Abstract
Two norcembranoidal diterpenes, 5-episinuleptolide acetate (1) and scabrolide D (2), were isolated from a Formosan octocoral identified as Sinularia sp. The structures of norcembranoids 1 and 2 were established by spectroscopic methods and by comparison of the spectral data with those of known analogues and 1 was proven to be a new natural product. Norcembranoid 1 was found to exhibit cytotoxicity toward a panel of tumor cells.
Keywords: Sinularia, norcembranoidal diterpene, cytotoxicity
1. Introduction
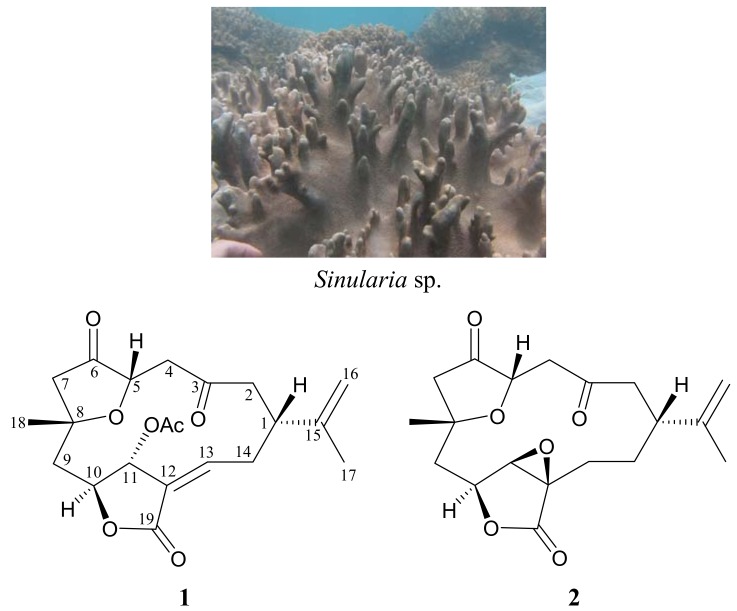
The search for bioactive natural products from marine organisms has been remarkably successful [1,2] and octocorals belonging to the genus Sinularia have proven to be rich sources of bioactive terpenoid analogues [3]. Among these terpenoid metabolites, the C19-norcembranoid diterpene derivatives played an important role [4]. In continuation of our search for new natural substances from the marine invertebrates collected off the waters of Taiwan at the intersection of the Kuroshio current and the South China Sea surface current, we have further isolated two norcembranoidal diterpenes, 5-episinuleptolide acetate (1) and scabrolide D (2), from an octocoral identified as Sinularia sp. (Figure 1). In this paper, we describe the isolation, structure determination and cytotoxicity of norcembranoids 1 and 2.
Figure 1.
The soft coral Sinularia sp. and the structures of 5-episinuleptolide acetate (1) and scabrolide D (2).
