Abstract
A series of new monoazo disperse dyes containing pyrazolopyrimidine moieties was synthesized by coupling malononitrile or 3-aminocrotononitrile with 4-hydroxy- benzenediazonium chloride. Treatment of the resulting products with hydrazine hydrate yields the corresponding 4-arylazoaminopyrazoles, which then react with either 2,4-pentanedione and enaminonitriles or aryl-substituted enaminoketones to give the target pyrazolopyrimidine monoazo disperse dyes. Structural assignments of the dyes were made using both NMR spectroscopic and X-ray crystallographic methods. A high temperature dyeing method, by microwave irradiation, was employed with polyester fabrics. Most of the dyed fabrics tested displayed moderate light fastness and excellent washing and perspiration fastness levels.
Keywords: aminopyrazoles; microwave irradiation; enaminone; disperse dyes; 2,4-pentanedione; enaminonitrile
1. Introduction
4-Arylazo-5-aminopyrazoles are readily obtainable, versatile compounds that have demonstrated antibiotic properties [1,2,3,4,5] and are used as dyes [6,7]. While a large number of arylazopyrazole dyes have been reported in the literature, very few condensed pyrazole derivatives carrying an arylazo function on the pyrazole ring have been reported [8,9,10,11,12,13]. The present study reports the synthesis of novel condensed 4-hydroxyphenylazopyrazolo[1,5-a]pyrimidine dyes, and their application as disperse dyes for polyester fabrics by a method using microwave irradiation as an energy source [14,15].
2. Results and Discussion
2.1. Synthesis
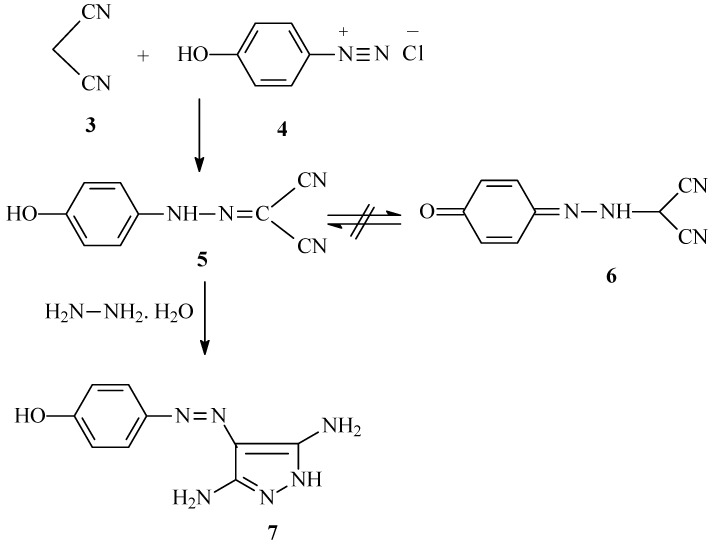
One of the sequences used for synthesis of the 4-hydroxyphenylazopyrazolo[1,5-a]pyrimidines is coupling of malononitrile (3) with p-hydroxybenzenediazonium chloride (4) to give arylhydrazone 5 (Scheme 1). 13C-NMR as well as NOE difference experiments show that this substance exists as phenol 5 rather than the cyclohexadienone tautomer 6, as its 13C-NMR spectrum does not contain resonances for a sp3 hybridized carbon, NOE experiments show that irradiation of the OH proton signal at 9.63 ppm causes an enhancement of the aryl proton signal at 6.78 ppm and vice versa. Hydrazone 5 reacts smoothly with hydrazine hydrate to yield the diaminopyrazole 7. It should be noted that although 7 was previously prepared using the same approach, evidence for its structural assignment was not provided in the earlier report [6].
Scheme 1.
Synthesis of 4-(3,5-diamino-1H-pyrazol-4-ylazo)-phenol (7).
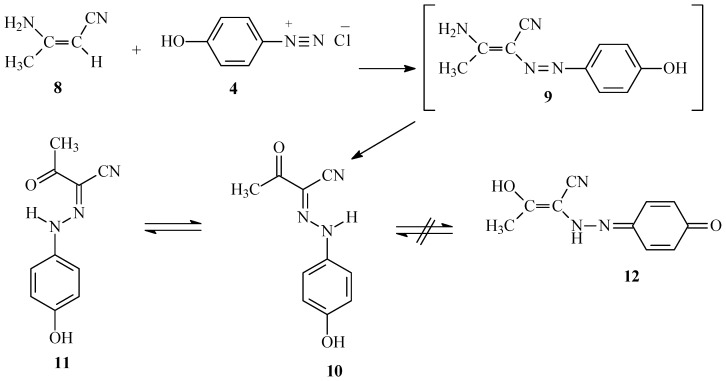
Similarly, 3-aminocrotononitrile 8 undergoes coupling with diazonium salt 4 to yield the hydrazone 10 rather than the quinohydrazone 12, via hydrolysis of the azo-intermediate 9 (Scheme 2). The possibility that 11 exists in the syn form because of the hydrogen bonding stabilization is considered unlikely based upon previous studies that show stereoelectronic factors dominate in such systems [16].
Scheme 2.
Synthesis of 2-[(4-hydroxyphenyl)-hydrazono]-3-oxo-butyronitrile (10).
NOE difference experiments were done to help establish the structure of 10. The results indicate that irradiation of the NH signal at 12.1 ppm causes an enhancement of the intensities of the aryl proton resonances at 7.39 and 6.80 ppm. In addition, irradiating the OH signal at 9.58 ppm does not promote any enhancement of these signals.
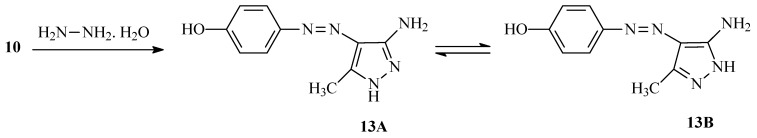
Reaction of hydrazone 10 with hydrazine hydrate (Scheme 3) generates the corresponding aminopyrazole that was found to exist as a 1:1 mixture of tautomers 13A and 13B according to a 1H-NMR experiment in DMSO-d6 solution at room temperature. NOE difference experiments show that irradiation of the NH signal at 11.94 ppm corresponding to 13A enhances the methyl proton signal at 2.36 ppm.
Scheme 3.
Synthesis of 4-(3-amino-5-methyl-1H-pyrazol-4-ylazo)-phenol (13A) and 4-(5-amino-3-methyl-1H-pyrazol-4-ylazo)-phenol (13B).
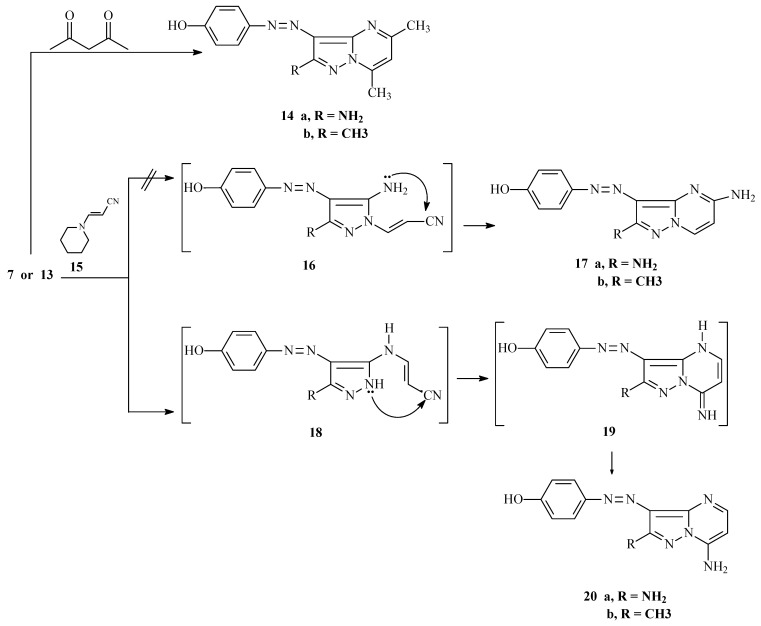
Pyrazoles 7 or 13 react with 2,4-pentanedione to yield 4-hydroxyphenylazopyrazolo[1,5-a]-pyrimidines 14a and 14b, both of which exist in their phenolic forms. This conclusion is also based on NOE difference experiments which demonstrate that irradiation of the OH signals at 9.78 and 9.96 ppm for 14a and 14b respectively, enhances the intensities of the respective ortho aryl proton signals 6.86 and 6.89 ppm.
