Abstract
A library of 24 6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides 10{1,2; 1–12} was prepared by a parallel solution-phase approach. The synthesis comprises a five-step transformation of itaconic acid (11) into 1-methyl and 1-phenyl substituted 6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxylic acids 17{1,2} followed by parallel amidation of 17{1,2} with a series of 12 aliphatic amines 18{1–12} to afford the corresponding carboxamides 10 in good overall yields and in 80–100% purity.
Keywords: parallel synthesis, pyrimidines, 2-(heteroaryl)ethylamines
1. Introduction
2-[(Hetero)aryl]ethylamines, such as dopamine, histamine, tryptamine, serotonin, and melatonin are representative chemical messengers playing a crucial role in biological processes [1]. Therefore, the preparation of libraries of their novel synthetic analogues is of particular interest and represents an important target in medicinal [2,3,4,5], synthetic organic, and combinatorial chemistry [6,7,8,9,10].
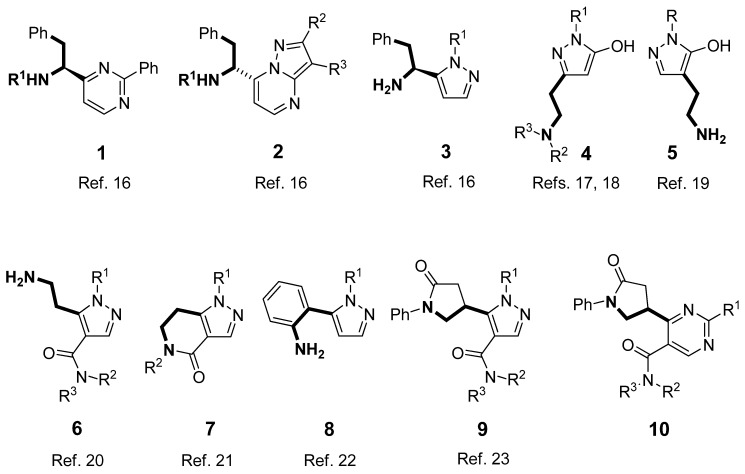
In the last two decades, alkyl 2-substituted 3-(dimethylamino)prop-2-enoates and related enaminones have proven to be easily available and versatile reagents for the preparation of various functionalized heterocycles [11,12,13,14,15]. Recently, a part of our research in this field has been focused on the synthesis of aminoethyl functionalized heterocycles. In this context, we first reported the synthesis of non-racemic 1-heteroaryl-2-phenylethylamines 1–3 from α-amino acid derived enaminoketones [16]. Further, the syntheses of the pyrazole analogues of histamine were developed: 2-aminoethyl substituted 1H-pyrazole derivatives 4–6 as the open-chain analogues [17,18,19,20] and 6,7-dihydro-1H-pyrazolo[4,3-c]pyridin-4(5H)-one derivatives 7 [21], 5-(2-aminophenyl)pyrazole derivatives 8 [22], and 5-(5-oxo-1-phenylpyrrolidin-3-yl)-1H-pyrazole-4-carboxamides 9 [23] as the conformationally constrained analogues of histamine. In continuation, we have focused our attention on 2-substituted 6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides 10 (Figure 1).
Figure 1.
Aminoethyl substituted heterocycles 1–10.
Herein, we report a parallel solution-phase synthesis of a library of 24 6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides 10{1,2; 1–12} as novel 2-heteroarylethylamine derivatives.
2. Results and Discussion
2.1. Synthesis of Title Compounds 10
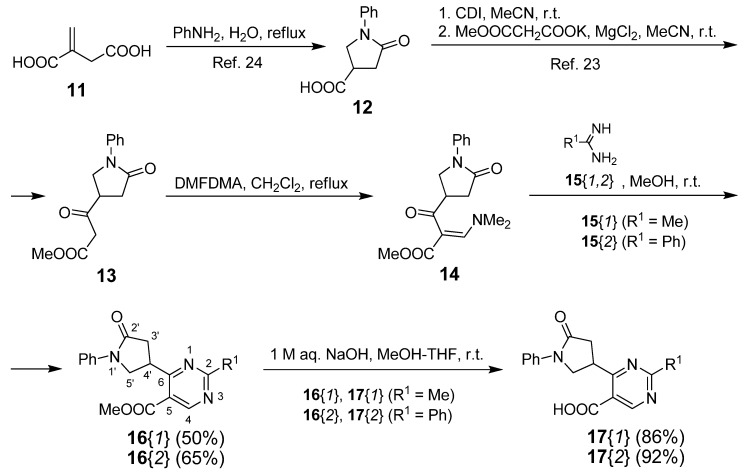
First, the starting compound 12 was prepared from commercially available itaconic acid (11) and aniline following the literature procedure [24]. Transformation of 12 into the enaminone 14 as the first key intermediate was performed following the literature protocol [23]: Masamune-Claisen condensation of 12 with 1,1'-carbonyldiimidazole (CDI) as activating agent in anhydrous acetonitrile at room temperature gave the β-keto ester 13, which when treated with N,N-dimethylformamide dimethylacetal (DMFDMA) in refluxing toluene gave the enaminone intermediate 14. Subsequent cyclisation of 14 with acetamidine 15{1} and benzamidine 15{2} afforded methyl 4-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxylates 16{1} and 16{2} in 50% and 65% yield, respectively. Finally, hydrolysis of 16{1} and 16{2} with 1 M aqueous NaOH in a mixture of methanol and THF at room temperature furnished the corresponding carboxylic acids 17{1} and 17{2} in 86% and 92% yield, respectively (Scheme 1).
Scheme 1.
Synthesis of 4-(5-Oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxylic Acids 17{1,2}.
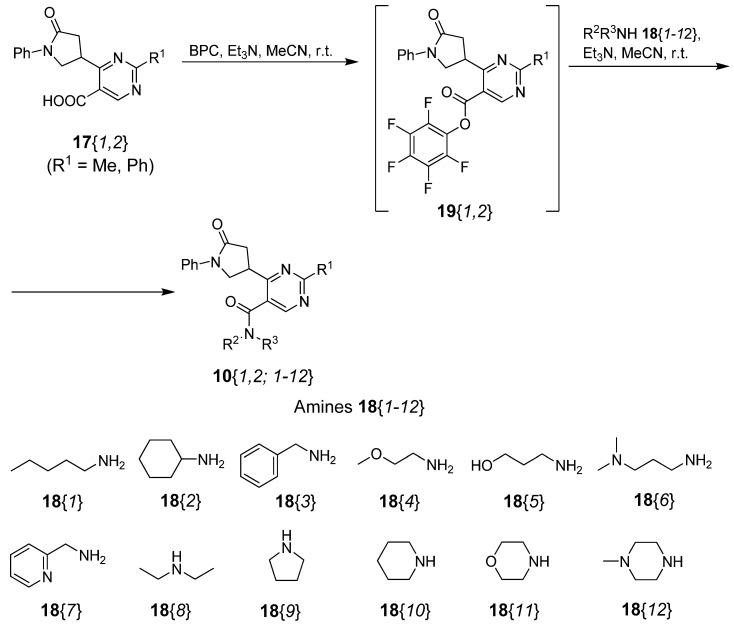
With the desired carboxylic acids 17{1,2} as the key-intermediates in our hands, a parallel solution-phase synthesis of 2-substituted 6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides 10 was studied. We supposed that the reaction conditions for the parallel amidation step as well as the workup protocol should be similar to those employed in the synthesis of closely related pyrazole analogues 9 [23]. Accordingly, bis(pentafluorophenyl) carbonate BPC was chosen as the reagent for activation of the carboxylic acids 17 and acetonitrile as the solvent. Preliminary amidations of 17{1,2} with benzylamine (18{3}) as the model primary amine proceeded smoothly to furnish the desired N-benzylcarboxamides 17{1; 3} and 17{2; 3}, which precipitated from the reaction mixtures and were isolated by filtration. Somewhat surprisingly, analogous amidations of 17{1,2} with diethylamine (18{8}) did not proceed to completion unless excess diethylamine (18{8}) was employed. The corresponding carboxamides 17{1; 8} and 17{2; 8} did not precipitate from the reaction mixtures and were isolated by evaporation of the reaction mixtures followed by purification by dry flash column chromatography (DFCC) [25,26] over aluminium oxide [27]. Consequently, the following procedure for parallel amidation was applied: the acids 17{1,2} were activated with triethylamine and bis(pentafluorophenyl) carbonate (BPC) in acetonitrile at room temperature to give the intermediate pentafluorophenyl esters 19{1,2}, which were subsequently treated with 1 equiv. of primary amines 18{1–7} or with 10 equiv. of secondary amines 18{8–12} at room temperature for 12 h. Nine products that precipitated from the reaction mixtures were isolated by filtration to afford carboxamides 10{1; 3,4} and 10{2; 1–7} in 28–100% yields and in 84–100% purity (Workup A). The rest of the products, which did not precipitate from the reaction mixtures, were isolated by evaporation of the reaction mixtures followed by purification of the residues by DFCC over aluminium oxide, and evaporation of the eluates to give compounds 10{1; 1,2,5–12} and 10{2; 8–12} in 40–100% yields and in 80–100% purity (Workup B). In this manner, all 24 carboxamides 10{1,2; 1–12} were successfully obtained in 18–100% yields and in 80–100% purity. Out of 24 library members, 17 were ≥95% pure and 7 were ≥80% pure (Scheme 2, Table 1).
Table 1.
Selected experimental data for compounds 10{1,2; 1–12}.
| Compd. | R1 | R2R3NH 18 | Workup [a] | Yield (%) | Purity (%) |
|---|---|---|---|---|---|
| 10{1; 1} | Me | 1-pentylamine 18{1} | B | 85 | 80 [b] |
| 10{1; 2} | Me | cyclohexylamine 18{2} | B | 69 | 100 [b] |
| 10{1; 3} | Me | benzylamine 18{3} | A | 77 | 100 [b,c] |
| 10{1; 4} | Me | 2-methoxyethylamine 18{4} | A | 28 | 100 [b,c] |
| 10{1; 5} | Me | 3-amino-1-propanol 18{5} | B | 94 | 81 [b] |
| 10{1; 6} | Me | 3-dimethylamino-1-propylamine 18{6} | B | 40 | 94 [b] |
| 10{1; 7} | Me | 2-picolylamine 18{7} | B | 76 | 100 [b] |
| 10{1; 8} | Me | diethylamine 18{8} | B | 100 | 100 [b] |
| 10{1; 9} | Me | pyrrolidine 18{9} | B | 79 | 100 [b] |
| 10{1; 10} | Me | piperidine 18{10} | B | 100 | 100 [b] |
| 10{1; 11} | Me | morpholine 18{11} | B | 99 | 100 [b] |
| 10{1; 12} | Me | 4-methylpiperazine 18{12} | B | 100 | 100 [b] |
| 10{2; 1} | Ph | 1-pentylamine 18{1} | A | 100 | 100 [b,c] |
| 10{2; 2} | Ph | cyclohexylamine 18{2} | A | 100 | 86 [b,c] |
| 10{2; 3} | Ph | benzylamine 18{3} | A | 77 | 100 [b,c] |
| 10{2; 4} | Ph | 2-methoxyethylamine 18{4} | A | 65 | 100 [b,c] |
| 10{2; 5} | Ph | 3-amino-1-propanol 18{5} | A | 98 | 84 [b,c] |
| 10{2; 6} | Ph | 3-dimethylamino-1-propylamine 18{6} | A | 71 | 100 [b] |
| 10{2; 7} | Ph | 2-picolylamine 18{7} | A | 89 | 87 [b,c] |
| 10{2; 8} | Ph | diethylamine 18{8} | B | 68 | 88 [b,c] |
| 10{2; 9} | Ph | pyrrolidine 18{9} | B | 100 | 100 [b] |
| 10{2; 10} | Ph | piperidine 18{10} | B | 100 | 100 [b] |
| 10{2; 11} | Ph | morpholine 18{11} | B | 95 | 100 [b] |
| 10{2; 12} | Ph | 4-methylpiperazine 18{12} | B | 100 | 100 [b] |
[a] Workup A: filtration of the reaction mixture; Workup B: evaporation of the reaction mixture, followed by DFCC purification. [b] Determined by LC-MS, 1H-NMR, and 13C-NMR. [c] Confirmed by elemental analysis. The found values for C, H, and N were within ±0.4% range with respect to the theoretical values.
