240 Hz), 192,3; HRMS (EI) Calcd for C21H21FN2OS (M+): 368.13586, found: 368.13640.
3-[4-(4-Fluorophenyl)piperazin-1-yl]-1-(benzo[b]thiophen-2-yl)-1-propanone (7c). To a solution of 5b (90 mg, 0.0.49 mmol) and 1-(4-fluorophenyl)piperazine (132 mg, 0.73 mmol) in dichloromethane (20 mL) a mixture of the inorganic supported reagent SiO2–MnO2 (4:1, 2.0 g) was added. After solvent removal the solid mixture was irradiated at 900 W for 10 min. Purification by recrystallization (EtOH/hexane), afforded 68 mg. (38%) of pure 7c; mp 117–118 °C; IRνmax (KBr): 2954 (C-H arom.), 2822 (C-H aliph.), 1660 (C=O), 1510 (C=C) cm−1; 1H-NMR (CDCl3) δ: 2.70 (t, 4H, 2′′-H and 6′′-H, J = 4.8 Hz); 2.95 (t, 2H, 3′-H, J = 7.4 Hz); 3.13 (t, 4H, 3′′-H and 5′′-H, J = 4.7 Hz); 3.26 (t, 2H, 2′-H, J = 7.4 Hz); 6.87 (dd , 2H, 2′′′-H and 6′′′-H, J = 4.6 Hz); 6.96 (t, 2H, 3′′′-H and 5′′′-H, J = 9.0 Hz); 7.45 (m, 2H, 5-H and 6-H, J = 1.4 Hz, J = 9.4 Hz); 7.89 (t, 2H, 4-H and 7-H, J = 7.0 Hz); 8.00 (s, 1H, 3-H); 13C-NMR (CDCl3) δ: 40.1; 53.3 (2C); 56.3 (2C); 118.5; 118.8; 121.0 (d, 2C, 3J = 7.6 Hz); 126.2; 128.2; 129.1; 130.7; 132.2; 142.2; 145.7; 146.7; 151.0; 158.7; 161.9 (d, 1J = 239 Hz); 196.5; HRMS (EI) Calcd for C21H21FN2OS (M+): 368.13586, found: 368.13750.
3-[4-(2-Methoxyphenyl)piperazin-1-yl]-1-(benzo[b]thiophen-2-yl)-1-propanone (7d). To a solution of 5b (77 mg, 0.42 mmol) and 1-(2-methoxyphenyl)piperazine (80 mg,0.42 mmol), in dichloromethane (20 mL) the inorganic supported reagent SiO2-MnO2 (4:1, 2.0 g) was added. After solvent removal, the solid mixture was irradiated at 900 W for 10 min. Purification by recrystallization (EtOH/hexane), afforded 78 mg. (50%) of pure 7d; mp: 45–47 °C; IRνmax. (KBr): 2918 (C-H Arom), 2817 (C-H aliph.), 1662 (C=O), 1594 (C=C); 1H-NMR (CDCl3): 2.70 (m 4H, 2′′-H and 6′′-H); 2.91 (t, 2H, 3′-H, J = 7.6 Hz); 3.10 (m, 4H, 3′′-H and 5′′-H), 3.21 (t, 2H, 2′-H, J = 7.6 Hz); 3.79 (s, 3H, 2′′′-OCH3); 6.78–6.95 (m, 4H, 3′′′-H and 6′′′-H); 7.34 (t, 2H, 6-H or 5-H, J = 8.0 Hz); 7.40 (t, 2H, 5-H or 6-H, J =.8.0 Hz); 7.82 (t, 2H, 4-H and 7-H, J = 9.2 Hz); 7.94 (s, 1H, 3-H); 13C-NMR (CDCl3) δ: 30.9; 50.8 (2C); 53.3; 53.5 (2C); 54.7; 111.3; 118.3; 121,1; 123,0; 125,0; 128,8; 129,1; 137,7; 138,1; 139,1; 139,2; 152.3; 193.4; HRMS (EI) Calcd for C22H24N2O2S (M+): 380.15585, found: 380.15460.
1-(Benzo[b]thiophen-2-yl)-3-(4-pyridin-2-yl)-piperazin-1-yl)-1-propanone (7e). To a solution of 5b (122 mg, 0.66 mmol) and 1-(2-methoxyphenyl)piperazine (162 mg, 0.99 mmol), in dichloromethane (20 mL) the inorganic supported reagent SiO2-MnO2 (4:1; 2.0 g) was added. After solvent removal, the solid mixture was irradiated at 900 W for 10 min. Purification by column chromatography (AcOEt) afforded pure 7e (163 mg, 71%); mp: 56–58 °C; IRνmax (KBr): 3030 (C-H Ar), 2964, (C-H Aliph.), 1661 (C=O), 1592 (Ar, C=C)cm−1; 1H-NMR (CDCl3) δ: 2.65 (t, 4H, 2′′-H and 6′′-H, J = 4.9 Hz); 2.93 (t, 2H, 3′-H, J = 7.4 Hz); 3.27 (t, 2H, 2′-H, J = 7.3 Hz); 3.56 (t, 4H, 3′′-H and 5′′-H, J = 4.9 Hz); 6.62 (m, 2H, 4′′′-H and 6′′′-H, J = 5.0 Hz); 7.45 (m, 3H, 5-H, 6-H and 5′′′-H); 7.88 (t, 2H, 4-H and 7-H, J = 7.8 Hz); 8.00 (s, 1H, 3-H); 8.19 (m, 1H, 3′′′-H); 13C-NMR (CDCl3) δ: 36.9; 45.2 (2C); 53.0 (2C); 53.3; 107.1; 113.4; 123.0; 125.0; 126.0; 127.5; 129.1; 137.5; 139.1; 142.5; 143.6; 147.9; 159.4; 193.4. HRMS (EI) Calcd for (M+): C20H21N3OS: 351.14053; found: 351.14090.
