Abstract
Iodine-methanol mediated oxidative-aromatization of 2-aryl-6,8-dibromo-2,3-dihydroquinolin-4(1H)-ones afforded the corresponding 2-aryl-6,8-dibromo-4-methoxy-quinolines in high yield and purity. The isomeric 1-(2-amino-3,5-dibromophenyl)-3-aryl-2-propen-1-ones reacted with iodine in methanol afford in a single pot operation the corresponding 2-aryl-6,8-dibromo-4-methoxyquinoline (major) and 2-aryl-6,8-dibromoquinolin-4(1H)-one (minor) products that were separated in sequence by column chromatography on silica gel. Suzuki-Miyaura cross-coupling of the 6,8-dibromo-4-methoxyquinoline derivatives with excess arylvinylboronic acids afforded the corresponding 2-aryl-6,8-bis(2-arylethenyl)-4-methoxyquinolines. The absorption and fluorescence properties of these compounds were also determined.
Keywords: 1-(2-amino-3,5-dibromophenyl)-3-aryl-2-propen-1-ones; 2-aryl-6,8-dibromo-2,3-dihydroquinolin-4(1H)-ones; 2-aryl-6,8-dibromoquinolin-4(1H)-ones; 2-aryl-6,8-dibromo-4-methoxyquinolines; Suzuki-Miyaura cross-coupling; 2-aryl-6,8-bis(2-aryl-ethenyl)-4-methoxyquinolines; photophysical properties
1. Introduction
2-Aryl-2,3-dihydroquinolin-4(1H)-ones are valuable precursors for the synthesis of 2-arylquinolin-4(1H)-ones and 4-substituted quinoline derivatives. The heterocyclic ring of 2-aryl-2,3-dihydro- quinolin-4(1H)-ones enables different degrees of unsaturation via C2,3 dehydrogenation to afford the potentially tautomeric NH-4-oxo derivatives [1,2,3] or oxidative aromatization to afford the 4-alkoxy-2-arylquinolines [3,4,5,6,7,8]. The 2-arylquinolin-4(1H)-ones formed through dehydrogenation using either iodobenzene diacetate under basic conditions in methanol [1] or thallium(III) p-tolylsulfonate in dimethoxyethane [3] were found to undergo base-mediated alkylation to afford the corresponding 4-alkoxy-2-arylquinolines. The tautomeric equilibrium between the incipient quinolin-4(1H)-one and its quinolinol isomer often leads to mixtures of N-alkylquinolinones and O-alkylquinolines, particularly when primary alkyl halides such as iodomethane are used as alkylating reagent [9]. An indirect, but sure-fire approach which involves oxidative aromatization of the 2-arylquinolin-4-(1H)-ones with phosphoryl halides to afford the corresponding 2-aryl-4-chloro/bromoquinolines, followed by dehalogenoalkoxylation, affords the 4-alkoxyquinolines, albeit in moderate yields [10]. The most convenient direct syntheses of 4-methoxy-2-arylquinoline derivatives developed to-date involves oxidative aromatization of 2-aryl-2,3-dihydroquinolin-4(1H)-ones using oxidative reagents such as thallium(III) nitrate [4] or [hydroxyl(tosyloxy)iodo]benzene [5] in trimethyl orthoformate in the presence of perchloric acid as catalyst, molecular iodine in refluxing methanol [3,6] or FeCl3·6H2O in methanol [7]. We required polysubstituted quinolines in which an electron-acceptor 2-aryl-4-methoxy-quinoline framework is linked to the π-conjugated spacer through positions 6 and 8 to comprise a donor-π-acceptor system. Such derivatives would not be easily accessible via the known classical methods such as the Skraup, Friedlander and Doebner-von Miller reactions. The 2-aryl-6,8-dibromo-4-methoxyquinolines appeared suitable candidates for metal catalyzed cross-coupling to afford the requisite donor-π-acceptor systems. This prompted us to prepare the 2-aryl-6,8-dibromo-4-methoxyquinolines to serve as substrates for the Suzuki-Miyaura cross-coupling with arylvinylboronic acids to afford the requisite polysubstituted quinolines with potential photophysical properties.
2. Results and Discussion
2.1. Synthesis of the 2-Aryl-6,8-dibromo-2,3-dihydrquinolin-4(1H)-ones
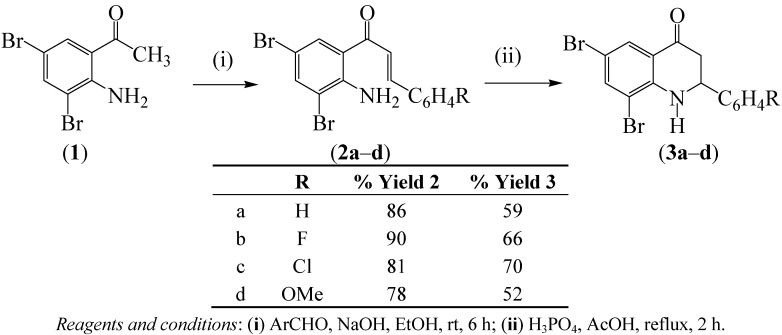
The known 2-aryl-6,8-dibromo-2,3-dihydroquinolin-4(1H)-ones [3,11] appeared to be suitable substrates for oxidative-aromatization to afford the requisite 2-aryl-6,8-dibromo-4-methoxyquinolines. The 2-aryl-2,3-dihydroquinolin-4(1H)-ones are conveniently accessible via acid- or base-mediated cyclization of the corresponding substituted 2-aminochalcones [12,13,14]. Base-promoted Claisen-Schmidt aldol condensation of 1-(2-amino-3,5-dibromophenyl)ethanone with benzaldehyde derivatives at low temperature (0–5 °C) previously afforded the corresponding 1-(2-amino-3,5-dibromophenyl)-3-aryl-2-propen-1-ones after 35–40 h [11]. In this investigation, we condensed 1-(2-amino-3,5-dibromophenyl)ethanone (1) with substituted benzaldehyde derivatives and found the reaction to proceed smoothly and rapidly at room temperature to afford the corresponding 1-(2-amino-3,5-dibromophenyl)-3-aryl-2-propen-1-ones 2a–d in high yield and purity within 6 h (Scheme 1). Compounds 2a–d were, in turn, subjected to acid-mediated cyclization with orthophosphoric-acetic acid mixture to afford the isomeric 2-aryl-6,8-dibromo-2,3-dihydroquinolin-4(1H)-ones 3a-d (Scheme 1). The 2-aryl-6,8-dibromo-2,3-dihydroquinolin-4(1H)-ones 3a–d were recently found to exhibit in vitro activity against the MCF-7 breast cancer cell line [11]. The 2-aryl-2,3-dihydroquinolin-4(1H)-ones were previously found to serve as antitumour agents by virtue of their capacity to bind to tubulin and thereby function as spindle toxins [15,16]. Moreover, some derivatives have also been found to act as microRNA inhibitors and also to control cell proliferation by altering the microRNA levels [17].
Scheme 1.
Synthesis of 2-aminochalcones 2a–d and their transformation into 3a–d.
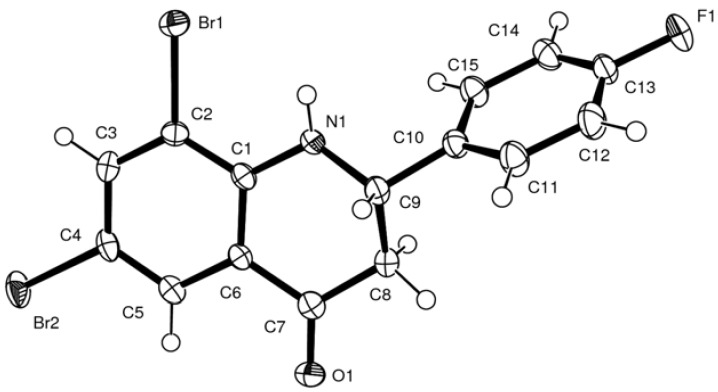
We also obtained crystals of 3b of suitable quality for X-ray diffraction and the geometry of these novel systems was thus further confirmed by X-ray diffraction (Figure 1) [18]. The six-membered ring is in a half-chair arrangement, with the 2-aryl ring deformed out of the plane of the quinolinone ring.
Figure 1.
X-ray crystal structure of 6,8-dibromo-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(1H)-one (3b) showing crystallographic numbering. For clarity, hydrogen atoms are not labeled.
2.2. Synthesis of the 4-Alkoxy-2-arylquinolines
2.2.1. Oxidative-aromatization of the 2,3-Dihydroquinolin-4(1H)-ones
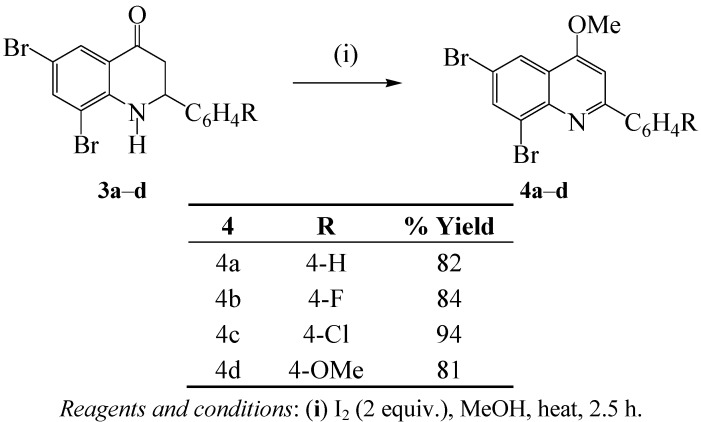
With the intent to synthesize 2,6,8-triaryl-4-methoxyquinolines for further transformation, we subjected systems 3 to oxidative aromatization using molecular iodine (2 equiv.) in methanol under reflux. We isolated the corresponding 2-aryl-6,8-tribromo-4-methoxyquinolines 4a–d in high yield and purity without the need for column chromatographic separation (Scheme 2). The analogous naturally occurring 4-methoxy-2-phenylquinoline and its 2-(methylene-dioxy-phenyl) derivative have been found to exhibit inhibitory activity against Mycobacterium tuberculosis H37Rv [19].