2. Results and Discussion
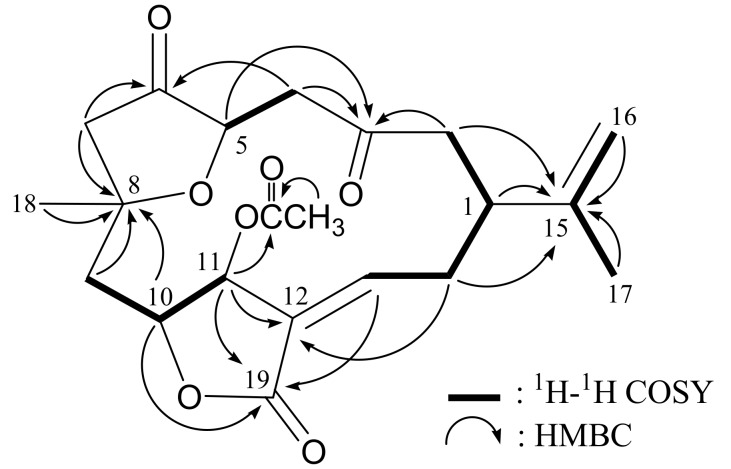
The molecular formula for norcembranoidal diterpene 1 was determined to be C21H26O7 (nine units of unsaturation) using HRESIMS (C21H26O7+Na, m/z 413.1574, calculated 413.1576). The IR spectrum of 1 showed strong bands at 1756, 1738 and 1719 cm–1, consistent with the presence of ester and ketone carbonyl groups. From the 1H- and 13C-NMR spectra (Table 1), 1 was found to possess an acetoxy group (δH 2.08, 3H, s; δC 171.2, C; 20.9, CH3), an ester group (δC 167.7, C-19) and two ketone carbonyls (δC 205.3, C-3; 214.5, C-6). Two additional unsaturated functionalities were indicated by 13C resonances at δC 127.6 (C-12), 147.2 (CH-13), 147.0 (C-15) and 110.4 (CH2-16), suggesting the presence of a trisubstituted olefin and an exocyclic carbon-carbon double bond. From the 1H–1H COSY spectrum of 1 (Table 1 and Figure 2), it was possible to differentiate among the separate spin systems of H-1/H2-2, H2-4/H-5, H2-9/H-10/H-11, H-13/H2-14/H-1 and H2-16/H3-17 (by allylic coupling). These data, together with the key HMBC correlations between protons and quaternary carbons of 1 (Table 1 and Figure 2), such as H2-2, H2-4, H-5/C-3; H2-4, H2-7/C-6; H2-7, H2-9, H-10, H3-18/C-8; H-11, H2-14/C-12; H-1, H2-2, H2-14, H2-16, H3-17/C-15; and H-10, H-11, H-13/C-19, permitted the elucidation of the carbon skeleton. The acetoxy group positioned at C-11 was confirmed from the HMBC correlations of H-11 (δH 5.47) and protons of an acetate methyl (δH 2.08) to the ester carbonyl carbon at δC 171.2 (C). Thus, 1 was revealed as a norcembranoidal diterpene possessing a γ-lactone ring, on the basis of the above analysis.
Table 1.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, 1H–1H COSY and HMBC correlations for norcembranoidial diterpene 1.
| Position | δΗ (J in Hz) | δC, Mult. | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 2.99 m | 39.6, CH | H2-2, H2-14 | C-15 |
| 2α β |
2.65 m 2.52 m |
45.9, CH2 | H-1, H-2β H-1, H-2α |
C-1, -3, -14, -15 C-1, -3, -14, -15 |
| 3 | 205.3, C | |||
| 4α β |
2.53 dd (16.4, 8.8) 2.82 dd (16.4, 2.4) |
43.1, CH2 | H-4β, H-5 H-4α, H-5 |
C-3, -5, -6 C-3, -5, -6 |
| 5 | 4.27 dd (8.8, 2.4) | 75.0, CH | H2-4 | C-3, -4 |
| 6 | 214.5, C | |||
| 7α β |
2.60 m 2.46 m |
51.1, CH2 | H-7β H-7α |
C-5, -6, -8, -9, -18 C-5, -6, -8, -9, -18 |
| 8 | 79.0, C | |||
| 9α β |
2.34 m 2.47 m |
41.8, CH2 | H-9β, H-10 H-9α, H-10 |
C-7, -8, -10, -11, -18 C-7, -8, -10, -11, -18 |
| 10 | 4.52 dt (6.8, 2.0) | 80.8, CH | H2-9, H-11 | C-8, -11, -19 |
| 11 | 5.47 br s | 76.4, CH | H-10 | C-9, -12, -13, -19, acetate carbonyl |
| 12 | 127.6, C | |||
| 13 | 6.45 ddd (10.8, 4.4, 1.2) | 147.2, CH | H2-14 | C-1, -11, -19 |
| 14α β |
2.20 ddd (14.8, 4.4, 3.6) 3.78 ddd (14.8, 10.8, 6.8) |
28.4, CH2 | H-1, H-13 H-1, H-13 |
C-1, -2, -12, -13, -15 C-1, -2, -12, -13, -15 |
| 15 | 147.0, C | |||
| 16a b |
4.86 s 4.79 s |
110.4, CH2 | H-16b, H3-17 H-16a, H3-17 |
C-1, -15, -17 C-1, -15, -17 |
| 17 | 1.80 s | 21.6, CH3 | H2-16 | C-1, -15, -16 |
| 18 | 1.42 s | 26.5, CH3 | C-7, -8, -9 | |
| 19 | 167.7, C | |||
| 11-OAc | 2.08 s | 171.2, C20.9, CH3 | Acetate carbonyl |
Figure 2.