1-(Benzo[b]thiophen-2-yl)-3-[4-(4-nitrophenyl)piperazin-1-yl]-1-propanone (7f).To a solution of 5b (90 mg, 0.49 mmol) and 1-(4-nitrophenyl)piperazine (152 mg, 0.73 mmol), in dichloromethane (20 mL) was added the inorganic supported reagent. The crude compound was purified by recrystallization (ethanol/hexane 4:1), to afford 131 mg (69%) of pure 7f; mp: 138–141 °C; IRνmax (KBr): 2949 (C-H arom.), 2833 (C-H aliph.), 1656 (C=O), 1601 (C=C), 1330 (NO2) cm−1; 1H-NMR (CDCl3) δ: 2.68 (t, 4H, 2′′-H and 6′′-H, J = 5.2 Hz), 2.96 (t, 2H, 3-H, J = 7.1 Hz), 3.26 (t, 2H, 2′-H, J = 7.1 Hz), 3.43 (t, 4H, 3′′-H and 5′′-H, J = 5.2 Hz), 6.82 (dd, 2H, 2′′′-H and 6′′′-H, Jm = 2.1 Hz, Jo = 8.4 Hz), 7.46 (m, 2H, 5-H and 6-H, J = 1.2 Hz, J = 3.4 Hz), 7.90 (t, 2H, 4-H and 7-H, J = 7.0 Hz), 8.00 (s, 1H, 3-H), 8.12 (dd, 2H, 3′′′-H and 5′′′-H, J = 2.0 Hz, J = 7.4 Hz); 13C-NMR (CDCl3) δ: 40.0, 50.1 (2C), 55.8 (2C), 56.2, 115.8 (2C), 126.2 (2C), 128.3, 129.1, 129.1, 130.7, 132.3, 141.6, 142.2, 145.6, 146.6, 157.9, 196.3; HRMS (EI) Calcd for C21H21NO3S (M+): 395.13037, found: 395.13237.
3.2.3. General Procedure for the Preparation of 1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-(4-arylpiperazin-1-yl)-1-propanol Derivatives 8a–f
1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-(4-phenyl-piperazin-1-yl)-1-propanol (8a). To a solution of 1-(4,7-dimethoxybenzo[b]thiophen-2-yl)-3-(4-phenylpiperazin-1-yl)-1-propanone (7a, 144 mg, 0.35 mmol) in methanol (20 mL), NaBH4 (57 mg, 1.5 mmol) was added and the mixture was vigorously stirred for 90 min at room temperature, after which the mixture was diluted with water (50 mL) and extracted with AcOEt (30 mL × 3). The combined organic layers were dried (Na2SO4), and removal of the solvent afforded 142 mg of a crude, which was further purified by recrystallization (EtOH/petroleum ether 2:1) to provide pure 8a (99 mg, 68.3% yield) as yellow pale crystals; mp 148–149 °C; IRνmax. (KBr): 3425(O-H), 3032 (C-H Ar), 2825 (C-H Aliph.), 1601 (C=C Ar) cm−1; 1H-NMR (CDCl3): δ 1.46–1.75 (bs,1H, OH), 1.96–2.2 (m, 2H, 2′-H), 2.65–2.83 (m, 6H, 3′-H and 2′′-H and 6′′-H), 3.23 (t, 4H, 3′′-H, and 5′′-H, J = 4.8 Hz), 3.90 (s, 3H, Ar-OMe,C-7), 3.94 (s, 3H, Ar-OMe, C-4), 5.29 (m, 1H, 1′-H), 6.63 (d, 1H, 5-H, J = 8.3 Hz), 6.65 (d, 1H, 6-H, J = 8.3 Hz), 6.82–7.11 (m, 4H, 2′′′-H, 4′′-H, 6′′-H, and OH), 7.26–7.33 (m, 3H, 3-H, 3′′′- and 5′′′-H); 13C-NMR (CDCl3): δ 33.3 (C-2), 49.2 (C-3′ and C-5′-Pip), 53.2 (C-2′ and C-6′-Pip), 55.9 (ArOMe, C-4′ or C-7′), 56.0 (ArOMe, C-7′ or C-4′), 56.4 (C-3), 72.3 (C-1), 103.9(C-5′BT), 104.6(C-6′BT), 115.9 (C-3′BT), 116.3 (C-2′′ and C-6′′-Ph), 120.1 (C-4′′-Ph), 129.2 (C-3′′ and C-5′′-Ph), 129.2 (C-7a), 132.0 (C-3a), 149.1 (C-4′BT), 149.5 (C-2′BT or C-7′BT), 149.7 (C-7′BT or C-2′BT), 151.1 (C-1′′-Ph); HRMS (EI) Calcd for C23H28N2O3S 412.18206 (M+), found: 412.18115.
