Abstract
The reaction of adamantane-1-carbohydrazide (1) with heterocyclic aldehydes, namely 5-(4-chlorophenyl)isoxazole-3-carboxaldehyde (2a), 5-(4-methylphenyl)isoxazole-3-carboxaldehyde (2b), 5-(4-methoxyphenyl)isoxazole-3-carboxaldehyde (2c), 1H-imidazole-2-carboxaldehyde and 2-butyl-4-chloro-1H-imidazole-5-carboxaldehyde, in ethanol, yielded the corresponding N′-heteroarylidene-1-adamantylcarbohydrazides 3a, 3b, 3c, 4 and 5, respectively, in good yields. The 4-acetyl-1,3,4-oxadiazoline analogues 6a‑c were prepared in 48–55% yields by heating their corresponding N′-heteroarylidene-1-adamantylcarbohydrazides 3a–c with acetic anhydride for two hours. Compounds 3a–c, 4, 5 and 6a–c were tested for in vitro activities against a panel of Gram-positive and Gram-negative bacteria and the yeast-like pathogenic fungus Candida albicans. Compounds 4 and 5 displayed potent broad-spectrum antimicrobial activity, while compounds 3a–c showed good activity against the Gram-positive bacteria.
Keywords: adamantane derivatives; 1,3,4-oxadiazoles; isoxazoles; antimicrobial activity
1. Introduction
The incorporation of an adamantyl moiety into a variety of molecules results in compounds with relatively high lipophilicity, which in turn can modify the biological availability of these molecules. In almost all cases, an adamantyl-bearing compound will be more lipophilic than the corresponding des-adamantyl analogue. Beyond increasing partition coefficients, the adamantyl group positively modulates the therapeutic index of many experimental compounds, through a variety of mechanisms [1,2]. Derivatives of adamantane have long been known for their antiviral activity against the influenza A [3,4,5,6] and HIV viruses [7,8,9]. Several adamantane derivatives were also associated with central nervous [10,11] and antimicrobial [12,13,14,15,16] properties. In addition, several 1,3,4-oxadiazole derivatives [17,18] and isoxazole derivatives [19,20] were reported to possess significant antimicrobial activity. In continuation to our interest in the chemical and pharmacological properties of adamantane derivatives [8,14,15,16,21,22,23,16,21], we report herein the synthesis, antimicrobial activity of new series of N’-heteroarylidene-1-adamantylcarbohydrazides and (±)-2-(1-adamantyl)-4-acetyl-5-[5-(4-substituted phenyl- 3-isoxazolyl)]-1,3,4-oxadiazolines.
2. Results and Discussion
2.1. Chemistry
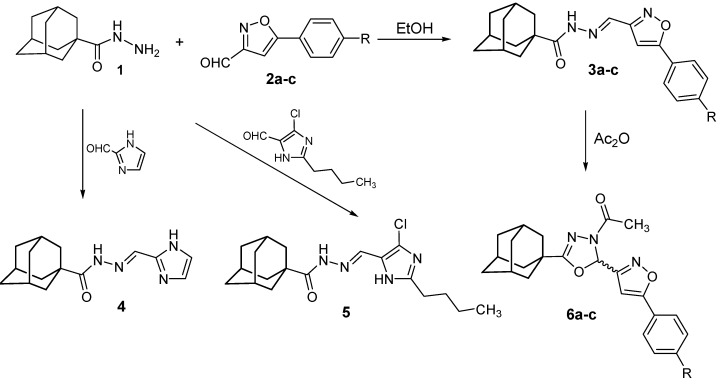
The adamantane-1-carbohydrazide (1) required as starting material, was prepared starting with adamantane-1-carboxylic acid via esterification with methanol to yield the methyl ester, which was subsequently reacted with hydrazine to yield adamantane-1-carboxylic acid hydrazide [21,24]. Adamantane-1-carbohydrazide (1) was next allowed to react in ethanol with heterocyclic aldehydes, namely 5-(4-chlorophenyl)isoxazole-3-carboxaldehyde (2a), 5-(4-methylphenyl)isoxazole-3-carbox-aldehyde (2b), 5-(4-methoxyphenyl)isoxazole-3-carboxaldehyde (2c), 1H-imidazole-2-carboxaldehyde and 2-butyl-4-chloro-1H-imidazole-5-carboxaldehyde, to yield the corresponding N′-heteroarylidene-1-adamantylcarbohydrazides 3a–c, 4 and 5, respectively, in good yield. The reaction of N-arylidene carboxylic acid hydrazides with acetic anhydride was reported to afford the corresponding N-acetyl-1,3,4-oxadiazoline derivatives [25,26,27,28]. Thus, compounds 3a–c were successfully cyclized to their 4-acetyl-1,3,4-oxadiazoline analogues 6a–c in 48–55% yield by heating with acetic anhydride for two hours (Scheme 1).