The 1H–1H COSY and key HMBC (protons→quaternary carbons) correlations for 1.
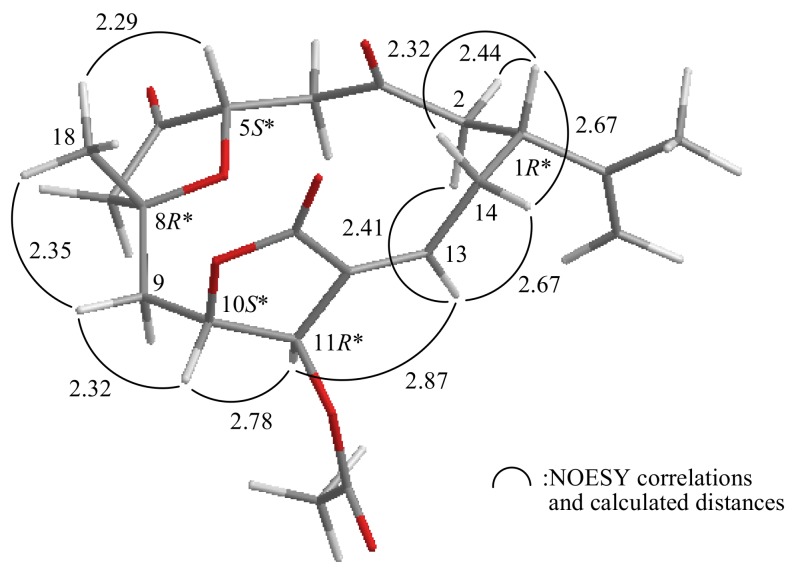
The relative configuration of 1 was elucidated mainly from a NOESY spectrum as being compatible with that of 1 offered by computer modeling (Figure 3), in which the close contacts of atoms in space calculated were consistent with the NOESY correlations [5]. Most naturally occurring cembrane-type natural products from soft coral belonging to the order Alcyonacea have the H-1 in the β-orientation [6]. In the NOESY experiment for 1, H-1 correlated with H2-14 and one proton of C-2 methylene (δH 2.52, H-2β), supporting this reasoning by molecular modeling analysis. H-13 showed correlations with H-2α (δH 2.65), H-11 and one proton of C-14 methylene (δH 2.20, H-14α), but not with H-1, indicating the E-configuration of the C-12/13 double bond. H-10 showed a correlation with H-11, as well as the lack of coupling was detected between these two protons, indicating the dihedral angle between H-10 and H-11 is approximately 90° and the configurations of chiral carbons C-10 and C-11 were assigned as S*- and R*-forms, respectively. One proton of C-9 methylene (δH 2.47) correlated with H-10 and H3-18, but not with H-11, and H3-18 showed a strong correlation with H-5, indicating that Me-18 and H-5 were β-oriented in 1. From the above evidences, the relative configuration of the chiarl carbons of 1 were assumed to be 1R*, 5S*, 8R*, 10S* and 11R*.
Figure 3.
The computer-generated model of 1 using MM2 force field calculations and the calculated distances (Å) between selected protons with key NOESY correlations.
By comparison of the spectral data with those of a known semisynthetic diterpene, compound 1 was elucidated unambiguously to be 5-episinuleptolide acetate [7]. To the best of our knowledge, norcembranoidal diterpene 1 was isolated for the first time from a natural source.