3-[4-(2-Fluorophenyl)piperazin-1-yl]-1-(4,7-dimethoxybenzo[b]thiophen-2-yl)-1-propanol (8b). Prepared from 7b (175 mg, 0.41 mmol) and sodium borohydride (46 mg, 1.20 mmol). The crude was purified by preparative chromatography (EtOH) to afford 111 mg (63%) of the title compound; mp 128–129 °C; IRνmax (KBr): 3422 (O-H), 2953 (C-H Aliph.), 1511 (ArC=C), 1485 (Ar C=C) cm−1; 1H-NMR (CDCl3): δ 1.35–1.82 (bs, 1H, OH), 1.97–2.22 (m, 2H, 2′-H), 2.76 (m, 6H, 2′′-H, 6′′-H and 3′-H), 3.14 (m, 4H, 3′′-H and 5′′-H), 3.90 (s, 3H, Ar-OMe), 3.94 (s, 3H, Ar-OMe), 5.27 (m, 1H, 1′-H), 6.63 (d, 1H, 5-H, J = 8.6 Hz), 6.67 (d, 1H, 6-H, J = 8.6 Hz), 6.91–7.12 (m, 4H, 3′′′-H, 4′′′-H, 5′′′-H, and 6′′′-H, Ar-F), 7.31 (s, 1H, 3-H); 13C-NMR (CDCl3): δ 33.2 (C-2), 50.6 (C-3′ and C-5′-Pip), 53.3 (C-2′ and C-6′-Pip), 55.9 (ArOMe, C-4′ or C-7′), 56.0 (ArOMe, C-7′ or C-4′), 56.4 (C-3), 72.2 (C-1), 103.9 (C-5′BT), 104.6 (C-6′BT), 115.9 (C-3′BT), 116.1 (d, C-3′′, 2JC-F = 21.0 Hz ), 119.0 (d, C-6′′, 3′J = 3.0 Hz), 122.7 (d, C-1′′, 2′JC-F = 8.0 Hz), 124.6 (d, C-5′′, 4JC-F = 3.8 Hz), 129.1 (C-7a), 132.0 (C-3a), 139.8 (d, C-4′′, 3J = 8.6 Hz), 148.8 (C-4′BT), 149.1 (C-2′BT or C-7′BT), 149.6 (C-7′BT or C-2′BT), 155.7 (d, C-2′′, 1JC-F = 245.0 Hz); HRMS (EI) Calcd for C23H27FN2O3S 430.17264 (M+), found: 430.17010.
3-[4-(4-Fluorophenyl)piperazin-1-yl]-1-(4,7-dimethoxy-benzo[b]thiophen-2-yl)-1-propanol (8c). Prepared from 3-[4-(4fluorophenyl)piperazin-1-yl]-1-(4,7-dimethoxybenzo[b]thiophen-2-yl)-1-propanone (7c, 50.0 mg, 0.116 mmol) and sodium borohydride (15 mg, 0.39 mmol). Crude yield quantitative. The compound was purified by preparative chromatography on silica gel (EtOH) (40 mg, 79.7%); mp 55–56 °C; IR νmax (KBr): 3419 (O-H), 2919 (C-H Aliph.), 1510 (ArC=C), 1485 (Ar C=C) cm−1; 1H-NMR (CDCl3): δ 1.50–1.72 (bs,1H, OH), 1.97–2.22 (m, 2H, 2′-H), 2.75 (m, 6H, 2′′-H and 6′′-H and 3′-H), 3.16 (t, 4H, 3′′-H and 5′′-H; J = 4.8 Hz), 3.91 (s, 3H, Ar-OMe), 3.95 (s, 3H, Ar-OMe), 5.27 (m, 1H, 1′-H), 6.63 (d, 1H, 5-H, J = 8.6 Hz), 6.67 (d, 1H, 6-H, J = 8.6 Hz), 6.84–6.90 (m, 2H, 2′′′-H and 6′′′-H Ar-F), 6.94–7.05 (m, 2H, 3′′′-H and 5′′′-H, Ar-F), 7.30 (d, 1H, 3-H, J = 1.1 Hz); 13C-NMR (CDCl3): δ 33.6 (C-2), 50.7 (C-3′-Pip and C-5′-Pip), 53.6 (C-2′ and C-6′-Pip), 56.2 (C-3), 56.4 (ArOMe, C-4′ or C-7′), 56.7 (ArOMe, C-7′ or C-4′), 72.6 (C-1), 104.2 (C-5′BT), 104.9 (C-6′BT), 115.9 (C-3′-BT), 116.2 (d, C-2′′ and C-6′′, 2JC-F = 11.3 Hz), 118.5 (d, C-3′′ and C-5′′, 3JC-F = 7.6 Hz), 129.8 (C-7a), 132.4 (C-4a), 148.0 (d, C-1′′, 4JC-F = 1.5 Hz), 149.1 (C-4′BT), 149.4 (C-2′BT), 150.0 (C-7′BT), 157.8 (d, C-4′′, 1JC-F = 239 Hz); HRMS (EI) Calcd for C23H27FN2O3S 430.17264 (M+), found: 430.17051.