Scheme 1.
Synthesis of compounds 3a–c, 4, 5 and 6a–c.
Attempted reaction of the imidazole derivatives 4 or 5 with acetic anhydride under the same conditions failed to yield their N-acetyl-1,3,4-oxadiazoline derivatives. Meanwhile, increasing the reaction time up to four hours was also unsuccessful and the reactions afforded unidentified dark tarry materials. The structures of newly synthesized compounds 3a–c, 4, 5 and 6a–c were confirmed by 1H-NMR, 13C-NMR and ESI-MS spectral data, in addition to the X-ray crystallography of compound 5 [29].
2.2. Antimicrobial Testing
The newly synthesized compounds 3a–c, 4, 5 and 6a–c were tested for their in vitro growth inhibitory activity against the standard strains of the Institute of Fermentation of Osaka (IFO) namely; Staphylococcus aureus IFO 3060, Bacillus subtilis IFO 3007, Micrococcus luteus IFO 3232 (Gram-positive bacteria), Escherichia coli IFO 3301, Pseudomonas aeuroginosa IFO 3448 (Gram-negative bacteria), and the yeast-like pathogenic fungus Candida albicans IFO 0583. The primary screening was carried out using the agar disc-diffusion method using Müller-Hinton agar medium [30]. The results of the preliminary antimicrobial testing of compounds 3a–c, 4, 5 and 6a-c (200 µg/8 mm disc), the antibacterial antibiotics gentamicin (100 µg/8 mm disc), ampicillin (100 µg/8 mm disc) and the antifungal drug clotrimazole (100 µg/8 mm disc) and the calculated log P values (Clog P) of the tested compounds (calculated using the CS ChemOffice Ultra, version 8.0, CambridgeSoft, Cambridge, MA, USA) are shown in Table 1.
Table 1.
Antimicrobial activity of compounds 3a–c, 4, 5 and 6a–c (200 µg/8 mm disc), the broad spectrum antibacterial antibiotics gentamicin (100 µg/8 mm disc), ampicillin (100 µg/8 mm disc) and the antifungal drug clotrimazole (100 µg/8 mm disc) against Staphylococcus aureus IFO 3060 (SA), Bacillus subtilis IFO 3007 (BS), Micrococcus luteus IFO 3232 (ML), Escherichia coli IFO 3301(EC), Pseudomonas aeuroginosa IFO 3448 (PA), and Candida albicans IFO 0583 (CA).
| Comp. No. | Clog P | Diameter of Growth Inhibition Zone (mm) * | |||||
|---|---|---|---|---|---|---|---|
| SA | BS | ML | EC | PA | C A | ||
| 3a | 4.96 | 19(16) † | 22(8) † | 17 | 11 | - | - |
| 3b | 4.74 | 18(16) † | 18(16) † | 14 | - | - | - |
| 3c | 4.24 | 17 | 18(16) † | 13 | - | - | - |
| 4 | 2.30 | 32(0.5) † | 26(0.5) † | 23(8) † | 16 | 11 | 17 |
| 5 | 3.89 | 28(1) † | 30(1) † | 22(4) † | 19(8) † | 14 | 16 |
| 6a | 5.20 | 12 | 11 | 11 | - | - | - |
| 6b | 4.95 | - | - | - | - | - | - |
| 6c | 4.48 | - | - | - | - | - | - |
| Gentamicin | 26(2) † | 25(2) † | 18(2) † | 20(0.5) † | 19(1) † | NT | |
| Ampicillin | 23(2) † | 21(0.5) † | 19(2) † | 17(2) † | 16(2) † | NT | |
| Clotrimazole | NT | NT | NT | NT | NT | 21 | |
* (-): Inactive (inhibition zone ≤ 10 mm); (NT): Not tested; † The figures shown in parentheses represent the MIC values (µg/mL).