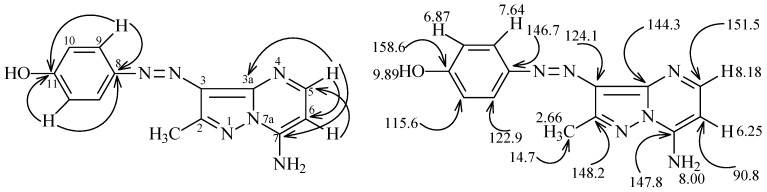
Similarly, 7 or 13 react with 3-piperidinylacrylonitrile 15 to produce the corresponding azopyrazolo[1,5-a]pyrimidines that might have either regioisomeric structure 17 or 20 (Scheme 4). The assignment of structures 20a and 20b was made by H-C correlations observed in HMBC 2-D experiments. The important HMBC correlations for 20b (Figure 1) are: (a) H5 at 8.18 ppm with C3a, C6 and C7 at 144.3, 90.8 and 147.8 ppm, respectively; (b) H6 at 6.25 ppm with C5 at 151.5 ppm; (c) H9 at 7.63 ppm with C8 and C11 at 146.7 and 158.6 ppm, respectively; and (d) H10 at 7.87 ppm with C8, C11 at 146.7 and 158.6 ppm, respectively. Further information came from the results of 1H-15N HMBC experiments, which show that N7a and N4 resonate at 205 and 235 ppm, respectively, this cross peak correlations exist for the shielded proton H6 at 6.25 ppm with N7a at 205 ppm (3J) (H6, N7a), and that the deshielded proton H5 at 8.18 ppm with N4 at 235 ppm (2J) (H5, N4).
Scheme 4.
Synthesis of compounds 14a,b and 20a,b.
Figure 1.
1H- and 13C-NMR resonance assignments of compound 20b.
Finally, the azopyrazoles 7 or 13 condense with enaminones 21a–e to yield structures which might be formulated as either 22 or 23. 15N-HMBC experiments for compound 22c revealed that the chemical shifts for N7a and N4 are 208.9 and 266.6 ppm, respectively, and that cross peak correlations exist for coupling of the shielded proton H6 at 7.24 ppm with N7a at 208.9 ppm (3J) (H6, N7a) and N4 at 266.6 ppm (3J) (H6, N4), and for coupling of the deshielded proton H5 at 8.58 ppm with only N4 at 266.6 ppm (2J) (H5, N4) (Scheme 5). These observations demonstrate that the azopyrazolo[1,5-a]pyrimidines have general structures 22.
Scheme 5.
Synthesis of pyrazolo[1,5-a]pyrimidine disperse dyes 22a–j.
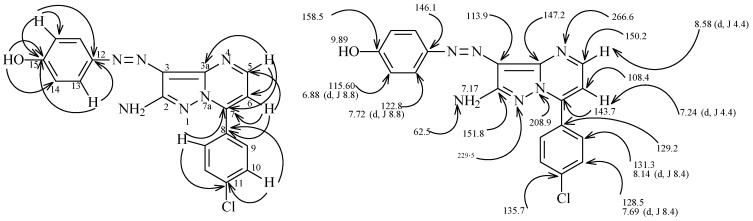
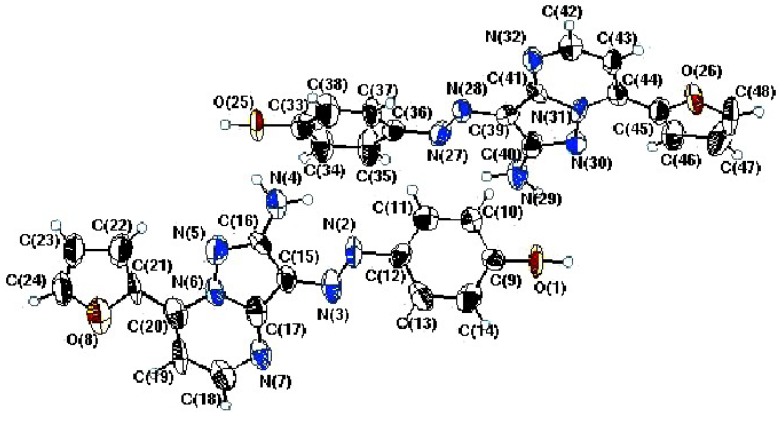
To confirm this conclusion, 2D NMR experiments were performed for 22c, giving the data displayed in Figure 2, and X-ray crystallographic analysis was done for 22i (Figure 3) [17]. Selected bond distances, angles and structure refinement in the crystal structure are given in Table 1 and Table 2, the data clearly show that the presence of N=N arylazo moiety and that the 7a-nitrogen (N31 in Figure 3) has sp2 hybridized character.
Figure 2.
1H and 13C NMR resonance assignments of compound 22c.
Figure 3.
ORTEP plot of the x-ray crystallographic structure of 22i.
Table 1.
Selected bond lengths and angles for 22i.
| Bond | Bond length (Å) | Bond | Bond angle (°) |
|---|---|---|---|
| N27—N28 | 1.286 (6) | N31—C44—C45 | 119.7 (6) |
| N31—N30 | 1.373 (6) | C36—N27—N28 | 114.3 (6) |
| N30—C40 | 1.345 (7) | N30—N31—C44 | 126.3 (6) |
| N32—C41 | 1.357 (6) | N31—C41—C39 | 103.7 (7) |
| N32—C42 | 1.324 (7) | C44—N31—C41 | 120.9 (6) |
| N27—C36 | 1.416 (7) | C44—N31—C41 | 120.9 (6) |
| N29—C40 | 1.345 (7) | C40—N29—H29A | 119.9(5) |
| N31—C41 | 1.437 (7) | C40—N29—H29B | 120.1(5) |
Table 2.
Crystal data and structure refinement for compound 22i.
| Chemical formula | C16H12N6O2 | Z | 4 |
|---|---|---|---|
| Formula weight | 320.312 | Temperature | 298 K |
| Crystal System | Triclinic | Radiation type | Mo Kα |
| Space group | P-1 | Measured reflections | 5804 |
| a | 8.4725 (4)Å | Independent reflections | 6544 |
| b | 8.5332 (5)Å | Observed reflections | 1068 |
| c | 22.111 (2)Å | Rint | 0.066 |
| α | 97.401 (3)° | R(all) | 0.329 |
| β | 92.591 (3)° | R(gt) | 0.054 |
| γ | 113.776 (7)° | wR(ref) | 0.094 |
| V | 1442.4 (2)Å3 | wR(all) | 0.270 |
| λ | 0.71073 | Parameters | 433 |
In addition, the azo moiety has E-geometry, thus enabling hydrogen bonding interaction between a hydrogen of the amino group (N29) and azo nitrogen (N27). Finally, the entire molecule is nearly planar, indicating that all atoms comprising the basic structure are sp2 hybridized.
