Abstract
Okara is a byproduct generated during tofu or soymilk production processes. Crude polysaccharide (yield 56.8%) was isolated by removing fat, protein and low molecular weight carbohydrates from initial okara. Crude okara polysaccharide was further divided into four soluble fractions and an insoluble residue fraction by extracting with 0.05 M EDTA + NH4 oxalate, 0.05 M NaOH, 1 M NaOH and 4 M NaOH, with yields of 7.7%, 3.6%, 20.7%, 16.0% and 27.9%, respectively. Arabinose, galactose, galacturonic acid, xylose and glucose (only for the insoluble fraction) were the major constituent sugars. The primary sugar residues of okara polysaccharides were 1,4-linked β-galactopyranose, 1,5- and 1,3-linked α-arabinofuranose, 1,5-linked α-xylofuranose, 1,2-linked, 1,2,4-linked and terminal α-rhamnopyranose (or fucopyranose), and 1,4-linked β-glucopyranose (only for the insoluble fraction), indicating okara polysaccharides might contain galactan, arabinan, arabinogalactan, xylogalacturonan, rhamnogalacturonan, xylan, xyloglucan and cellulose.
Keywords: okara, polysaccharide, structure, isolation
1. Introduction
Okara is the residue obtained from ground soybean after removing the water-extractable fraction used to produce tofu or soymilk. About 1.2 kg of fresh okara is produced from 1 kg of soybean processed for manufacturing tofu [1]. Huge quantities of okara are produced worldwide. In Japan about 800,000 tons, in Korea approximately 310,000 tons, and in China about 2,800,000 tons of okara are produced from the tofu industry every year [2,3]. These huge quantities of okara produced annually pose a significant disposal problem. Okara is sometimes used as animal feed but most is dumped and burned as waste.
Polysaccharides from dietary fibre are important among functional compounds due to the well-known role that dietary fibre plays in many physiological processes and in the prevention of different diseases [4]. In recent years, there has been a trend to find new sources of dietary fibre that can be used as ingredients in the food industry. Dry okara contains about 50% dietary fibre and 25% protein, so it is an excellent dietary fibre source and as such could be added to different foods [5,6,7]. Many studies have been undertaken on chemical components, nutrition and utilization of okara [8]. However, few reports have been found in the literature on the structural characterisation of polysaccharides in okara [9,10,11]. Knowledge of the structure may promote to explain the relationship of function and structure of okara polysaccharides and allow the use of okara in different functional foods. The aim of this work was to investigate the structural characteristics of polysaccharides from okara through sequential extraction with different extractive solutions.
2. Results and Discussion
2.1. Isolation and Fractionation of Okara Polysaccharides
Generally, okara contains about 50% dietary fibre, 25% protein, 10% lipid, 4% low molecular weight carbohydrates and 4% ash [1,5,6,7]. Other soy components that are also likely present in okara include isoflavones, lignans, phytosterols, coumestans, saponins and phytates. The chemical composition of okara will depend on the amount of water phase extracted from the ground soybean and whether further water was added to extract residual extractable components. It also depends on the cultivar of soybean and the production methods [8]. The okara in our study had 46.3% dietary fibre, 17.8% protein, 5.9% lipid, 3.9% ash, 2.6% reducing sugar, 0.22% flavone and 6.7% moisture.