The antimicrobial activity results of the synthesized compounds (Table 1) revealed that the tested compounds showed varying degrees of inhibition against the tested microorganisms. In general, it was observed that the antibacterial activity is not dependent on their lipophilicity. The antibacterial activity of the tested N’-heteroarylidene-1-adamantylcarbohydrazides 3a–c, 4 and 5 mainly depended on the heterocyclic nucleus. The imidazole derivatives 4 and 5 displayed higher and broad-spectrum activity than the isoxazole derivatives 3a–c, which exhibited potent or moderate activity against the tested Gram-positive bacteria. The cyclization of the N’-heteroarylidene analogues 3a–c to their 4-acetyl-1,3,4-oxadiazoline analogues 6a–c resulted in a dramatic decrease in the antibacterial activity, and only compound 6a retained marginal activity against the tested Gram-positive bacteria (Table 1). The minimal inhibitory concentration (MIC) for the active compounds 3a–c, 4 and 5 against the same microorganism used in the primary screening was carried out using the microdilution susceptibility method in Müller-Hinton Broth and Sabouraud Liquid Medium [31]. The MIC of the compounds 3a-c, 4 and 5, the antibacterial antibiotics gentamicin and ampicillin trihydrate which are shown in Table 1, were in accordance with the results obtained in the primary screening.
3. Experimental
3.1. General
Melting points (°C) were measured in open glass capillaries using a Branstead 9001 electrothermal melting point apparatus and are uncorrected. NMR spectra were obtained on a Brüker AC 500 Ultra Shield NMR spectrometer (Brüker BioSpin AG, Fällanden, Switzerland) operating at 500.13 MHz for 1H and 125.76 MHz for 13C; the chemical shifts are expressed in δ (ppm) downfield from tetramethylsilane (TMS) as internal standard; coupling constants (J) are expressed in Hz. Electrospray ionization mass spectra (ESI-MS) were recorded on an Agilent 6410 Triple Quad tandem mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) at 4.0 and 3.5 kV for positive and negative ions, respectively. Elemental analyses (C, H, N) were in full agreement with the proposed structures within ±0.4% of the theoretical values. Monitoring the reactions and checking the purity of the final products were carried out by thin layer chromatography (TLC) using silica gel precoated aluminum sheets (60 F254, Merck) and visualization with ultraviolet light (UV) at 365 and 254 nm. The bacterial strains and Candida albicans fungus were obtained from the Institute of Fermentation of Osaka (IFO, Osaka, Japan). The reference drugs gentamicin (CAS 1405-41-0), ampicillin trihydrate (CAS 7177-48-2) and clotrimazole (CAS 23593-75-1) were obtained from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany).
3.2. N′-Heteroarylidene-1-adamantylcarbohydrazides 3a–c, 4 and 5
The appropriate heterocyclic aldehyde 2a–c (0.01 mol) was added to a solution of adamantane-1-carbohydrazide 1 (1.94 gm, 0.01 mol) in ethanol (10 mL), and the mixture was heated under reflux for 3 hours and allowed to stand at room temperature for overnight. The separated precipitate was filtered, washed with water, dried and crystallized from ethanol (compounds 3a–c) or aqueous ethanol (compounds 4, 5).