Scheme 2.
Parallel synthesis of 6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides 10.
2.2. Structure Determination
The structures and purities of novel compounds 10{1,2; 1–12}, 16{1,2}, and 17{1,2} were determined by spectroscopic methods (IR, 1H-NMR and 13C-NMR, MS, HRMS), by LC-MS, and by elemental analyses for C, H, and N. Spectral and analytical data for novel compounds 10{1,2; 1–12}, 16{1,2}, and 17{1,2} were in agreement with the proposed structures. Correlation of NMR data for compounds 10{1,2; 1–12}, 16{1,2}, and 17{1,2} revealed very good agreement of chemical shifts and coupling constants for the core nuclei (Table 2).
Table 2.
Selected NMR data for compounds 10{1,2; 1–12}.
| Compd. | δ (ppm) | 3 JH–H (Hz) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-H | 2'-Ha | 2'-Hb | 3'-H | 4'-Ha | 4'-Hb | 2'a-2'b | 2'a-3' | 2'b-3' | 3'-4'a | 3'-4'b | 4'a-4'b | ||
| 16{1} | 9.11 | 4.15 | 4.24 | 4.70 | 2.96 | 3.17 | 9.6 | 6.4 | 8.4 | 9.1 | 7.3 | 16.9 | |
| 16{2} | 9.28 | 4.16 | 4.36 | 4.78 | 3.04 | 3.29 | 9.7 | 5.4 | 8.1 | 8.9 | 6.2 | 16.9 | |
| 17{1} | 9.05 | 4.00 | 4.23 | 4.56 | 2.92 [a] | 9.8 | 5.4 | 8.5 | [a] | [a] | [a] | ||
| 17{2} | 9.25 | 4.06 | 4.35 | 4.73 | 2.96 | 3.03 | 9.9 | 4.1 | 7.9 | 4.9 | 8.6 | 16.7 | |
| 10{1; 1} | 8.62 | 4.14 | 4.23 | 4.28 | 2.90 | 3.15 | 9.3 | 6.5 | 7.3 | 8.8 | 7.3 | 17.0 | |
| 10{1; 2} | 8.60 | 4.16 | 4.22 | 4.27 | 2.91 | 3.15 | 9.1 | 6.5 | 8.7 | 8.8 | 7.5 | 16.9 | |
| 10{1; 3} | 8.63 | 4.09 | 4.18 | 4.28 | 2.83 | 3.10 | 9.5 | 6.8 | 8.9 | 9.0 | 7.7 | 16.9 | |
| 10{1; 4} | 8.65 | 4.15 | 4.21 | 4.28 | 2.90 | 3.16 | 9.4 | 7.1 | 8.6 | 8.9 | 7.9 | 16.8 | |
| 10{1; 5} | 8.65 | 4.16 | 4.22 | 4.31 | 2.92 | 3.12 | 9.6 | 6.8 | 8.9 | 9.0 | 7.7 | 16.9 | |
| 10{1; 6} | 8.61 | 4.14 | 4.25 | 4.43 | 2.92 | 3.18 | 9.6 | 6.7 | 8.4 | 9.1 | 7.8 | 16.9 | |
| 10{1; 7} | 8.78 | 4.16 | 4.22 | 4.34 | 2.93 | 3.19 | 9.5 | 7.1 | 8.9 | 9.0 | 8.0 | 16.9 | |
| 10{1; 8} | 8.49 | 4.13 | 4.20 | 3.84 | 2.88 | [b] | 8.5 | 8.5 | 8.5 | 8.8 | [a] | 16.7 | |
| 10{1; 9} | 8.56 | 4.17 | 4.21 | 3.96 | 2.90 | 3.17 | 9.5 | 8.4 | 7.6 | 9.0 | 8.7 | 16.9 | |
| 10{1;10} | 8.47 | 4.19 [a] | 3.90 | 2.90 | 3.19 | [a] | [a] | [a] | [a] | [a] | [a] | ||
| 10{1; 11 | 8.48 | 4.19 [a] | 3.91 | 2.90 | 3.18 | [a] | [a] | [a] | [a] | [a] | [a] | ||
| 10{1;12} | 8.47 | 4.17 [a] | 3.89 | 2.90 | 3.19 | [a] | [a] | [a] | [a] | [a] | [a] | ||
| 10{2; 1} | 8.77 | 4.14 | 4.31 | 4.35 | 2.95 | 3.23 | 9.1 | 5.2 | 8.6 | 6.5 | 8.7 | 16.9 | |
| 10{2; 2} | 8.75 | 4.15 | ~4.3 [a] | 2.95 | 3.23 | 9.6 | 4.8 | [a] | 8.8 | 6.5 | 16.9 | ||
| 10{2; 3} | 8.81 | 4.14 | 4.31 | 4.39 | 2.96 | 3.26 | 9.6 | 5.8 | 8.8 | 8.8 | 6.7 | 16.9 | |
| 10{2; 4} | 8.82 | 4.17 | 4.32 | 4.37 | 2.98 | 3.27 | 9.4 | 5.8 | 8.8 | 8.6 | 6.8 | 16.8 | |
| 10{2; 5} | 8.80 | 4.17 | 4.31 | 4.38 | 2.98 | 3.21 | 9.6 | 5.6 | 8.9 | 8.7 | 6.5 | 16.9 | |
| 10{2; 6} | 8.77 | 4.20 | 4.28 | 4.51 | 3.00 | 3.29 | 9.7 | 5.8 | 8.1 | 8.9 | 6.7 | 16.9 | |
| 10{2; 7} | 8.96 | 4.19 | 4.32 | 4.44 | 3.00 | 3.29 | 9.6 | 6.1 | 8.3 | 8.9 | 7.1 | 16.9 | |
| 10{2; 8} | 8.65 | 4.22 | 4.24 | 3.92 | 2.95 | [a] | [a] | [a] | [a] | 8.9 | [a] | 16.9 | |
| 10{2; 9} | 8.72 | 4.24 | 4.27 | 4.06 | 2.97 | 3.28 | 9.7 | 6.8 | 8.2 | 8.9 | 7.8 | 16.9 | |
| 10{2;10} | 8.63 | 4.24 | 4.24 | 3.98 | 2.96 | 3.27 | [a] | [a] | [a] | [a] | [a] | [a] | |
| 10{2;11} | 8.64 | 4.23 | 4.25 | 3.99 | 2.97 | 3.27 | [a] | [a] | [a] | 8.3 | 7.0 | 16.3 | |
| 10{2;12} | 8.64 | 4.25 | 4.25 | 3.98 | 2.98 | 3.29 | [a] | [a] | [a] | [a] | [a] | [a] | |
[a] Multiplet or broad singlet; [b] Overlapped by other signals.
Since the products 10{1,2; 1–12} were isolated as racemic mixtures [28], we also tried to find suitable conditions for separation of the enantiomers of compounds 10 by HPLC using analytical chiral stationary phase column Chiralcel® OD-H (0.46 cm × 25 cm) and n-hexane/isopropanol as mobile phase. To our pleasant surprise, all 24 racemic compounds were resolved under these conditions. Most probably, the results obtained on analytical column should be applicable in (semi)preparative separation of enantiomers of 10{1,2; 1−12}, while these separation conditions could also serve as a important information for separation of analogous racemic compounds (Table 3).
Table 3.
Analytical data for separation of enantiomers of racemic compounds 10{1,2; 1–12}.
| Compound | n-hexane:i-PrOH | R t (min) | ||
|---|---|---|---|---|
| Enantiomer A | Enantiomer B | |||
| 10{1; 1} | 50:50 | 4.084 | 5.078 | |
| 10{1; 2} | 50:50 | 9.987 | 16.650 | |
| 10{1; 3} | 50:50 | 8.208 | 12.734 | |
| 10{1; 4} | 50:50 | 5.321 | 7.031 | |
| 10{1; 5} | 50:50 | 3.828 | 4.542 | |
| 10{1; 6} | 50:50 | 5.083 | 5.477 | |
| 10{1; 7} | 50:50 | 7.577 | 8.352 | |
| 10{1; 8} | 50:50 | 5.728 | 6.380 | |
| 10{1; 9} | 50:50 | 6.960 | 9.471 | |
| 10{1; 10} | 50:50 | 5.798 | 7.185 | |
| 10{1; 11} | 50:50 | 8.509 | 9.619 | |
| 10{1; 12} | 50:50 | 7.206 | 7.928 | |
| 10{2; 1} | 50:50 | 4.409 | 5.515 | |
| 10{2; 2} | 50:50 | 4.537 | 5.840 | |
| 10{2; 3} | 50:50 | 11.292 | 29.227 | |
| 10{2; 4} | 50:50 | 6.462 | 7.522 | |
| 10{2; 5} | 80:20 | 14.864 | 18.975 | |
| 10{2; 6} | 50:50 | 6.160 | 19.660 | |
| 10{2; 7} | 50:50 | 10.778 | 12.764 | |
| 10{2; 8} | 50:50 | 5.293 | 24.904 | |
| 10{2; 9} | 50:50 | 9.284 | 10.960 | |
| 10{2; 10} | 50:50 | 7.102 | 8.212 | |
| 10{2; 11} | 80:20 | 14.864 | 18.975 | |
| 10{2; 12} | 50:50 | 14.429 | 19.625 | |
Finally, some physicochemical properties of compounds 10{1,2; 1–12} were calculated to estimate their drug-likeness. The compounds have molecular weight (MW) between 160 and 500, number of atoms between 20 and 70, CLogP between –0.4 and 5.6, number of hydrogen bond donors (HBD) ≤ 5, number of hydrogen bond acceptors (HBA) ≤ 10, and polar surface area (PSA) bellow 140 Ǻ2 [29,30]. These calculated physicochemical properties compliant with Lipinski’s rule of five indicate promising drug-likeness of the synthesized compounds 10{1,2; 1–12} (Table 4).
Table 4.
Calculated physicochemical properties of compounds 10{1,2; 1–12} [a].
| Compound | MW (g·mol–1) | No. of atoms | CLogP | No. of HBD | No. of HBA | PSA (Ǻ2) |
|---|---|---|---|---|---|---|
| 10{1; 1} | 366 | 53 | 2.82 | 1 | 6 | 74 |
| 10{1; 2} | 378 | 54 | 2.73 | 1 | 6 | 74 |
| 10{1; 3} | 386 | 51 | 2.67 | 1 | 6 | 74 |
| 10{1; 4} | 354 | 48 | 0.90 | 1 | 7 | 83 |
| 10{1; 5} | 354 | 48 | 0.46 | 2 | 7 | 94 |
| 10{1; 6} | 381 | 55 | 1.39 | 1 | 7 | 77 |
| 10{1; 7} | 387 | 50 | 1.17 | 1 | 7 | 86 |
| 10{1; 8} | 352 | 50 | 1.63 | 0 | 6 | 65 |
| 10{1; 9} | 350 | 48 | 1.20 | 0 | 6 | 65 |
| 10{1; 10} | 364 | 51 | 1.76 | 0 | 6 | 65 |
| 10{1; 11} | 366 | 49 | 0.73 | 0 | 7 | 75 |
| 10{1; 12} | 379 | 53 | 1.29 | 0 | 7 | 69 |
| 10{2; 1} | 428 | 60 | 4.42 | 1 | 6 | 74 |
| 10{2; 2} | 440 | 61 | 4.33 | 1 | 6 | 74 |
| 10{2; 3} | 448 | 58 | 4.27 | 1 | 6 | 74 |
| 10{2; 4} | 416 | 55 | 2.50 | 1 | 7 | 83 |
| 10{2; 5} | 416 | 55 | 2.06 | 2 | 7 | 94 |
| 10{2; 6} | 443 | 62 | 2.99 | 1 | 7 | 77 |
| 10{2; 7} | 449 | 57 | 2.77 | 1 | 7 | 86 |
| 10{2; 8} | 414 | 57 | 3.22 | 0 | 6 | 65 |
| 10{2; 9} | 412 | 55 | 2.80 | 0 | 6 | 65 |
| 10{2; 10} | 426 | 58 | 3.36 | 0 | 6 | 65 |
| 10{2; 11} | 428 | 56 | 2.33 | 0 | 7 | 75 |
| 10{2; 12} | 441 | 60 | 2.89 | 0 | 7 | 69 |
[a] Calculated with ChemBioDraw Ultra v11.0.