Norcembranoidial diterpene 2 was identified as scabrolide D, which had been isolated from a Formosan octocoral Sinularia scabra. Its spectral data were in full agreement with those previously reported [8].
Cytotoxicity of the norcembranoidal diterpenes 1 and 2 toward K562 (human erythromyeloblastoid leukemia), MOLT-4 (human acute lymphoblastic leukemia), HTC-116 (human acute promyelocytic leukemia), DLD-1 (human colorectal adenocarcinoma), T-47D (human breast ductal carcinoma) and MDA-MB-231 (human breast adenocarcinoma) cells was studied, and the results are shown in Table 2. These data showed that compound 1 (5-episinuleptolide acetate) exhibited modest cytotoxicity towards all the cells.
Table 2.
Cytotoxic data of norcembranoidal diterpenes 1 and 2.
| Compounds | Cell lines IC50 (μg/mL) | |||||
|---|---|---|---|---|---|---|
| K562 | MOLT-4 | HTC-116 | DLD-1 | T-47D | MDA-MB-231 | |
| 1 | 0.67 | 0.59 | 4.09 | 0.92 | 3.09 | 2.95 |
| 2 | NA | NA | NA | NA | NA | NA |
| Doxorubicin a | 0.15 | 0.01 | 1.11 | 0.22 | 0.40 | 1.30 |
a Doxorubicin was used as positive control. NA = not active at 20 μg/mL for 72 h.
3. Experimental
3.1. General Procedures
Optical rotation values were measured with a Jasco-P1010 digital polarimeter. Infrared spectra were obtained on a Varian Diglab FTS 1000 FT-IR spectrophotometer. NMR spectra were recorded on a Varian Mercury Plus 400 FT-NMR at 400 MHz for 1H and 100 MHz for 13C in CDCl3 at 25 °C. Proton chemical shifts were referenced to the residual CHCl3 signal (δH 7.26 ppm). 13C-NMR spectra were referenced to the center peak of CDCl3 at δC 77.1 ppm. ESIMS and HRESIMS data were recorded on Bruker APEX II mass spectrometer. Column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck) and spots were visualized by spraying with 10% H2SO4 solution followed by heating. HPLC was performed using a system comprised of a Hitachi L-7100 pump, a Hitachi L-7455 photodiode array detector and a Rheodyne 7725 injection port. A normal phase column (Hibar 250 × 10 mm, Merck, silica gel 60, 5 μm) was used for HPLC.
3.2. Animal Material
Specimens of the soft coral Sinularia sp. were collected by hand using scuba equipment off the coast of Sansiantai, Taitung County, Taiwan in October 13, 2011, and stored in a freezer (−20 °C) until extraction. This organism was identified by comparison with previous descriptions [9]. A voucher specimen was deposited in the National Museum of Marine Biology and Aquarium, Taiwan.
3.3. Extraction and Isolation
The freeze-dried and mince material of Sinularia sp. (wet weight 1.30 kg, dry weight 328 g) was extracted with ethyl acetate (EtOAc) at 25 °C (2 L × 10). The EtOAc extract left after removal of the solvent (11.4 g) was separated by silica gel and eluted using n-hexane/EtOAc in a stepwise fashion from 100:1 to pure EtOAc to yield 13 fractions A–M. Fraction H was separated by normal-phase HPLC (NP-HPLC) using a mixture of n-hexane and EtOAc (9:4) as the mobile phase to afford compounds 1 (29.5 mg) and 2 (4.4 mg).
5-Episinuleptolide acetate (1): [α] −81 (c 0.5, CHCl3); IR (neat) νmax 1756, 1738, 1719 cm−1; 1H- (CDCl3, 400 MHz) and 13C- (CDCl3, 100 MHz) NMR data, see Table 1; ESIMS m/z 413 [M + Na]+; HRESIMS: m/z 413.1574 (calcd for C21H26O7 + Na, 413.1576).
Scabrolide D (2): [α] −67 (c 0.2, CHCl3); IR (neat) νmax 1775, 1762, 1711 cm−1; 1H- and 13C-NMR spectral data were found to be in full agreement with those of reported previously [8].