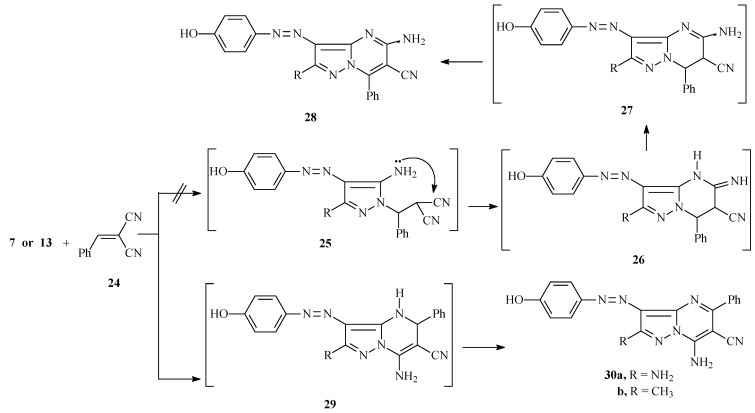
It has been reported that reaction of the diaminopyrazole 7 with benzylidenemalononitrile 24 results in the formation of azopyrazolo[1,5-a]pyrimidines 28, whose structural assignment was made based on the results of a previous investigation carried out by Elfahham et al. [18]. However, we observed that 7 reacts with 24 to yield the regioisomeric azopyrazolo[1,5-a]pyrimidines 30a, whose structure was determined by using 2D NMR spectroscopic methods. Similarly the reaction of 24 with 13 afforded the corresponding azopyrazolo[1,5-a]pyrimidine 30b (Scheme 6).
Scheme 6.
Synthesis of compounds 30a,b.
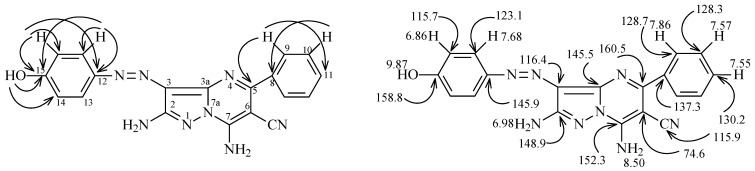
The 1H- and 13C-NMR signal assignments and H-C correlations in the HMBC 2-D experiment of 30a are displayed in Figure 4. Specific data from these measurements show that H9 at 9.87 ppm correlates with C5 and C11 at 160.4 and 130.2 ppm, respectively, H10 at 7.57 ppm correlates with C8 at 137.3 ppm, H13 at 7.68 ppm correlates with C12, C13 and C15 at 145.9, 123.0, 158.8 ppm, respectively, H14 at 6.86 ppm correlates with C12, C14 and C15 at 145.9, 115.8, 158.8 ppm, respectively, and OH at 9.87 ppm correlates with C14 and C15 at 115.8 and 158.8, respectively.
Figure 4.
1H and 13C NMR signal assignments of 30a.
The regioisomerism assignment of 30a was confirmed by comparison of the pyrimidine carbons of 30b in the 13C-NMR which appear at almost the same positions at δ = 160.9, 74.3, 150.4 ppm respectively. The structure of 30b was confirmed by 15N-HSQC and 15N-HMBC. Thus, 15N-HSQC shows the NH2 at δ = 87 ppm, and in 15N-HMBC the CH3 protons correlates with N1 at δ = 260 ppm and the NH2 protons correlates with N7a at δ = 195 ppm (which is close to the N7a chemical shift of 20b and 22c).
2.2. Dyeing and Fastness Properties
The 4-hydroxyphenylazopyrazoles 7, 13, and their pyrimidine derivatives 22a–d, and 22f–h were explored as dyes for polyester fabrics at 1%–6% shades using the high temperature dyeing method (HT) at 130 °C for 60 min with microwave heating as the energy source. The physical and analytical data for the dyed fabrics, given in Table 3 and Table 4, show that use of microwave irradiation leads to a large increase in dye uptake and dyeing rates along with enhancements in performances of dye leveling and color homogeneity as compared by conventional method.
Table 3.
Color strengths of monoazo disperse dyes on polyester fabrics.
| DyeNo | Molecular weight | Color shade on polyester | Color strength (K/S)% Dye o.m.f. | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 7 | 234 | Yellowish brown | 0.44 | 0.84 | 1.00 | 1.16 | 1.49 | 1.76 |
| 22a | 330 | Yellow | 7.72 | 9.78 | 10.03 | 13.03 | 13.33 | 16.88 |
| 22b | 344 | Yellow | 14.59 | 15.72 | 15.81 | 17.76 | 21.18 | 24.13 |
| 22c | 364 | Yellow | 10.72 | 14.92 | 16.23 | 17.26 | 21.96 | 24.42 |
| 22d | 320 | Yellowish brown | 15.44 | 21.08 | 23.02 | 25.30 | 26.25 | 28.07 |
| 13 | 217 | Pale brown | 2.18 | 4.35 | 4.88 | 6.43 | 12.86 | 13.10 |
| 22f | 329 | Yellowish orange | 22.12 | 23.55 | 24.04 | 24.41 | 25.20 | 25.67 |
| 22g | 343 | Orange | 20.82 | 20.93 | 21.92 | 22.18 | 23.43 | 23.65 |
| 22h | 363 | Orange | 14.17 | 16.52 | 17.82 | 19.94 | 20.12 | 23.42 |
Table 4.
Fastness properties of monoazo disperse dyes on polyester fabrics.
| Dye | Dye o.m.f. % | Wash fastness a,b | Persipiration fastness | Light fastness | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline | Acedic | ||||||||||
| Alt | SC | SW | Alt | SC | SW | Alt | SC | SW | |||
| 7 | 1% | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 4 |
| 22a | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22b | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | |
| 22c | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22d | 4–5 | 4–5 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | |
| 13 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | |
| 22f | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | |
| 22g | 5 | 5 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | |
| 22h | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | |
| 7 | 2% | 5 | 5 | 5 | 5 | 5 | 5 | 4–5 | 4 | 5 | 3–4 |
| 22a | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22b | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | |
| 22c | 5 | 5 | 5 | 5 | 5 | 5 | 4–5 | 4 | 5 | 3 | |
| 22d | 5 | 4 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | |
| 13 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5–6 | |
| 22f | 5 | 5 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 2–3 | |
| 22g | 5 | 5 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | |
| 22h | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | |
| 7 | 3% | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 |
| 22a | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22b | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22c | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | |
| 22d | 5 | 4–5 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | |
| 13 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 22f | 5 | 5 | 4–5 | 5 | 4–5 | 5 | 5 | 5 | 5 | 2–3 | |
| 22g | 5 | 5 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | |
| 22h | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | |
| 7 | 4% | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 |
| 22a | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22b | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22c | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22d | 5 | 3–4 | 3–4 | 5 | 4–5 | 5 | 5 | 4–5 | 5 | 3 | |
| 13 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4–5 | 5 | 5–6 | |
| 22f | 5 | 5 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 2–3 | |
| 22g | 4–5 | 5 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22h | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | |
| 7 | 5% | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 |
| 22a | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22b | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22c | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | |
| 22d | 4–5 | 3–4 | 3–4 | 5 | 5 | 5 | 5 | 5 | 5 | 2 | |
| 13 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5–6 | |
| 22f | 5 | 5 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 2–3 | |
| 22g | 5 | 5 | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | |
| 22h | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | |
| 7 | 6% | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 |
| 22a | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | |
| 22b | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 | |
| 22c | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | |
| 22d | 4–5 | 4 | 4 | 5 | 4–5 | 5 | 5 | 5 | 5 | 2 | |
| 13 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5–6 | |
| 22f | 5 | 5 | 4–5 | 4–5 | 4–5 | 4–5 | 5 | 4–5 | 5 | 2–3 | |
| 22g | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | |
| 22h | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | |
a ISO CO2/CO41; b Alt = alteration; SC = staining on cotton; SW = staining on wool.