After removing the fat, protein and low molecular weight carbohydrates from okara, the crude okara polysaccharide (COP) was obtained which accounted for 56.8% of the initial okara. COP was sequentially treated with 0.05 M ethylenediaminetetraacetic acid disodium (EDTA) + NH4 oxalate, 0.05 M NaOH, 1 M NaOH and 4 M NaOH to provide four soluble fractions (OP1~OP4) and an insoluble residue (OP5). The yields of OP1~OP5 were 7.7%, 3.6%, 20.7%, 16.0% and 27.9% of COP, respectively. The isolation and fractionation of okara polysaccharides have been described by Yamaguchi et al., Huisman et al., and Mateos-Aparicio et al. [9,10,11,12]. The latter obtained 77.2 g cell wall material (CWM) from 100 g okara by delivering fat, protein and low molecular weight carbohydrates. They sequentially extracted CWM with 0.05 M cyclohexanediamine tetraacetic acid (CDTA) + NH4 oxalate, 0.05 M NaOH, 1 M KOH, 4 M KOH and 0.3% NaClO2/acetic acid to leave a cellulose-rich residue, and the yields of the six fractions were 16.7%, 2.9%, 20.0%, 13.6%, 19.7% and 21.9% of CWM, respectively [11]. Their results showed that chelating agent (CDTA) and strong alkali extracted the most okara polysaccharide. The study of Huisman et al. revealed that chelating agent soluble solids was the major extract, yielding 38% of the polysaccharides present in the water unextractable solids from soybean meal [10]. Our study showed strong alkali soluble solids was the major extract, and the yield of chelating agent (EDTA) soluble solids just was 7.7% of okara. The difference was partly due to the okara material used. In our study, the okara was obtained by the Chinese method, and in the study of Mateos-Aparicio et al., okara was obtained by the Japanese method, i.e., the rehydrated soybean was cooked before grinding and filtering. In the study of Huisman et al., soybean meal, the residue of oil extraction, was used as the raw material. Another reason might be the difference of chelating agent, CDTA and EDTA. Therefore, the isolation and fractionation of polysaccharides were influenced by the okara materials and extraction reagents.
2.2. Monosaccharide Compositions of Okara Polysaccharides
The monosaccharide compositions of okara, crude okara polysaccharide and five polysaccharide fractions are shown in Table 1. The main neutral sugars of okara polysaccharides are arabinose, galactose and xylose, and the minor sugars are rhamnose, glucose, fucose and mannose. Okara polysaccharides also contain a considerable amount of galacturonic acid. Mateos-Aparicio et al. and Tsubaki et al. also reported galactose, arabinose and galacturonic acid as the major sugars [11,13]. However, in the study of Mateos-Aparicio et al., a maximum amount of arabinose was present in the cellulose-rich residue (equivalent to OP5), and more xylose existed in the 4 M NaOH fraction [11].
Table 1.
Monosaccharide compositions of okara polysaccharides.
| Sample | Monosaccharide compositions (mol%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ara | Rha | Fuc | Xyl | Man | Gal | Glc | GalA | |
| Okara | 23.6 | 5.1 | 3.3 | 12.8 | 3.1 | 26.1 | 5.8 | 20.3 |
| COP | 27.4 | 4.1 | 2.8 | 13.9 | 2.2 | 29.0 | 2.4 | 18.1 |
| OP1 | 24.2 | 3.9 | 1.9 | 5.7 | 2.8 | 24.4 | 1.6 | 35.4 |
| OP2 | 28.7 | 6.4 | 3.6 | 10.2 | nd | 14.0 | 1.4 | 35.7 |
| OP3 | 26.7 | 4.5 | 3.3 | 11.8 | 1.0 | 31.2 | 3.1 | 18.4 |
| OP4 | 22.8 | 2.7 | 2.1 | 12.7 | 4.4 | 41.8 | 3.4 | 10.1 |
| OP5 | 15.5 | 5.4 | 1.3 | 17.1 | 1.0 | 14.6 | 26.8 | 18.2 |
COP: crude okara polysaccharide; OP1: fraction extracted with EDTA-ammonium oxalate; OP2: fraction extracted with 0.05 M NaOH; OP3: fraction extracted with 1 M NaOH; OP4: fraction extracted with 4 M NaOH; OP5: residue left after sequential extraction; nd: not detected.
The high values of arabinose (22~28%), galactose (14~41%), galacturonic acid (10~35%) and xylose (5~17%) are probably due to pectins and hemicelluloses. Some soybean cell wall polysaccharides have already been partly elucidated during the past years, and include arabinans, galactans, arabinogalactans, xylogalacturonans, and rhamnogalacturonans, etc. [10]. Because okara mainly comes from the water unextractable cell wall material of soybean, it might contain similar polysaccharides as soybean to some extent. Yamaguchi et al. has reported a pectic polysaccharide from okara, which comprised regions of galacturonan and rhamnogalacturonan carrying side chains composed mainly of homogeneous arabinan and galactan. The galacturonan regions were distributed at both the reducing and nonreducing ends of the polysaccharide [9].