3.4. Molecular Mechanics Calculations
Implementation of the MM2 force field [5] in CHEM3D PRO software from CambridgeSoft Corporation (Cambridge, MA, USA; ver. 9.0, 2005) was used to calculate molecular models.
3.5. Cytotoxicity Testing
The cytotoxicity of norcembranoidal diterpenes 1 and 2 was assayed with a modification of the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric method. Cytotoxicity assays were carried out according to previously described procedures [10,11].
4. Conclusions
Cembrane- and norcembrane-type natural products are major constituents of the extracts of Sinularia spp. octocorals distributed in the waters off Taiwan [8,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Our continuing studies on the chemical constituents of a wild-type soft coral Sinularia sp. has led to the isolation of a new natural product, 5-episinuleptolide acetate (1), which was found to exhibit modest cytotoxicity against K562, MOLT-4, HTC-116, DLD-1, T-47D and MDA-MB-231 tumor cells. This study suggested that 5-episinuleptolide acetate (1) is worthy of further biomedical investigation. The study material Sinularia sp. has begun to be transplanted to culturing tanks with a flow-through sea water system located in the National Museum of Marine Biology and Aquarium, Taiwan for the extraction of additional natural products in order to establish a stable supply of bioactive material.
Acknowledgments
This work was supported by grants from the National Dong Hwa University; the National Museum of Marine Biology and Aquarium (Grant No. 10120022); the Division of Marine Biotechnology, Asia-Pacific Ocean Research Center, National Sun Yat-sen University, (Grant No. 00C-0302-05); the Department of Health Clinical Trial and Research Center of Excellence (Grant No. DOH101-TD-C-111-004); and the National Science Council (Grant No. NSC 101-2325-B-291-001, 100-2325-B-291-001, 101-2320-B-291-001-MY3 and 98-2320-B-291-001-MY3), Taiwan, awarded to Y.-H.K. and P.-J.S.
Footnotes
Sample Availability: Not available.
References
- 1.Molinski T.F., Dalisay D.S., Lievens S.L., Saludes J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 2.Rocha J., Peixe L., Gomes N.C.M., Calado R. Cnidarians as a new marine bioactive compounds–An overview of the last decade and future steps for bioprospecting. Mar. Drugs. 2011;9:1860–1886. doi: 10.3390/md9101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W.-T., Li Y., Guo Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sinica B. 2012;2:227–237. doi: 10.1016/j.apsb.2012.04.004. [DOI] [Google Scholar]
- 4.Li Y., Pattenden G. Novel macrocyclic and polycyclic norcembranoid diterpenes from Sinularia species of soft coral: structural relationships and biosynthetic speculations. Nat. Prod. Rep. 2011;28:429–440. doi: 10.1039/c0np00029a. [DOI] [PubMed] [Google Scholar]
- 5.Allinger N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977;99:8127–8134. doi: 10.1021/ja00467a001. [DOI] [Google Scholar]
- 6.Rodríguez A.D., Li Y., Dhasmana H., Barnes C.L. New marine cembrane diterpenoids isolated from the Caribbean gorgonian Eunicea mammosa. J. Nat. Prod. 1993;56:1103–1113. doi: 10.1021/np50097a014. [DOI] [Google Scholar]