Scheme 2.
Iodine/methanol-mediated oxidative aromatization of 3a–d.
2.2.2. Oxidative-cyclization of 1-(2-Amino-3,5-dibromophenyl)-3-aryl-2-propen-1-ones
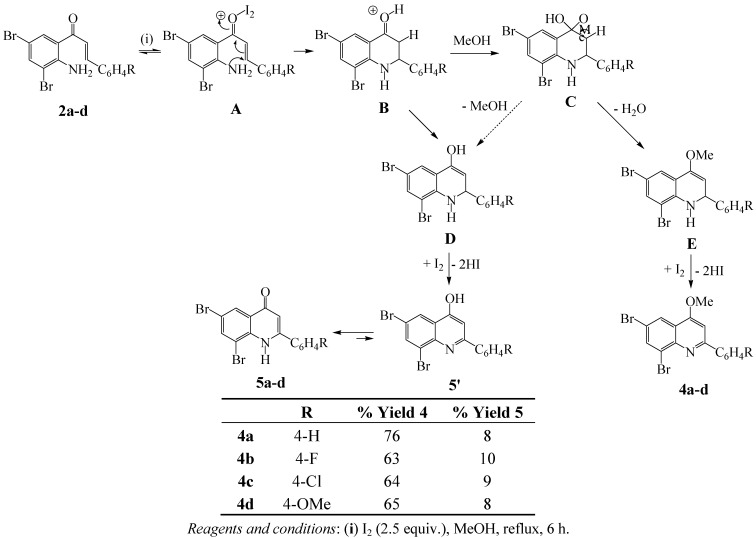
A direct one-pot synthesis of the 2-aryl-4-methoxyquinolines from the isomeric 2'-aminochalcones involving FeCl3.6H2O mediated oxidative cyclization in methanol has been described in the literature [20]. However, according to the authors oxidizing agents such as Mn(OAc)2, CAN, PCC, NBS, Co(NO3)2–K2S2O8 and iodine in methanol all failed to effect oxidative cyclization of the 2'-aminochalcones [20]. We envisioned that the electrophilic and oxidative potential of iodine would promote cyclization and oxidative aromatization of systems 2a–d to afford compounds 4a–d. Based on this hypothesis, we subjected the 2'-aminochalcones 2 to iodine (2.5 equiv.) in methanol under reflux. Interestingly in our case, we isolated in each case by column chromatography on silica gel the corresponding (major) 2-aryl-6,8-dibromo-4-methoxyquinolines 4a–d and (minor) 2-aryl-6,8-dibromo-quinolin-3(1H)-one derivatives 5a–d in sequence (Scheme 3). We rationalize the formation of products 4a–d and 5a–d as a consequence of the initial iodine-mediated cyclization to form the incipient 2-aryl-2,3-dihydroquinolin-4(1H)-one. Protonation of the 2-aryl-2,3-dihydro-quinolin-4(1H)-one occurs to afford B, which may undergo enolization to D or nucleophilic addition by methanol to yield a hemiacetal C. Extrusion of water from C yields the enolether intermediate E, which then undergoes iodine-promoted oxidative aromatization to afford 4. Iodine-promoted oxidative aromatization of the enol derivative D (which may also be a consequence of extrusion of methanol from C), on the other hand, affords the potentially tautomeric quinolinol derivatives 5'. The 4-quinolinol versus 4-quinolinone tautomeric equilibrium has been found by spectroscopic and X-ray crystallography means to favour the preponderance of the NH-4-oxo tautomer 5 in solution, gas and solid state [21]. Whereas systems 5b–d are described in this investigation for the first time, the 6,8-dibromo-2-phenylquinolin-4(1H)-one 5a was previously isolated as a minor product from the reaction of 3a with excess bromine (4 equiv.) in chloroform [22].
Scheme 3.
Direct one-pot iodine-methanol-mediated oxidative-cyclization of 2.
2.3. Suzuki-Miyaura Cross-Coupling of the 2-Aryl-6,8-dibromo-4-methoxyquinolines
Bromoquinolines are of interest to chemists as precursors for heterocyclic compounds with multifunctionality giving a wide variety of compounds through, e.g., couplings [23,24] and metal exchange reactions [25,26]. A one-pot borylation of 8-bromo/chloroquinolines with bis(pinacolato)-diboron and subsequent Suzuki-Miyaura cross-coupling of the resultant 8-quinoline-boronic acids with aryl halides, for example, previously afforded the 8-arylquinolines in high yields [23]. However, lack of selectivity was observed for 8-bromo-6-chloroquinoline with 1 equiv. of bis(pinacolato)diboron and the authors, in turn, used an excess of bis(pinacolato)diboron (2.2 equiv.) and phenylbromide to afford 6,8-diphenylquinoline in 94% yield [23]. Dibromoquinolines such as 5,7-dibromoquinoline and 8-benzyloxy-5,7-dibromoquinoline, on the other hand, undergo Suzuki cross-coupling with less or no selectivity compared to many of the other dibromoheteroaromatics [24,27]. Our initial trial to effect the Suzuki-Miyaura cross-coupling of 6,8-dibromo-4-methoxy-2-phenylquinoline (1a) and 4-fluoro-phenylvinylboronic acid (1 equiv.) using Pd(PPh3)4 as Pd(0) source and K2CO3 as a base in DMF-water (3:1, v/v) resulted in the recovery of the starting material. The failure of Pd(PPh3)4 to promote the cross-coupling is probably due to the inhibiting role of the extra PPh3 generated in the second equilibrium {SPd(0)(PPh3)3 ⇄ SPd(0)(PPh3)2 + PPh3 (K2/[PPh3] << 1); S = solvent} to afford the reactive low ligated 14-electron species (Pd(0)(PPh3)2) [28]. Conversely, the oxidative addition performed by the palladium(0) complex (Pd(0)(PPh3)2Cl−) generated by the reduction of dichlorobis-(triphenylphosphine)palladium(II) (PdCl2(PPh3)2) is reported to be more than 30 times faster than that performed from Pd(0)(PPh3)4 [28].
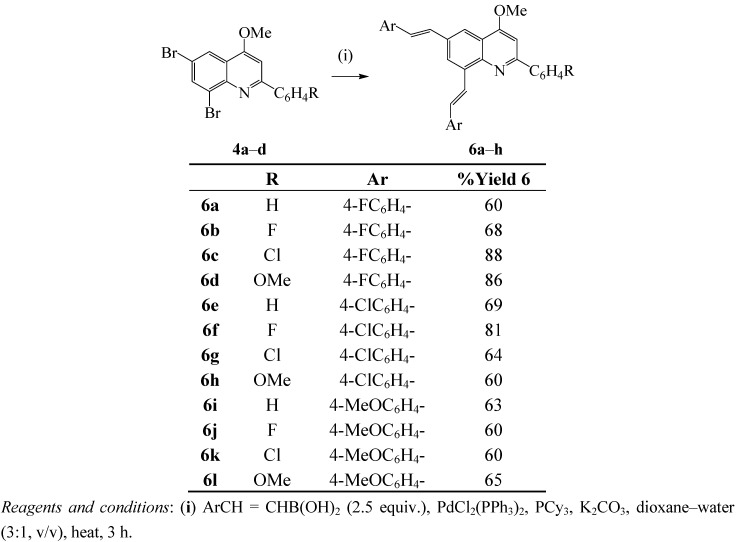
Application of dichlorobis-(triphenylphosphine)palladium(II) (PdCl2(PPh3)2 as Pd(0) catalyst source, on the other hand, led to incomplete conversion of 4a to afford the cross-coupled product 6a in 55% yield after 18 h. The prolonged reaction times and reduced yield prompted us to use PdCl2(PPh3)2–tricyclohexylphosphine catalyst complex in DMF-water (3:1, v/v) and an excess of arylboronic acid (2.5 equiv.) in the presence of K2CO3 as a base in analogy with the literature precedents [23,24,27] and we achieved complete conversion of 4a to 6a within 3 h. Alkylphosphine ligands, are known to coordinate with palladium and increase its electron density more so than arylphosphines and, in turn, accelerate the oxidative addition and reductive elimination steps in the catalytic cycle [28,29]. Extension of these reaction conditions to other dibromoquinolines 4 using 4-substituted phenyvinylboronic acids as coupling partners afforded the corresponding 2-aryl-6,8-bis(2-arylethenyl)-4-methoxyquinolines 6a–l in high yield without the need for column chromatography (Scheme 4).
Scheme 4.
One-pot Suzuki-Miyaura cross-coupling of 4 with arylvinylboronic acids.