2.2.1. Color Strength
The data given in Table 3 reveal that the color strengths (K/S) of the dyed polyester fabrics are directly proportional to the amounts of the dyes applied (% o.m.f.). The hues of the fabrics treated with the azo dyes were found to vary from yellowish-brown to orange in a manner that depends on the dye structures. Differences in the color strengths typically depend on substitution present in the arylazopyrazolopyrimidine disperse dyes [19,20,21]. The data in Table 3 clearly show that the magnitude of color strength obtained using dye 22d is much larger than those for 22a–c and 22f–h.
2.2.2. Wash Fastness
The polyester samples, dyed with the disperse dyes 7, 13, 22a–d, and 22f–h using the high temperature dyeing method, were subjected to washing at 95 °C. The rates of leaching of the dyes from the fiber in the presence of soap (or synthetic detergent) solutions of various degrees of alkalinity are factors that determine fastness to washing. Table 4 showed that the washing fastness is excellent with respect to most of the tested compounds except compound 22d (1%–6% shades) showed good results. Data showed that the magnitude of dye removal depends on the molecular size of the dye molecule, compound specific interactions between dye and substrate, wash liquor; molecular geometry; and concentration in substrate.
2.2.3. Light Fastness
The light fastness of each of the dyes was measured by employing the standard method for determination of color fastness of textiles. Several reports [22,23,24] suggest that fading of azo dyes is mainly a consequence of decomposition of the –N=N– moiety by either oxidation, reduction or photolysis. The rates of these processes should be sensitive to the chemical structure of the dye, the type of substrate and treatment conditions. Since the dyed substrate employed in this study is a polyester fabrics (i.e., non-proteinic), the fading process likely occurs by oxidation [25]. The ease of oxidation of azo linkages should be a function of electron density. Therefore, electron donating substituents on this moiety should increase the fading rate while electron withdrawing groups should decrease the rate. This proposal is in agreement with the observed results (Table 4) which demonstrate that the presence of a methyl group in dyes 22b and 22g causes a decrease of light fastness to 3. On the other hand, the chlorine atom in dyes 22c and 22h is associated with an increase of light fastness to 4 and 6, respectively.
2.2.4. Perspiration Fastness
The resistance to the action of human perspiration, referred to as the fastness to perspiration, is evaluated by treatment of a dyed material under alkaline and acidic conditions. The results of these tests (Table 4) using the dyed polyester indicate that the magnitude of dye removal under the influence of perspiration is dependent upon the more polar character of the dye molecule giving lower magnitudes of the dye removal (e.g., polyester fabrics dyed with 22h have excellent perspiration fastness owing to the presence of the electronegative chlorine atom). In addition the results obtained showed that dyed fabrics have good fastness to perspiration may be due to: (a) the absence of solubilizing groups, which renders solubility, and wash ability of the dye-out of the fabrics, (b) the size of the dye molecule is considered relatively big, (c) the good intra-fiber diffusion of the dye molecules inside the fabrics.
3. Experimental
3.1. General
All melting points were recorded on a Gallenkamp apparatus and are uncorrected. IR spectra were recorded in KBr disks on a Perkin Elmer System 2000 FT-IR spectrophotometer. 1H- and 13C-NMR spectra were recorded on a Bruker DPX 400 MHz super-conducting NMR spectrometer. Mass spectra were measured on a VG Auto-spec-Q instrument (high resolution, high performance, tri-sector 5189 GC/MS/MS) and by LC MS using an Agilent 1100 series LC/MSD with API-ES/APCI ionization mode. Microanalyses were performed on a LECO CH NS-932 Elemental Analyzer. The microwave oven used is a single mode cavity Explorer Microwave (CEM Corporation, Matthews, NC, USA) and irradiate in heavy-walled Pyrex tube (capacity 10 mL and 80 mL for dyeing). The color strengths (K/S) of the dyed polyester fabrics and the color fastness to light were evaluated at the Dyeing, Printing and Textile Auxiliaries Department, Textile Research Division, National Research Centre (NRC), Giza, Egypt. X-ray crystallography was carried out on a Kappa CCD Enraf Nonius FR 590 diffractometer, National Research Centre, Giza, Egypt.
3.2. General Procedure for the Synthesis of Azo Disperse Dyes
4-(3,5-Diamino-1H-pyrazol-4-ylazo)-phenol (7). This compound was prepared according to the literature [6]. Mp 245–246 °C, 1H-NMR (DMSO-d6) δ 9.52 (s, 1H, OH), 7.52 (d, 2H, J = 8.8 Hz), 6.77 (d, 2H, J = 8.8 Hz), 6.46 (br, 1H, NH), 5.87 (br, 4H, 2NH2). 13C-NMR (DMSO-d6) δ 156.8, 146.4, 121.8, 115.5, 115.3, 115.2, 112.9. λmax (DMF)/nm 369.
2-[(4-Hydroxyphenyl)-hydrazono]-3-oxobutyronitrile (10). p-Aminophenol (10.9 g, 0.1M) was dissolved in concentrated HCl (30 mL) and water (20 mL) cooled in ice and then NaNO2 (7 g) in water (50 mL) was added in portions. A mixture of 3-aminocrotononitrile (8.2 g, 0.1 mole), NaOAc (20 g), ethanol (15 mL), and water (50 mL) was prepared separately and cooled in ice. The diazonium salt solution was added slowly to the second solution, with ice cooling. The cooled reaction was stirred for 0.5 h and filtered to give brown crystals, which were crystallized from alcohol/water. Brown solid (7.0 g, 85%). Mp 214–215 °C. 1H-NMR (DMSO-d6) δ 12.18 (s, 1H, NH), 9.61 (s, 1H, OH), 7.39 (d, 1H, J = 8.8 Hz), 6.81 (d, 1H, J = 8.8 Hz), 2.37 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 192.6, 155.3, 134.1, 118.4, 115.9, 111.8, 111.3, 24.5. IR: 3199, 3073, 2922, 2213, 1631, 1599, 1465, 1440, 1367, 1329, 1286, 1268, 1226, 1208, 952, 835 cm–1. MS (EI) m/z (%) = 203 ([M]+, 100), 121 (53), 108 (93). HRMS: m/z (EI) for C10H9N3O2; calcd. 203.0689; found: 203.0689.
4-(3-Amino-5-methyl-1H-pyrazol-4-ylazo)-phenol (13A) and 4-(5-Amino-3-methyl-1H-pyrazol-4-ylazo)-phenol (13B). A mixture of 10 (2.03 g, 10 mmol), hydrazine hydrate (2.5 mL) in ethanol (20 mL) was stirred at reflux for 3–4 h. The solvent was removed under vacuum and the formed solid was collected and crystallized from ethanol/water. Brown solid (1.75 g, 86%). Mp 239–240 °C. 1H-NMR (DMSO-d6) δ 11.94 (br, 1H, NH, 13A), 11.51 (br, 1H, NH, 13B), 9.92 (s, 2H, 2OH, 13A,13B), 7.56 (d, 4H, J = 8.4 Hz, 13A,13B), 6.81 (d, 4H, J = 8.4 Hz, 13A,13B), 6.75 (br, 2H, NH2), 5.84 (br, 2H, NH2), 2.36 (s, 6H, 2CH3). 13C-NMR (DMSO-d6) δ 157.9, 148.2, 146.0, 140.6, 122.3, 115.5, 115.2, 18.5. λmax (DMF)/nm 376. IR: 3399, 3267, 3213, 1629, 1590, 1530, 1461, 1386, 1256, 1095, 838 cm–1. MS (EI) m/z (%) =217 ([M]+, 100), 124 (60), 93 (12). HRMS: m/z (EI) for C10H11N5O; calcd. 217.0958; found: 217.0958.
3.3. Synthesis of Compounds 14a–b, 20a,b and 22a–j
General procedure: a mixture of 7 (0.218 g, 1 mmol) or 13 (0.217 g, 1 mmol), acetylacetone, 2-piperidinylacrylonitrile or enaminones 21a–e (1 mmol) in acetic acid (20 mL) and sod. acetate (0.12 g, 1.5 mmol) was refluxed for 1 h. The reaction mixture was poured onto ice water (50 mL) filtered and crystallized from the DMF solvent.