N′-[5-(4-Chlorophenyl)isoxazol-3-yl)methylidene]adamantane-1-carbohydrazide (3a): M.p.: 223–225 °C, Yield: 3.46 gm (90%). 1H-NMR (DMSO-d6): δ 1.71 (s, 6H, adamantane-H), 1.89 (s, 6H, adamantane-H), 2.02 (s, 3H, adamantane-H), 7.37 (s, 1H, isoxazole-H), 7.60 (d, 2H, Ar-H, J = 8.0 Hz), 7.98 (d, 2H, Ar-H, J = 8.0 Hz), 8.52 (s, 1H, CH=N), 11.21 (s, 1H, NH). 13C-NMR: δ 27.48, 35.95, 38.13, 39.99 (adamantane-C), 97.82, 161.25, 168.36 (isoxazole-C), 125.28, 127.58, 129.31, 135.29 (Ar-C), 136.14 (CH=N), 173.52 (C=O). ESI-MS, m/z (Rel. Int.): 384.9 (M− +2, 34), 382.9 (M−, 100).
N′-[5-(4-Methylphenyl)isoxazol-3-yl)methylidene]adamantane-1-carbohydrazide (3b): M.p.: 201–203 °C, Yield: 3.13 gm (86%). 1H-NMR (DMSO-d6): δ 1.71 (s, 6H, adamantane-H), 1.89 (s, 6H, adamantane-H), 2.02 (s, 3H, adamantane-H), 2.37 (s, 3H, CH3), 7.22 (s, 1H, isoxazole-H), 7.34 (d, 2H, Ar-H, J = 7.5 Hz), 7.83 (d, 2H, Ar-H, J = 7.5 Hz), 8.52 (s, 1H, CH=N), 11.20 (s, 1H, NH). 13C-NMR: δ 20.97 (CH3), 27.18, 35.95, 38.14, 38.98 (adamantane-C), 96.51, 161.08, 169.64 (isoxazole-C), 123.78, 125.70, 129.74, 136.36 (Ar-C), 140.55 (CH=N), 173.53 (C=O). ESI-MS, m/z (Rel. Int.): 362.2 (M−, 100).
N′-[5-(4-Methoxyphenyl)isoxazol-3-yl)methylidene]adamantane-1-carbohydrazide (3c): M.p.: 199–201 °C, Yield: 3.26 gm (86%). 1H-NMR (DMSO-d6): δ 1.71 (s, 6H, adamantane-H), 1.89 (s, 6H, adamantane-H), 2.02 (s, 3H, adamantane-H), 3.83 (s, 3H, OCH3), 7.08 (d, 2H, Ar-H, J = 7.0 Hz), 7.15 (s, 1H, isoxazole-H), 7.89 (d, 2H, Ar-H, J = 7.0 Hz), 8.51 (s, 1H, CH=N), 11.19 (s, 1H, NH). 13C-NMR: δ 27.48, 35.95, 38.14, 38.98 (adamantane-C), 55.34 (OCH3), 95.60, 161.05, 169.54 (isoxazole-C), 114.61, 119.12, 127.49, 160.98 (Ar-C), 136.44 (CH=N), 173.51 (C=O). ESI-MS, m/z (Rel. Int.): 378.2 (M−, 100).
N′-[(1H-Imidazol-2-yl)methylidene]adamantane-1-carbohydrazide (4): M.p.: 210–212 °C, Yield: 1.69 gm (62%). 1H-NMR (DMSO-d6): δ 1.70–1.76 (m, 6H, adamantane-H), 1.88–1.91 (m, 6H, adamantane-H), 2.01 (s, 3H, adamantane-H), 7.08–7.42 (m, 2H, imidazole-H), 8.31 (s, 1H, CH=N), 10.81 (s, 1H, NH), 13.67 (s, 1H, imidazole-NH). 13C-NMR: δ 27.53, 35.99, 38.29, 38.68 (adamantane-C), 128.95, 129.37, 138.58 (imidazole-C), 141.33 (CH=N), 173.10 (C=O). ESI-MS, m/z (Rel. Int.): 271.1 (M−, 100).