In terms of five polysaccharide fractions, their monosaccharide compositions have some differences. Fractions OP1 and OP2 contain more galacturonic acid (35%) in comparison with other fractions, indicating the two fractions include a large number of pectic polymers that was easily extracted by EDTA/ammonium oxalate through the formation of a chelated complex and by dilute alkali. Fractions OP3 and OP4 contain more galactose than other fractions, indicating galactans and arabinogalactans were easily extracted by strong alkali. The amounts of glucose and xylose in fraction OP5 were the highest in five the fractions, suggesting the insoluble fibre of okara might be composed of celluloses, xylans and xyloglucans. Arabinose is one of the main sugars and the amount is similar in fractions OP1~OP4, thus arabinose-containing polysaccharides are in a great quantity and almost similar in the four soluble fractions. Xylose content gradually increases from OP1 to OP5. This might be due to the xylogalacturonan regions described previously in the hull of the pea by Le Goff et al. [14], in apple by Schols et al. [15], and in soybean meal by Huisman et al. [10].
2.3. Methylation Analysis of Okara Polysaccharides
Methylation results (Table 2) showed that the main structural features for all okara polysaccharide fractions are →4)Hexp(1→ and →5)Penf (1→, suggesting galactose and glucose (for OP5) residues are mainly 1,4-linked pyranoses, and arabinose and xylose residues are primary 1,5-linked furanoses based on their sugar compositions.
Table 2.
Methylation results of okara polysaccharide fractions.
| Structural feature | COP | OP1 | OP2 | OP3 | OP4 | OP5 |
|---|---|---|---|---|---|---|
| Penf(1→ | 2.46 | 7.57 | 3.71 | 9.41 | 1.46 | 0.39 |
| Penp(1→ | 1.03 | 2.11 | 0.84 | 1.68 | 1.00 | nd |
| dHexp (1→ | 1.43 | 3.96 | 2.21 | 1.84 | 2.45 | 0.21 |
| →5)Penf (1→ | 7.37 | 12.76 | 17.56 | 11.42 | 7.91 | 4.55 |
| →5)Penf (1→ | 5.27 | 2.78 | 3.07 | 5.29 | 6.20 | 13.65 |
| Hexp(1→ | 2.39 | 4.22 | 4.56 | 2.77 | 3.97 | 0.40 |
| →3)Penf(1→ | 1.79 | 4.84 | 3.97 | 2.37 | 2.35 | 0.22 |
| →2)dHexp(1→ | 1.01 | 1.31 | 1.85 | nd | 0.82 | nd |
| →2,4)Hexp(1→ | nd | nd | nd | nd | nd | 0.43 |
| →2,4)dHexp(1→ | 1.49 | 2.21 | 3.33 | 1.15 | 0.80 | 0.37 |
| →4)Hexp(1→ | 40.47 | 30.94 | 48.67 | 53.07 | 51.87 | 4.31 |
| →4)Hexp(1→ | 27.61 | 2.49 | 1.03 | 1.16 | 7.22 | 69.17 |
| →2)Hexp(1→ | nd | nd | nd | nd | 1.07 | nd |
| →6)Hexp(1→ | 0.64 | nd | 1.95 | 0.88 | 0.82 | nd |
| →6)Hexp(1→ | nd | nd | 0.54 | nd | nd | nd |
| →3,4)Hexp(1→ | nd | nd | 0.67 | 0.28 | nd | 0.40 |
| →2,6)Hexp(1→ | 0.48 | 0.60 | 0.50 | 0.20 | nd | 0.41 |
| →4,6)Hexp(1→ | nd | nd | nd | 0.10 | 1.13 | nd |
| →4,6)Hexp(1→ | 2.12 | 0.92 | nd | 0.68 | 5.16 | 0.86 |
| →4,6)Hexp(1→ | 0.59 | 0.72 | 1.18 | nd | 0.40 | 0.95 |
Pen: pentose; Hex: hexose; dHex: deoxy hexose;. f: furanose; p: pyranose; nd: not detected.