The analogous aryl, alkenyl and alkynyl substituted quinoline derivatives have been found to serve as potent inhibitors of tyrosine kinase PDGF-RTK [30], and anti-retroviral agents [31] or LTD4 receptor antagonists [32]. The 4-alkoxy-3,6-diarylquinolines, on the other hand, are reported to exhibit potent and selective agonism of the somatostatin receptor subtype 2 (sst2) and to represent promising agents for the treatment of diabetic retinopathy and proliferative diseases [33]. Likewise, the 6- or 8-aryl substituted 2,4-dimethoxyquinolines were found to exhibit high activity against the agriculturally important nematode, Haemonchus contortus with potency comparable to that of the commercially available levamisole [34]. Systems 6a–l are also analogues of the 2-aryl-3,4-bis(phenylethenyl) quinolines and the 3,4-bis(alkynyl)-2-arylquinolines with potential to serve as molecular organic materials in nanomaterials or building blocks for polyquinolines or quinoline-based copolymers with enhanced photonic and electronic properties [35].
2.4. Photophysical Property Studies of Systems 6
Polysubstituted quinolines 6a–l comprise an electron-deficient quinoline framework as an electron-acceptor linked to the 4-substituted aryl ring via a π-conjugated spacer to comprise a donor-π-acceptor system. Preliminary absorption and fluorescence properties of systems 6a–l were determined in chloroform at room temperature.
2.4.1. UV-Vis Absorption Properties of 4-Methoxyquinoline Derivatives 6
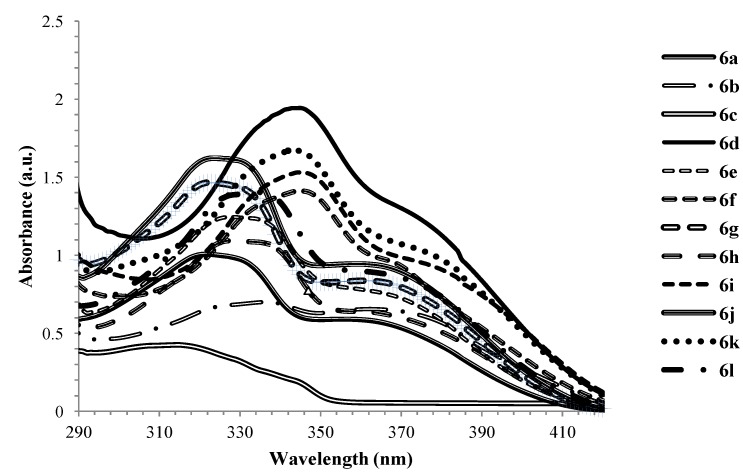
The electronic absorption spectra of the 4-methoxyquinoline derivatives 6a–l were acquired in CHCl3 and the compounds absorb in the region μ 310–390 nm (Figure 1). The absorption spectra of these compounds are characterized by intense absorption peaks near 310–350 nm and the less intense ones around 360–380 nm. The band in the region 310–350 nm is due to π–π* transition attributed to the conjugated quinoline ring of the molecule in analogy with the assignment for π-styrylquinolines [36], whereas the lowest energy band which is largely of charge transfer character is due to the 2-aryl group.
Both the absorption maxima and wavelength are influenced by the variation of substituents on the arylvinyl and the 2-aryl groups. Compounds 6d, 6f, 6i and 6k showed strong UV-vis absorption intensities at around 340 nm reflecting the following trend in intensity: 6d > 6k > 6i > 6f. A combination of the strong electron donating 2-(4-methoxyphenyl) group and moderately donating 4-fluorophenylvinyl substituents in 6d seem to increase the electron density of the quinoline ring thus the π-π* transition. A combination of the relatively less donating 2-(4-chlorophenyl) group or 2-phenyl group and the two 4-methoxyvinyl substituents on the fused benzo ring also enhance the absorptivities for 6f and 6i, respectively. Enhanced intensity comparable to that of 6f but at different wavelength is also observed for 6h bearing the 2-(4-methoxyphenyl) group and the two 4-chlorophenylvinyl groups. Except for the 2-(4-methoxyphenyl) substituted derivative 6d, it seems the presence of 4-fluorophenyl moieties in 6a, b and c led to reduced absorption intensities than for derivatives bearing chloro atoms on the styryl groups. It appears the presence of the chloro atoms increase the electron affinity of the quinoline ring than the fluoro atoms.
Figure 1.
UV-Vis spectra of 6a–l in CHCl3 (2.0 × 10−5 mol/L).
2.4.2. The Fluorescence Properties of 4-Methoxyquinoline Derivatives
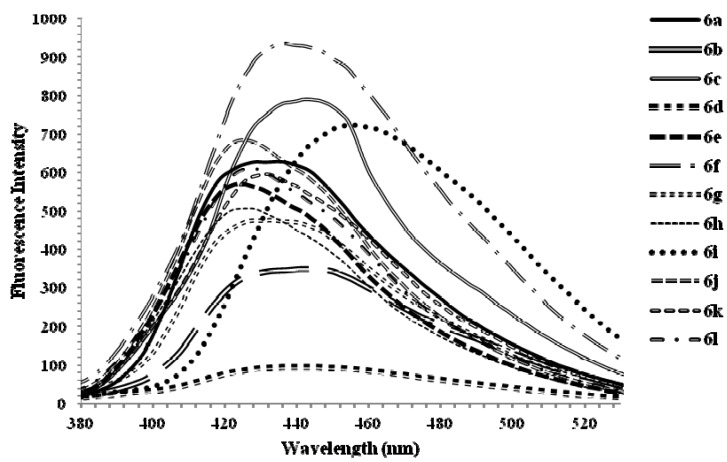
The fluorescence properties of compounds 6a–l have been studied at room temperature in the moderately polar chloroform at the excitation wavelength of 355 nm (Figure 2).
Figure 2.
The fluorescence emission spectra of 6a–l (excitation wavelength λex = 355 nm) in CHCl3 (2.0 × 10−7 mol/L) at rt.
The fluorescence spectra of these compounds show similar pattern and are characterized by a single emission band in the region 410–490 nm, which are attributed to π-π* transition of the conjugated system. The fluorescence patterns of compounds 6a–l are affected by the presence of the halo substituents on the styryl and 2-phenyl groups [37]. Systems 6c and 6f with mixed halides (F and Cl) on either the 2-phenyl or styryl groups show increased emission and slight bathochromic shifts. A combination of the 2-(4-methoxyphenyl) and 4-fluorophenylvinyl derivative 6d significantly reduces the emission intensity than the other derivatives bearing the 4-fluorophenylvinyl substituents. Increased emission and pronounced red shift effect are also observed for 6i bearing the 2-phenyl and 4-methoxystyryl groups. The increased intensities and bathochromic shifts are presumably the result of increased π-electron delocalization along the vinylene bridge and/or the 2-aryl group towards the electron-deficient quinoline ring.
3. Experimental
3.1. General
Melting points were recorded on a Thermocouple digital melting point apparatus and are uncorrected. IR spectra were recorded as powders using a Bruker VERTEX 70 FT-IR Spectrometer with a diamond ATR (attenuated total reflectance) accessory by using the thin-film method. For column chromatography, Merck kieselgel 60 (0.063–0.200 mm) was used as stationary phase. NMR spectra were obtained as CDCl3 solutions using Varian Mercury 300 MHz NMR spectrometer and the chemical shifts are quoted relative to the solvent peaks. Low- and high-resolution mass spectra were recorded at an ionization potential of 70eV using Micromass Autospec-TOF (double focusing high resolution) instrument. The synthesis and characterization of substrate 1 have been described elsewhere [38]. The UV-vis spectra were recorded on a Perkin Elmer Lambda 35 UV/vis spectrometer while emission spectra were taken using a Perkin Elmer LS 45 fluorescence spectrometer.
3.2. Typical Procedure for the Synthesis of the 2-Amino-4,6-dibromochalcones 2a–d
1-(2-Amino-3,5-dibromophenyl)-3-phenyl-2-propen-1-one (2a). A stirred mixture of 2-amino-3,5-dibromoacetophenone (1, 2.00 g, 6.80 mmol) and benzaldehyde (0.721 g, 6.80 mmol) and sodium hydroxide (0.60 g) in ethanol (30 mL) was stirred at room temperature for 6 h and then quenched with an ice-cold water. The resulting precipitate was filtered and then taken-up into chloroform. The chloroform solution was dried over MgSO4, filtered and then evaporated under reduced pressure to afford 2a as a yellow solid (2.23 g, 86% yield), m.p. 120–123 °C (ethanol); νmax (ATR) 688.9, 771.1, 974.0, 1186.1, 1336.5, 1514.9, 1560.6, 1592.5, 1644.8 3294.5, 3400.0, 3462.4 cm−1; δH (CDCl3) 6.92 (br s, 2H), 7.41–7.43 (3H), 7.48 (d, J 15.6 Hz, 1H), 7.62–7.65 (m, 2H), 7.70 (d, J 1.8 Hz, 1H), 7.92 (d, J 15.6 Hz, 1H), 7.92 (d, J 1.8 Hz, 1H); δC (CDCl3) 106.1, 111.8, 120.7, 121.9, 128.5, 129.0, 130.6, 132.6, 134.7, 139.1, 144.7, 146.7, 146.8, 190.1; m/z 380 (100, MH+); HRMS (ES): MH+, found 379.9284. C15H12NO79Br2+ requires 379.9286.