3. Experimental
3.1. General Methods
Melting points were determined on a Stanford Research Systems MPA100 OptiMelt automated melting point system (Sunnyvale, CA, USA). The NMR spectra were obtained on a Bruker Avance III UltraShield 500 plus (Karlsruhe, Germany) at 500 MHz for 1H and 126 MHz for the 13C nucleus, using DMSO-d6 and CDCl3 with TMS as the internal standard, as solvents. Mass spectra were recorded on a Agilent 6224 Accurate Mass TOF LC/MS spectrometer (Santa Clara, CA, USA), IR spectra on a Perkin-Elmer Spectrum BX FTIR spectrophotometer (Waltham, MA, USA). Microanalyses were performed on a Perkin-Elmer CHN Analyzer 2400 II (Waltham, MA, USA). Drying of the compounds 10 and 17 was performed in a Büchi drying oven (Flawil, Switzerland). Dry flash column chromatography (DFCC) was performed on Aluminium Oxide Fluka for Chromatography, cat. # 06310, type 506 C weakly acidic, 0.05–0.15 mm, pH 6.0 ± 0.5 (Buchs, Switzerland).
For LC-MS/MS experiments, liquid chromatograph Perkin Elmer Series 200 from Perkin Elmer (Shelton, CT, USA) with UV detector and 3200 QTRAP LC/MS/MS System equipped with ESI and APCI ion sources from Applied Biosystems/MDS Sciex (Foster City, CA, USA) were used. HPLC column was Gemini, dimensions 150 mm × 4.6 mm, 3 μm particles from Phenomenex (Torrance, CA, USA). Mobile phase was a gradient of acetonitrile (A) and deionised water (B): 0 min-10% A, 25 min-100% A, 3 min equilibration time with initial mobile phase (10% A) was allowed for column equilibration. Mobile phase flow was 1 mL/min. Injection volume was 20 μL. Signal was recorded using UV detector at 254 nm and mass spectra were recorded using positive (ESI+) and negative (ESI−) ionization mode simultaneously. Mass range was from 70 to 500 amu. Electrospray ion source (ESI) conditions were as follows: cone voltage 5500 V (ESI+) and −4500 V (ESI−), respectively, ion source temperature 4,000 °C, curtain gas N2 pressure was set to 10 psi, nebulizer gas N2 pressure was set to 20 psi and turbo gas (air) pressure was set to 40 psi. Declustering potential 30 V and entrance potential 10 V was used, respectively.
Itaconic acid (11), 1,1'-carbonyldiimidazole, N,N-dimethylformamide dimethylacetal (DMFDMA), acetamidine hydrochloride 15{1}, benzamidine 15{2}, bis(pentafluorophenyl) carbonate (BPC), and amines 18{1–12} are commercially available (Sigma-Aldrich). 5-Oxo-1-phenylpyrrolidin-3-carboxylic acid (12) [24] and methyl 3-oxo-3-(5-oxo-1-phenylpyrrolidin-3-yl)propanoate (13) [23] were prepared according to the literature procedures.
Parallel stirring and filtrations were carried out on Mettler-Toledo Bohdan MiniBlock™ Compact Shaking and Washing Station and Vacuum Collection Base (2 × 12 positions, Vortex stirring, 400 r.p.m. in all cases). Parallel evaporations were carried out on Büchi Syncore® Polyvap parallel evaporator (24 positions, Vortex stirring, 400 r.p.m. in all cases). Parallel drying was carried out on Hettlab IR-Dancer Infra-Red Vortex-Evaporator (42 positions, Vortex stirring, 400 r.p.m. in all cases).
3.2. Synthesis of Methyl 3-(Dimethylamino)-2-(5-oxo-1-phenylpyrrolidine-3-carbonyl)acrylate (14)
This compound was prepared according to a slightly modified literature procedure [23]. A mixture of β-keto ester 13 [23] (5.2 g, 20 mmol), anhydrous toluene (20 mL), and DMFDMA (2.8 g, 3 mL, 20 mmol) was stirred at 60 °C for 3 h. Volatile components were evaporated in vacuo to give the crude 14 as a yellow oil in quantitative yield.
3.3. Synthesis of Methyl 2-Methyl-6-(5-Oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxylate (16{1})
Cold (0 °C) solution of t-BuOK (2.3 g, 20 mmol) in anhydrous methanol (20 mL) was added to a cold (0 °C) solution of acetamidine hydrochloride (15{1}, 1.9 g, 20 mmol) in methanol (20 mL) and the mixture was stirred at 0 °C for 5 min. The suspension was filtered with suction through a fritted funnel and the precipitated KCl was washed with anhydrous methanol (2 × 10 mL) to afford a solution of the free acetamidine 15{1} (20 mmol) in methanol. This was added to a solution of the crude enaminone 13 (20 mmol) in methanol (100 mL) and the mixture was stirred at room temperature for 18 h. The precipitate was collected by filtration, and washed with methanol (2 × 10 mL) to give 16{1}. Yield: 3.2 g (50%) of white solid; m.p. 131–133 °C. 1H-NMR (500 MHz, DMSO-d6): δ 2.77 (3H, s, 2-CH3); 2.96 (1H, dd, J = 9.1, 16.9 Hz, 4'-Ha); 3.17 (1H, dd, J = 7.3, 16.9 Hz, 4'-Hb); 3.97 (3H, s, OCH3); 4.15 (1H, dd, J = 6.4, 9.6 Hz, 2'-Ha); 4.24 (1H, dd, J = 8.4, 9.5 Hz, 2'-Hb); 4.70 (1H, quintet, J = 8.3 Hz, 3'-H); 7.14 (1H, br t, J = 7.4 Hz, p-Ph); 7.37 (2H, br t, J = 8.0 Hz, o-Ph); 7.63 (2H, br d, J = 7.9 Hz, m-Ph); 9.06 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 26.5, 35.5, 38.0, 52.9, 53.3, 120.1, 120.3, 124.8, 129.0, 139.3, 159.5, 165.2, 170.1, 171.2, 172.7. LC-MS: Rt = 13.217 min, m/z = 312 (MH+), area% = 80. m/z (HRMS) Found: 312.1345 (MH+). C17H18N3O3 requires: m/z = 312.1343. (Found: C, 65.22; H, 5.46; N, 13.16. C17H17N3O3 requires: C, 65.58; H, 5.50; N, 13.50.); νmax (KBr) 3420, 1718, 1693, 1598, 1572, 1545, 1480, 1397, 1306, 1268, 1097, 818, 764, 693 cm−1.
3.4. Synthesis of Methyl 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxylate (16{2})
Benzamidine 15{2} (2.4 g, 20 mmol) was added to a solution of the crude enaminone 14 (20 mmol) in anhydrous methanol (100 mL) and the mixture was stirred at room temperature for 72 h. The precipitate was collected by filtration, and washed with methanol (2 × 30 mL) to give 16{2}. Yield: 4.9 g (65%) of white solid; m.p. 146–147 °C. 1H-NMR (500 MHz, CDCl3): δ 3.04 (1H, dd, J = 8.9, 16.9 Hz, 4'-Ha); 3.29 (1H, dd, J = 6.2, 16.9 Hz, 4'-Hb); 4.00 (3H, s, OCH3); 4.16 (1H, dd, J = 5.4, 9.7 Hz, 2'-Ha); 4.36 (1H, dd, J = 8.1, 9.6 Hz, 2'-Hb); 4.78 (1H, tt, J = 5.9, 8.4 Hz, 3'-H); 7.16 (1H, t, J = 7.4 Hz, p-Ph); 7.38 (2H, br t, J = 8.0 Hz, o-Ph); 7.47–7.55 (3H, m, 3H of Ph); 7.64 (2H, br d, J = 7.7 Hz, m-Ph); 8.50–8.52 (2H, m, m-Ph); 9.28 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 35.9, 38.0, 52.9, 53.6, 120.2, 120.3, 124.8, 128.9, 129.0, 129.2, 132.2, 136.3, 139.3, 160.1, 165.1, 166.2, 170.5, 173.0. LC-MS: Rt = 19.692 min, m/z = 374 (MH+), area% = 100. m/z (ESI) = 374 (MH+). m/z (HRMS) Found: 374.1499 (MH+). C22H20N3O3 requires: m/z = 374.1499. (Found: C, 69.61; H, 5.44; N, 11.07. C22H19N3O3·⅓H2O requires: C 69.59; H 5.23; N 11.07.); νmax (KBr) 3484, 1717, 1676, 1569, 1478, 1406, 1311, 1281, 1196, 1108, 836, 766, 692 cm−1.
3.5. Synthesis of 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxylic Acid (17{1})
A mixture of the ester 16 (13 mmol), 1 M aqueous NaOH (30 mL), methanol (30 mL), and THF (30 mL) was stirred at room temperature for 5 h. Methanol and THF were removed by evaporation in vacuo (35 °C, 100 mbar), the aqueous residue was acidified with concentrated hydrochloric acid to pH ~1, and the product was extracted with dichloromethane (4 × 200 mL). The combined organic phases were dried over anhydrous Na2SO4, filtered, and the filtrate was evaporated in vacuo to give 17{1}. Yield: 3.33 g (86%) of a pale yellow solid; m.p. 70–82 °C. 1H-NMR (500 MHz, DMSO-d6): δ 2.66 (3H, s, 2-CH3); 2.88–2.96 (2H, m, 4'-CH2); 4.00 (1H, dd, J = 5.4, 9.8 Hz, 2'-Ha); 4.23 (1H, dd, J = 8.5, 9.7 Hz, 2'-Hb); 4.56 (1H, dq, J = 5.6, 8.0 Hz, 3'-H); 7.13 (1H, br t, J = 7.4 Hz, p-Ph); 7.37 (2H, br t, J = 8.0 Hz, o-Ph); 7.65 (2H, br d, J = 7.8 Hz, m-Ph); 9.05 (1H, s, 4-H); 13.78 (1H, s, COOH). 13C (126 MHz, DMSO-d6): δ 26.0, 34.4, 37.4, 52.6, 119.6, 121.0, 124.0, 128.7, 139.3, 159.1, 166.0, 169.6, 170.0, 172.3. LC-MS: Rt = 13.7 min, m/z = 296 (M–H+), area% = 100. m/z (ESI) = 296 (M–H+). m/z (HRMS) Found: 298.1189 (MH+). C16H16N3O3 requires: m/z = 298.1186. (Found: C 64.51; H 5.16; N 13.95. C16H15N3O3 requires: C 64.64; H 5.09; N 14.13.); νmax (KBr) 3418, 1700, 1676, 1597, 1542, 1500, 1400, 1265, 762, 692 cm−1.