1-(4,7-Dimethoxy-benzo[b]thiophen-2-yl)-3-[4-(2-methoxyphenyl)piperazin-1-yl]-1-propanol (8d). Prepared from 1-(4,7-dimethoxybenzo[b]thiophen-2-yl)-3-[4-(2-methoxyphenyl)piperazin-1-yl]-1-propanone (7d, 80 mg, 0.18 mmol) and sodium borohydride (207 mg, 5.4 mmol), in methanol (20 mL). Crude yield quantitative. The crude was purified by preparative chromatography (EtOH) to afford 75 mg of the tile compound (93.4%) as pale yellow crystals; mp 55–56 °C; IRνmax. (KBr): 3415 (O-H), 3029 (C-H Ar), 2934 (C-H Aliph.), 1257 (C-O) cm−1; 1H-NMR (CDCl3): δ 1.52–1.70 (bs, 1H, OH), 1.95–2.2 (m, 2H, 2′-H), 2.65–2.92 (m, 6H, 3-H and 2′′-H and 6′′-H), 3.12 (m, 4H, 3′′-H, 5′′-H), 3.86 (s, 3H, 2′′′-Ar-OMe), 3.90 (s, 3H, Ar-OMe, C-7), 3.94 (s, 3H, Ar-OMe, C-4), 5.28 (m, 1H, 1′-H), 6.63 (d, 1H, 5-H, J = 8.3 Hz), 6.67 (d, 1H, 6-H, J = 8.3 Hz), 6.85–7.06 (m, 4H, 3′′′-H, 4′′′-H, 5′′′-H, and 6′′′-H Ar-OMe), 7.31 (d, 1H, 3-H J = 1.1 Hz); 13C-NMR (CDCl3): δ 33.1 (C-2), 50.6 (C-3′ and C-5′-Pip), 53.3 (C-2′ and C-6′-Pip), 55.3 (ArOMe), 55.8 (ArOMe, C-4′ or C-7′), 55.9 (ArOMe, C-7′ or C-4′), 56.5 (C-3), 72.7 (C-1), 103.7 (C-5′BT), 104.4 (C-6′BT), 111.0 (C-3′′), 115.8 (C-3′BT), 118.3 (C-6′′), 121.0 (C-5′′), 123.2 (C-4′′), 129.4 (C-7a), 132.0 (C-3a), 140.9 (C-1′′), 148.7 (C-2′BT), 149.0 (C-4′BT), 149.8 (C-2′′), 152.2 (C-7′BT); HRMS (EI) Calcd for C24H30N2O4S 442.19263 (M+), found: 442.19009.
1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-(4-pyridin -2-yl)-piperazin-1-yl)-1-propanol (8e). Prepared from 1-(4,7-dimethoxybenzo[b]thiophen-2-yl)-3-(4-pyridin-2-yl)-piperazin-1-yl)-1-propanone (7e, 117 mg, 0.28 mmol) and sodium borohydride (40 mg, 1.05 mmol). Quantitative yield. The crude was purified by preparative chromatography (EtOH) to afford 90 mg (76.6%) of pure 8e; mp 157–158 °C; IRνmax. (KBr): 3422 (O-H), 2832 (C-H Aliph.), 1596 (ArC=N), 1485 (Ar C=C) cm−1; 1H-NMR (CDCl3): 1.49–1.71 (bs, 1H, OH), 1.92–2.25 (m, 2H, 2′-H), 2.6–2.8 (m, 6H, 2′′-H and 6′′-H, 3′-H), 3.47 (m, 4H, 3′′-H and 5′′-H, ), 3.90 (s, 3H, Ar-OMe), 3.95 (s, 3H, Ar-OMe), 5.28 (m, 1H, 1′-H), 6.63–6.67 (m, 4H, 5-H, 6-H and 3′′′-H, 5′′′-H), 7.31 (s, 1H, 3-H), 7.49 (td, 1H, 4′′′-H, Jo = 6.7 Hz, Jm = 2.0 Hz), 8.2 (dd, 1H, 6′′′-H, Jo = 5.4 Hz, Jm = 2.0 Hz); 13C-NMR (CDCl3): δ 33.2(C-2), 45.2 (C-3′ and C-5′-Pip), 53.0 (C-2′ and C-6′-Pip), 55.8 (ArOMe, C-4′ or C-7′), 56.0 (ArOMe, C-7′ or C-4′), 56.5 (C-3), 72.2 (C-1), 103.8 (C-5′BT), 104.5 (C-6′BT), 107.1 (C-3′′),113.3 (C-5′′),115.9 (C-3′BT), 129.3 (C-7a), 132.0 (C-3a), 137.6 (C-4′′), 148.0 (C-6′′), 148.8 (C-2′BT), 149.0 (C-4′BT), 149.7 (C-7′BT), 159.3 (C-2′′); HRMS (EI) Calcd for C22H27N3O3S 413.17731 (M+), found: 413.17593.