4-(2-Amino-5,7-dimethyl-pyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (14a). Yellow solid (0.22 g, 77%). Mp 287–288 °C. 1H-NMR (DMSO-d6) δ 9.81 (s, 1H, OH), 7.65 (dd, 2H, J = 7.2 and 1.8 Hz), 7.05 (br, 2H, NH2), 6.89 (s, 1H), 6.85 (dd, 2H, J = 7.2 and 1.8 Hz), 2.57 (s, 3H), 2.52 (s, 3H). 13C-NMR (DMSO-d6) δ 159.7, 158.2, 151.6, 146.1, 145.9, 145.0, 122.7, 115.6, 113.7, 109.1, 24.1, 16.4. IR: 3414, 3281, 1624, 1564, 1444, 1360, 1200, 1138, 837 cm−1. MS (EI) m/z (%) = 282 ([M]+, 100), 189 (40), 161 (20). HRMS: m/z (EI) for C14H14N6O; calcd. 282.1223; found: 282.1223.
4-(2,5,7-Trimethylpyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (14b). Greenish yellow solid (0.20 g, 72%). Mp > 350 °C. 1H-NMR (DMSO-d6) δ 9.98 (s, 1H, OH), 7.68 (dd, 2H, J = 7.2 and 1.8 Hz), 7.04 (s, 1H), 6.89 (dd, 2H, J = 7.2 and 1.8 Hz), 2.70 (s, 3H), 2.66 (s, 3H), 2.59 (s, 3H). 13C-NMR (DMSO-d6) δ 161.4, 159.1, 146.8, 146.4, 145.7, 144.0, 124.5, 123.3., 115.6, 110.1, 24.4, 16.2, 15.3. IR: 3427, 3061, 1617, 1596, 1562, 1495, 1448, 1405, 1370, 1263, 1116, 849 cm−1. MS (EI) m/z (%) = 281 ([M]+, 100), 188 (90), 160 (50). HRMS: m/z (EI) for C15H15N5O; calcd. 281.1271; found: 281.1271.
4-(2,7-Diaminopyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (20a). Reddish brown solid (0.19 g, 70%). Mp 248–249 °C. 1H-NMR (DMSO-d6) δ 9.76 (s, 1H, OH), 8.02 (d, 1H, J = 5.6 Hz), 7.68 (br, 2H, NH2), 7.61 (d, 2H, J = 8.8 Hz), 6.89 (br, 2H, NH2), 6.84 (d, 2H, J = 8.8 Hz), 6.20 (d, 1H, J = 5.6 Hz) 13C-NMR (DMSO-d6) δ 157.8, 151.2, 149.5, 146.9, 146.7, 146.2, 122.3, 115.5, 113.8, 91.5. IR: 3456, 3330, 2972, 1632, 1485, 1452, 1373, 1159, 954, 835 cm−1. MS (EI) m/z (%) = 269 ([M]+, 100), 176 (33), 148 (30). HRMS: m/z (EI) for C12H11N7O; calcd. 269.1019; found: 269.1019.
4-(7-Amino-2-methylpyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (20b). Brown solid (0.20 g, 75%). Mp 291–292 °C. 1H-NMR (DMSO-d6) δ 9.89 (s, 1H, OH), 8.18 (d, 1H, J = 5.4 Hz), 8.00 (br, 2H), 7.64 (dd, 2H, J = 7.6 and 1.8 Hz), 6.87 (dd, 2H, J = 7.6 and 1.8 Hz), 6.25 (d, 1H, J = 5.4 Hz) 2.66 (s, 3H). 13C-NMR (DMSO-d6) δ 158.6, 151.5, 148.2, 147.8, 146.7, 144.3, 124.1, 122.9, 115.6, 90.8, 14.7. IR: 3476, 3347, 3182, 1639, 1596, 1481, 1450, 1369, 1322, 1275, 1251, 1145, 828 cm−1. MS (EI) m/z (%) = 268 ([M]+, 100), 175 (55), 147 (40). HRMS: m/z (EI) for C13H12N6O; calcd. 268.1067; found: 268.1067.
4-(2-Amino-7-phenylpyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (22a). Orange solid (0.25 g, 76%). Mp 301–302 °C. 1H-NMR (DMSO-d6) δ 9.88 (s, 1H, OH), 8.58 (d, 1H, J = 4.8 Hz), 8.08 (m, 2H,), 7.71 (d, 2H, J = 8.8 Hz) 7.61 (m, 3H), 7.21 (d, 1H, J = 4.8 Hz), 7.14 (br, 2H, NH2), 6.87 (d, 2H, J =8.8 Hz). 13C-NMR (DMSO-d6) δ 158.5, 151.8, 150.3, 147.3, 146.1, 144.9, 130.9, 130.5, 129.5, 128.5, 122.9, 115.6, 113.9, 108.6. λmax (DMF)/nm 385. IR: 3432, 3318, 3167, 1627, 1550, 1450, 1271, 1242, 832 cm−1. MS (EI) m/z (%) = 330 ([M]+, 100), 237 (25), 115 (30). Anal. calcd. for C18H14N6O (330.3): C 65.44; H 4.27; N 25.44. Found: C 65.34; H 4.20; N 25.37.
4-(2-Amino-7-p-methylphenylpyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (22b). Reddish brown solid (0.29 g, 84%). Mp 309–310 °C. 1H-NMR (DMSO-d6) δ 9.88 (s, 1H, OH), 8.54 (d, 1H, J = 4.4 Hz), 8.02 (d, 2H, J = 8.4 Hz), 7.72 (d, 2H, J = 8.8 Hz), 7.41 (d, 2H, J = 8.4 Hz), 7.19 (d, 1H, J = 4.4 Hz), 7.14 (br, 2H, NH2), 6.88 (d, 2H, J = 8.8 Hz), 2.43 (s, 3H). 13C-NMR (DMSO-d6) δ 158.4, 151.8, 150.1, 147.3, 146.1, 144.9, 141.1, 129.4, 129.0, 127.5, 122.8, 115.6, 113.8, 108.2, 21.1. λmax (DMF)/nm 385. IR: 3421, 3309, 2917, 1602, 1548, 1447, 1328, 1232, 1095, 833 cm−1. MS (EI) m/z (%) = 344 ([M]+, 80), 196 (30), 129 (100). HRMS: m/z (EI) for C19H16N6O; calcd. 344.1380; found: 344.1380.
4-(2-Amino-7-p-chlorophenylpyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (22c). Orange solid (0.28 g, 78%). Mp 306–307 °C. 1H-NMR (DMSO-d6) δ 9.89 (s, 1H, OH), 8.58 (d, 1H, H5, J = 4.4 Hz), 8.14 (d, 2H, H9, J = 8.4 Hz), 7.72 (d, 2H, H13, J = 8.8 Hz) 7.69 (d, 2H, H10, J = 8.4 Hz), 7.24 (d, 1H, H6, J = 4.4 Hz), 7.17 (br, 2H, NH2), 6.88 (d, 2H, H14, J = 8.8 Hz). 13C-NMR (DMSO-d6) δ 158.5, 151.8, 150.2, 147.2, 146.1, 143.7, 135.7, 131.3, 129.2, 128.5, 122.8, 115.6, 113.9, 108.4. λmax (DMF)/nm 383. IR: 3418, 3308, 3180, 1616, 1601, 1549, 1487, 1450, 1331, 1271, 1237, 1088, 842 cm−1. MS (EI) m/z (%) = 364 ([M]+, 100), 271 (32), 149 (42). HRMS: m/z (EI) for C18H13ClN6O; calcd. 364.0833; found: 364.0833.