3.6. Synthesis of 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxylic Acid (17{2})
A mixture of the ester 16 (13 mmol), 1 M aqueous NaOH (30 mL), methanol (30 mL), and THF (30 mL) was stirred at room temperature for 5 h. Methanol and THF were removed by evaporation in vacuo (35 °C, 100 mbar) and the aqueous residue was acidified with concentrated hydrochloric acid to pH ~1. The precipitate was collected by filtration to give 17{2}. Yield: 4.34 g (92%) of a pale yellow solid; m.p. 251–253 °C. 1H-NMR (500 MHz, DMSO-d6): δ 2.96 (1H, dd, J = 4.9, 16.7 Hz, 4'-Ha); 3.03 (1H, dd, J = 8.6, 16.7 Hz, 4'-Hb); 4.06 (1H, dd, J = 4.1, 9.9 Hz, 2'-Ha); 4.35 (1H, dd, J = 7.9, 9.9 Hz, 2'-Hb); 4.73 (1H, br septet, J = 4.2 Hz, 3'-H); 7.13 (1H, br t, J = 7.4 Hz, p-Ph); 7.37 (2H, br t, J = 8.0 Hz, o-Ph); 7.52 (2H, br t, J = 8.0 Hz, o-Ph); 7.57 (1H, br t, J = 7.3 Hz, p-Ph); 7.69 (2H, br d, J = 7.8 Hz, m-Ph); 8.43 (2H, br d, J = 7.2 Hz, m-Ph); 9.25 (1H, s, 4-H); 13.84 (1H, s, COOH). 13C (126 MHz, DMSO-d6): δ 35.0, 37.7, 52.8, 119.5, 121.2, 123.9, 128.4, 128.7, 128.8, 131.9, 136.2, 139.5, 159.8, 164.3, 165.9, 170.8, 172.6. LC-MS: Rt = 18.192 min, m/z = 358 (M–H+), area% = 100. m/z (ESI) = 358 (M–H+). m/z (HRMS) Found: 360.1346 (MH+). C21H18N3O3 requires: m/z = 360.1343. (Found: C 69.38; H 4.65; N 11.38. C21H17N3O3·¼H2O requires: C 69.37; H 4.84; N 11.56.); νmax (KBr) 3420, 2364, 1708, 1654, 1567, 1500, 1424, 1312, 1257, 1183, 756, 697 cm−1.
3.7. Parallel Synthesis of 2-Substituted 6-(5-Oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides 10{1,2; 1–12}
Two MiniBlocksTM were equipped with 12 fritted vessels each and mounted on a compact stirring and washing station. The reaction vessels were charged with carboxylic acids 17{1} (12 × 149 mg, 12 × 0.5 mmol) and 17{2} (12 × 180 mg, 12 × 0.5 mmol), anhydrous acetonitrile (24 × 5 mL), BPC (24 × 236 mg, 24 × 0.6 mmol), and triethylamine (24 × 0.14 mL, 24 × 1 mmol) and the mixtures were stirred at room temperature for 30 min. Then, the amines 18{1–7} (2 × 12 × 0.5 mmol) and amines 18{8–12} (2 × 4 × 5 mmol) were added and stirring at room temperature was continued for 12 h. The reaction mixtures were filtered to afford 10{1; 3,4} and 10{2; 1–7} (Workup A). The filtrates containing the products 10{1; 1,2,5–12} and 10{2; 8–12} were evaporated in vacuo (40 °C/2 mbar) and the residues (resins) were dissolved in dichloromethane (15 × 2.5 mL) and purified sequentially by DFCC over aluminium oxide (5 g, d = 15 mm) by gradient elution with a) EtOAc (30 mL) and b) EtOAc–EtOH (5:1, 50 mL). The combined eluates were evaporated in vacuo (60 °C/1 mbar) to afford compounds 10{1; 1,2,5–12} and 10{2; 8–12} (Workup B). The following compounds were prepared in this manner.
3.7.1. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-pentylpyrimidine-5-carboxamide (10{1; 1})
Prepared from 17{1} and 1-pentylamine (18{1}), workup B. Yield: 204 mg (85%) of yellow-brown resin. 1H-NMR (500 MHz, CDCl3): δ 0.92 (3H, dd, J = 4.7, 9.0 Hz, CH3 of C5H11), 1.35–1.40 (4H, m, 2CH2 of C5H11), 1.64 (2H, quintet, J = 7.3 Hz, CH2 of C5H11), 2.74 (3H, s, 2-CH3), 2.90 (1H, dd, J = 8.8, 17.0 Hz, 4'-Ha), 3.15 (1H, dd, J = 7.3, 17.0 Hz, 4'-Hb), 3.45 (2H, br q, J = 7.3 Hz, CH2NH), 4.14 (1H, dd, J = 6.5, 9.3 Hz, 2'-Ha), 4.23 (1H, t, J = 8.8 Hz, 2'-Hb), 4.28 (1H, br q, J = 8.2 Hz, 3'-H), 6.24 (1H, s, NH), 7.15 (1H, br t, J = 7.4 Hz, p-Ph), 7.36 (2H, br t, J = 7.9 Hz, m-Ph), 7.59 (2H, br d, J = 7.6 Hz, o-Ph), 8.62 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 14.2, 22.5, 26.3, 29.3, 29.4, 35.7, 38.3, 40.6, 53.9, 120.6, 125.1, 126.5, 129.1, 139.1, 154.7, 165.9, 168.3, 169.8, 172.9. LC-MS: Rt = 14.733 min, m/z = 367 (MH+), area% = 80. m/z (ESI) = 365 (M−H+). m/z (HRMS) Found: 365.1987 ([M−H]−). C21H25N4O2 requires: m/z = 365.1983. νmax (KBr) 3460, 2356, 1651, 1516, 1501, 985, 760, 694 cm−1.
3.7.2. N-Cyclohexyl-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 2})
Prepared from 17{1} and (cyclohexylamine 18{2}), workup B. Yield: 228 mg (69%) of yellow-brown resin. 1H-NMR (500 MHz, CDCl3): δ 1.16–1.32 (4H, m, 4H of C6H11), 1.43 (2H, br tq, J = 3.5, 12.1 Hz, 2H of C6H11), 1.68 (1H, br td, J = 3.5, 12.8 Hz, 2H of C6H11), 1.75–1.81 (2H, m, 2H of C6H11), 2.05 (2H, br dd, J = 2.2, 12.3 Hz, 2H of C6H11), 2.74 (3H, s, 2-CH3), 2.91 (1H, dd, J = 8.8, 16.9 Hz, 4'-Ha ), 3.15 (1H, dd, J = 7.5, 16.9 Hz, 4'-Hb), 3.95 (1H, ttd, J = 3.9, 7.8, 14.5 Hz, 1H of C6H11), 4.16 (1H, dd, J = 6.5, 9.1 Hz, 2'-Ha), 4.22 (1H, t, J = 8.7 Hz, 2'-Hb), 4.27 (1H, quintet, J = 8.0 Hz, 3'-H), 6.04 (1H, J = 6.8 Hz, NH), 7.16 (1H, br t, J = 7.8 Hz, p-Ph), 7.36 (2H, br t, J = 8.0 Hz, m-Ph), 7.59 (2H, br d, J = 7.7 Hz, o-Ph), 8.60 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 25.0, 25.6, 26.2, 33.2, 35.7, 38.3, 49.6, 53.9, 120.6, 125.1, 126.7, 129.1, 139.1, 154.6, 165.1, 168.2, 169.7, 173.0. LC-MS: Rt = 14.483 min, m/z = 379 (MH+), area% = 100. m/z (ESI) = 379 (MH+). m/z (HRMS) Found: 379.2131 (MH+). C22H27N4O2 requires: m/z = 379.2129. νmax (KBr) 3418, 2934, 1634, 1516, 1501, 1400, 1281, 1150, 1007, 839, 762, 694 cm−1.
3.7.3. N-Benzyl-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 3})
Prepared from 17{1} and benzylamine (18{3}), workup A. Yield: 150 mg (77%) of white solid; m.p. 153–155 °C. 1H-NMR (500 MHz, CDCl3): δ 2.72 (3H, s, 2-CH3), 2.83 (1H, dd, J = 9.0, 16.9 Hz, 1H, 4'-Ha), 3.10 (1H, dd, J = 7.7, 16.9 Hz, 4'-Hb), 4.09 (1H, dd, J = 6.8, 9.5 Hz, 2'-Ha), 4.18 (1H, t, J = 8.9 Hz, 2'-Hb), 4.28 (1H, quintet, J = 7.9 Hz, 3'-H), 4.59 and 4.63 (2H, 2dd, 1:1, J = 5.5, 14.5 Hz, CH2Ph), 6.72 (1H, t, J = 5.3 Hz, NH), 7.14 (1H, br t, J = 7.4 Hz, p-Ph), 7.31–7.38 (7H, m, m-Ph, Ph'), 7.55 (2H, br d, J = 7.4 Hz, o-Ph), 8.63 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 26.4, 35.6, 38.3, 44.6, 53.9, 120.5, 125.0, 126.2, 128.2, 128.3, 129.1, 129.2, 137.6, 139.2, 155.0, 165.9, 168.3, 170.0, 172.9. LC-MS: Rt = 13.808 min, m/z = 387 (MH+), area% = 100. m/z (ESI) = 385 (M–H+). m/z (HRMS) Found: 385.1669 ([M−H]−). C23H21N4O2 requires: m/z = 385.167. (Found: C 71.30; H 5.83; N 14.44. C23H22N4O2 requires: C 71.48; H 5.74; N 14.50); νmax (KBr) 3422, 3269, 1706, 1634, 1560, 1498, 1446, 1396, 1308, 1279, 1218, 824, 756, 691 cm−1.
3.7.4. N-(2-Methoxyethyl)-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 4})
Prepared from 17{1} and 2-methoxyethylamine (18{4}), workup A. Yield: 48 mg (28%) of white solid; m.p. 101–103 °C. 1H-NMR (500 MHz, CDCl3): δ 2.74 (3H, s, 2-CH3), 2.90 (1H, dd, J = 8.9, 16.8 Hz, 1H, 4'-Ha), 3.16 (1H, dd, J = 7.9, 16.8 Hz, 4'-Hb), 3.39 (3H, s, OCH3), 3.57–3.59 and 3.63–3.66 (4H, 2m, 1:1, CH2CH2), 4.15 (1H, dd, J = 7.1, 9.4 Hz, 2'-Ha), 4.21 (1H, t, J = 8.6 Hz, 2'-Hb), 4.28 (1H, quintet, J = 8.1 Hz, 3'-H), 6.25 (1H, s, NH), 7.15 (1H, br t, J = 7.4 Hz, p-Ph), 7.36 (2H, br t, J = 8.0 Hz, m-Ph), 7.61 (2H, br d, J = 8.5 Hz, o-Ph), 8.65 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 26.3, 35.7, 38.3, 40.1, 53.8, 59.1, 70.9, 120.4, 120.4, 124.9, 126.2, 129.0, 129.0, 139.2, 155.0, 166.0, 168.1, 169.9, 172.8. LC-MS: Rt = 10.167 min, m/z = 355 (MH+), area% = 100. m/z (ESI) = 355 (MH+). m/z (HRMS) Found: 389.1387 ([M+Cl]−). C19H22ClN4O3 requires: m/z = 389.1386. (Found: C 64.17; H 6.22; N 15.67. C19H22N4O3 requires: C 64.39; H 6.26; N 15.81); νmax (KBr) 3422, 3269, 1706, 1634, 1560, 1498, 1446, 1396, 1308, 1279, 1218, 824, 756, 691 cm−1.