1-(2-Amino-3,5-dibromophenyl)-3-(4-fluorophenyl)-2-propen-1-one (2b). A mixture of 1 (2.00 g, 6.80 mmol), 4-fluorobenzaldehyde (0.85 g, 6.80 mmol) and NaOH (0.60 g) in ethanol (30 mL) afforded 2b as a yellow solid (2.45 g, 90%), m.p. 149–150 °C (ethanol) (Lit. 118–120 °C [11]).
1-(2-Amino-3,5-dibromophenyl)-3-(4-chlorophenyl)-2-propen-1-one (2c). A mixture of 1 (2.00 g, 6.80 mmol), 4-chlorobenzaldehyde (0.96 g, 6.80 mmol) and NaOH (0.60 g) in ethanol (30 mL) afforded 2c as an orange solid (2.30 g, 81%), m.p. 145–148 °C (ethanol) (Lit. 124–128 °C [11]).
1-(2-Amino-3,5-dibromophenyl)-3-(4-methoxyphenyl)-2-propen-1-one (2d). A mixture of 1 (2.00 g, 6.80 mmol), 4-methoxybenzaldehyde (0.93 g, 6.80 mmol) and NaOH (0.60 g) in ethanol (30 mL) afforded 2d as a yellow solid (2.25 g, 78%), m.p. 139–141 °C (ethanol) (Lit. 130–135 °C [11]).
3.3. Typical Procedure for the Synthesis of 3a–d
A stirred mixture of 2a (3.00 g, 7.87 mmol) and orthophosphoric acid (20 mL) in glacial acetic acid (40 mL) was heated under reflux for 2 h. The mixture was allowed to cool to room temperature and then quenched with an ice-cold water. The product was extracted into chloroform and the combined organic phases were washed thoroughly with saturated solution of sodium carbonate and then dried over MgSO4. The solvent was evaporated under reduced pressure and the residue was recrystallized from ethanol to afford 3a as a light yellow solid (1.78 g, 59%), m.p. 135–136 °C (Lit. 131–133 °C [34]). The following products were prepared in this fashion: 3b (1.97 g, 66%), m.p. 127–129 °C (Lit. 126–129 °C [35]); 3c (2.10 g, 70%), m.p. 133–135 (Lit. 138–140 °C [11]) and 3d (1.57 g, 52% yield), m.p. 143–145 °C (Lit. 149–151 °C [35]).
3.4. Typical Procedure for the Aromatization of 3a–d with Iodine in Methanol
6,8-Dibromo-4-methoxy-2-phenylquinoline (4a). A stirred mixture of 3a (1.00 g, 2.62 mmol) and iodine (1.33 g, 5.25 mmol) in methanol (20 mL) was refluxed for 3 hours. The mixture was quenched with an ice-cold solution of saturated sodium thiosulphate and the product was extracted into chloroform. The combined chloroform phases were dried over MgSO4, filtered and then evaporated under reduced pressure to afford 4a as a white solid (0.84 g, 82%) m.p. 174–176 °C (ethanol); νmax (ATR) 776, 868, 1002, 1219, 1357, 1477, 1576 cm−1; δH (CDCl3) 4.12 (s, 3H), 7.24 (s, 1H), 7.46–7.55 (m, 3H), 8.10 (d, J = 1.8 Hz, 1H), 8.20 (d, J = 1.5 Hz, 1H), 8.23 (d, J = 1.5 Hz, 1H), 8.28 (d, J = 1.8 Hz, 1H); δC (CDCl3) 56.1, 98.5, 118.3, 122.5, 124.1, 127.6, 128.8, 129.9, 136.2, 139.2, 144.8, 159.0, 162.1; m/z 394 (100%, C16H12NO79Br81Br+); HRMS (ES): MH+, found 391.9277. C16H12NO79Br2+ requires 391.9286.
2,6,8-Dibromo-2-(4-fluorophenyl)-4-methoxyquinoline (4b). A stirred mixture of 3b (1.00 g, 2.50 mmol) and iodine (1.27 g, 5.01 mmol) in methanol (20 mL) afforded 4b as a white solid (0.86 g, 84%) m.p. 172–175 °C (ethanol); νmax (ATR) 718, 828, 1002, 1218, 1361, 1481, 1583 cm−1; δH (300 MHz, CDCl3) 4.08 (s, 3H), 7.12 (s, 1H), 7.18 (t, J = 8.4 Hz, 2H), 8.07 (d, J = 2.1 Hz, 1H), 8.16–8.23 (m, 3H); δC (75 MHz, CDCl3) 56.0, 98.0, 115.8 (d, 2JCF = 21.6 Hz), 118.3, 122.1, 124.1, 129.4 (d, 3JCF = 8.3 Hz), 135.2 (d, 4JCF = 3.2 Hz), 136.3, 144.6, 157.7, 162.1, 164.1 (d, 1JCF = 248.5 Hz); m/z 412 (100%, C16H11NO79Br81BrF+); HRMS (ES): MH+, found 409.9175. C16H11NO79Br2F+ requires 409.9151.
6,8-Dibromo-2-(4-chlorophenyl)-4-methoxyquinoline (4c). A stirred mixture of 3c (0.89 g, 2.14 mmol) and iodine (1.09 g, 4.28 mmol) in methanol (20 mL) afforded 4c as a white solid (0.85 g, 94%) m.p. 198–200 °C (ethanol); νmax (ATR) 692, 820, 1000, 1093, 1219, 1357, 1437, 1479, 1588 cm−1; δH (CDCl3) 4.10 (s, 3H), 7.16 (s, 1H), 7.62 (d, J 8.7 Hz, 2H), 8.09 (d, J 2.1 Hz, 1H), 8.15 (d, J = 8.7 Hz, 2H), 8.26 (d, J = 2.1 Hz, 1H); δC (CDCl3) 56.1, 98.1, 118.6, 122.2, 124.1, 125.9, 128.8, 129.0, 136.1, 136.4, 137.5, 144.7, 157.6, 162.2; m/z 428 (100%, C16H11NO37Cl79Br2+ or C16H11NO35Cl 79Br81Br+); HRMS (ES): MH+, found 425.8974. C16H11NO35Cl79Br2+ requires 425.8974.
6,8-Dibromo-4-methoxy-2-(4-methoxyphenyl)quinoline (4d). A stirred mixture of 3d (1.00 g, 2.42 mmol) and iodine (1.23 g, 4.84 mmol) in methanol (20 mL) afforded 4d as a white solid (0.82 g, 81%) m.p. 193–194 °C (ethanol); νmax (ATR) 1001, 1031, 1176, 1220, 1244, 1363, 1486, 1576 cm−1; δH (CDCl3) 3.88 (s, 3H), 4.06 (s, 3H), 7.02 (d, J = 9.0 Hz, 2H), 7.16 (s, 1H), 8.07 (d, J = 2.1 Hz, 1H), 8.18 (d, J = 9.0 Hz, 2H), 8.23 (d, J = 2.1 Hz, 1H); δC (CDCl3) 55.4, 56.0, 97.8, 114.2, 117.8, 122.0, 124.0, 125.7, 129.0, 131.7, 136.1, 144.8, 158.5, 161.3, 162.0; m/z 424 (100%, C17H14NO279Br81Br); HRMS (ES): MH+, found 421.9378. C17H14NO279Br2+ requires 421.9391.
3.5. Typical Procedure for the One-pot Cyclization and Aromatization of 2 with Iodine in Methanol
Preparation of 6,8-dibromo-4-methoxy-2-phenylquinoline (4a) and 6,8-dibromo-2-phenylquinolin-4(1H)-one (5a). A stirred mixture of 3a (0.50 g, 1.32 mmol) and iodine (0.83 g, 3.28 mmol) in methanol (20 mL) was refluxed for 5 h. The mixture was quenched with an ice-cold solution of saturated sodium thiosulphate and the product was extracted into chloroform. The combined chloroform phases were dried over MgSO4, filtered and then evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (40% ethyl acetate-hexane) to afford 4a and 5a in sequence.
6,8-Dibromo-4-methoxy-2-phenylquinoline (4a). White solid (0.390 g, 76%), Rf 0.77.
6,8-Dibromo-2-phenylquinolin-4(1H)-one (5a). White solid (0.04 g, 8%) m.p. 212–213 °C (Lit. 213 °C [22]), Rf 0.37; νmax (ATR) 734, 843, 866, 1349, 1383, 1491, 1537, 1620, 3389 cm−1; δH (CDCl3) 6.60 (s, 1H), 7.56–7.59 (m, 3H), 7.66–7.71 (m, 2H), 7.96 (d, J = 2.1 Hz, 1H), 8.48 (d, J = 2.1 Hz, 1H), 8.64 (br s, 1H); δC (CDCl3) 109.0, 112.3, 116.9, 126.3, 127.3, 128.7, 129.8, 131.3, 133.8, 136.1, 137.3, 149.5, 177.0; m/z 380 (100%, C15H10NO79Br81Br+); HRMS (ES): MH+, found 377.9128. C15H10NO79Br2+ requires 377.9129.
6,8-Dibromo-2-(4-fluorophenyl)-4-methoxyquinoline (4b) and 6,8-dibromo-2-(4-fluorophenyl)quinolin-4(1H)-one (5b). A stirred mixture of 3b (0.50 g, 1.26 mmol) and iodine (0.79 g, 3.14 mmol) in methanol (20 mL) afforded 4b and 5b in sequence.