As far as OP1 is concerned, the linkage types for pentose reidues are mainly →5)Penf(1→ (12.76%), Penf(1→ (7.57%) and →3)Penf(1→ (4.84%), indicating arabinose and xylose residues are mainly terminal, 1,5- and 1,3-linked. The primary linkage type of deoxyhexose is dHexp (1→, suggesting most of rhamnose (or fucose) residues locate at the nonreducing terminals of polysaccharides. The predominant linkage type for galactose residues is 1,4-linked (30.94%). According to the data of methylation analysis and sugar composition, OP1 may comprise an arabinan, containing 1,5- and 1,3-linked arabinofuranose residues; a galactan, containing 1,4-linked galactopyranose residues; a xylogalacturonan, containing 1,5-linked xylofuranose residues; and a rhamnogalacturonan, containing 1,2-linked with highly branching at C-4 rhamnopyranose residues.
Compared with the methylation result of OP1, the main differences of OP2, OP3 and OP4 are mole ratios of Penf(1→, →5)Penf (1→ and →4)Hexp(1→. OP2 has more →5)Araf (1→ and fewer Araf (1→, indicating the branching degree of arabinan is lower than that of OP1. OP3 and OP4 have more →5)Xylf (1→ and fewer galacturonic acid in comparison with OP1, suggesting the two fractions might contain xylans. The primary structural feature of OP5 is →4)Glcp(1→, indicating cellulose is the main component of OP5. Furthermore, OP5 could also contain a few of xylans and xyloglucans due to a considerable amount of →5)Xylf (1→.
2.4. NMR of Okara Polysaccharides
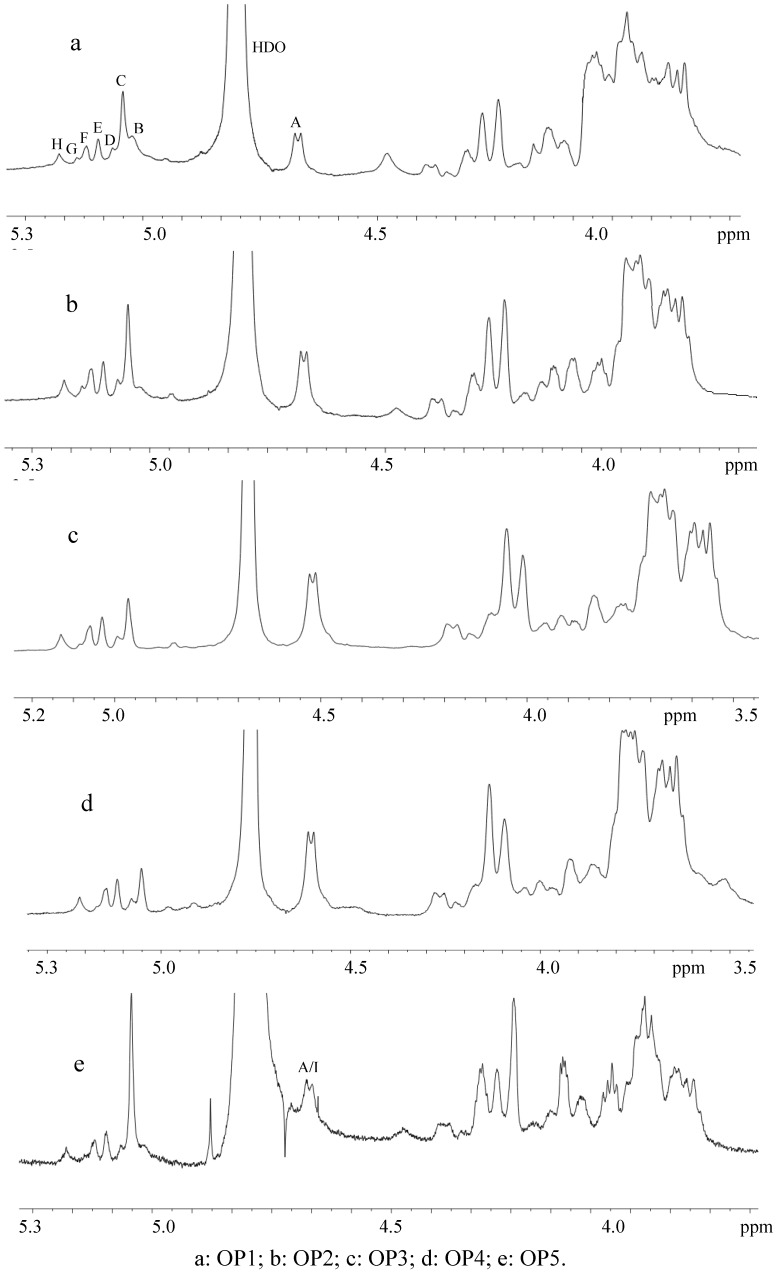
The configuration of the anomeric protons of sugar residues in polysaccharides can be identified from their 1H-NMR spectra according to their chemical shift (δ) and coupling constant (3J1,2). In generally, anomeric protons with δ > 5.0 and 3J1,2 < 4.0 Hz are in an α configuration, and anomeric protons with δ < 5.0 and 3J1,2 > 6.0 Hz are in a β configuration. The 1H-NMR spectra of OP1, OP2, OP3 and OP4 (Figure 1) show that the chemical shifts and 3J1,2 of most anomeric protons (B~H) are higher than 5.0 and lower than 4.0 Hz, suggesting these anomeric protons are of the α configuration. Anomeric proton A has a strong signal at δ 4.6, and its 3J1,2 is about 8.0 Hz, indicating A is in a β configuration. In terms of the sugar composition and methylation analysis of OP1~OP4, it can be deduced that A is the anomeric proton of →4)β-Galp(1→ residue. Thus, it can also be inferred that arabinose, xylose, rhamnose, fucose and glucouronic acid are of the α anomeric configuration. With respect to OP5, there are at least two peaks (A, I) at δ 4.56~4.66, indicating more than two anomeric protons are β configurations. According to methylation result of OP5, it could be deduced that I is the anomeric proton of →4)β-Glcp(1→ residue.