- 7.Li Y., Pattenden G. Biomimetic syntheses of ineleganolide and sinulochmodin C from 5-episinuleptolide via sequences of transannular Michael reactions. Tetrahedron. 2011;67:10045–10052. doi: 10.1016/j.tet.2011.09.040. [DOI] [Google Scholar]
- 8.Sheu J.-H., Ahmed A.F., Shiue R.-T., Dai C.-F., Kuo Y.-H. Scabrolides A–D, four new norditerpenoids isolated from the soft coral Sinularia scabra. J. Nat. Prod. 2002;65:1904–1908. doi: 10.1021/np020280r. [DOI] [PubMed] [Google Scholar]
- 9.Bayer F.M. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981;94:902–947. [Google Scholar]
- 10.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 11.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., Currens M.J., Seniff D., Boyd M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 12.Chao C.-H., Chou K.-J., Huang C.-Y., Wen Z.-H., Hsu C.-H., Wu Y.-C., Dai C.-F., Sheu J.-H. Bioactive cembranoids from the soft coral Sinularia crassa. Mar. Drugs. 2011;9:1955–1968. doi: 10.3390/md9101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y.-S., Lee N.-L., Lu M.-C., Su J.-H. Two new cembrane-based diterpenoids from the marine soft coral Sinularia crassa. Molecules. 2012;17:5422–5429. doi: 10.3390/molecules17055422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y., Su H.-J., Chen Y.-H., Wen Z.-H., Sheu J.-H., Su J.-H. Anti-inflammatory cembranoids from the Formosan soft coral Sinularia discrepans. Arch. Pharm. Res. 2011;34:1263–1267. doi: 10.1007/s12272-011-0804-x. [DOI] [PubMed] [Google Scholar]
- 15.Duh C.-Y., Wang S.-K., Tseng H.-K., Sheu J.-H., Chiang M.Y. Novel cytotoxic cembranoids from the soft coral Sinularia flexibilis. J. Nat. Prod. 1998;61:844–847. doi: 10.1021/np980021v. [DOI] [PubMed] [Google Scholar]
- 16.Duh C.-Y., Wang S.-K., Tseng H.-K., Sheu J.-H. A novel cytotoxic biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 1998;39:7121–7122. doi: 10.1016/S0040-4039(98)01512-3. [DOI] [Google Scholar]
- 17.Hsieh P.-W., Chang F.-R., McPhail A.T., Lee K.-H., Wu Y.-C. New cembranolide analogues from the Formosan soft coral Sinularia flexibilis and their cytotoxicity. Nat. Prod. Res. 2003;17:409–418. doi: 10.1080/14786910310001617677. [DOI] [PubMed] [Google Scholar]
- 18.Su J.-H., Lin Y.-F., Lu Y., Yeh H.-C., Wang W.-H., Fan T.-Y., Sheu J.-H. Oxygenated cembranoids from the cultured and wild-type soft corals Sinularia flexibilis. Chem. Pharm. Bull. 2009;57:1189–1192. doi: 10.1248/cpb.57.1189. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y.-S., Chen C.-H., Liaw C.-C., Chen Y.-C., Kuo Y.-H., Shen Y.-C. Cembrane diterpenoids from the Taiwanese soft coral Sinularia flexibilis. Tetrahedron. 2009;65:9157–9164. doi: 10.1016/j.tet.2009.09.031. [DOI] [Google Scholar]
- 20.Lo K.-L., Khalil A.T., Kuo Y.-H., Shen Y.-C. Sinuladiterpenes A–F, new cembrane diterpenes from Sinularia flexibilis. Chem. Biodivers. 2009;6:2227–2235. doi: 10.1002/cbdv.200800298. [DOI] [PubMed] [Google Scholar]
- 21.Lo K.-L., Khalil A.T., Chen M.-H., Shen Y.-C. New cembrane diterpenes from Taiwanese soft coral Sinularia flexibilis. Helv. Chim. Acta. 2010;93:1329–1335. doi: 10.1002/hlca.200900386. [DOI] [Google Scholar]
- 22.Chen B.-W., Su J.-H., Dai C.-F., Sung P.-J., Wu Y.-C., Lin Y.-T., Sheu J.-H. Two new cembranes from a Formosan soft coral Sinularia facile. Bull. Chem. Soc. Jpn. 2011;84:1371–1373. doi: 10.1246/bcsj.20110186. [DOI] [Google Scholar]