6,8-Dibromo-2-(4-fluorophenyl)-4-methoxyquinoline (4b). White solid (0.32 g, 63%), Rf 0.80.
6,8-Dibromo-2-(4-fluorophenyl)quinolin-4(1H)-one (5b). White solid (0.05 g, 10%), m.p. 225–227 °C, Rf 0.50; νmax (ATR) 719, 831, 1221, 1490, 1543, 1579, 1614, 3388 cm−1; δH (CDCl3) 6.54 (s, 1H), 7.28 (t, J = 8.4 Hz, 2H), 7.69 (t, J = 8.4 Hz, 2H), 7.97 (d, J = 2.1 Hz, 1H), 8.47 (d, J = 2.1 Hz, 1H), 8.56 (br s, 1H); δC (CDCl3) 109.0, 112.3, 117.0, 117.1 (d, 2JCF = 22.2 Hz), 127.2, 128.5 (d, 3JCF = 8.6 Hz), 128.7, 129.9 (d, 4JCF = 3.4 Hz), 136.0, 137.4, 148.6, 164.5 (d, 1JCF = 251.6 Hz), 176.9; m/z 396 (100, MH+); HRMS (ES): MH+, found 395.9035. C15H9NFO79Br2+ requires 395.9031.
6,8-Dibromo-2-(4-chlorophenyl)-4-methoxyquinoline (4c) and 6,8-dibromo-2-(4-chlorophenyl)quinolin-4(1H)-one (5c). A stirred mixture of 3c (0.50 g, 1.20 mmol) and iodine (0.77 g, 3.02 mmol) in methanol (20 mL) afforded 4c and 5c in sequence.
6,8-Dibromo-2-(4-chlorophenyl)-4-methoxy-quinoline (4c). White solid (0.32 g, 64%), Rf 0.85.
6,8-Dibromo-2-(4-chlorophenyl)quinolin-4(1H)-one (5c). White solid (0.05 g, 9%), m.p. 237–239 °C, Rf 0.29; νmax (ATR) 825, 1095, 1383, 1491, 1543, 1618, 3385 cm−1; δH (CDCl3) 6.55 (s, 1H), 7.55 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 8.4 Hz, 2H), 7.96 (d, J = 2.1 Hz, 1H), 8.45 (d, J = 2.1 Hz, 1H), 8.56 (br s, 1H); δC (CDCl3) 109.2, 112.3, 117.1, 127.3, 127.7, 128.8 (2 × C), 130.1, 132.3, 136.0, 137.5, 148.4, 176.9; m/z 414 (100%, C15H9NO37Cl79Br2+); HRMS (ES): MH+, found 411.8737. C15H9NO35Cl 79Br2+ requires 411.8739.
6,8-Dibromo-4-methoxy-2-(4-methoxyphenyl)quinoline (4d) and 6,8-dibromo-2-(4-methoxyphenyl)-quinolin-4(1H)-one (5d). A stirred mixture of 3d (0.50 g, 1.22 mmol) and iodine (0.77 g, 3.04 mmol) in methanol (20 mL) afforded 4d and 5d in sequence.
6,8-Dibromo-4-methoxy-2-(4-methoxyphenyl)quinoline (4d). White solid (0.33 g, 65%), Rf 0.74.
6,8-Dibromo-2-(4-methoxyphenyl)quinolin-4(1H)-one (5d). White solid (0.04 g, 8%), m.p. 200–201 °C, Rf 0.26; νmax (ATR) 719, 824, 1030, 1179, 1250, 1490, 1510, 1609, 3388 cm−1; δH (CDCl3) 3.88 (s, 3H), 6.54 (s, 1H), 7.06 (d, J = 8.7 Hz, 2H), 7.62 (d, J = 8.7 Hz, 2H), 7.93 (d, J = 2.1 Hz, 1H), 8.45 (d, J = 2.1 Hz, 1H), 8.59 (br s, 1H); δC (CDCl3) 55.6, 108.1, 112.2, 115.1, 116.7, 125.8, 127.2, 127.7, 128.7, 136.0, 137.1, 149.3, 162.1, 176.9; m/z 410 (100%, C16H12NO279Br81Br+); HRMS (ES): MH+, found 407.9236. C16H12NO279Br2+ requires 407.9235.
3.6. Typical Procedure for the Suzuki-Miyaura Cross Coupling of 3 with Arylvinylboronic Acids
6,8-Bis[2-(4-fluorophenyl)ethenyl]-4-methoxy-2-phenylquinoline (6a). A mixture of 4a (0.35 g, 0.892 mmol), 4-fluorophenylvinylboronic acid (0.37 g, 2.23 mmol), PdCl2(PPh3)2 (0.031 g, 0.045 mmol), PCy3 (0.025 g, 0.089 mmol) and K2CO3 (0.246 g, 1.178 mmol) in dioxane/water (20 mL) in a two-necked flask equipped with a stirrer bar, rubber septum and a condenser was flushed for 20 minutes with argon gas. A balloon filled with argon gas was then connected to the top of the condenser and the mixture was heated with stirring at 80–90 °C under argon atmosphere for 3 h. The mixture was allowed to cool to room temperature and then poured into an ice-cold water. The resulting precipitate was filtered and taken-up into chloroform. The organic solution was washed with brine, dried over anhydrous MgSO4, filtered and then evaporated under reduced pressure to afford 6a as a yellow solid (0.25 g, 60%), m.p. 201–203 °C (ethanol); νmax (ATR) 667, 769, 814, 831, 852, 961, 876, 1044, 1207, 1380, 1483, 1575, 1576, 1590 cm−1; δH (CDCl3) 4.13 (s, 3H), 7.08 (t, J = 8.7 Hz, 2H), 7.10 (t, J = 8.7 Hz, 2H), 7.18 (d, J = 16.5 Hz, 1H), 7.23 (s, 1H), 7.27 (d, J = 16.5 Hz, 1H), 7.42 (d, J = 16.5 Hz, 1H), 7.47–7.58 (m, 5H), 7.65 (t, J = 8.7 Hz, 2H), 8.14 (d, J = 1.8 Hz, 1H), 8.17 (d, J = 1.8 Hz, 1H), 8.23 (dd, J = 1.5 and 8.4 Hz, 2H), 8.48 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.7, 98.9, 115.6 (d, 2JCF = 21.4 Hz), 115.7 (d, 2JCF = 21.4 Hz), 119.2, 120.9, 123.5, 125.1, 127.4,128.0, 128.1 (d, 3JCF = 8.0 Hz), 128.2, 128.4 (d, 3JCF = 8.3 Hz), 128.8, 129.0, 129.4, 133.4 (d, 4JCF = 3.5 Hz), 133.8, 134.2 (d, 4JCF = 3.5 Hz), 135.6, 140.1, 146.4, 156.9, 162.4 (d, 1JCF = 245.6 Hz), 162.4 (d, 1JCF = 246.2 Hz), 162.8; m/z 476 (100, MH+); HRMS (ES): MH+, found 476.1823. C32H24NOF2+ requires 476.1826.
6,8-Bis[2-(4-fluorophenyl)ethenyl]-2-(4-fluorophenyl)-4-methoxyquinoline (6b). Yellow solid (0.28 g, 68%), m.p. 182–183 °C (ethanol); νmax (ATR) 717, 825, 1001, 1218, 1360, 1438, 1481, 1542, 1582 cm−1; δH (CDCl3) 4.15 (s, 3H), 7.08 (t, J = 8.7 Hz, 2H), 7.11 (t, J = 8.7 Hz, 2H), 7.19 (s, 1H), 7.20 (d, J = 16.5 Hz, 1H), 7.22 (t, J = 8.7 Hz, 2H), 7.29 (d, J = 16.5 Hz, 1H), 7.42 (d, J = 16.5 Hz, 1H), 7.56 (t, J = 8.7 Hz, 2H), 7.65 (t, J = 8.7 Hz, 2H), 8.17 (d, J = 1.8 Hz, 1H), 8.20 (d, J = 8.7 Hz, 2H), 8.23 (s, 1H), 8.43 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.7, 97.6, 115.6 (d, 2JCF = 21.3 Hz), 115.7 (d, 2JCF = 21.6 Hz), 115.8 (d, 2JCF = 21.6 Hz), 119.3, 120.8, 123.7, 125.0, 128.1 (d, 3JCF = 8.0 Hz), 128.2 (2 × C), 128.4 (d, 3JCF = 8.0 Hz), 129.2, 129.3 (d, 3JCF = 8.6 Hz), 133.4 (d, 4JCF = 3.5 Hz), 133.9, 134.1 (d, 4JCF = 3.2 Hz), 135.6, 136.3 (d, 4JCF = 3.0 Hz), 146.4, 155.9, 162.3 (d, 1JCF = 245.3 Hz), 162.4 (d, 1JCF = 245.9 Hz), 163.0, 163.8 (d, 1JCF = 247.3 Hz); m/z 412 (100, MH+); HRMS (ES): MH+, found 411.9178. C32H23NOF3+ requires 411.9178.