1-(4,7-Dimethoxybenzo[b]thiophen-2-yl)-3-[4-(4-nitrophenyl)piperazin-1-yl]-1-propanol (8f). Prepared from 1-(4,7-dimethoxybenzo[b]thiophen-2-yl)-3-[4-(4-nitrophenyl)piperazin-1-yl]-1-propanone (7f, 58 mg, 0.127 mmol) and sodium borohydride (19 mg, 0.5 mmol). Quantitative yield. Purified by preparative chromatography (EtOH) to afford 42 mg (72%) of pure 8f; mp 173–174 °C; IRνmax (KBr): 3420 (O-H), 3030 (C-H Ar), 2918 (C-H Aliph.), 1596 (NO2), 1327 (NO2), 1487 (C=C Ar) cm−1;1H-NMR (CDCl3): δ 1.52–1.72 (bs, 1H, OH), 1.95–2.22 (m, 2H, 2′-H), 2.72 (m, 6H, 2′′-H, 6′′′-H and 3-H), 3.47 (t, 4H, 3′′-H and 5′′-H; J = 5.0 Hz), 3.93 (s, 3H, Ar-OMe), 3.97 (s, 3H, Ar-OMe), 5.26 (m, 1H, 1′-H), 6.63 (d, 1H, 5-H, J = 8.8 Hz), 6.66 (d, 1H, 6-H, J = 8.8 Hz), 6.84 (d, 2H, 2′′′-H Ar-NO2 and 6′′′-H Ar-NO2, J = 9.4 Hz), 7.33 (s, 1H, 3-H), 8.23 (d, 2H, 3′′′-H Ar-NO2 and 5′′′-H, Ar-NO2, J = 9.4 Hz); 13C-NMR (CDCl3): δ 33.7 (C-2), 47.4 (C-3′, C-5′-Pip), 53.0 (C-2′,C-6′-Pip), 56.2 (ArOMe, C-4′ or C-7′), 56.4 (ArOMe, C-7′ or C-4′), 56.6 (C-3), 72.4 (C-1), 104.4 (C-5′BT), 105.0 (C-6′BT), 113.3 (C-2′′ and C-6′′ Ar-NO2), 116.5 (C-3′BT), 126.3 (C-3′′ and C-5′′ Ar-NO2), 129.8 (C-7a), 132.4 (C-3a), 139.2 (C-4′′-Ar-NO2), 149.1 (C-4′BT), 149.5 (C-2′BT or C-7′BT), 149.6 (C-7′BT or C-2′BT), 155.1 (C-1′′-Ar-NO2); HRMS (EI) Calcd for C23H27N3O5S 457.16714 (M+), found: 457.16591.
3.3. Biological Methods
Radioligand Binding Assays
For binding assays, male Sprague-Dawley rats (Rattus norvegicus albinus) weighing about 200–250 g were killed by decapitation, and their brains were rapidly removed and dissected (cerebral cortex for 5-HT1AR). Binding assays were performed by a modification of the procedure previously described by Srinivas et al. [27] The cerebral cortex was homogenized in about 10 volumes of ice-cold 50 mM Tris-HCl buffer (pH 7.4) containing 0.32 M sucrose using a glass-teflon homogenizer and an Ultra Turrax T8 IKA Labortechnik (5 × 30 s). Then the homogenate was centrifuged at 2.264 g for 10 min at 4 °C (Heraeus Sepatech Suprafuge 22 ultracentrifuge). The pellet was discarded and the supernatant was centrifuged at 56.599 g for 30 min at 4 °C. The pellet was resuspended in 20 volumes of ice-cold 50 mM Tris-HCl buffer (pH 7.4) and centrifugated again at 56.599 g for 30 min at the same temperature as before. The pellet was then washed once by resuspension in fresh buffer and centrifuged as before. Finally the pellet was splitted and frozen until the day of experiment. The final pellet was resuspended in ice-cold 50 mM Tris-HCl buffer (pH 7.4). Fractions of 50 μL of the final membrane suspension (about 127 μg of protein) were incubated at 37 °C for 30 min with 5 nM [3H]-8-OH-DPAT (170 Ci/mmol), in the presence or absence of the competing drug, in a final volume of 200 μL of assay. Nonspecific binding was determined with 10 μM 8-OH-DPAT. All incubations were terminated by the addition of 2 mL of ice-cold Tris-HCl buffer (pH 7.4). The reaction mixture was rapidly filtrated through GF/C filters (pre-soaked in buffer containing 0.3% polyethyleneimine) under vacuum and washed twice with 2 mL of ice-cold buffer. Filters were dried and transferred to scintillation vials containing 4 mL of scintillation cocktail (PPO 4 g/L; POPOP 0.1 g/L in toluene) and allowed to equilibrate overnight before counting in a liquid scintillation counter at 28% efficiency.
3.4. Molecular Modeling
3.4.1. 5.HT1A Receptor Modeling
The aminoacid sequence for the human 5-HT1AR was obtained from SWISS-PROT [28] (access number: P08908). Moreover, as a template for comparative modeling was used three-dimensional crystal structure of bovine rhodopsin obtained from the Protein Data Bank (www.rcsb.org/pdb) (PDB code: 2R4R) [29]. The initial alignment between 2R4R and the sequence of 5-HT1A were made with the program Clustal W [30].