N′-[(2-Butyl-4-chloro-1H-imidazol-5-yl)methylidene]adamantane-1-carbohydrazide (5): M.p.: 188.90 °C, Yield: 2.58 gm (71%). 1H-NMR (DMSO-d6): δ 0.86 (t, 3H, CH3, J = 7.0 Hz), 1.05–1.08 (m, 2H, CH2CH3), 1.25–1.29 (m, 2H, CH2CH2CH3), 1.58–1.61 (m, 2H, CH2CH2CH2CH3), 1.69 (s, 6H, adamantane-H), 1.86 (s, 6H, adamantane-H), 2.0 (s, 3H, adamantane-H), 8.27 (s, 1H, CH=N), 10.72 (s, 1H, NH), 12.76 (s, 1H, imidazole-NH). 13C-NMR: δ 13.51 (CH3), 18.49 (CH2CH3), 21.51 (CH2CH2CH3), 27.28 (CH2CH2CH2CH3), 27.56, 29.80, 36.01, 38.33 (adamantane-C), 120.73, 130.45, 134.97 (imidazole-C), 150.67 (CH=N), 172.91 (C=O). ESI-MS, m/z (Rel. Int.): 363.2 (M− +2, 30), 361.2 (M−, 100).
3.3. (±)-2-(1-Adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines 6a–c
A mixture of the appropriate N’-heteroarylidene-1-adamantylcarbohydrazides 3a–c (5.0 mmol) and acetic anhydride (8 mL) was heated under reflux for 2 hours. The excess acetic anhydride was then evaporated under reduced pressure and ice-water (50 mL) was added to the resulted oily or sticky residue and refrigerated for 2 hours. The separated solid was filtered, washed with water, dried and crystallized from aqueous ethanol to yield the products 6a–c in 48–55% yield.
(±)-2-(1-Adamantyl)-4-acetyl-5-[5-(4-chlorophenyl-3-isoxazolyl)]-1,3,4-oxadiazoline (6a): M.p.: 131–133 °C, Yield: 2.34 gm (55%). 1H-NMR (DMSO-d6): δ 1.70 (br. s, 3H, adamantane-H), 1.89–2.01 (m, 12H, adamantane-H), 2.09 (s, 3H, COCH3), 7.18 (s, 1H, isoxazole-H), 7.19 (s, 1H, oxadiazole-H), 7.62 (d, 2H, Ar-H, J = 8.5 Hz), 7.95 (d, 2H, Ar-H, J = 8.5 Hz). 13C-NMR: δ 21.02 (COCH3), 26.93, 30.66, 35.67, 38.38 (adamantane-C), 83.96, 163.50 (oxadiazole-C), 98.62, 164.50, 169.50 (isoxazole-C), 124.50, 127.57, 120.37, 134.50 (Ar-C), 171.98 (C=O). ESI-MS, m/z (Rel. Int.): 451.3 (M+ +2+Na, 22), 449.3 (M+ +Na, 100).
(±)-2-(1-Adamantyl)-4-acetyl-5-[5-(4-methylphenyl-3-isoxazolyl)]-1,3,4-oxadiazoline (6b): M.p.: 126–128 °C, Yield: 2.07 gm (51%). 1H-NMR (DMSO-d6): δ 1.69 (s, 6H, adamantane-H), 1.86 (s, 6H, adamantane-H), 1.99 (s, 3H, adamantane-H), 2.17 (s, 3H, COCH3), 2.37 (s, 3H, CH3), 7.03 (s, 1H, isoxazole-H), 7.16 (s, 1H, oxadiazole-H), 7.34 (d, 2H, Ar-H, J = 7.5 Hz), 7.79 (d, 2H, Ar-H, J = 7.5 Hz). 13C-NMR: 20.91 (COCH3), 20.97 (CH3), 26.94, 33.44, 35.69, 38.37 (adamantane-C), 83.68, 161.06 (oxadiazole-C), 97.33, 163.87, 168.59 (isoxazole-C), 123.64, 125.66, 129.76, 140.72 (Ar-C), 170.52 (C=O). ESI-MS, m/z (Rel. Int.): 429.3 (M+ +1+Na, 100), 406.3 (M+, 22).