2-(4-Chlorophenyl)-6,8-bis[2-(4-fluorophenyl)ethenyl]-4-methoxyquinoline (6c). Yellow solid (0.36 g, 88%), m.p. 228–229 °C (ethanol); νmax (ATR) 816, 849, 1010, 1221, 1251, 1557, 1575 cm−1; δH (CDCl3) 4.13 (s, 3H), 7.08 (t, J = 8.7 Hz, 2H), 7.11 (t, J = 8.7 Hz, 2H), 7.17 (s, 1H), 7.18 (d, J = 16.5 Hz, 1H), 7.27 (d, J = 16.5 Hz, 1H), 7.41 (d, J = 16.5 Hz, 1H), 7.51(d, J = 9.0 Hz, 2H), 7.54 (t, J = 8.7 Hz, 2H), 7.64 (t, J = 8.7 Hz, 2H), 8.14 (s, 1H), 8.15 (d, J = 8.7 Hz, 2H), 8.18 (s, 1H), 8.43 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.7, 97.5, 115.6 (d, 2JCF = 21.4 Hz), 115.7 (d, 2JCF = 21.6 Hz), 119.2, 130.0, 123.7, 124.9, 128.1 (d, 3JCF = 8.0 Hz), 128.2 (d, 2JCF = 8.0 Hz), 128.4, 128.7, 128.8, 128.9, 129.2, 133.4 (d, 4JCF = 3.1 Hz), 134.0, 134.1 (d, 4JCF = 3.5 Hz), 135.5, 135.6, 138.5, 146.3, 155.6, 162.3 (d, 1JCF = 245.3 Hz), 162.5 (d, 1JCF = 246.2 Hz), 163.0; m/z 510 (100, MH+); HRMS (ES): MH+, found 510.1428. C32H23NO35ClF2+ requires 510.1436.
6,8-Bis[2-(4-fluorophenyl)ethenyl]-4-methoxy-2-(4-methoxyphenyl)quinoline (6d). Yellow solid (0.36 g, 86%), m.p. 224-225 °C (ethanol); νmax (ATR) 777, 792, 852, 939, 961, 1209, 1228, 1292, 1483, 1507, 1558, 1601 cm−1; δH (CDCl3) 3.90 (s, 3H), 4.12 (s, 3H), 7.06 (d, J = 9.0 Hz, 2H), 7.08 (t, J = 8.7 Hz, 2H), 7.10 (t, J = 8.7 Hz, 2H), 7.18 (s, 1H), 7.17 (d, J = 16.5 Hz, 1H), 7.25 (d, J = 16.5 Hz, 1H), 7.41 (d, J = 16.5 Hz, 1H), 7.54 (t, J = 8.7 Hz, 2H), 7.65 (t, J = 8.7 Hz, 2H), 8.13 (s, 1H), 8.15 (s, 1H), 8.19 (d, J = 9.0 Hz, 2H), 8.46 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.4, 55.7, 97.3, 114.5, 115.6 (d, 2JCF = 21.7 Hz), 115.7 (d, 2JCF = 21.7 Hz), 119.3, 120.7, 123.6, 125.3, 127.9, 128.0 (d, 3JCF = 8.0 Hz), 128.3 (d, 3JCF = 8.0 Hz), 128.4, 128.7, 128.9, 132.7, 133.4, 133.5 (d, 4JCF = 3.4 Hz), 134.3 (d, 4JCF = 3.4 Hz), 135.4, 146.5, 156.6, 160.8, 162.3 (d, 1JCF = 245.3 Hz), 162.4 (d, 1JCF = 245.8 Hz), 162.7; m/z 506 (100, MH+); HRMS (ES): MH+, found 506.1932. C33H26NO2F2+ requires 506.1932.
6,8-Bis[2-(4-chlorophenyl)ethenyl]-4-methoxy-2-phenylquinoline (6e). Yellow solid (0.31 g, 69%), m.p. 187–188 °C (ethanol); νmax (ATR) 698, 777, 810, 903, 1011, 1272, 1288, 1360, 1489, 1579 cm−1; δH (CDCl3) 4.13 (s, 3H), 7.22 (s, 1H), 7.23 (s, 2H), 7.35 (d, J = 8.7 Hz, 2H), 7.37 (d, J = 8.7 Hz, 2H), 7.40 (d, J = 16.5 Hz, 1H), 7.49 (d, J = 8.7 Hz, 2H), 7.54 (d, J = 8.7 Hz, 2H), 7.55 (d, J = 16.5 Hz, 1H), 7.60 (d, J = 8.7 Hz, 2H), 8.15 (d, J = 1.8 Hz, 1H), 8.16 (d, J = 1.8 Hz, 1H), 8.20 (dd, J = 1.8 and 8.4 Hz, 2H), 8.53 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.7, 97.9, 119.67, 120.9, 123.7, 126.0, 127.5, 127.7, 128.0 (2 × C), 128.7, 128.8, 128.9, 129.0, 129.1, 129.5, 133.1, 133.3, 133.6, 135.5, 135.7, 136.5, 140.1, 146.5, 157.1, 162.9; m/z 508 (100, MH+); HRMS (ES): MH+, found 508.1247. C32H24NO35Cl2+ requires 508.1235.
6,8-Bis[2-(4-chlorophenyl)ethenyl]-2-(4-fluorophenyl)-4-methoxyquinoline (6f). Yellow solid (0.36 g, 81%), m.p. 213–214 °C (ethanol); νmax (ATR) 813, 826, 839, 860, 1217, 1308, 1511, 1589 cm−1; δH(CDCl3) 4.12 (s, 3H), 7.15 (s, 1H), 7.22 (d, J = 8.4 Hz, 2H), 7.23 (t, J = 8.7 Hz, 2H), 7.35 (d, J = 16.5 Hz, 2 × H), 7.36 (t, J = 8.7 Hz, 2H), 7.37 (d, J = 16.5 Hz, 1H), 7.49 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 8.4 Hz, 2H), 8.13 (d, J = 1.8 Hz, 1H), 8.15 (d, J = 1.8 Hz, 1H), 8.19 (t, J = 8.7 Hz, 2H), 8.48 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.7, 97.6, 115.6 (d, 2JCF = 21.4 Hz), 119.6, 120.8, 123.8, 125.8, 127.7, 128.0, 128.1, 128.8, 128.9, 129.0, 129.2, (d, 3JCF = 8.3 Hz), 133.2, 133.4, 133.7, 135.4, 135.7, 136.2 (d, 4JCF = 3.1 Hz), 136.4, 146.4, 155.9, 162.9, 163.8 (d, 1JCF = 247.6 Hz); m/z 526 (100, MH+); HRMS (ES): MH+, found 526.1143. C32H23NO35Cl2F+ requires 526.1141.
2-(4-Chlorophenyl)-6,8-bis[2-(4-chlorophenyl)ethenyl]-4-methoxyquinoline (6g). Yellow solid (0.28 g, 64%), m.p. 217–219 °C (ethanol); νmax (ATR) 822, 846, 969, 1012, 1151, 1161, 1211, 1361, 1491, 1591 cm−1; δH (DMSO-d6) 4.18 (s, 3H), 7.47 (d, J = 8.7 Hz, 2H), 7.52 (s, 4H), 7.60 (d, J = 8.4 Hz, 2H), 7.65 (s, 1H), 7.67 (d, J = 16.5 Hz, 1H), 7.71 (d, J = 8.7 Hz, 2H), 7.73 (d, J = 8.7 Hz, 2H), 8.14 (d, J = 1.5 Hz, 1H), 8.40 (d, J = 8.7 Hz, 2H), 8.44 (d, J = 16.5 Hz, 1H), 8.48 (d, J = 1.5 Hz, 1H); δC (DMSO-d6) 56.8, 99.0, 120.2, 120.9, 124.1, 125.8, 128.5, 128.6, 128.7, 128.8, 129.2 (2 × C), 129.4, 129.5, 129.6 (2xC), 132.6, 134.3, 134.9, 135.2, 136.5, 137.0, 138.5, 145.9, 155.5, 163.1; m/z 542 (100, MH+); HRMS (ES): MH+, found 542.0842. C32H23NO35Cl3+ requires 542.0845.
6,8-Bis[2-(4-chlorophenyl)ethenyl]-4-methoxy-2-(4-methoxyphenyl)quinoline (6h). Yellow solid (0.26 g, 60%), m.p. 244–246 °C (ethanol); νmax (ATR) 785.7, 805, 957, 975, 1170, 1215, 1292, 1363, 1489, 1588 cm−1; δH (CDCl3) 3.90 (s, 3H), 4.12 (s, 3H), 7.06 (d, J = 8.7 Hz, 2H), 7.10 (d, J = 16.5 Hz, 1H), 7.17 (s, 1H), 7.22 (s, 2H), 7.36 (d, J = 16.5 Hz, 1H), 7.37 (d, J = 8.4 Hz, 2H), 7.39 (d, J = 16.5 Hz, 1H), 7.49 (d, J = 8.7 Hz, 2H), 7.60 (d, J = 8.4 Hz, 2H), 8.13 (d, J = 1.8 Hz, 1H), 8.14 (d, J = 1.8 Hz, 1H), 8.18 (d, J = 8.7 Hz, 2H), 8.51 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.4, 55.7, 97.4, 114.2, 119.7, 120.7, 123.7, 126.1, 127.7, 127.8, 127.9, 128.0, 128.7, 128.8, 128.9, 129.1, 132.7, 133.1, 133.2, 133.3, 135.3, 135.8, 136.6, 146.6, 156.7, 160.9, 162.8; m/z 538 (100, MH+); HRMS (ES): MH+, found 538.1348. C33H26NO235Cl2+ requires 538.1341.