3.7.5. N-(3-Hydroxypropyl)-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 5})
Prepared from 17{1} and 3-amino-1-propanol (18{5}), workup B. Yield: 205 mg (94%) of yellow-brown resin. 1H-NMR (500 MHz, CDCl3): δ 1.85 (2H, quintet, J = 6.3 Hz, CH2CH2CH2), 2.73 (3H, s, 2-CH3), 2.92 (1H, dd, J = 9.0, 16.9 Hz, 4'-Ha), 3.12 (1H, dd, J = 7.7, 16.9 Hz, 4'-Hb), 3.62 (2H, ddd, J = 2.2, 5.6, 11.5 Hz, CH2NH), 3.81 (2H, t, J = 5.5 Hz, CH2OH), 4.16 (1H, dd, J = 6.8, 9.6 Hz, 2'-Ha), 4.22 (1H, t, J = 8.9 Hz, 2'-Hb), 4.31 (1H, quintet, J = 7.9 Hz, 3'-H), 6.40 (1H, br s, NH), 7.16 (1H, br t, J = 7.5 Hz, p-Ph), 7.19 (1H, br t, J = 5.0 Hz, OH), 7.36 (2H, br t, J = 8.0 Hz, m-Ph), 7.58 (2H, d, J = 7.8 Hz, o-Ph), 8.65 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 26.2, 31.4, 35.6, 38.4, 38,7, 54.0, 61.2, 120.7, 125.2, 126.3, 129.1, 139.0, 155.0, 166.4, 168.3, 169.8, 173.1. LC-MS: Rt = 9.183 min, m/z = 355 (MH+), area% = 81. m/z (ESI) = 353 ([M−H]−). m/z (HRMS) Found: 353.1623 ([M−H]−). C19H21N4O3 requires: m/z = 353.1619. νmax (KBr) 3444, 2370, 1645, 1517, 1501, 1309, 984, 838, 761, 691 cm−1.
3.7.6. N-(3-Dimethylaminopropyl)-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 6})
Prepared from 17{1} and 3-(dimethylamino)propylamine (18{6}), workup B. Yield: 115 mg (40%) of yellow resin. 1H-NMR (500 MHz, CDCl3): δ 1.79 (2H, br quintet, J = 5.8 Hz, CH2CH2CH2), 2.28 (6H, s, NMe2), 2.54 (2H, t, J = 5.7 Hz, CH2NMe2), 2.74 (3H, s, 2-CH3), 2.92 (1H, dd, J = 9.1, 16.9 Hz, 4'-Ha), 3.18 (1H, dd, J = 7.8, 16.9 Hz, 4'-Hb), 3.56 (2H, tq, J = 6.0, 7.0 Hz, CH2NH), 4.14 (1H, dd, J = 6.7, 9.6 Hz, 2'-Ha), 4.25 (1H, dd, J = 8.4, 9.6 Hz, 2'-Hb), 4.43 (1H, quintet, J = 8.2 Hz, 3'-H), 7.15 (1H, br t, J = 7.4 Hz, p-Ph), 7.36 (2H, br t, J = 8.0 Hz, m-Ph), 7.62 (2H, br d, J = 7.7 Hz, o-Ph), 8.61 (1H, s, NH), 8.78 (1H, br s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 24.6, 26.2, 35.8, 37.6, 38.5, 43.7, 53.9, 56.0, 120.5, 125.0, 126.0, 129.0, 139.2, 155.3, 166.5, 168.2, 169.7, 173.0. LC-MS: Rt = 1.867 min, m/z = 382 (MH+), area% = 94. m/z (ESI) = 353 (MH+). m/z (HRMS) Found: 382.2242 (MH+). C21H28N5O2 requires: m/z = 382.2238. νmax (KBr) 3444, 2356, 1651, 1503, 1312, 1163, 1008, 985, 838, 762, 693 cm−1.
3.7.7. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-((pyridin-2-yl)methyl)pyrimidine-5-carboxamide (10{1; 7})
Prepared from 17{1} and 2-picolylamine (18{7}), workup B. Yield: 214 mg (76%) of yellow-brown resin. 1H-NMR (500 MHz, CDCl3): δ 2.76 (3H, s, 2-CH3), 2.93 (1H, dd, J = 9.0, 16.9 Hz, 4'-Ha), 3.19 (1H, dd, J = 8.0, 16.9 Hz, 4'-Hb), 4.16 (1H, dd, J = 7.1, 9.5 Hz, 2'-Ha), 4.22 (1H, t, J = 8.9 Hz, 2'-Hb), 4.34 (1H, quintet, J = 8.9 Hz, 3'-H), 4.74 and 4.78 (2H, 2dd, 1:1, J = 4.8, 16.5 Hz, CH2NH), 7.15 (1H, br t, J = 7.4 Hz, p-Ph), 7.28 (1H, dd, J = 5.3, 7.2 Hz, 5''-H), 7.36 (3H, br t, J = 8.0 Hz, m-Ph, NH), 7.58–7.64 (3H, m, o-Ph, 3''-H), 7.75 (1H, dt, J = 1.7, 7.7 Hz, 4''-H), 8.53 (1H, br d, J = 4.6 Hz, 6''-H), 8.78 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 26.3, 35.7, 38.4, 44.7, 53.9, 120.5, 122.8, 123.2, 125.0, 126.3, 129.1, 137.7, 139.2, 149.1, 155.0, 155.2, 165.9, 168.3, 170.0, 173.0. LC-MS: Rt = 11.775 min, m/z = 388 (MH+), area% = 100. m/z (ESI) = 388 (MH+). m/z (HRMS) Found: 388.1769 (MH+). C22H22N5O2 requires: m/z = 388.1768. νmax (KBr) 3452, 1654, 1515, 1500, 1405, 1311, 986, 760, 696 cm−1.
3.7.8. N,N-(Diethyl)-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 8})
Prepared from 17{1} and diethylamine (18{8}), workup B. Yield: 219 mg (100%) of yellow resin. 1H-NMR (500 MHz, CDCl3): δ 1.14 and 1.29 (6H, 2t, 1:1, J = 7.1 Hz, 2CH2CH3), 2.75 (3H, s, 2-CH3), 2.86 (1H, dd, J = 8.8, 16.7 Hz, 4'-Ha), 3.15–3.27 (3H, m, 4'-Hb, CH2CH3), 3.53–3.67 (2H, m, CH2CH3), 3.83 (1H, quintet, J = 8.6 Hz, 3'-H), 4.12 (1H, br t, J = 8.5 Hz, 2'-Ha), 4.20 (1H, br t, J = 8.6 Hz, 2'-Hb), 7.17 (1H, br t, J = 7.4 Hz, p-Ph), 7.37 (2H, br t, J = 8.0 Hz, m-Ph), 7.60 (2H, br d, J = 7.8 Hz, o-Ph), 8.49 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 13.1, 14.5, 26.1, 36.1, 39.9, 43.7, 120.5, 125.2, 127.3, 129.1, 139.0, 153.7, 166.2, 166.6, 168.9, 172.5. LC-MS: Rt = 13.242 min, m/z = 353 (MH+), area% = 100. m/z (ESI) = 353 (MH+). m/z (HRMS) Found: 353.1973 (MH+). C20H25N4O2 requires: m/z = 353.1972. νmax (KBr) 3413, 2346, 1637, 1516, 1500, 1430, 1310, 995, 761, 691 cm−1.
3.7.9. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-(pyrrolidin-1-yl)pyrimidine-5-carboxamide (10{1; 9})
Prepared from 17{1} and pyrrolidine (18{9}), workup B. Yield: 205 mg (79%) of yellow resin. 1H-NMR (500 MHz, CDCl3): δ 1.90–2.08 (4H, m, 4H of pyrrolidine), 2.75 (3H, s, 2-CH3), 2.88 (1H, dd, J = 9.0, 16.8 Hz, 4'-Ha), 3.17 (1H, dd, J = 8.7, 16.8 Hz, 4'-Hb), 3.23–3.29 (1H, m, 1H of pyrrolidine), 3.30–3.37 (1H, m, 1H of pyrrolidine), 3.68 (2H, br t, J = 6.9 Hz, 2H of pyrrolidine), 3.97 (1H, quintet, J = 8.4 Hz, 3'-H), 4.15 (1H, br t, J = 8.9 Hz, 2'-Ha), 4.21 (1H, dd, J = 7.6, 9.5 Hz, 2'-Hb), 7.17 (1H, br t, J = 7.4 Hz, p-Ph), 7.37 (2H, br t, J = 8.0 Hz, m-Ph), 7.60 (2H, d, J = 7.4 Hz, o-Ph), 8.56 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 24.6, 26.1, 26.4, 36.2, 38.3, 46.3, 49.5, 53.8, 120.6, 125.2, 127.5, 129.1, 138.9, 154.4, 165.4, 166.5, 169.1, 172.7. LC-MS: Rt = 12.258 min, m/z = 351 (MH+), area% = 100. m/z (ESI) = 351 (MH+). m/z (HRMS) Found: 351.1815 (MH+). C20H23N4O2 requires: m/z = 351.1816. νmax (KBr) 3422, 2362, 1638, 1516, 1426, 997, 764, 670 cm−1.
3.7.10. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-(piperidin-1-yl)pyrimidine-5-carboxamide (10{1; 10})
Prepared from 17{1} and piperidine (18{10}), workup B. Yield: 201 mg (100%) of yellow resin. 1H-NMR (500 MHz, CDCl3): δ 1.54 (2H, br s, 2H of piperidine), 1.72 (4H, br s, 4H of piperidine), 2.76 (3H, s, 2-CH3), 2.81–2.99 (1H, m, 4'-Ha), 3.13–3.25 (1H, m, 4'-Hb), 3.29 (2H, br s, 2H of piperidine), 3.77 (2H, br s, 2H of piperidine), 3.90 (1H, quintet, J = 8.5 Hz, 3'-H), 4.19 (2H, br s, 2'-CH2), 7.17 (1H, br t, J = 7.4 Hz, p-Ph), 7.38 (2H, br t, J = 8.0 Hz, m-Ph), 7.60 (2H, br d, J = 7.7 Hz, o-Ph), 8.47 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 24.4, 25.8, 26.1, 27.0, 36.2, 43.3, 48.9, 120.6, 125.2, 126.8, 129.1, 139.0, 154.2, 165.5, 166.4, 169.0, 172.6. LC-MS: Rt = 12.55 min, m/z = 365 (MH+), area% = 100. m/z (ESI) = 365 (MH+). m/z (HRMS) Found: 365.197 (MH+). C21H25N4O2 requires: m/z = 365.1972. νmax (KBr) 3438, 2325, 1630, 1515, 1500, 1431, 1288, 1000, 760, 692 cm−1.
3.7.11. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-(morpholin-4-yl)pyrimidine-5-carboxamide (10{1; 11})
Prepared from 17{1} and morpholine (18{11}), workup B. Yield: 228 mg (99%) of yellow resin. 1H-NMR (500 MHz, CDCl3): δ 2.76 (3H, s, 2-CH3), 2.86–2.95 (1H, m, 4'-Ha), 3.11–3.23 (1H, m, 4'-Hb), 3.32–3.43 (2H, m, 2H of morpholine), 3.60–3.71 (2H, m, 2H of morpholine), 3.79–3.88 (4H, m, 4H of morpholine), 3.91 (1H, quintet, J = 8.4 Hz, 3'-H), 4.12–4.25 (2H, m, 2'-CH2), 7.17 (1H, br t, J = 7.4 Hz, o-Ph), 7.38 (2H, br t, J = 8.0 Hz, m-Ph), 7.60 (2H, br d, J = 7.7 Hz, o-Ph), 8.48 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 26.2, 36.2, 42.7, 45.8, 48.1, 67.0, 120.6, 125.2, 125.8, 129.1, 138.9, 154.5, 165.9, 166.9, 169.5, 172.4. LC-MS: Rt = 10.342 min, m/z = 367 (MH+), area% = 100. m/z (ESI) = 367 (MH+). m/z (HRMS) Found: 367.1766 (MH+). C20H23N4O3 requires: m/z = 367.1765. νmax (KBr) 3454, 2326, 1633, 1516, 1501, 1428, 1284, 1117, 984, 840, 762, 696 cm−1.