(±)-2-(1-Adamantyl)-4-acetyl-5-[5-(4-methoxyphenyl-3-isoxazolyl)]-1,3,4-oxadiazoline (6c): M.p.: 119–121 °C, Yield: 2.02 gm (48%). 1H-NMR (DMSO-d6): δ 1.70 (s, 6H, adamantane-H), 1.89 (s, 6H, adamantane-H), 2.0–2.02 (m, 3H, adamantane-H), 2.17 (s, 3H, COCH3), 3.83 (s, 3H, OCH3), 6.95 (s, 1H, isoxazole-H), 7.08 (s, 1H, oxadiazole-H), 7.14 (d, 2H, Ar-H, J = 8.5 Hz), 7.89 (d, 2H, Ar-H, J = 8.5 Hz). 13C-NMR: 20.0 (COCH3), 27.48, 33.44, 35.95, 38.14 (adamantane-C), 55.36 (OCH3), 83.71, 163.89 (oxadiazole-C), 96.39, 166.49, 169.55 (isoxazole-C), 114.62, 118.97, 127.49, 161.06 (Ar-C), 173.53 (C=O). ESI-MS, m/z (Rel. Int.): 422.4 (M+, 100).
3.4. Determination of the Antimicrobial Activity by the Agar Disc-Diffusion Method [30]
Sterile filter paper discs (8 mm diameter) were moistened with the compound solution in dimethylsulphoxide of specific concentration (200 µg/disc), the antibacterial antibiotics gentamicin and ampicillin trihydrate (100 µg/disc) and the antifungal drug clotrimazole (100 µg/disc) were carefully placed on the agar culture plates that had been previously inoculated separately with the microorganisms. The plates were incubated at 37 °C, and the diameter of the growth inhibition zones were measured after 24 hours in case of bacteria and 48 h in case of Candida albicans.
3.5. Determination of Minimal Inhibitory Concentrations (MICs) [31]
Compounds 3a–c, 4, 5, gentamicin and ampicillin trihydrate were dissolved in dimethylsulphoxide at the concentration of 128 µg/mL. The twofold dilutions of the solution were prepared (128, 64, 32, 16, 8, 4, 2, 1 and 0.5 µg/mL). The microorganism suspensions at 106 CFU/mL (colony forming unit/mL) concentrations were inoculated to the corresponding wells. The plates were incubated at 36 °C for 24. The MIC values were determined as the lowest concentration that completely inhibited visible growth of the microorganism as detected by unaided eye.
4. Conclusions
In this study, the synthesis and characterization of a series of N′-heteroarylidene-1-adamantylcarbohydrazides (compounds 3a–c, 4, 5) and 2-(1-adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines (compounds 6a–c) was described. The structure-antimicrobial activity relationship studies revealed that the N’-heteroarylidene-1-adamantyl-carbohydrazides were viable leads for further studies.
Acknowledgements
The financial support of the Deanship of Scientific Research and the Research Center of the College of Pharmacy, King Saud University is greatly appreciated. The authors are grateful to El-Sayed E. Habib, Department of Microbiology, Faculty of Pharmacy, University of Mansoura, Egypt, for performing the antimicrobial testing.
Footnotes
Sample Availability: Contact the corresponding author.
References
- 1.Lamoureux G., Artavia G. Use of the adamantane structure in medicinal chemistry. Curr. Med. Chem. 2010;17:2967–2978. doi: 10.2174/092986710792065027. [DOI] [PubMed] [Google Scholar]
- 2.Liu J., Obando D., Liao V., Lifa T., Codd R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011;46:1949–1963. doi: 10.1016/j.ejmech.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 3.Davies W.L., Grunnert R.R., Haff R.F., McGahen J.W., Neumeyer E.M., Paulshock M., Watts J.C., Wood T.R., Hermann E.C., Hoffmann C.E. Antiviral activity of 1-adamantamine (amantadine) Science. 1964;144:862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- 4.Vernier V.G., Harmon J.B., Stump J.M., Lynes T.L., Marvel M.P., Smith D.H. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol. Appl. Pharmacol. 1969;15:642–665. doi: 10.1016/0041-008X(69)90066-0. [DOI] [PubMed] [Google Scholar]