The models were generated using the program MODELLER [31]. MODELLER produces comparative models that meet space restrictions with simultaneous optimization of energy CHARMM [32]. The shape of space constraints was obtained by statistical analysis of the relationship between pairs of homologous structures from a database of sequence alignments of 416 known 3D structures of proteins in 105 families. The comparative models were verified by validation programs PROCHECK [33,34]. The ligands were optimized to get its lowest energy conformation with the program SPARTAN 2. In all cases the ligands were protonated on the aliphatic nitrogen atom in the piperazine ring.
3.4.2. Molecular Docking
The grids of the maps that represent the 5-HT1AR in the docking process were calculated with AutoGrid (included in the program AutoDock) [35]. The grids were chosen large enough to include not only the active site but significant portions of the surrounding area. The points of the grids were therefore 60 × 60 × 60 Å for the 5-HT1AR with a grid spacing of 0.375 Å (about one quarter of the length of a carbon-carbon bond) and as is known the importance of D116, the cubic grids were centered on the ligand binding site. The automated docking studies were carried out using the program AutoDock 3.0.5. Lamarckian genetic algorithms were applied to model the interaction between the 5-HT1AR and the residue D116. For the local search were used pseudo-Solis and Wets algorithms. For all docking parameters, were used standard values as described above, except the number of independent docking runs for each docking simulation. To find aspects of computing time and data size on the one hand, and convergence criteria and statistical significance on the other, 200 independent docking runs were performed for each case. The cluster analysis was performed on the docking results using a tolerance for half-square deviation of 0.5 Å. All calculations were performed in a PC Cluster Intel Pentium 4 3.0 GHz operating system that runs on Red Hat Enterprise Linux version 4.
3.4.3. Binding Model Analysis
To select the most likely conformations of the complexes given by AutoDock were used quantitative and qualitative considerations. First, was elected the conformation with the lowest docking energy as a starting point. Then the chosen conformation was analyzed qualitatively based on the orientation/location of the serotonin agonist in relation to the receptor. In the event that the orientation/location of the chosen conformation did not agree with the corresponding model of agonist binding of 5-HT1AR, the docked complex was discarded and analyzed the next conformation with the lowest final docking energy. This procedure was repeated until they find a shape in harmony with the appropriate mode of binding of 5-HT.
4. Conclusions
A successful strategy for the synthesis of novel functionalized 3-[(4-aryl)piperazin-1-yl]arylpropan-1-one 6a–f and 7a–f along with the reduced derivative series 8a–f is reported. The series 6a–f and 7a–f were obtained using microwave Michael addition of commercial arylpiperazines on the corresponding benzo[b]thiophenenones 5a,b. The series 6a–f displayed lower affinity values for the 5-HT1A receptor, in comparison with their corresponding reduced derivatives 8a–f. On the other hand, the 7a–f family exhibited better activities compared to compounds 6 and 8a–f.
As it was expected, the 2-methoxy derivatives 6d, 8d, 7d ranked among the four best results obtained, along with compound 7e. On the contrary, the para-substitution exemplified by the fluoro- and nitro-6c,f, 7f, and 8f apparently cause repulsive interactions in the binding site according to our docking results, confirming the detrimental effect expected for para-substituents on the phenyl ring of the arylpiperazine moiety. Regards to the 4,7-dimethoxy substitution pattern on the benzo[b]thiophene ring, we concluded that this disubstitution might be relevant in order to explain the poor activity displayed by these compounds. The more promising compound 7e, having a Ki value of 2.30 μM, will help us in the design of compounds with additional modifications of future series in the exploration of potentially 5-HT1A activity.
Acknowledgments
Authors wish to thank to Fondo Nacional de Ciencia y Tecnología FONDECYT Research Projects Nº 1050289 and 1090169.
Footnotes
Sample Availability: Samples of the compounds 6-b, 8-a, 8-d, 8-e are available from the authors.