Figure 1 shows the 1H-NMR spectra of OP1~OP4 are somewhat similar, which conforms to the sugar composition and methylation analysis data. The result suggests the polysaccharides in OP1~OP4 could be similar to some extent, and the differences might be the molecular size, branch degree, and the ratio of different sugar residues of polysaccharides. OP5 has a different 1H-NMR spectrum with other four fractions, which might be caused by the large amount of cellulose residues in OP5.
3. Experimental
3.1. Materials
Fresh okara was obtained from a tofu production line of Henan Xiaobao Douye Co. Ltd., Xinxiang, China. Soybean (Glycine max L.), north-east variety (China) was soaked, rinsed, and ground, and the okara was filtered off according to guidelines for the Chinese method. Okara was freeze-dried (LGJ-18 freeze-dryer, Beijing Sihuan) and ground to fine powder (80 mesh). Standard sugars were purchased from Sigma. The other chemicals and reagents used were of analytical grade.
Figure 1.
500-MHz 1H-NMR spectra of okara polysaccharides.
3.2. Isolation and Sequential Extraction of Polysaccharides from Okara
Okara polysaccharide was isolated and extracted according to the methods of Huisman et al. and Mateos-Aparicio et al. [10,11]. Okara (20 g) was defatted in a Soxhlet system by extraction with diethyl ether solvent. Defatted okara was treated with 1.5% (w/v) sodium dodecyl sulfate solution containing 10 mM 1,4-dithioerythreitol for 3 h at room temperature. After centrifugation (5,000 r/min, 30 min), this extraction was repeated three times to remove the protein. The final residue was washed twice with distilled water and freeze-dried. Afterwards, the dried residue (14.40 g) were extracted with 85% ethanol for 1 h at 60 °C. After centrifugation (5,000 r/min, 30 min), this extraction was repeated twice to deliver low molecular weight carbohydrates. The residue was freeze-dried and 11.35 g crude okara polysaccharide (COP) was obtained.
COP (10 g) was sequentially extracted with 0.05 M EDTA + 0.05 M NH4 oxalate in 0.05 M sodium acetate buffer, pH 5.0 (four times 300 mL) at 70 °C for 1 h to separate the OP1 fraction. The remnant was then extracted with 0.05 M NaOH, 4 °C, 1 h (OP2 fraction), 1 M NaOH + 0.02 M NaBH4, room temperature, 2 h (OP3 fraction) and 4 M NaOH + 0.02 M NaBH4, room temperature, 2 h (OP4 fraction). Each extraction was done three times. Each extract was neutralised with glacial acetic acid, concentrated at 50 °C in low-pressure, dialysed with 12–14 KDa membrane against distilled water and freeze-dried. The residue of OP4 extraction was neutralised with acetic acid, followed by centrifugation. The precipitation was taken out with distilled water and dialysed with 12–14 KDa membrane against distilled water. The remain was freeze-dried to obtain OP5 fraction.