- 5.Togo Y., Hornick R.B., Dawkins A.T. Studies on induced influenza in man. I. Double blind studies designed to assess prophylactic efficacy of amantadine hydrochloride against A2/Rockville/1/65 strain. J. Am. Med. Assoc. 1968;203:1089–1094. doi: 10.1001/jama.203.13.1089. [DOI] [PubMed] [Google Scholar]
- 6.Zoidis G., Kolocouris N., Naesens E., De Clercq E. Design and synthesis of 1,2-annulated adamantane piperidines with anti-influenza virus activity. Bioorg. Med. Chem. 2009;17:1534–1541. doi: 10.1016/j.bmc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Van Derpoorten K., Balzarini J., De Clercq E., Poupaert J.H. Anti-HIV activity of N-1-adamantyl-4-aminophthalimide. Biomed. Pharmacother. 1997;51:464–468. doi: 10.1016/S0753-3322(97)82327-X. [DOI] [PubMed] [Google Scholar]
- 8.El-Emam A.A., Al-Deeb O.A., Al-Omar M.A., Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thione. Bioorg. Med. Chem. 2004;12:5107–5113. doi: 10.1016/j.bmc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Balzarini J., Orzeszko B., Mauri J.K., Orzeszko A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007;42:993–1003. doi: 10.1016/j.ejmech.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.10. Abou-Gharbia M.A., Childers W.E., Jr., Fletcher H., McGaughey G., Patel U., Webb M.B., Yardley J., Andree T., Boast C., Kucharik R.J., Jr., et al Synthesis and SAR of adatanserin: novel adamantly aryl- and heteroarylpiperazines with dual serotonin 5-HT1A and 5-HT2 activity as potential anxiolytic and antidepressant agents. J. Med. Chem. 1999;42:5077–5094. doi: 10.1021/jm9806704. [DOI] [PubMed] [Google Scholar]
- 11.Owen J.C.E., Whitton P.S. Effect of amantadine and budipune on antidepressant drug-evoked changes in extracellular dopamine in the frontal cortex of freely moving rats. Brain Res. 2006;1117:206–212. doi: 10.1016/j.brainres.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 12.Omar K., Geronikaki A., Zoumpoulakis P., Camoutsis C., Soković M., Ćirić A., Glamoćlija J. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg. Med. Chem. 2010;18:426–432. doi: 10.1016/j.bmc.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 13.Orzeszko A., Kamińska B., Starościak B.J. Synthesis and antimicrobial activity of new adamantane derivatives III. Il Farmaco. 2002;57:619–624. doi: 10.1016/S0014-827X(02)01199-0. [DOI] [PubMed] [Google Scholar]
- 14.14. Al-Deeb O.A., Al-Omar M.A., El-Brollosy N.R., Habib E.E., Ibrahim T.M., El-Emam A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]acetic acids, 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]propionic acids and related derivatives. Arzneim.-Forsch./Drug Res. 2006;56:40–47. doi: 10.1055/s-0031-1296699. [DOI] [PubMed] [Google Scholar]
- 15.Kadi A.A., El-Brollosy N.R., Al-Deeb O.A., Habib E.E., Ibrahim T.M., El-Emam A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazole. Eur. J. Med. Chem. 2007;42:235–242. doi: 10.1016/j.ejmech.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Al-Omar M.A., Al-Abdullah E.S., Shehata I.A., Habib E.E., Ibrahim T.M., El-Emam A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 5-(1-adamantyl)-4-arylideneamino-3-mercapto-1,2,4-triazoles and related derivative. Molecules. 2010;15:2526–2550. doi: 10.3390/molecules15042526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamolo M.G., Zampieri D., Vio L., Fermeglia M., Ferrone M., Pricl S., Scialino G., Banfi E. Antimycobacterial activity of new 3-substituted 5-(pyridin-4-yl)-3H-1,3,4-oxadiazol-2-one and 2-thione derivatives. Preliminary molecular modeling investigations. Bioorg. Med. Chem. 2005;13:3797–3809. doi: 10.1016/j.bmc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Macaev F., Rusu G., Bogrebnoi S., Gudima A., Stingaci E., Vlad L., Shvets N., Kandemirli F., Dimoglo A., Reynolds R. Synthesis of novel 5-aryl-2-thio-1,3,4-oxadiazoles and the study of their structure-anti-mycobacterial activities. Bioorg. Med. Chem. 2005;13:4842–4850. doi: 10.1016/j.bmc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Andrzejak V., Muccioli G.G., Body-Malapel M., El Bakali J., Djouina M., Renault N., Chavatte P., Desreumaux P., Lambert D.M., Millet R. New FAAH inhibitors based on 3-carboxamido-5-aryl-isoxazole scaffold that protect against experimental colitis. Bioorg. Med. Chem. 2011;19:3777–3786. doi: 10.1016/j.bmc.2011.04.057. [DOI] [PubMed] [Google Scholar]