3.7.12. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-(4-methylpiperazin-1-yl)pyrimidine-5-carboxamide (10{1; 12})
Prepared from 17{1} and 4-methylpiperazine (18{12}), workup B. Yield: 203 mg (100%) of yellow resin. 1H-NMR (500 MHz, CDCl3): δ 2.38 (3H, s, 4''-CH3), 2.39 (1H, br s, 1H of piperazine), 2.46 (1H, br s, 1H of piperazine), 2.53–2.65 (2H, m, 2H of piperazine), 2.76 (3H, s, 2-CH3), 2.82–2.98 (1H, m, 4'-Ha), 3.12–3.25 (1H, m, 4'-Hb), 3.40 (2H, m, 2H of piperazine), 3.78–3.87 (1H, m, 1H of piperazine), 3.89 (1H, quintet, J = 8.4 Hz, 3'-H), 3.90–3.97 (1H, m, 1H of piperazine), 4.17 (2H, m, 2'-CH2), 7.17 (1H, br t, J = 7.4 Hz, p-Ph), 7.38 (2H, br t, J = 8.0 Hz, m-Ph), 7.60 (2H, br d, J = 7.7 Hz, o-Ph), 8.47 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 26.2, 31.1, 36.2, 41.8, 45.7, 45.9, 47.3, 54.7, 55.3, 120.5, 125.2, 126.1, 129.1, 139.0, 154.5, 165.7, 166.7, 169.4, 172.4. LC-MS: Rt = 9.683 min, m/z = 380 (MH+), area% = 100. m/z (ESI) = 380 (MH+). m/z (HRMS) Found: 380.2085 (MH+). C21H26N5O2 requires: m/z = 380.2081. νmax (KBr) 3448, 2365, 1636, 1500, 1292, 1154, 986, 764, 694 cm−1.
3.7.13. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-N-pentyl-1-phenylpyrimidine-5-carboxamide (10{2; 1})
Prepared from 17{2} and 1-pentylamine (18{1}), workup A. Yield: 214 mg (100%) of white solid; m.p. 122–126 °C. 1H-NMR (500 MHz, CDCl3): δ 0.93 (3H, t, J = 7.0 Hz, CH3 of C5H11), 1.36–1.41 (4H, m, 2CH2 of C5H11), 1.62–1.68 (2H, m, CH2 of C5H11), 2.95 (1H, dd, J = 8.7, 16.9 Hz, 4'-Ha), 3.23 (1H, dd, J = 6.5, 16.9 Hz, 4'-Hb), 3.46 (2H, q, J = 6.3 Hz, CH2 of C5H11), 4.14 (1H, dd, J = 5.2, 9.1 Hz, 2'-Ha), 4.31 (1H, t, J = 8.6 Hz, 3'-H), 4.32–4.37 (1H, m, 2'-Hb), 6.39 (1H, s, NH), 7.14 (1H, br t, J = 7.4 Hz, p-Ph), 7.35 (2H, br t, J = 8.0 Hz, m-Ph), 7.44–7.52 (3H, m, m,p-Ph), 7.59 (2H, br d, J = 7.8 Hz, o-Ph), 8.44 (2H, br d, J = 7.0 Hz, o-Ph), 8.77 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 14.2, 22.5, 29.3, 29.4, 35.9, 38.3, 40.5, 54.1, 120.5, 125.0, 126.6, 128.8, 128.9, 129.0, 131.8, 136.6, 139.2, 155.4, 165.3, 165.9, 168.5, 173.1. LC-MS: Rt = 20.192 min, m/z = 429 (MH+), area% = 100. m/z (ESI) = 429 (MH+). m/z (HRMS) Found: 429.2289 (MH+). C26H29N4O2 requires: m/z = 429.2285. (Found: C 72.10; H 6.44; N 12.90. C26H28N4O2 requires (428.5): C 72.87; H 6.59; N 13.07.); νmax (KBr) 3436, 2340, 1677, 1656, 1537, 1435, 1409, 1308, 755, 693 cm−1.
3.7.14. N-Cyclohexyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 2})
Prepared from 17{2} and cyclohexylamine (18{2}), workup A. Yield: 225 mg (100%) of white solid; m.p. 196–199 °C. 1H-NMR (500 MHz, CDCl3): δ 1.21–1.32 (3H, m, 3H of C6H11), 1.40–1.48 (2H, m, 2H of C6H11), 1.65–1.71 (1H, m, 1H of C6H11), 1.76–1.82 (2H, m, 2H of C6H11), 2.03–2.10 (2H, m, 2H of C6H11), 2.95 (1H, dd, J = 8.8, 16.9 Hz, 4'-Ha), 3.23 (1H, dd, J = 6.5, 16.9 Hz, 4'-Hb), 3.96 (1H, tdd, J = 4.0, 8.0, 11.5 Hz, 1H of C6H11), 4.15 (1H, q, J = 4.8 Hz, 2'-Ha), 4.27–4–35 (2H, m, 2'-Hb, 3'-H), 6.22 (1H, d, J = 7.3 Hz, NH), 7.13 (1H, br t, J = 7.4 Hz, p-Ph), 7.35 (2H, br t, J = 8.0 Hz, m-Ph), 7.43–7.52 (3H, m, p,m-Ph), 7.59 (2H, br d, J = 7.8 Hz, o-Ph), 8.44 (2H, br d, J = 7.0 Hz, o-Ph), 8.75 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 25.1, 25.6, 33.2, 35.9, 38.2, 49.6, 54.0, 120.4, 124.9, 126.8, 128.8, 128.9, 129.0, 131.8, 136.6, 139.2, 155.4, 165.1, 165.2, 168.4, 173.0. LC-MS: Rt = 20.275 min, m/z = 441 (MH+), area% = 86. m/z (ESI) = 441 (MH+). m/z (HRMS) Found: 441.2287 (MH+). C27H29N4O2 requires: m/z = 441.2285. (Found: C 73.66; H 6.13; N 12.53. C27H28N4O2 (440.5) requires: C 73.61; H 6.41; N 12.72.); νmax (KBr) 3411, 2342, 1691, 1633, 1567, 1431, 1400, 1316, 1229, 756, 717, 693 cm–1.
3.7.15. N-Benzyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 3})
Prepared from 17{2} and benzylamine (18{3}), workup A. Yield: 173 mg (77%) of white solid; m.p. 169–171 °C. 1H-NMR (500 MHz, CDCl3): δ 2.96 (1H, dd, J = 8.8, 16.9 Hz, 4'-Ha), 3.26 (1H, dd, J = 6.7, 16.9 Hz, 4'-Hb), 4.14 (1H, dd, J = 5.8, 9.6 Hz, 2'-Ha), 4.31 (1H, t, J = 8.8 Hz, 2'-Hb), 4.39 (1H, ddd, J = 6.4, 8.3, 12.5 Hz, 3'-H), 4.64 and 4.68 (2H, 2dd, J = 5.7, 14.6 Hz, CH2Ph'), 6.48 (1H, t, J = 5.4 Hz, NH), 7.15 (1H, br t, J = 7.4 Hz, p-Ph), 7.32–7.41 (7H, m, m-Ph, Ph'), 7.45–7.52 (3H, m, m,p-Ph), 7.60 (2H, br d, J = 7.9 Hz, o-Ph), 8.46 (2H, br d, J = 7.0 Hz, o-Ph), 8.81 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 35.9, 38.2, 44.6, 54.1, 120.4, 124.9, 126.2, 128.2, 128.3, 128.9, 129.0, 129.1, 129.3, 131.9, 136.5, 137.5, 139.3, 155.4, 165.5, 165.8, 168.8, 173.0. LC-MS: Rt = 19.4 min, m/z = 449 (MH+), area% = 100. m/z (ESI) = 449 (MH+). m/z (HRMS) Found: 449.198 (MH+). C28H25N4O2 requires: m/z = 449.1972. (Found: C 74.63; H 5.43; N 12.38. C28H24N4O2 (448.5) requires: C 74.98; H 5.39; N 12.49.); νmax (KBr) 3418, 1679, 1662, 1570, 1498, 1434, 1305, 760, 692 cm−1.
3.7.16. N-(2-Methoxyethyl)-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 4})
Prepared from 17{2} and 2-methoxyethylamine (18{4}), workup A. Yield: 138 mg (65%) of white solid; m.p. 164–168 °C. 1H-NMR (500 MHz, CDCl3): δ 2.98 (1H, dd, J = 8.6, 16.8 Hz, 4'-Ha), 3.27 (1H, dd, J = 6.8, 16.9 Hz, 4'-Hb), 3.40 (3H, s, OCH3), 3.60 (2H, t, J = 4.9 Hz, CH2OMe), 3.66–3.69 (2H, t, J = 5.0 Hz, CH2NH), 4.17 (1H, dd, J = 5.8, 9.4 Hz, 2'-Ha), 4.32 (1H, t, J = 8.8 Hz, 2'-Hb), 4.34–4.40 (1H, m, 3'-H), 6.60 (1H, s, NH), 7.14 (1H, br t, J = 7.4 Hz, p-Ph), 7.36 (2H, br t, J = 8.0 Hz, m-Ph), 7.45–7.53 (3H, m, p,m-Ph), 7.62 (2H, br d, J = 7.7 Hz, o-Ph), 8.46 (2H, dd, J = 1.4, 8.1 Hz, o-Ph), 8.82 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 35.9, 38.3, 40.1, 54.0, 59.1, 70.9, 120.4, 124.9, 126.4, 128.8, 128.9, 129.1, 131.9, 136.6, 139.3, 155.6, 165.4, 166.0, 168.5, 173.0. LC-MS: Rt = 15.017 min, m/z = 417 (MH+), area% = 100. m/z (ESI) = 417 (MH+). m/z (HRMS) Found: 415.1779 ([M–H]–). C24H23N4O3 requires: m/z = 415.1776. (Found: C 68.03; H 5.43; N 13.11. C24H24N4O3·⅖H2O (423.7) requires: C 68.04; H 5.90; N 13.23.); νmax (KBr) 3466, 2934, 1682, 1663, 1568, 1432, 1306, 1122, 761, 693 cm−1.
3.7.17. N-(3-Hydroxypropyl)-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 5})
Prepared from 17{2} and 3-amino-1-propanol (18{5}), workup A. Yield: 206 mg (98%) of white solid; 145–146 °C. 1H-NMR (500 MHz, CDCl3): δ 1.86 (2H, quintet, J = 5.7 Hz, CH2CH2CH2), 2.98 (1H, dd, J = 8.7, 16.9 Hz, 4'-Ha), 3.21 (1H, dd, J = 6.5, 16.9 Hz, 4'-Hb), 3.65 (2H, br q, J = 6.1 Hz, CH2NH), 3.82 (2H, t, J = 5.5 Hz, CH2OH), 4.17 (1H, dd, J = 5.6, 9.6 Hz, 2'-Ha), 4.31 (1H, t, J = 8.9 Hz, 2'Hb), 4.38 (1H, ddd, J = 6.4, 8.3, 12.2 Hz, 3'-H), 7.12 (1H, br s, NH); 7.14 (1H, br t, J = 7.4 Hz, p-Ph), 7.35 (2H, br t, J = 8.0 Hz, m-Ph), 7.44–7.52 (3H, m, m,p-Ph), 7.60 (2H, br d, J = 7.7 Hz, o-Ph), 8.44 (2H, dd, J = 1.5, 8.5 Hz, o-Ph), 8.80 (1H, s, 4-H), OH exchanged. 13C-NMR (126 MHz, CDCl3): δ 31.5, 35.9, 38.4, 38.7, 54.1, 61.3, 120.6, 125.1, 126.3, 128.8, 128.9, 129.1, 131.9, 136.5, 139.2, 155.7, 165.3, 166.4, 168.6, 173.2. LC-MS: Rt = 13.117 min, m/z = 417 (MH+), area% = 84. m/z (ESI) = 417 (MH+). m/z (HRMS) Found: 417.192 (MH+). C24H25N4O3 requires: m/z = 417.1921. (Found: C 68.23; H 5.60; N 13.21. C24H24N4O3·⅓H2O (422.5) requires: C 68.24; H 5.89; N 13.27.); νmax (KBr) 3458, 2343, 1682, 1646, 1568, 1432, 1402, 1306, 1071, 762, 694 cm−1.