3.3. Determination of Monosaccharide Composition
Okara polysaccharide samples were subjected to methanolysis (1 M methanolic HCl, 24 h, 85 °C), after addition of internal standard (mannitol). The resulting mixtures of methyl glycosides were trimethylsilylated (hexamethyldisilazane-trimethylchlorosilane-pyridine 1:1:5, 30 min, room temperature), and then analyzed by GLC on an EC-1 column (30 m × 0.32 mm, Alltech), using a Chrompack CP9002 gas chromatograph (temperature program 140–240 °C, 4 °C/min) [16].
3.4. Methylation Analysis
Okara polysaccharide samples were permethylated using CH3I and solid NaOH in DMSO, as described earlier [17]. After hydrolysis with 2 M TFA (2 h, 120 °C), the partially methylated monosaccharides were reduced with NaBD4 (2 h, room temperature). Then neutralisation with HOAc and removal of boric acid by co-evaporation with methanol, followed by acetylation with 1:1 acetic anhydride: pyridine (30 min, 120 °C), a mixture of partially methylated alditol acetates were yielded, which was analysed by GLC-EI-MS on an EC-1 column (30 m × 0.25 mm) using a GCMS-QP2010. Plus instrument (Shimadzu, Japan) and temperature gradient (140–250 °C at 8 °C /min).
3.5. NMR Analysis
Resolution-enhanced 500-MHz 1H-NMR spectra of okara polysaccharide samples were recorded in D2O on a Varian-500 spectrometer at a probe temperature of 300 K. Prior to analysis, samples were exchanged twice in D2O (99.9 atom% D, Cambridge Isotope Laboratories, Inc., Andover, MA, USA) with intermediate lyophilization, and then dissolved in 0.6 mL D2O.
4. Conclusions
Okara contains 56.8% crude polysaccharides after removing fat, protein and low molecular weight carbohydrates from the initial okara. Crude okara polysaccharides can be further partitioned into five fractions by extracting with 0.05 M EDTA + NH4 oxalate, 0.05 M NaOH, 1 M NaOH and 4 M NaOH, and their yields were 7.7%, 3.6%, 20.7%, 16.0% and 27.9%, respectively. The main monosaccharides of okara polysaccharides are arabinose, galactose, galacturonic acid and xylose. OP5 also contains a great amount of glucose. The primary sugar residues of okara polysaccharides are 1,4-linked β-galactopyranose, 1,5- and 1,3-linked α-arabinofuranose, 1,5-linked α-xylofuranose, 1,2-linked, 1,2,4-linked and terminal α-rhamnopyranose (or fucopyranose), and 1,4-linked β-glucopyranose (only for OP5). These results indicate okara polysaccharides might contain galactan, arabinan, arabinogalactan, xylogalacturonan, rhamnogalacturonan, xylan, xyloglucan and cellulose.
Acknowledgements
This work was supported by Key Scientific and Technological Project of Henan Province (102102110031) and Overseas Student Science and Technology Activities Project of Ministry of Human Resources and Social Security.
Footnotes
Sample Availability: Samples of the compounds okara polysaccharides (OP1~OP5) are available from the authors.