4-Methoxy-6,8-bis[2-(4-methoxyphenyl)ethenyl]-2-phenylquinoline (6i). Green solid (0.28 g, 63%), m.p. 195–196 °C (ethanol); νmax (ATR) 696, 775, 953, 1035, 1286, 1303, 1508, 1575, 1589, 1604 cm−1; δH (CDCl3) 3.85 (s, 3H), 3.86 (s, 3H), 4.14 (s, 3H), 6.94 (t, J = 8.7 Hz, 2H), 6.95 (t, J = 8.7 Hz, 2H), 7.16 (d, J = 16.5 Hz, 1H), 7.24 (d, J = 16.5 Hz, 1H), 7.25 (s, 1H), 7.28 (d, J = 16.5 Hz, 1H), 7.44 (d, J = 16.5 Hz, 1H), 7.53 (d, J = 8.7 Hz, 2 × 2H), 7.64 (d, J = 8.7 Hz, 2H), 8.12 (d, J = 1.5 Hz, 1H), 8.20 (d, J = 1.5 Hz, 1H), 8.24 (dd, J = 1.8 and 8.4 Hz, 2H), 8.46 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.3 (2 × C), 55.6, 97.7, 114.1, 114.2, 118.4, 120.9, 123.2, 123.3, 126.5, 127.4, 127.8, 128.1, 128.7, 128.8, 129.2, 129.6, 130.1, 131.0, 134.3, 135.9, 140.3, 146.2, 156.4, 159.2, 157.4, 162.8; m/z 500 (100, MH+); HRMS (ES): MH+, found 500.2237. C34H30NO3+ requires 500.2235.
2-(4-Fluorophenyl)-4-methoxy-6,8-bis[2-(4-methoxyphenyl)ethenyl]quinoline (6j). Green solid (0.27 g, 60%), m.p. 180–181 °C (ethanol); νmax (ATR) 788, 957, 1173, 1305, 1486, 1509, 1584, 1602 cm−1; δH (CDCl3) 3.84 (s, 3H), 3.86 (s, 3H), 4.07 (s, 3H), 6.92 (d, J = 8.7 Hz, 2H), 6.95 (d, J = 8.7 Hz, 2H), 7.08 (s, 1H), 7.11 (d, J = 16.5 Hz, 1H), 7.20 (t, J = 8.7 Hz, 2H), 7.23 (d, J = 16.5 Hz, 1H), 7.28 (d, J = 16.5 Hz, 1H), 7.51 (d, J = 8.7 Hz, 2H), 7.62 (d, J = 8.7 Hz, 2H), 8.03 (d, J = 1.5 Hz, 1H), 8.13 (d, J = 1.5 Hz, 1H), 8.19 (t, J = 8.7 Hz, 2H), 8.38 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.3 (2 × C), 55.6, 57.2, 114.1, 114.2, 115.5 (d, 2JCF = 21.3 Hz), 118.3, 120.8, 123.1, 123.3, 126.4, 127.8, 128.1, 128.8, 129.2, (d, 3JCF = 8.3 Hz), 129.6, 130.1, 130.9, 134.3, 135.8, 136.3 (d, 4JCF = 3.0 Hz), 146.1, 155.2, 159.3, 159.4, 162.8, 163.7 (d, 1JCF = 247.1 Hz); m/z 518 (100, MH+); HRMS (ES): MH+, found 518.2136. C34H29NO3F+ requires 518.2131.
2-(4-Chlorophenyl)-4-methoxy-6,8-bis[2-(4-methoxyphenyl)ethenyl]quinoline (6k). Green solid (0.26 g, 60%), m.p. 201–203 °C (ethanol); νmax (ATR) 844, 899, 1011, 1083, 1129, 1304, 1461, 1484, 1574, 1604 cm−1; δH (CDCl3) 3.85 (s, 3H), 3.86 (s, 3H), 4.12 (s, 3H), 6.95 (d, J = 8.7 Hz, 2H), 6.96 (d, J = 8.7 Hz, 2H), 7.14 (d, J = 16.5 Hz, 1H), 7.16 (s, 1H), 7.27 (d, J = 16.5 Hz, 1H), 7.43 (d, J = 16.5 Hz, 1H), 7.51 (d, J = 8.7 Hz, 2H), 7.52 (d, J = 8.7 Hz, 2H), 7.63 (d, J = 8.7 Hz, 2H), 8.09 (d, J = 1.5 Hz, 1H), 8.15 (d, J = 1.5 Hz, 1H), 8.16 (d, J = 8.7 Hz, 2H), 8.39 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.3 (2 × C), 55.7, 97.3, 114.1, 114.2, 118.4, 121.0, 123.1, 123.4, 126.4, 127.8, 128.1, 128.7, 128.9, 129.0, 129.8, 130.1, 130.9, 134.6, 135.3, 136.0, 138.7, 146.2, 155.1, 159.3, 159.4, 162.9; m/z 534 (100, MH+); HRMS (ES): MH+, found 534.1829534.1829. C34H29NO335Cl+ requires 534.1836.
4-Methoxy-2-(4-methoxyphenyl)-6,8-bis[2-(4-methoxyphenyl)ethenyl]quinoline (6l). Green solid (0.28 g, 65%), m.p. 181–182 °C (ethanol); νmax (ATR) 807.3, 1027, 1102, 1244, 1303, 1361, 1508, 1605 cm−1; δH (CDCl3) 3.85 (s, 3H), 3.87 (s, 3H), 3.91 (s, 3H), 4.11 (s, 3H), 6.93 (d, J = 8.7 Hz, 2H), 6.96 (d, J = 8.7 Hz, 2H), 7.07 (d, J = 8.7 Hz, 2H), 7.14 (d, J = 16.2 Hz, 1H), 7.15 (s, 1H), 7.26 (d, J = 16.2 Hz, 1H), 7.43 (d, J = 16.5 Hz, 1H), 7.53 (d, J = 8.7 Hz, 2H), 7.65 (d, J = 8.7 Hz, 2H), 8.07 (d, J = 1.5 Hz, 1H), 8.16 (d, J = 1.5 Hz, 1H), 8.21 (d, J = 8.7 Hz, 2H), 8.44 (d, J = 16.5 Hz, 1H); δC (CDCl3) 55.3 (2 × C), 55.4, 55.6, 97.1, 114.0, 114.1, 114.2, 118.4, 120.7, 123.3, 123.5, 126.6, 127.8, 128.1, 128.6, 128.7, 129.5, 130.2, 131.1, 132.9, 134.0, 135.8, 146.3, 156.0, 159.3, 159.4, 160.8, 162.7; m/z 530 (100, MH+); HRMS (ES): MH+, found 530.1842. C35H32NO4+ requires 530. 1842.
3.7. Crystal Structure Solution and Refinement
X-ray quality crystals of the title compound 3b were obtained by slow crystallization from ethanol solution. Intensity data were collected on a Bruker APEX II CCD area detector diffractometer with graphite monochromated Mo Kα radiation (50 kV, 30 mA) using the Bruker APEX 2 [39] data collection software. The collection method involved ω-scans of width 0.5° and 512 × 512 bit data frames. Data reduction was carried out using the program Bruker SAINT+ [40]. The crystal structure was solved by direct methods using Bruker SHELXTL [41]. Non-hydrogen atoms were first refined isotropically followed by anisotropic refinement by full matrix least-squares calculations based on F2 using SHELXTL. Hydrogen atoms were first located in the difference map then positioned geometrically and allowed to ride on their respective parent atoms. Diagrams and publication material were generated using SHELXTL, PLATON [42] and ORTEP-3 [43]. Crystallographic parameters are summarized in Table 1.
Table 1.
Crystal data and structure refinement for compound 3b.
| Empirical formula | C15H10Br2FNO |
| Formula weight | 399.06 |
| Temperature | 173(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P2(1)/n |
| Unit cell dimensions | a = 13.0752(6) Å α = 90°b = 8.0086(3) Å β = 111.8230(10)°c = 14.3026(6) Å γ = 90° |
| Volume | 1390.35(10) Å3 |
| Z | 4 |
| Density (calculated) | 1.906 Mg/m3 |
| Absorption coefficient | 5.835 mm−1 |
| F(000) | 776 |
| Crystal size | 0.41 × 0.40 × 0.20 mm3 |
| Theta range for data collection | 1.80 to 27.99°. |
| Index ranges | −17 ≤ h ≤ 17, −10 ≤ k ≤ 10, −18 ≤ l ≤ 16 |
| Reflections collected | 17097 |
| Independent reflections | 3358 [R(int) = 0.0768] |
| Completeness to theta = 27.00° | 99.9% |
| Absorption correction | Integration |
| Max. and min. transmission | 0.3882 and 0.1983 |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 3358 / 0 / 185 |
| Goodness-of-fit on F2 | 0.922 |
| Final R indices [I>2sigma(I)] | R1 = 0.0277, wR2 = 0.0561 |
| R indices (all data) | R1 = 0.0391, wR2 = 0.0584 |
| Largest diff. peak and hole | 0.470 and −0.709 e.Å−3 |
4. Conclusions
The generality, brevity and operational simplicity of iodine/methanol–mediated oxidative aromatization reaction of the 2-aminochalcones or their 2,3-dihydroquinolin-4(1H)-one isomers and the accompanying high yields make this methodology a suitable alternative to metal–catalyzed aromatization and subsequent methylation or oxidative aromatization–dechloromethoxylation of related derivatives. Elaboration of the 6,8-dibromoquinoline scaffold via Suzuki-Miyaura cross-coupling with arylvinylboronic acids, on the other hand, afforded polysubstituted quinoline derivatives that would not be easily accessible via the known classical methods such as the Skraup, Friedlander and Doebner-von Miller reactions. The absorption and fluorescent properties of these compounds showed a strong correlation with the substituent groups on the styryl and the 2-phenyl groups with the halo substituents shifting both the absorption and emission maxima to shorter wavelengths. Many styrylquinolines prove to be dyes with enhanced photo- and electroluminescent properties and they find application in medicine and pharmacology [44]. The preliminary photophysical properties of compounds 6 serve as a prelude to compounds with potential photonic or electronic properties.