3.7.18. N-(3-Dimethylaminopropyl)-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 6})
Prepared from 17{2} and 3-(dimethylamino)propylamine (18{6}), workup A. Yield: 158 mg (71%) of white solid; 111–114 °C. 1H-NMR (500 MHz, CDCl3): δ 1.78–1.84 (2H, m, CH2CH2CH2), 2.29 (6H, s, NMe2), 2.54 (2H, t, J = 5.7 Hz, CH2NMe2), 3.00 (1H, dd, J = 8.9, 16.9 Hz, 4'-Ha), 3.29 (1H, dd, J = 6.7, 16.9 Hz, 4'-Hb), 3.54–3.65 (2H, m, CH2NH), 4.17 (1H, dd, J = 5.8, 9.7 Hz, 2'-Ha), 4.36 (1H, dd, J = 8.1, 9.7 Hz, 2'-Hb), 4.51 (1H, tt, J = 6.6, 8.5 Hz, 3'-H), 7.14 (1H, br t, J = 7.4 Hz, p-Ph), 7.37 (2H, br t, J = 8.0 Hz, m-Ph), 7.46–7.53 (3H, m, p,m-Ph), 7.64 (2H, br d, J = 7.7 Hz, o-Ph), 8.48 (2H, dd, J = 1.6, 7.9 Hz, o-Ph), 8.77 (1H, s, 4-H), 8.85 (1H, br s, NH). 13C-NMR (126 MHz, CDCl3): δ 23.7, 36.2, 38.9, 40.4, 44.6, 53.7, 58.3, 120.4, 124.8, 126.4, 128.7, 128.8, 129.0, 131.2, 137.2, 139.5, 159.6, 164.1, 168.2, 171.3, 173.8. LC-MS: Rt = 9.342 min, m/z = 444 (MH+), area% = 100. m/z (ESI) = 444 (MH+). m/z (HRMS) Found: 444.2401 (MH+). C26H30N5O2 requires: m/z = 444.2394. νmax (KBr) 3446, 2946, 1689, 1631, 1570, 1431, 754, 692 cm−1.
3.7.19. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-2-phenyl-N-((pyridin-2-yl)methyl)pyrimidine-5-carboxamide (10{2; 7})
Prepared from 17{2} and 2-picolylamine (18{7}), workup A. Yield: 204 mg (89%) of gray solid, m.p. 160–165 °C. 1H-NMR (500 MHz, CDCl3): δ 3.00 (1H, dd, J = 8.9, 16.9 Hz, 4'-Ha), 3.29 (1H, dd, J = 7.1, 16.9 Hz, 4'-Hb), 4.19 (1H, dd, J = 6.1, 9.6 Hz, 2'-Ha) 4.32 (1H, dd, J = 8.3, 9.6 Hz, 2'-Hb), 4.44 (1H, t, J = 7.0, 8.4 Hz, 3'-H), 4.76 and 4.80 (2H, 2dd, 1:1, J = 4.7, 17.5 Hz, CH2NH), 7.14 (1H, br t, J = 7.4 Hz, p-Ph), 7.25 (1H, br dd, J = 5.2, 7.1 Hz, 5''-H), 7.33–7.38 (3H, m, p,m-Ph), 7.46–7.53 (3H, m, m-Ph, NH), 7.62 (2H, br d, J = 7.7 Hz, o-Ph), 7.73 (2H, dt, J = 1.7, 7.6 Hz, 3''-H, 4''-H), 8.49 (2H, dt, J = 1.5, 8.1 Hz, o-Ph), 8.54 (1H, br d, J = 4.6 Hz, 6''-H), 8.96 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 35.9, 38.4, 44.7, 54.1, 120.4, 122.4, 123.0, 124.9, 126.4, 128.8, 128.9, 129.0, 131.8, 136.6, 137.3, 139.3, 149.2, 155.1, 156.0, 165.4, 165.9, 168.5, 173.0. LC-MS: Rt = 15.65 min, m/z = 450 (MH+), area% = 87. m/z (ESI) = 450 (MH+). m/z (HRMS) Found: 448.1785 ([M−H]−). C27H22N5O2 requires: m/z = 448.1779. (Found: C 70.65; H 5.00; N 15.09. C27H23N5O2∙½H2O (458.5) requires: C 70.73; H 5.28; N 15.27.); νmax (KBr) 3472, 1682, 1662, 1569, 1434, 1404, 1307, 758, 693 cm−1.
3.7.20. N,N-(Diethyl)-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 8})
Prepared from 17{2} and diethylamine (18{8}), workup B. Yield: 148 mg (68%) of yellowish resin. 1H-NMR (500 MHz, CDCl3): δ 1.17 and 1.32 (6H, 2t, 1:1, J = 7.1 Hz, 2CH3CH2), 2.95 (1H, dd, J = 8.9, 16.9 Hz, 4'-Ha), 3.24–3.34 (3H, m, 4'-Hb, CH2CH3), 3.60 and 3.66 (2H, 2 septets, J = 7.2 Hz, CH2CH3), 3.92 (1H, quintet, J = 8.0 Hz, 3'-H), 4.22 and 4.24 (2H, 2dd, 1:1, J = 10.0, 12.5 Hz, 2'-CH2), 7.16 (1H, br t, J = 7.4 Hz, p-Ph), 7.38 (2H, br t, J = 8.0 Hz, m-Ph), 7.46–7.54 (3H, m, p,m-Ph), 7.64 (2H, br d, J = 7.9 Hz, o-Ph), 8.47 (2H, dd, J = 1.8, 8.0 Hz, o-Ph), 8.65 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 11.6, 36.2, 38.8, 42.3, 53.9, 120.3, 124.8, 126.8, 128.8, 128.9, 129.1, 131.4, 137.2, 139.5, 159.8, 164.5, 168.6, 170.7, 173.6. LC-MS: Rt = 18.008 min, m/z = 415 (MH+), area% = 88. m/z (ESI) = 415 (MH+). m/z (HRMS) Found: 415.2121 (MH+). C25H27N4O2 requires: m/z = 415.2129. (Found: C 69.44; H 6.41; N 12.62. C25H26N4O2·H2O (432.5) requires: C 69.42; H 6.53; N 12.95.); νmax (KBr) 3410, 2364, 1665, 1638, 1616, 1500, 1393, 1366, 1312, 751, 717, 690 cm−1.
3.7.21. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-2-phenyl-N-(pyrrolidin-1-yl)pyrimidine-5-carboxamide (10{2; 9})
Prepared from 17{2} and pyrrolidine (18{9}), workup B. Yield: 208 mg (100%) of yellow resin. 1H-NMR (500 MHz, CDCl3): δ 1.93–2.10 (4H, m, 4H of pyrrolidine), 2.97 (2H, dd, J = 8.9, 16.9 Hz, 4'-Ha), 3.28 (1H, dd, J = 7.8, 16.9 Hz, 4'-Hb), 3.29–3.36 and 3.37–3.43 (2H, 2m, 1:1, 2H of pyrrolidine), 3.72 (2H, t, J = 7.0 Hz, 2H of pyrrolidine), 4.06 (1H, quintet, J = 8.1 Hz, 3'-H), 4.24 (1H, dd, J = 6.8, 9.6 Hz, 2'-Ha), 4.27 (1H, dd, J = 8.2, 9.7 Hz, 2'-Hb), 7.16 (1H, br t, J = 7.4 Hz, p-Ph), 7.38 (2H, br t, J = 8.0 Hz, m-Ph), 7.45–7.53 (3H, m, m,p-Ph), 7.63 (2H, br d, J = 7.7 Hz, o-Ph), 8.47 (2H, dd, J = 1.8, 8.2 Hz, o-Ph), 8.72 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 24.6, 26.4, 36.3, 38.3, 46.3, 49.5, 53.9, 120.4, 120.5, 125.1, 127.8, 128.7, 128.9, 129.1, 131.7, 136.7, 139.2, 155.2, 159.6, 164.8, 172.7. LC-MS: Rt = 17.075 min, m/z = 413 (MH+), area% = 100. m/z (ESI) = 413 (MH+). m/z (HRMS) Found: 413.1975 (MH+). C25H25N4O2 requires: m/z = 413.1972. νmax (KBr) 3431, 2361, 1636, 1500, 1418, 983, 754, 704, 668 cm−1.
3.7.22. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-2-phenyl-N-(piperidin-1-yl)pyrimidine-5-carboxamide (10{2; 10})
Prepared from 17{2} and piperidine (18{10}), workup B. Yield: 214 mg (100%) of yellow resin. 1H-NMR (500 MHz, CDCl3): δ 1.51–1.61 (2H, m, 2H of piperidine), 1.74 (4H, br s, 4H of piperidine), 2.96 (1H, br s, 4'-Ha), 3.27 (1H, br s, 4'-Hb), 3.34 (2H, br s, 2H of piperidine), 3.79 (2H, br s, 2H of piperidine), 3.98 (1H, quintet, J = 7.9 Hz, 3'-H), 4.24 (2H, br s, 2'-CH2), 7.16 (1H, br t, J = 7.4 Hz, p-Ph), 7.38 (2H, br t, J = 7.9 Hz, m-Ph), 7.47–7.54 (3H, m, m,p-Ph), 7.64 (2H, br d, J = 7.8 Hz, o-Ph), 8.47 (2H, dd, J = 1.9, 7.9 Hz, o-Ph), 8.63 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 22.6, 22.9, 36.0, 38.6, 44.6, 54.0, 120.4, 124.7, 127.0, 128.6, 128.7, 129.0, 131.2, 137.2, 139.4, 159.6, 164.2, 168.4, 170.9, 173.8. LC-MS: Rt = 18.608 min, m/z = 427 (MH+), area% = 100. m/z (ESI) = 427 (MH+). m/z (HRMS) Found: 427.2135 (MH+). C26H27N4O2 requires: m/z = 427.2129. νmax (KBr) 3438, 2326, 1630, 1515, 1500, 1431, 1288, 1000, 760, 692 cm−1.
3.7.23. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-N-(morhpolin-4-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 11})
Prepared from 17{2} and morpholine (18{11}), workup B. Yield: 204 mg (95%) of yellow resin. 1H-NMR (500 MHz, CDCl3): δ 2.97 (1H, dd, J = 8.3, 16.3 Hz, 4'-Ha), 3.27 (1H, br dd, J = 7.0, 16.3 Hz, 4'-Hb), 3.38–3.49 (2H, m, 2H of morpholine), 3.63–3.72 (2H, m, 2H of morpholine), 3.80–3.92 (4H, m, 4H of morpholine), 3.99 (1H, quintet, J = 7.7 Hz, 3'-H), 4.23 and 4.25 (2H, 2br d, 1:1, 2'-CH2), 7.17 (1H, br t, J = 7.4 Hz, p-Ph), 7.38 (2H, br t, J = 8.0 Hz, m-Ph), 7.47–7.55 (3H, m, m,p-Ph), 7.64 (2H, br d, J = 7.7 Hz, o-Ph), 8.47 (2H, dd, J = 1.5, 8.0 Hz, o-Ph), 8.64 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 35.9, 38.6, 43.3, 54.0, 64.2, 120.4, 124.8, 126.7, 128.6, 128.7, 129.0, 131.2, 137.1, 139.3, 159.7, 164.2, 168.5, 170.8, 173.8. LC-MS: Rt = 16.042 min, m/z = 429 (MH+), area% = 100. m/z (ESI) = 429 (MH+). m/z (HRMS) Found: 429.1921 (MH+). C25H25N4O3 requires: m/z = 429.1921. νmax (KBr) 3449, 2366, 1669, 1607, 1500, 1368, 1313, 1125, 752, 717,689 cm−1.