- 20.Changtam C., Hongmanee P., Suksamrarn A. Isoxazole analogs of curcuminoids with highly potent multidrug-resistant antimycobacterial activity. Eur. J. Med. Chem. 2010;45:4446–4457. doi: 10.1016/j.ejmech.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 21.El-Emam A.A., Ibrahim T.M. Synthesis, anti-inflammatory and analgesic activity of certain 3-(1-adamantyl)-4-substituted-5-mercapto-1,2,4-triazole derivatives. Arzneim.-Forsch./Drug Res. 1991;41:1260–1264. [PubMed] [Google Scholar]
- 22.Kadi A.A., Al-Abdullah E.S., Shehata I.A., Habib E.E., Ibrahim T.M., El-Emam A.A. Synthesis, antimicrobial and anti-inflammatory activities of novel 5-(1-adamantyl)-1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010;45:5006–5011. doi: 10.1016/j.ejmech.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Al-Abdullah E.S., Shehata I.A., Al-Deeb O.A., El-Emam A.A. Microwave-assisted dehydrosulphurization: An efficient, solvent-free synthesis of 5-(1-adamantyl)-2-arylamino-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. Heterocycles. 2007;71:379–388. doi: 10.3987/COM-06-10906. [DOI] [Google Scholar]
- 24.Ficarra R., Ficarra P., Tommasini A., Fenech G., Pizzimenti F.C., Bisignano G. 1-Adamantanecarboxylic acid hydrazides with presumed antimicrobial activity. Boll. Chim. Farmaceutico. 1984;123:317–321. [PubMed] [Google Scholar]
- 25.Hassan G.S., El-Emam A.A., Gad L.M., Barghash A.M. Synthesis, antimicrobial and antiviral testing of some new 1-adamantyl analogues. Saudi Pharm. J. 2010;18:123–128. doi: 10.1016/j.jsps.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii M., Jorge S.D., de Oliveira A.A., Palace-Berl F., Sonehara I.Y., Pasqualoto K.F.M., Tavares L.C. Synthesis, molecular modeling and preliminary biological evaluation of a set of 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazole as potential antibacterial, anti-Trypanosomacruzi and antifungal agent. Bioorg. Med. Chem. 2011;19:6292–6301. doi: 10.1016/j.bmc.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Hamdi H., Passarelli V., Romerosa A. Synthesis, spectroscopy and electrochemistry of new 4-(4-acetyl-5-substituted-4,5-dihydro-1,3,4-oxodiazol-2-yl)methoxy)-2H-chromen-2-ones as a novel class of potential antibacterial and antioxidant derivatives. Compt. Rend. Chim. 2011;14:548–555. [Google Scholar]
- 28.Joshi S.D., Vagdevi H.M., Vaidya V.P., Gadaginamath G.S. Synthesis of new 4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: A novel class of potential antibacterial and antitubercular agents. Eur. J. Med. Chem. 2008;43:1989–1996. doi: 10.1016/j.ejmech.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Al-Tamimi A.-M.S., Bari A., Al-Omar M.A., El-Emam A.A., Ng S.W. N’-[(2-n-Butyl-4-chloro-1H-imidazol-5-yl)methylidene]adamantane-1-carbohydrazide sesquihydrate ethanol hemisolvate. Acta Cryst. 2010;E66:o2131. doi: 10.1107/S1600536810029260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C., Yolken R.H. In: Manual of Clinical Microbiology. Wood G.L., Washington J.A., editors. American Society for Microbiology; Washington, DC, USA: 1995. [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards (NCCLS). Approved standard document M7A . NCCLS; Villanova, PA, USA: 1985. [Google Scholar]