References and Notes
- 1.Caliendo G., Santagada V., Perisutti E., Fiorino F. Derivatives as 5HT1A receptor ligands—Past and present. Curr. Med. Chem. 2005;12:1721–1735. doi: 10.2174/0929867054367220. [DOI] [PubMed] [Google Scholar]
- 2.Elhwuegi A.S. Progress in neuro-psychopharmacology and biological psychiatry. Prog. Neuro-Psychoph. 2004;28:435–451. doi: 10.1016/j.pnpbp.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Czopek A., Byrtus H., Kołaczkowski M., Pawłowski M., Dybała M., Nowak G., Tatarczynska E., Wesołowska A., Chojnacka-Wójcik E. Synthesis and pharmacological evaluation of new 5-(cyclo)alkyl-5-phenyl-and 5-spiroimidazolidine-2,4-dione derivatives. Novel 5-HT1A receptor agonist with potential antidepressant and anxiolytic activity. Eur. J. Med. Chem. 2010;45:1295–1303. doi: 10.1016/j.ejmech.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 4.Herold F., Chodkowski A., Izbicki Ł., Król M., Kleps J., Tur1o J., Nowak G., Stachowicz K., Dybała M., Siwek A. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity, Part 1. Eur. J. Med. Chem. 2009;44:1710–1717. doi: 10.1016/j.ejmech.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Savitz J., Lucki I., Drevets W.C. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akimova E., Lanzenberger R., Kasper S. The serotonin-1A receptor in anxiety disorders. Biol. Psychiat. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Dounay A.B., Barta N.S., Bikker J.A., Borosky S.A., Campbell B.M., Crawford T., Denny L., Evans L.M., Gray D.L., Lee P., et al. Synthesis and pharmacological evaluation of aminopyrimidine series of 5-HT1A partial agonists. Bioorg. Med. Chem. Lett. 2009;19:1159–1163. doi: 10.1016/j.bmcl.2008.12.087. [DOI] [PubMed] [Google Scholar]
- 8.Pessoa-Mahana H., Araya-Maturana R., Saitz B.C., David Pessoa-Mahana C. A synthetic overview of antidepressants with 5-HT1A binding affinities. Mini. Rev. Med. Chem. 2003;3:77–93. doi: 10.2174/1389557033405278. [DOI] [PubMed] [Google Scholar]
- 9.Bromidge S.M., Bertani B., Borriello M., Bozzoli A., Faedo S., Gianotti M., Gordon L.J., Hill M., Zucchelli V., Watson J.M., et al. 8-[2-(4-Aryl-1-piperazinyl)ethyl]-2H-1,4-benzoxazin-3(4H)-ones: Dual-acting 5-HT1A/B/D receptor antagonists and serotonine re-uptake inhibitors. Part II. Bioorg. Med. Chem. Lett. 2009;19:2338–2342. doi: 10.1016/j.bmcl.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 10.Butini S., Campiani G., Franceschini S., Trotta F., Kumar V., Guarino E., Borrelli G., Fiorini I., Novellino E., Fattorusso C., et al. Discovery of bishomo(hetero)arylpiperazines as novel multifunctional ligands targeting dopamine D3 and serotonin 5-HT1A and 5-HT2A receptors. J. Med. Chem. 2010;53:4803–4807. doi: 10.1021/jm100294b. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Esparza J., Oficialdegui A.M., Pérez-Silanes S., Heras B., Orús L., Palop J.A., Lasheras B., Roca J., Mourelle M., Bosch A., et al. New 1-aryl-3-(4-arylpiperazin-1-yl)propane derivatives with dual action at 5-HT1A serotonin receptors and serotonin transporter as a new class of antidepressants. J. Med. Chem. 2001;44:418–428. doi: 10.1021/jm001059j. [DOI] [PubMed] [Google Scholar]
- 12.Orús L., Pérez-Silanes S., Oficialdegui A.M., Martínez-Esparza J., Del Castillo J.C., Mourelle M., Langer T., Guccione S., Donzella G., Krovat E.M., et al. Synthesis and molecular modeling of new 1-aryl-3-[4-(arylpiperazin-1-yl]-1-propane derivatives with high affinity at the serotonin transporter and at 5-HT1A receptors. J. Med. Chem. 2002;45:4128–4139. doi: 10.1021/jm0111200. [DOI] [PubMed] [Google Scholar]
- 13.Berrade L., Aisa B., Ramirez M.J., Galiano S., Guccione S., Moltzau L.R., Levy F.O., Nicoletti F., Battaglia G., Molinaro G., et al. Novel benzo[b]thiophene derivatives as new potential antidepressants with rapid onset of action. J. Med. Chem. 2011;54:3086–3090. doi: 10.1021/jm2000773. [DOI] [PubMed] [Google Scholar]
- 14.Pessoa-Mahana H., González C.M., González S.M., Pessoa-Mahana C.D., Araya-Maturana R., Ron H.N., Saitz B.C. Solvent-free microwave-promoted Michael addition of aza-nucleophiles to benzo[b]thiophen-2-yl-2-propenone. ARKIVOC. 2009;xi:316–325. [Google Scholar]
- 15.Pessoa-Mahana H., Kosche C.J., Ron H.N., Recabarren-Gajardo G., Saitz B.C., Araya-Maturana R., Pessoa-Mahana C.D. Solvent-free microwave synthesis of 3-[4-benzo[b]thiophene-2-carbonyl)-1-piperazynil-1-benzo[b]thiophen-2-yl-1-propanones. New hetero bis-ligands with potential 5-HT1A serotonergic activity. Heterocycles. 2008;75:1913–1929. doi: 10.3987/COM-08-11326. [DOI] [Google Scholar]