3.7.24. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-N-(4-methylpiperazin-1-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 12})
Prepared from 17{2} and 4-methylpiperazine (18{12}), workup B. Yield: 225 mg (100%) of yellow resin. 1H-NMR (500 MHz, CDCl3): δ 2.37 (3H, s, 4''-CH3), 2.36–2.42 (1H, m, 1H of piperazine), 2.46 (1H, br s, 1H of piperazine), 2.53–2.64 (2H, m, 2H of piperazine), 2.92–3.03 (1H, m, 4'-Ha), 3.24–3.34 (1H, m, 4'-Hb), 3.45 (2H, br s, 2H of piperazine), 3.85 (1H, br s, 1H of piperazine), 3.95 (1H, br s, 1H of piperazine), 3.98 (1H, quintet, J = 7.9 Hz, 3'-H), 4.25 (2H, br s, 2'-CH2), 7.17 (1H, br t, J = 7.4 Hz, p-Ph), 7.38 (2H, br t, J = 8.0 Hz, m-Ph), 7.46–7.54 (3H, m, m,p-Ph), 7.63 (2H, d, J = 7.8 Hz, o-Ph), 8.47 (2H, dd, J = 1.5, 8.0 Hz, o-Ph), 8.64 (1H, s, 4-H). 13C-NMR (126 MHz, CDCl3): δ 36.3, 42.0, 46.1, 47.5, 54.8, 55.5, 120.5, 125.1, 126.4, 128.7, 128.9, 129.1, 131.8, 136.6, 139.1, 155.2, 165.0, 165.8, 166.9, 172.5. LC-MS: Rt = 9.392 min, m/z = 442 (MH+), area% = 100. m/z (ESI) = 380 (MH+). m/z (HRMS) Found: 442.2238 (MH+). C26H28N5O2 requires: m/z = 442.2238. νmax (KBr) 3456, 2340, 1637, 1500, 1421, 1298, 1168, 983, 760, 696 cm−1.
4. Conclusions
2-Substituted 6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides 10 as a novel type of conformationally constrained 2-(heteroaryl)ethylamines are available in six-steps from itaconic acid (11). The synthetic pathway consists of two parts: (a) a five-step preparation of pyrimidine-5-carboxylic acids 17{1,2} as the key-intermediates and (b) combinatorial solution-phase BPC-mediated amidation of 17{1,2} with primary and secondary amines 18{1–12} to give the title compounds 10{1,2; 1–12} in good overall yields and purity upon simple workup. The method is general and substrate-independent. All 24 amidations proceeded smoothly and no major differences in reactivity was observed with respect to the C(2) substituent in the pyrimidine-5-carboxylic acids 17. On the other hand, the secondary amines 18{8–12} were less reactive in these amidations than the primary amines 18{1–hy,lip98u7}. Consequently, a 10-fold excess of secondary amines 18{8–12} was employed in order to assure completion of the amidation reaction. Besides, preparation of the 2-chloro analogue of 16, e.g., by treatment of 14 with methyl carbamimidate followed by demethylation and chlorination, would enable functionalization at position 2 in the pyrimidine ring, either by SNAr reaction, or by cross-coupling reaction. These results also indicate that the above synthetic method could serve as a useful tool for the preparation of novel compound libraries for pharmaceutical and other practical applications.
Acknowledgements
The financial support from Boehringer-Ingelheim Pharma (Biberach, Germany) and from the Slovenian Research Agency through grant P1-0179 is gratefully acknowledged. We acknowledge with thanks the financial contributions of pharmaceutical companies Krka d.d. (Novo mesto, Slovenia) and Lek d.d. a new Sandoz Company (Ljubljana, Slovenia), which made the purchase of Mettler-Toledo Bohdan MiniBlock™ Compact Shaking and Washing Station and Vacuum Collection Base possible.
Footnotes
Sample Availability: Samples of the compounds 16{1,2}, 17{1,2}, and 10{1,2; 1-12} are available from the authors.
References and Notes
- 1.Patrick G.L. An Introduction to Medicinal Chemistry, 4th ed. Oxford University Press; Oxford, UK: 2009. pp. 1–752. [Google Scholar]
- 2.Takahashi T., Miyazawa M. N-Caffeoyl serotonin as selective COX-2 inhibitor. Bioorg. Med. Chem. Lett. 2012;22:2494–2496. doi: 10.1016/j.bmcl.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Zefirova O.N., Baranova T.Y., Lyssenko K.A., Zefirov N.A., Zyk N.V., Vassiliev P.M., Yakovlev D.S., Spasov A.A. Synthesis and biological testing of conformationally restricted serotonin analogues with bridgehead moieties. Mendeleev Commun. 2012;22:75–77. doi: 10.1016/j.mencom.2012.03.007. [DOI] [Google Scholar]
- 4.Bonner L.A., Laban U., Chemel B.R., Juncosa J.I., Lill M.A., Watts V.J., Nichols D.E. Mapping the catehol binding site in dopamine D1 receptors: Synthesis and evaluation of two parallel series of bicyclic dopamine analogues. ChemMedChem. 2011;6:1024–1040. doi: 10.1002/cmdc.201100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlotos D.P., Attia M.I., Julius J., Sethi S.S., Witt-Enderby P.A. 2-[(2,3-Dihydro-1H-indol-1-yl)methyl]melatonin analogues: A novel class of MT2-Selective melatonin receptor antagonists. J. Med. Chem. 2009;52:826–833. doi: 10.1021/jm800974d. [DOI] [PubMed] [Google Scholar]
- 6.Dolle R.E. Solid-phase Synthesis of Heterocyclic Systems (Heterocycles Containing One Heteroatom) In: Nicolaou K.C., Hanko R., Hartwig W., editors. Handbook of Combinatorial Chemistry. Drugs, Catalysts, Materials. Vol. 2. Wiley-VCH Verlag GmbH; Weinheim, Germany: 2002. pp. 725–742. [Google Scholar]
- 7.Pernerstorfer J. Molecular Design and Combinatorial Compound Libraries. In: Nicolaou K.C., Hanko R., Hartwig W., editors. Handbook of Combinatorial Chemistry. Drugs, Catalysts, Materials. Vol. 2. Wiley-VCH Verlag GmbH; Weinheim, Germany: 2002. pp. 725–742. [Google Scholar]
- 8.Dolle R.E., le Bourdonnec B., Morales G.A., Moriarty K.J., Salvino J.M. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2007. J. Comb. Chem. 2008;10:753–802. doi: 10.1021/cc800119z. [DOI] [PubMed] [Google Scholar]
- 9.Dolle R.E., le Bourdonnec B., Goodman A.J., Morales G.A., Thomas C.J., Zhang W. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2008. J. Comb. Chem. 2009;11:739–790. doi: 10.1021/cc9000828. [DOI] [PubMed] [Google Scholar]
- 10.Dolle R.E., le Bourdonnec B., Worm K., Morales G.A., Thomas C.J., Zhang W. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2009. J. Comb. Chem. 2010;12:765–806. doi: 10.1021/cc100128w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanovnik B., Svete J. Synthesis of heterocycles from alkyl 3-(dimethylamino)propenoates and related enaminones. Chem. Rev. 2004;104:2433–2480. doi: 10.1021/cr020093y. [DOI] [PubMed] [Google Scholar]
- 12.Svete J. Ex-chiral pool enaminones in the synthesis of functionalised heterocycles. Monatsh. Chem. 2004;135:629–641. doi: 10.1007/s00706-003-0133-y. [DOI] [Google Scholar]
- 13.Svete J. Utilisation of chiral enaminones and azomethine imines in the synthesis of functionalised pyrazoles. ARKIVOC. 2006;vii:35–56. [Google Scholar]
- 14.Bevk D., Svete J., Stanovnik B. Modern Approaches to the Synthesis of O- and N-Heterocycles. Vol. 3. Research Signpost; Kerala, India: 2007. Enaminones and Related Compounds in the Synthesis of Pyrazoles; pp. 73–88. [Google Scholar]
- 15.Stanovnik B., Grošelj U. Dialkyl acetone-1,3-dicarboxylates and their mono- and bis(dimethylamino) methylidene derivatives in the synthesis of heterocyclic systems. Adv. Heterocycl. Chem. 2010;100:145–174. doi: 10.1016/S0065-2725(10)10005-1. [DOI] [Google Scholar]
- 16.Pirc S., Bevk D., Golobič A., Stanovnik B., Svete J. Transformation of amino acids into nonracemic 1-(heteroaryl)ethanamines by the enamino ketone methodology. Helv. Chim. Acta. 2006;89:30–44. doi: 10.1002/hlca.200690010. [DOI] [Google Scholar]
- 17.Kralj D., Grošelj U., Meden A., Dahmann G., Stanovnik B., Svete J. A simple synthesis of 4-(2-aminoethyl)-5-hydroxy-1H-pyrazoles. Tetrahedron. 2007;63:11213–11222. doi: 10.1016/j.tet.2007.07.052. [DOI] [Google Scholar]
- 18.Kralj D., Novak A., Dahmann G., Grošelj U., Meden A., Svete J. One-pot parallel solution-phase synthesis of 1-substituted 4-(2-aminoethyl)-1H-pyrazol-5-ols. J. Comb. Chem. 2008;10:664–670. doi: 10.1021/cc8000794. [DOI] [PubMed] [Google Scholar]
- 19.Grošelj U., Kralj D., Wagger J., Dahmann G., Stanovnik B., Svete J. Synthesis of 3-(2-aminoethyl)-5-hydroxy-1H-pyrazole derivatives. ARKIVOC. 2012;iii:49–65. [Google Scholar]
- 20.Kralj D., Friedrich M., Grošelj U., Kiraly-Potpara S., Meden A., Wagger J., Dahmann G., Stanovnik B., Svete J. A synthesis of 1-substituted 5-[2-(acylamino)ethyl]-1H-pyrazole-4-carboxamides. Tetrahedron. 2009;65:7151–7162. [Google Scholar]
- 21.Žerovnik D., Grošelj U., Kralj D., Malavašič Č., Bezenšek J., Dahmann G., Stare K., Meden A., Stanovnik B., Svete J. Synthesis of 1,5,6,7-tetrahydro-4H-pyrazolo[4,3-c]pyridin-4-ones as conformationally constrained pyrazole analogues of histamine. Synthesis. 2010:3363–3373. [Google Scholar]
- 22.Janjić M., Prebil R., Grošelj U., Kralj D., Malavašič Č., Golobič A., Stare K., Dahmann G., Stanovnik B., Svete J. A simple synthesis of 5-(2-aminophenyl)-1H-pyrazoles. Helv. Chim. Acta. 2011;94:1703–1717. doi: 10.1002/hlca.201100055. [DOI] [Google Scholar]
- 23.Perdih P., Baškovč J., Dahmann G., Grošelj U., Kočar D., Novak A., Stanovnik B., Svete J. Parallel synthesis of 1-substituted 5-(5-oxopyrrolidin-3-yl)-1H-pyrazole-4-carboxamides. Synthesis. 2011:2822–2832. [Google Scholar]
- 24.Paytash P.L., Sparrow E., Gathe J.C. The reaction of itaconic acid with primary amines. J. Am. Chem. Soc. 1950;72:1415–1416. doi: 10.1021/ja01159a520. [DOI] [Google Scholar]
- 25.Harwood L.M., Moody C.J. Experimental Organic Chemistry, Principles and Practice. Blackwell Science; Oxford, UK: 1989. ‘Dry Flash’ Column Chromatography; pp. 185–188. [Google Scholar]
- 26.Harwood L.M. Dry-Column” Flash Chromatography. Aldrichimica Acta. 1985;18:25–25. [Google Scholar]
- 27.Since satisfactory results were obtained with evapotative workup and DFCC, the use of scavenging reagents such as solid supported tosyl chloride or propionyl chloride was not explored. Besides, covalent binding of scavenging reagents to products containing hydroxy and amino functions would probably make the isolation of products more difficult.
- 28.The above method is applicable for the synthesis of libraries of racemic compounds 10 for primary testing and screening. However, for a larger scale synthesis of certain enantiomerically pure final products 10, a modified ‘chiral pool’ synthesis of non-racemic 10 utilizing enantiomerically pure starting compound 12 should be developed.
- 29.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 30.Ghose A.K., Viswandhan V.N., Wendoloski J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999;1:55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]