4-(2-Amino-7-furanpyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (22d). Red solid (0.26 g, 80%). Mp 292–293 °C. 1H-NMR (DMSO-d6) δ 9.87 (s, 1H, OH), 8.55 (d, 1H, J = 5.2), 8.19 (m, 2H), 7.71 (dd, 2H, J = 7.2 and 1.6), 7.43 (d, 1HJ = 5.2), 7.24 (br, 2H, NH2), 6.94 (dd, 1H, J = 3.6,1.6), 6.87 (dd, 2H, J 7.2 and 1.6). 13C-NMR (DMSO-d6) δ 158.5, 151.9, 149.3, 147.4, 146.9, 146.0, 143.0, 133.8, 122.9, 120.0, 115.6, 113.5, 113.3, 103.1. λmax (DMF)/nm 378. IR: 3449, 3411, 2923, 1595, 1449, 1316, 1009, 824 cm−1. MS (EI) m/z (%) = 320 ([M]+, 100), 227 (30), 116 (15). HRMS: m/z (EI) for C16H12N6O2 calcd. 320.1016; found: 320.1016.
4-(2-Amino-7-thiophenepyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (22e). Brown solid (0.27 g, 80%). Mp 276–277 °C. 1H-NMR (DMSO-d6) δ 9.93 (s, 1H, OH), 8.54 (d, 1H, J = 3.6), 8.52 (d, 1H, J = 4.8), 8.16 (dd, 1H, J = 4.8 and 1.2), 7.73 (m, 3H), 7.40 (dd, 1H, J = 4.8 and 1.2), 7.30 (br, 2H, NH2), 6.88 (d, 2H, J = 8.8). 13C-NMR (DMSO-d6) δ 158.7, 151.5, 149.2, 147.1, 145.9, 138.2, 134.9, 132.4, 129.9, 128.0, 122.8, 115.7, 113.5, 104.5. λmax (DMF)/nm 370, IR: 3437, 3107, 3023, 1602, 1549, 1500, 1450, 1414, 1330, 1242, 1183, 839 cm−1. MS (EI) m/z (%) = 336 ([M]+, 100), 243 (50), 188 (20). Anal. calcd. for C16H12N6OS (336.4): C 57.13; H 3.60; N 24.98; S 9.53. Found: C 57.04; H 3.48; N 24.87; S 9.48.
4-(2-Methyl-7-phenylpyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (22f). Yellow solid (0.28 g, 85%). Mp 297–298 °C. 1H-NMR (DMSO-d6) δ 10.05 (s, 1H, OH), 8.79 (d, 1H, J = 4.2 Hz), 8.11 (m, 2H), 7.73 (dd, 2H, = J 8.4 and 1.8 Hz), 7.65 (m, 3H), 7.38(d, 1H, J = 4.2 Hz), 6.92 (dd, 2H, J = 8.4 and 1.8 Hz), 2.68 (s, 3H).13C-NMR (DMSO-d6) δ 159.5, 152.1, 147.8, 146.4, 144.7, 144.6, 136.1, 131.5, 128.8, 128.6, 125.1, 123.5, 115.7, 109.4, 14.9. λmax (DMF)/nm 372. IR: 3163, 3066, 1587, 1542, 1482, 1331, 1228, 1263, 1083, 819 cm−1. MS (EI) m/z (%) = 329 ([M]+, 100), 236 (70), 182 (30). Anal. calcd. for C19H15N5O (329.4): C 69.29; H 4.59, N 21.26. Found: C 69.27; H 4.67; N 21.25.
4-(2-Methyl-7-p-methylphenylpyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (22g). Reddish brown solid (0.28 g, 83%). Mp 267–268 °C. 1H-NMR (DMSO-d6) δ 10.01 (s, 1H, OH), 8.73 (d, 1H, J = 4.4 Hz), 8.03 (d, 2H, J = 8.0 Hz), 7.70 (d, 2H, J = 8.8 Hz), 7.42 (d, 2H, J = 8.0 Hz), 7.33 (d, 1H, J = 4.4 Hz), 6.89 (d, 2H, J = 8.8 Hz), 2.66 (s, 3H), 2.42 (s, 3H). 13C-NMR (DMSO-d6) δ 159.9, 152.5, 148.2, 147.0, 146.4, 145.3, 141.9, 130.1, 129.6, 127.6, 125.5, 123.9, 116.2, 109.5, 21.6, 15.5. λmax (DMF)/nm 373. IR: 3418, 3144, 2923, 1602, 1542, 1502, 1274, 1143, 841cm−1. MS (EI) m/z (%) = 343 ([M]+, 100), 250 (53), 196 (33). HRMS: m/z (EI) for C20H17N5O; calcd. 343.1428; found: 343.1427.
4-(2-Methyl-7-p-chlorophenylpyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (22h). Orange solid (0.29 g, 80%). Mp 259–260 °C. 1H-NMR (DMSO-d6) δ 10.06 (s, 1H, OH), 8.79 (d, 1H, J = 4.2 Hz), 8.16 (d, 2H, J = 8.8 Hz), 7.72 (m, 4H), 7.4 (d, 1H, J = 4.2 Hz), 6.92 (d, 2H, J = 8.8 Hz), 2.68 (s, 3H).13C-NMR (DMSO-d6) δ 159.4, 152.1, 147.8, 146.5, 145.9, 144.7, 131.2, 130.1, 129.6, 128.6, 125.0, 123.4, 115.7, 109.4, 14.9. λmax (DMF)/nm 373. IR: 3430, 3162, 3053, 1598, 1548, 1491, 1452, 1353, 1269, 1241, 1145, 836 cm−1. MS (EI) m/z (%) = 363 ([M]+, 100), 270 (75), 216 (30). Anal. calcd. for C19H14ClN5O (363.8): C 62.73; H 3.88, N 19.25. Found: C 62.90; H 3.87; N 19.30.
4-(2-Methyl-7-furanepyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (22i). Red crystals were obtained from DMF, (0.25 g, 78%). Mp 284–285 °C. 1H-NMR (DMSO-d6) δ 10.06 (s, 1H, OH), 8.76 (d, 1H, J = 4.8 Hz), 8.21 (m, 2H), 7.72 (dd, 2H, J = 7.0 and 1.8 Hz), 7.56 (d, 1H, J = 4.8 Hz), 6.93 (m, 1H), 6.91 (dd, 2H, J = 7.0 and 1.8 Hz), 2.76 (s, 3H). 13C-NMR (DMSO-d6) δ 159.5, 151.3, 148.0, 147.7, 146.5, 144.5, 142.7, 134.7, 124.9, 123.5, 120.4, 115.7, 113.5, 104.0, 15.1. λmax (DMF)/nm 373. IR: 3157, 3035, 2978, 1596, 1569, 1522, 1450, 1350, 1265, 1236, 1145, 1015, 842 cm−1. MS (EI) m/z (%) = 319 ([M]+, 100), 226 (45), 198 (30). Anal. calcd. for C17H13N5O2 (319.3): C 63.94; H 4.10, N 21.93. Found: C 63.93; H 4.08; N 21.78.
4-(2-Methyl-7-thiophenepyrazolo[1,5-a]pyrimidin-3-ylazo)-phenol (22j). Reddish brown solid (0.24 g, 72%). Mp 278–279 °C. 1H-NMR (DMSO-d6) δ 10.04 (s, 1H, OH), 8.73 (d, 1H, J = 4.8 Hz), 8.57 (dd, 1H, J = 4.2 and 1.2 Hz), 8.15 (dd, 1H, J = 4.8 and 1.2 Hz), 7.86 (d, 1H, J = 4.8 Hz), 7.72 (dd, 2H, J = 7.2 and 1.8 Hz), 7.40 (dd, 1H, , J = 4.6 and 3.6 Hz), 6.92(dd, 2H, J = 7.2 and 1.8 Hz), 2.76 (s, 3H). 13C-NMR (DMSO-d6) δ 159.4, 151.2, 147.7, 146.5, 144.5, 139.2, 135.3, 132.8, 129.5, 127.8, 124.9, 123.5, 115.7, 105.4, 15.1. λmax (DMF)/nm 376, IR: 3407, 3097, 2972, 1592, 1542, 1500, 1448, 1330, 1237, 1145, 1008, 843 cm−1. MS (EI) m/z (%) = 335 ([M]+, 100), 242 (70), 188 (30). Anal. calcd. for C17H13N5OS (335.4): C 60.88; H 3.91, N 20.88; S 9.56. Found: C 61.01; H 3.92; N 21.01; S 9.44.