- 23.Shih H.-J., Tseng Y.-J., Huang C.-Y., Wen Z.-H., Dai C.-F., Sheu J.-H. Cytotoxic and anti-inflammatory diterpenoids from the Dongsha atoll soft coral Sinularia flexibilis. Tetrahedron. 2012;68:244–249. doi: 10.1016/j.tet.2011.10.054. [DOI] [Google Scholar]
- 24.Ahmed A.F., Wen Z.-H., Su J.-H., Hsieh Y.-T., Wu Y.-C., Hu W.-P., Sheu J.-H. Oxygenated cembranoids from a Formosan soft coral Sinularia gibberosa. J. Nat. Prod. 2008;71:179–185. doi: 10.1021/np070356p. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A.F., Tai S.-H., Wen Z.-H., Su J.-H., Wu Y.-C., Hu W.-P., Sheu J.-H. A C-3 methylated isocembranoid and 10-oxocembranoids from a Formosan soft coral, Sinularia grandilobata. J. Nat. Prod. 2008;71:946–951. doi: 10.1021/np7007335. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y., Huang C.-Y., Lin Y.-F., Wen Z.-H., Su J.-H., Kuo Y.-H., Chiang M.Y., Sheu J.-H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008;71:1754–1759. doi: 10.1021/np8003563. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y., Su J.-H., Huang C.-Y., Liu Y.-C., Kuo Y.-H., Wen Z.-H., Hsu C.-H., Sheu J.-H. Cembranoids from the soft corals Sinularia granosa and Sinularia querciformis. Chem. Pharm. Bull. 2010;58:464–466. doi: 10.1248/cpb.58.464. [DOI] [PubMed] [Google Scholar]
- 28.Cheng S.-Y., Chuang C.-T., Wen Z.-H., Wang S.-K., Chiou S.-F., Hsu C.-H., Dai C.-F., Duh C.-Y. Bioactive norditerpenoids from the soft coral Sinularia gyrosa. Bioorg. Med. Chem. 2010;18:3379–3386. doi: 10.1016/j.bmc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed A.F., Shiue R.-T., Wang G.-H., Dai C.-F., Kuo Y.-H., Sheu J.-H. Five novel norcembranoids from Sinularia leptoclados and S. parva. Tetrahedron. 2003;59:7337–7344. doi: 10.1016/S0040-4020(03)01138-4. [DOI] [Google Scholar]
- 30.Tseng Y.-J., Ahmed A.F., Dai C.-F., Chiang M.Y., Sheu J.-H. Sinulochmodins A–C, three novel terpenoids from the soft coral Sinularia lochmodes. Org. Lett. 2005;7:3813–3816. doi: 10.1021/ol051513j. [DOI] [PubMed] [Google Scholar]
- 31.Tseng Y.-J., Ahmed A.F., Hsu C.-H., Su J.-H., Dai C.-F., Sheu J.-H. New norcembranoids from the soft coral Sinularia lochmodes. J. Chin. Chem. Soc. 2007;54:1041–1044. doi: 10.1002/jccs.200700150. [DOI] [Google Scholar]
- 32.Su J.-H., Ahmed A.F., Sung P.-J., Chao C.-H., Kuo Y.-H., Sheu J.-H. Manaarenolides A–I, diterpenoids from the soft coral Sinularia manaarensis. J. Nat. Prod. 2006;69:1134–1139. doi: 10.1021/np050483q. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed A.F., Su J.-H., Kuo Y.-H., Sheu J.-H. Scabrolides E–G, three new norditerpenoids from the soft coral Sinularia scabra. J. Nat. Prod. 2004;67:2079–2082. doi: 10.1021/np040112u. [DOI] [PubMed] [Google Scholar]
- 34.Su J.-H., Wen Z.-H. Bioactive cembrane-based diterpenoids from the soft coral Sinularia triangular. Mar. Drugs. 2011;9:944–951. doi: 10.3390/md9060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheu J.-H., Chang K.-C., Sung P.-J., Duh C.-Y., Shen Y.-C. Chemical constituents of a Formosan soft coral Sinularia sp. J. Chin. Chem. Soc. 1999;46:253–257. doi: 10.1002/jccs.199900040. [DOI] [Google Scholar]