- 16.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H., Shindyalov I.N., Bourne P.E. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bissantz C., Kuhn B., Stahl M. A medicinal chemist’s guide to molecular interactions. J. Med. Chem. 2010;53:5061–5084. doi: 10.1021/jm100112j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zlatovic M.V., Sukalovic V.V., Schneider C., Roglic G.M. Interaction of arylpiperazine ligands with the hydrophobic part of the 5-HT1A receptor binding Site. Bioorg. Med. Chem. 2006;14:2994–3001. doi: 10.1016/j.bmc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Santana L., Uriarte E., Fall Y., Teijeira M., Teran C., Garcia-Martinez E., Tolf B.R. Synthesis and structure–activity relationships of new arylpiperazines: para substitution with electron-withdrawing groups decrease binding to 5-HT1A and D2A receptors. Eur. J. Med. Chem. 2002;37:503–510. doi: 10.1016/S0223-5234(02)01357-0. [DOI] [PubMed] [Google Scholar]
- 20.Martinez J., Perez S., Oficialdegui A.M., Heras B., Orus L., Villanueva H., Palop J.A., Roca J., Mourelle M., Bosch A., et al. New 3-[4-(aryl)piperazin-1-yl]-1-(benzo[b]thiophen-3-yl)propane derivatives with dual action at 5-HT1A serotonin receptors and serotonin transporter as a new class of antidepressants. Eur. J. Med. Chem. 2001;36:55–61. doi: 10.1016/S0223-5234(00)01198-3. [DOI] [PubMed] [Google Scholar]
- 21.Glennon R.A., Naiman N.A., Pierson M.E., Titeler M., Lyon R.A., Weisberg E. NAN-190: An arylpiperazine analog that antagonizes the stimulus effects of the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) Eur. J. Pharmacol. Mol. Pharmacol. 1988;154:339–341. doi: 10.1016/0014-2999(88)90212-9. [DOI] [PubMed] [Google Scholar]
- 22.Perrone R., Berardi F., Colabufo N.A., Tortorella V., Fiorentini F., Olgiati V., Vanotti E., Govoni S. Mixed 5-HT1A/D-2 activity of a new model of arylpiperazines: 1-Aryl-4-[3-(1,2-dihydronaphthalen-4-yl)-n-propyl]piperazines. 1. Synthesis and structure-activity relationship. J. Med. Chem. 1994;37:99–104. doi: 10.1021/jm00027a012. [DOI] [PubMed] [Google Scholar]
- 23.Mokrosz J.L., Paluchowska M.H., Chojnacka-Wojcik E., Filip M., Charakchieva-Minol S., Deren-Wesolek A., Mokrosz M.J. Structure-activity relationship studies of central nervous system agents. 13. 4-[3-(Benzotriazol1-1-y1)propyll-1-(2 methoxyphenyl)piperazine,a new putative 5-HT1A receptor antagonist, and its analogs. J. Med. Chem. 1994;37:2754–2760. doi: 10.1021/jm00043a014. [DOI] [PubMed] [Google Scholar]
- 24.Oficialdegui A.M., Martinez J., Perez S., Heras B., Irurzun M., Palop J.A., Tordera R., Lasheras B., del Rio J., Monge A. Design, synthesis and biological evaluation of new 3-[(4-aryl)piperazin-1-yl]-1-arylpropane derivatives as potential antidepressants with a dual mode of action: Serotonin reuptake inhibition and 5-HT1A receptor antagonism. Farmaco. 2000;55:345–353. doi: 10.1016/S0014-827X(00)00050-1. [DOI] [PubMed] [Google Scholar]
- 25.Orús L., Sainz Y., Perez S., Oficialdegui A.M., Martinez J., Lasheras B., del Río J., Monge A. New 3-[4-(aryl)piperazin-1-yl]-1-(benzo[b]thiophen-2-yl)propane derivatives with dual action at 5-HT1A serotonin receptors and serotonin transporter as a new class of antidepressants. Pharmazie. 2002;57:355–357. [PubMed] [Google Scholar]
- 26.Leopoldo M., Lacivita E., Colabufo N.A., Niso M., Berardi F., Perrone R. Bivalent ligand approach on 4-[2-(3-Methoxyphenyl)ethyl]-1-(2-methoxyphenyl) piperazine: Synthesis and binding affinities for 5-HT7 and 5-HT1A receptors. Med. Chem. Lett. 2007;15:5316–5321. doi: 10.1016/j.bmc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Srinivas B.N., Subhash M.N., Vinod K.Y. Cortical 5-HT(1A) receptor downregulation by antidepressants in rat brain. Neurochem. Int. 2001;38:573–579. doi: 10.1016/S0197-0186(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 28.Bairoch A.A.R., Wu C.H., Barker W.C., Boeckmann B., Ferro S., Gasteiger E., Huang H., Lopez R., Magrane M., Martin M.J., et al. The Universal Protein Resource (UniProt) Nucleic Acids Res. 2005;33:154–159. doi: 10.1093/nar/gki070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palczewski K., Kumasaka T., Hori T., Behnke C.A., Motoshima H., Fox B., Le Trong I., Teller D.C., Okada T., Stenkamp R.E., et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 30.Shpaer E.G., Robinson M., Yee D., Candlin J.D., Mines R., Hunkapiller T. Sensitivity and selectivity in protein similarity searches. Genomics. 1996;38:179–191. doi: 10.1006/geno.1996.0614. [DOI] [PubMed] [Google Scholar]
- 31.Sali A., Blundell T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 32.Brooks B.R., Bruccoleri R.E., Olafson D.J., States D.J., Swaminathan S., Karplus M. CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J. Comp. Chem. 1983;4:187–217. [Google Scholar]
- 33.Laskowski R.A., MacArthur M.W., Moss D.S., Thornton J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993;26:283–291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- 34.Morris A.L., MacArthur M.W., Hutchinson E.G., Thornton J.M. Stereochemical quality of protein structure coordinates. Proteins. 1992;12:345–364. doi: 10.1002/prot.340120407. [DOI] [PubMed] [Google Scholar]
- 35.Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., Olson A.J. Automated docking using a lamarckian genetic algorithm and and empirical binding free energy function. J. Comp. Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]