3.4. General Procedure for the Preparation of 30a–b
A mixture of 7 (0.218 g, 1 mmol) or 13 (0.217 g, 1 mmol) in ethanol (20 mL), 5 drops of piperidine, and benzylidenemalononitrile (0.154 g, 1 mmol) was refluxed for 6 h, then the reaction mixture was poured into ice water, and neutralized by HCl, filtered off and recrystallized from ethanol.
2,5-Diamino-3-(4-hydroxyphenylazo)-7-phenylpyrazolo[1,5-a]pyrimidine-6-carbonitrile (30a). Reddish brown solid (0.22 g, 60%). Mp 195–196 °C. 1H-NMR (DMSO-d6) δ 9.87 (br, s, 1H, OH), 8.50 (br, 2H, NH2), 7.86 (dd, 2H, J = 8.4 and 1.4 Hz), 7.68 (d, 2H, J = 8.8 Hz), 7.57–7.55 (m, 3H), 6.98 (br, 2H, NH2), 6.86 (d, 2H, J = 8.8). 13C-NMR (DMSO-d6) δ 160.5, 158,8, 152.3, 148.9, 145.9, 145.5, 137.4, 130.2, 128.7, 128.3, 123.1, 116.4, 115.9, 115.7, 74.6. IR: 3429, 2919, 2850, 2213, 1624, 1471, 1367, 1130, 844 cm−1. MS (EI) m/z (%) = 370 ([M]+, 90), 321 (100), 265 (23). HRMS: m/z (EI) for C19H14N8O; calcd. 370.1285; found: 370.1285.
5-Amino-3-(4-hydroxyphenylazo)-2-methyl-7-phenylpyrazolo[1,5-a]pyrimidine-6-carbonitrile (30b). Yellow solid (0.23g, 63%). Mp 302–303 °C. 1H-NMR (DMSO-d6) δ 9.97 (br, s, 1H, OH), 9.00 (br, 2H, NH2), 7.87 (dd, 2H, J = 8.4 and 1.4 Hz), 7.67(d, 2H, J = 8.4 Hz), 7.57–7.54 (m, 3H), 6.89 (d, 2H, J = 8.4), 2.68 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 160.9, 159.5, 150.4, 148.7, 146.3, 143.9, 137.3, 130.2, 128.7, 128.3, 126.1, 123.5, 116.1, 115.7, 74.3, 15.5. IR: 3431, 3304, 3235, 3166, 2213, 1643, 1593, 1449, 1287, 1148, 842 cm−1. MS (EI) m/z (%) = 369 ([M]+, 100), 276 (43), 222 (18). HRMS: m/z (EI) for C20H15N7O; calcd. 369.1332; found: 369.1332.
3.5. High Temperature Dyeing Method (HT)
3.5.1. Materials
Scoured and bleached 100% polyester (150, 130 g/m2, 70/2 denier) was obtained from El-Shourbagy Co., Cairo, Egypt. The fabric was treated before dyeing with a solution containing nonionic detergent (Hostapal CV, Clariant-Egypt, 5 g/L) and sodium carbonate (2 g/L) in a ratio of 50:1 at 60 °C for 30 min, thoroughly washed with water, and air dried at room temperature.
3.5.2. Dyeing
A dispersion of the dye was produced by dissolving the appropriate amount of dye (1%–6% shades) in 1 cm3 acetone and then added dropwise with stirring to the dyebath (liquor ration 20:1) containing sodium lignin sulphonate as dispersing agent. The pH of the dyebath was adjusted to 5.5 using aqueous acetic acid and the wetted-out polyester fabrics were added. Dyeing was performed by raising the dyebath temperature to 130 °C under pressure in a microwave oven at a rate of 20 °C/min, holding at this temperature for 60 min and rapidly cooling to 50 °C. After dyeing, the fabrics were thoroughly washed and subjected to surface reduction clearing [(5 g NaOH + 6 g sodium hydrosulphite)/L]. The samples were heated in this solution for 10 min at 60 °C and then thoroughly washed and air-dried.
3.6. Color Measurements and Analyses
3.6.1. Color Measurements of the Dyed Fabrics
The color yields of the dyed samples were determined by using the light reflectance technique performed on a Perkin-Elmer (Lambda 3B) UV/VIS Spectrophotometer. The color strengths, expressed as K/S values, were determined by applying the Kubelka-Mink equation:
| K/S = [(1 − R)2/2R] − [(1 − Ro)2/2Ro] |
where R = decimal fraction of the reflectance of the dyed fabric, Ro = decimal fraction of the reflectance of the undyed fabric, K = absorption coefficient, and S = scattering coefficient.
3.6.2. Color Fastness Tests
3.6.2.1. Fastness to Washing
After washing using 2 g/L of the nonionic detergent Hostapal CV at 80 °C for 15 min, the dyed fabrics were tested by using ISO standard methods [26]. A specimen of dyed polyester fabric was stitched between two pieces of undyed cotton and wool fabrics, all of equal length, and then washed at 95 °C for 30 min. The staining on the undyed adjacent fabrics was assessed according to the following gray scale: 1—poor, 2—fair, 3—moderate, 4—good, 5—excellent.
3.6.2.2. Fastness to Perspiration
The samples were prepared by stitching a piece of dyed polyester fabric between two pieces of cotton and wool fabrics, all of equal length, and then immersed in the acid or alkaline solution for 30 min. The staining on the undyed adjacent fabrics was assessed according to the following gray scale: 1—poor, 2—fair, 3—moderate, 4—good, 5—excellent. The acid solution (pH = 3.5) contains sodium chloride (10 g/L), sodium dihydrogen orthophosphate (1 g/L) and histidine monohydrochloride (0.25 g/L). The alkaline solution (pH = 8) contains sodium chloride (10 g/L), disodium orthophosphate (1 g/L) and histidine monohydrochloride (0.25 g/L).
3.6.2.3. Fastness to Light
Light fastness was determined by exposing the dyed polyester fabrics on a Xenotest 150 (Original Hanau, city, country). Chamber temperature: 25–30 °C, black panel temperature: 60 °C, relative humidity: 50–60%, dark glass UV filter system) for 40 h. The changes in color were assessed according to the following blue scale: 1—poor, 3—moderate, 4—good, 6—very good, 8—excellent.
4. Conclusions
This study describes the synthesis of some new monoazo disperse pyrazolopyrimidine dyes, which involves initial coupling of malononitrile or 3-aminocrotononitrile with 4-hydroxybenzenediazonium chlorides, subsequent treatment of the hydrazone products with hydrazine hydrate gave the corresponding 4-hydroxyphenylazoaminopyrazoles that were then treated with either 2,4-pentandione or arylenaminoketones to give the target pyrazolopyrimidine monoazo disperse dyes. The dyes produced in this manner were then applied to polyester fabrics using the HT dyeing method assisted by microwave irradiation. The dyed fabrics, which display yellowish brown to orange hues, were found to have moderate fastness to light and excellent fastness levels to washing and perspiration.
Acknowledgments
The support of the Public Authority for Applied Education and Training, for financing through project (HS-08-02) and the facilities of AnaLab (SAF) at Kuwait University are gratefully acknowledged.
Footnotes
Sample Availability: Samples of compounds 7, 10, 13, 14a, 14b, 20a, b, 22a–j and 30a, 30b are available from the authors.