References and Notes
- 1.Li B., Zhang Y., Yang H., Li R. Effect of drying methods on functional properties of bean curd dregs. J. Henan Ins. Sci. Technol. (Nat. Sci. Ed.) 2008;36:64–66. [Google Scholar]
- 2.Ohno A., Ano T., Shoda M. Production of the antifungal peptide antibiotic, iturin by NB22 in solid state fermentation. J. Ferment. Bioeng. 1993;75:23–27. doi: 10.1016/0922-338X(93)90172-5. [DOI] [Google Scholar]
- 3.Ahn S.H., Oh S.C., Choi I., Han G., Jeong H., Kim K., Yoon Y., Yang I. Environmentally friendly wood preservatives formulated with enzymatic-hydrolyzed okara, copper and/or boron Salts. J. Hazard. Mater. 2010;178:604–611. doi: 10.1016/j.jhazmat.2010.01.128. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez R., Jiménez A., Fernández-Bolaños J., Guillén R., Heredia A. Dietary fibre from vegetable products as source of functional ingredients. Trends Food Sci. Tech. 2006;17:3–15. doi: 10.1016/j.tifs.2005.10.002. [DOI] [Google Scholar]
- 5.Van der Riet W.B., Wight A.W., Cilliers J.J.L., Datel J.M. Food chemical investigation of tofu and its byproduct okara. Food Chem. 1989;34:193–202. doi: 10.1016/0308-8146(89)90140-4. [DOI] [Google Scholar]
- 6.Redondo-Cuenca A., Villanueva-Suarez M.J., Mateos-Aparicio I. Soybean seeds and its by-product okara as sources of dietary fiber. Measurement by AOAC and Englyst Methods. Food Chem. 2008;108:1099–1105. doi: 10.1016/j.foodchem.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 7.Mateos-Aparicio I., Redondo-Cuenca A., Villanueva-Suárez M.J., Zapata-Revilla M., Tenorio-Sanz M. Pea pod, broad bean pod and okara, potential sources of functional compounds. LWT-Food Sci. Technol. 2010;43:1467–1470. doi: 10.1016/j.lwt.2010.05.008. [DOI] [Google Scholar]
- 8.O'Toole D.K. Characteristics and use of okara, the soybean residue from soy milk production—A review. J. Agric. Food Chem. 1999;47:363–371. doi: 10.1021/jf980754l. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi F., Ota Y., Hatanaka C. Extraction and purification of pectic polysaccharides from soybean okara and enzymatic analysis of their structures. Carbohydr. Polym. 1996;30:265–273. doi: 10.1016/S0144-8617(96)00046-X. [DOI] [Google Scholar]
- 10.Huisman M.M.H., Schols H.A., Voragen A.G.J. Cell wall polysaccharides from soybean (Glycine max.) meal. Isolation and characterisation. Carbohydr. Polym. 1998;37:87–95. doi: 10.1016/S0144-8617(97)00111-2. [DOI] [Google Scholar]
- 11.Mateos-Aparicio I., Redondo-Cuenca A., Villanueva-Suárez M.J. Isolation and characterisation of cell wall polysaccharides from legume by-products: Okara (soymilk residue), pea pod and broad bean pod. Food Chem. 2010;122:339–345. doi: 10.1016/j.foodchem.2010.02.042. [DOI] [Google Scholar]
- 12.Mateos-Aparicio I., Mateos-Peinado C., Jimenez-Escrig A., Rupérez P. Multifunctional antioxidant activity of polysaccharide fractions from the soybean byproduct okara. Carbohydr. Polym. 2010;82:245–250. doi: 10.1016/j.carbpol.2010.04.020. [DOI] [Google Scholar]
- 13.Tsubake S., Nakauchi M., Ozaki Y., Azuma J. Microwave Heating for Solubilization of Polysaccharide and Polyphenol from Soybean Residue (Okara) Food Sci. Technol. Res. 2009;15:307–314. doi: 10.3136/fstr.15.307. [DOI] [Google Scholar]
- 14.Le Goff A., Renard C.M.G.C., Bonnin E., Thibault J.-F. Extraction, purification and chemical characterisation of xylogalacturonans from pea hulls. Carbohydr. Polym. 2001;45:325–334. doi: 10.1016/S0144-8617(00)00271-X. [DOI] [Google Scholar]
- 15.Schols H.A., Vierhuis E., Baks E.J., Voragen A.G.J. Different populations of pectic hairy regions occur in apple cell walls. Carbohydr. Res. 1995;275:343–360. doi: 10.1016/0008-6215(95)00155-M. [DOI] [PubMed] [Google Scholar]
- 16.Kamerling J.P., Vliegenthart J.F.G. Mass spectrometry. In: Lawson A.M., editor. Clinical Biochemistry—Principles, Methods, Applications. Vol. 1. Walter de Gruyter; Berlin, Germany: 1989. pp. 176–263. [Google Scholar]
- 17.Needs P.W., Selvendran R.R. Avoiding oxidative degradation during sodium hydroxide/methyl iodide-mediated carbohydrate methylation in dimethyl sulfoxide. Carbohydr. Res. 1993;245:1–10. doi: 10.1016/0008-6215(93)80055-J. [DOI] [Google Scholar]