References and Notes
- 1.Ertan N. Synthesis of some hetarylazopyrazolone dyes and solvent effects on their absorption spectra. Dyes Pigments. 1999;44:41–48. doi: 10.1016/S0143-7208(99)00066-2. [DOI] [Google Scholar]
- 2.Khalil A.K., Hassan M.A., Mohamed M.M., El-Sayed A.M. Metal salt-catalyzed diazocoupling of 3-substituted-1H-pyrazol-2-in-5-ones in aqueous medium. Dyes Pigments. 2005;66:241–245. doi: 10.1016/j.dyepig.2004.10.005. [DOI] [Google Scholar]
- 3.Genin M.J., Biles C., Keiser B.J., Poppe S.M., Swaney S.M., Tarpley W.G. Novel 1,5- diphenylpyrazole nonnucleoside HIV-1 reverse transcriptase inhibitors with enhanced activity versus the delavirdine-resistant P236L mutant: Lead identification and sar of 3- and 4-substituted derivatives. J. Med. Chem. 2000;43:1034–1040. doi: 10.1021/jm990383f. [DOI] [PubMed] [Google Scholar]
- 4.Kandil S.S., Abdel-Hay F.I., Issa R.M. Thermal studies of cobalt(II), nickel(II) and copper(II) complexes of 4-(sulfonylazido phenylazo)pyrazolones. J. Therm. Anal. Calor. 2001;63:173–180. doi: 10.1023/A:1010148706396. [DOI] [Google Scholar]
- 5.Tsai P.C., Wang I.J. Synthesis and solvatochromic properties of some disazo dyes derived from pyrazolo[1,5-a]pyrimidine derivatives. Dyes Pigments. 2005;64:259–264. doi: 10.1016/j.dyepig.2004.05.013. [DOI] [Google Scholar]
- 6.Rangnekar D.W., Puro S.S. Synthesis and dyeing characteristics of new 2-methyl-3-arylazo-6-phenyl-7-amino- and 7-acetamidopyrazolo[1,5-a]pyrimidines. Ind. J. Fibre Textile Res. 1990;15:37–38. [Google Scholar]
- 7.Rangnekar D.W., Puro S.S. Synthesis and dyeing characteristics of 3,6-bis(arylazo)pyrazolo-[1,5-a]pyrimidines. Ind. J. Fibre Textile Res. 1990;15:23–25. [Google Scholar]
- 8.Krystof V., Cankar P., Frysova I., Slouka J., Kontopidis G., Dzubak P., Hajduch M., Srovnal J., de Azevedo W.F.J., Orsag M., et al. 4-Arylazo-3,5-diamino-1H- pyrazole CDK inhibitors: SAR study, crystal structure in complex with CDK2, selectivity, and cellular effects. J. Med. Chem. 2006;49:6500–6509. doi: 10.1021/jm0605740. [DOI] [PubMed] [Google Scholar]
- 9.Ho Y.W. Synthesis of some new azo pyrazolo[1,5-a]pyrimidine-thieno[2,3-b]pyridine derivatives and their application as disperse dyes. Dyes Pigments. 2005;64:223–230. doi: 10.1016/j.dyepig.2004.06.007. [DOI] [Google Scholar]
- 10.Tsai P.C., Wang I.J. Synthesis and solvatochromic properties of 3,6-bis-hetarylazo dyes derived from pyrazolo[1,5-a]pyrimidine. Dyes Pigments. 2008;76:575–581. doi: 10.1016/j.dyepig.2007.01.005. [DOI] [Google Scholar]
- 11.Karcı F., Demirçalı A. Synthesis of disazo pyrazolo[1,5-a]pyrimidines. Dyes Pigments. 2007;74:288–297. doi: 10.1016/j.dyepig.2006.02.007. [DOI] [Google Scholar]
- 12.Tsai P.C., Wang I.J. A facile synthesis of some new pyrazolo[1,5-a]pyrimidine heterocyclic disazo dyes and an evaluation of their solvatochromic behaviour. Dyes Pigments. 2007;74:578–584. doi: 10.1016/j.dyepig.2006.03.022. [DOI] [Google Scholar]
- 13.Sayed A.Z., Aboul-Fetouh M.S., Nassar H.S. Synthesis, biological activity and dyeing performance of some novel azo disperse dyes incorporating pyrazolo[1,5-a]pyrimidines for dyeing of polyester fabrics. J. Mol. Struct. 2012;1010:146–151. doi: 10.1016/j.molstruc.2011.11.046. [DOI] [Google Scholar]
- 14.Al-Etaibi A.M., Al-Awadi N.A., El-Apasery M.A., Ibrahim M.R. Synthesis of some novel pyrazolo[1,5-a]pyrimidine derivatives and their application as disperse dyes. Molecules. 2011;16:5182–5193. doi: 10.3390/molecules16065182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Etaibi A.M., El-Apasery M.A., Mahmoud H.M., Al-Awadi N.A. One-pot synthesis of disperse dyes under microwave irradiation: Dyebath reuse in dyeing of polyester fabrics. Molecules. 2012;17:b4266–b4280. doi: 10.3390/molecules17044266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Dusouqui O.M.E., Abdelkhalik M.M., Al-Awadi N.A., Dib H.H., George B.J., Elnagdi M.H. Chemistry of 2-arylhydrazonals: Utility of substituted 2-arylhydrazono-3-oxoalkanals as precursors for 3-oxoalkanonitriles, 3-aminoisoxazole and 1,2,3- and 1,2,4-triazoles. J. Chem. Res. 2006;5:295–302. [Google Scholar]
- 17.CCDC 828902 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; Email: deposit@ccdc.cam.ac.uk).
- 18.Elfahham H.A., Elgemeie G.E.H., Ibraheim Y.R., Elnagdi M.H. Studies on 3,5-diaminopyrazoles: New routes for the synthesis of new pyrazoloazines and pyrazoloazoles. Liebig. Ann. Chem. 1988;8:819–822. [Google Scholar]
- 19.Andrews B.A.K., Blanchard E.J., Reinhardt R.M. Fabric whiteness retention in durable press finishing with citric acid. Text. Chem. Color. 1993;25:52–54. [Google Scholar]
- 20.El-Kholy Y.M., Abdel-Hafiz S.A., Ahmed S.H. Synthesis and dyeing properties of novel disperse dyes. Part 2: Pyrazole disperse dye derivatives. J. Soc. Dyers Colour. 1998;114:45–48. [Google Scholar]
- 21.Peters R.H., Sumner H.H. The affinities of vat dyes in relation to their constitutions. J. Soc. Dyers Colour. 1955;71:130–138. [Google Scholar]
- 22.Giles C.H., Rahman S.M.K. Studies on high fastness to light in coloring matters in hydrophobic substrates. Disperse dyes in cellulose acetates and polyesters. Textile Res. J. 1961;31:1012–1019. doi: 10.1177/004051756103101203. [DOI] [Google Scholar]
- 23.Shakra S., Ghattas A.A.G. The effect of hydrogen bond on the light fastness of azo dyes. Kolorisztikai Ertesito. 1979;21:287–294. [Google Scholar]
- 24.Ashkar S.M., El-Apasery M.A., Touma M.M., Elnagdi M.H. Synthesis of some novel biologically active disperse dyes derived from 4-methyl-2,6-dioxo-1-propyl-1,2,5,6-tetrahydropyridine-3-carbonitrile as coupling component and their colour assessment on polyester fabrics. Molecules. 2012;17:8822–8831. doi: 10.3390/molecules17088822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chipalkatti H.R., Desai N.F., Giles C.H., Macaulay N. The influence of the substrate upon the light fading of azo dyes. J. Soc. Dyers Colour. 1954;70:487–501. [Google Scholar]
- 26.Chrysler L.P. Methods of Test for Color Fastness of Textiles and Leather. 7th. Bradford; London, UK: 1990. pp. 89–94. [Google Scholar]