Acknowledgments
The authors are grateful to the University of South Africa for financial assistance. MMM and TAK thank the College of Science, Engineering and Technology’s “Cultivating Woman Leaders in Sciences Program” for BSc Hon. Research Assistantship.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Prakash O., Kumar D., Saini R.K., Singh S.P. Hypervalent iodine oxidation of 2-aryl-1,2,3,4-tetrahydro-4-quinolones: An easy access to 2-aryl-4-quinolones. Synth. Commun. 1994;24:2167–2172. doi: 10.1080/00397919408010231. [DOI] [Google Scholar]
- 2.Singh O.V., Kapil R.S. A new route to 2-aryl-4-quinolones via thallium(III) p-tolylsulfonate-mediated oxidation of 2-aryl-1,2,3,4-tetrahydro-4-quinolones. Synth. Commun. 1993;23:277–283. doi: 10.1080/00397919308009779. [DOI] [Google Scholar]
- 3.Mphahlele M.J., Oyeyiola F.A. Suzuki-Miyaura cross-coupling of 2-aryl-6,8-dibromo-1,2,3,4-tetrahydroquinolin-4-ones and subsequent dehydrogenation and oxidative aromatization of the resulting 2,6,8-triaryl-1,2,3,4-tetrahydroquinolones. Tetrahedron. 2011;67:6819–6825. doi: 10.1016/j.tet.2011.06.085. [DOI] [Google Scholar]
- 4.Singh O.V., Kapil S. Oxidation of 2-aryl-1,2,3,4-tetrahydro-4-quinolones: A novel entry for the synthesis of 2- and 3-arylquinoline alkaloids. Synlett. 1992:751–752. doi: 10.1055/s-1992-21481. [DOI] [Google Scholar]
- 5.Varma R.S., Kumar D. Hypervalent iodine oxidation of 2-aryl-1,2,3,4-tetrahydro-4-quinolones: An expedient route to naturally occurring 4-alkoxy-2-arylquinolines. Tetrahedron Lett. 1998;39:9113–9116. doi: 10.1016/S0040-4039(98)02059-0. [DOI] [Google Scholar]
- 6.Mphahlele M.J., Hlatshwayo S.M., Mogamisi F.K., Tsanwani M., Mampa R.M. Iodine-methanol–promoted aromatization of 2-aryl-1,2,3,4-tetrahydro-4-quinolones to 2-aryl-4-methoxyquinolines. J. Chem. Res. 1999:706–707. [Google Scholar]
- 7.Kumar K.H., Muralidharan D., Perumal P.T. Hypervalent iodine oxidation of 2-aryl-1,2,3,4-tetrahydro-4-quinolones: An expedient route to naturally occurring 4-alkoxy-2-arylquinolines. Tetrahedron Lett. 2004;45:7903–7906. doi: 10.1016/j.tetlet.2004.08.144. [DOI] [Google Scholar]
- 8.Goodwin S., Smith A.F., Velasquez A.A., Horning E.C. Alkaloids of Lunasia amara. Isolation studies. J. Am. Chem. Soc. 1959;81:6209–6213. doi: 10.1021/ja01532a026. [DOI] [Google Scholar]
- 9.Ko T.-C., Hour M.-J., Lien J.-C., Teng C.-M., Lee K.-H., Kuo S.-C., Huang L.-J. Synthesis of 4-alkoxy-2-phenylquinoline derivatives as potent antiplatelet agents. Bioorg. Med. Chem. Lett. 2001;11:279–282. doi: 10.1016/S0960-894X(00)00652-1. [DOI] [PubMed] [Google Scholar]
- 10.See review by Mphahlele M.J. Synthesis of 2-arylquinolin-4(1H)-ones and their transformation to N-alkylated and O-alkylated derivatives. J. Heterocycl. Chem. 2010;47:1–14.
- 11.Bheemanapalli L.N., Kaur A., Arora R., Sangeeta, Akkinepally R.R., Javalli N.M. Synthesis, Evaluation of 6,8-dibromo-2-aryl-2,3-dihydroquinolin-4(1H)-ones in MCF-7 (breast cancer) cell lines and their docking studies. Med. Chem. Res. 2012;21:1741–1750. doi: 10.1007/s00044-011-9688-z. [DOI] [Google Scholar]
- 12.Donnelly J.A., Farrell D.F. Chalcone derivatives as precursors of 1,2,3,4-tetrahydro-4-quinolones. Tetrahedron. 1990;46:885–894. doi: 10.1016/S0040-4020(01)81369-7. [DOI] [Google Scholar]
- 13.Donnelly J.A., Farrell D.F. The chemistry of 2'-amino analogs of 2'-hydroxychalcone and its derivatives. J. Org. Chem. 1990;55:1757–1761. doi: 10.1021/jo00293a017. [DOI] [Google Scholar]
- 14.Tokes A.L., Litkei G. Schmidt reaction on 2-aryl-1,2,3,4-tetrahydro-4-quinolone. Synth. Commun. 1993;23:895–902. doi: 10.1080/00397919308013286. [DOI] [Google Scholar]
- 15.Xia Y., Yang Z.-Y., Xia P., Batow K.F., Tachibana Y., Kuo S.-C., Hamel E., Hackl T., Lee K.-H. Antitumor agents. 181. Synthesis and biological evaluation of 6,7,2',3',4'-substituted-1,2,3,4-tetrahydro-2-phenyl-4-quinolones as a new class of antimitotic antitumor agents. J. Med. Chem. 1988;41:1155–1162. doi: 10.1021/jm9707479. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S.-X., Feng J., Kuo S.-C., Brossi A., Hamel E., Tropsha A., Lee K.-H. Antitumor Agents. 199. Three-dimensional quantitative structure-activity relationship study of the colchicine binding site ligands using comparative molecular field analysis. J. Med. Chem. 2000;43:167–176. doi: 10.1021/jm990333a. [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekhar S., Pushpavalli S.N.C.V.L., Chatla S., Mukhopadhyay D., Changanna B., Vijeender K., Srihari P., Reddy C.R., Ramaiah M.J., Bhadra U. aza-Flavanones as potent cross-species microRNA inhibitors that arrest cell cycle. Bioorg. Med. Chem. Lett. 2012;22:645–648. doi: 10.1016/j.bmcl.2011.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Compound 3b crystallizes in the monoclinic space group P2(1)/c [a = 10.2571(2), b = 13.2887(2), c = 16.7681(3) Å; α = 103.289(1)°, β = 99.454(1)°, γ = 96.939(1)°]. CCDC910000 contains the cif file for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre. Available online: http://www.ccdc.cam.ac.uk/data_request/cif
- 19.Alguinaldo A.M., Dalangin-Mallari V.M., Macabeo A.P.G., Byrne L.T., Abe F., Yamauchi T., Franzblau S.G. First detection of the plasmid-mediated quinolone resistance determinant qnrA in Enterobacteriaceae clinical isolates in Japan. Int. J. Antimicrob. Agents. 2007;29:738–746. doi: 10.1016/j.ijantimicag.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Kumar K.H., Perumal P.T. A novel one-pot oxidative cyclization of 2'-amino and 2'-hydroxychalcones employing FeCl3·6H2O-methanol. Synthesis of 4-alkoxy-2-aryl-quinolines and flavones. Tetrahedron. 2007;63:9531–9535. [Google Scholar]
- 21.Mphahlele M.J., El-Nahas A.M. Tautomeric 2-arylquinolin-4(1H)-one derivatives- spectroscopic, X-ray and quantum chemical structural studies. J. Mol. Struct. 2004;688:129–136. doi: 10.1016/j.molstruc.2003.10.003. [DOI] [Google Scholar]
- 22.Janzso G., Philbin E.M. Bromination reactions of 2-phenyltetrahydroquinolones. Tetrahedron Lett. 1971:3075–3076. doi: 10.1016/S0040-4039(01)97094-7. [DOI] [Google Scholar]
- 23.Zhang Y., Gao J., Li W., Lee H., Lu B.Z., Senanayake C.H. Synthesis of 8-arylquinolines via one-pot Pd-catalyzed borylation of quinoline-8-yl halides and subsequent Suzuki–Miyaura coupling. J. Org. Chem. 2011;76:6394–6400. doi: 10.1021/jo200904g. [DOI] [PubMed] [Google Scholar]
- 24.Piala A., Mayi D., Handy S.T. Studies of one-pot double couplings on dibromoquinolines. Tetrahedron. 2011;67:4147–4154. doi: 10.1016/j.tet.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahin A., Cajmak O., Demirtas I., Okten S., Tutar A. Efficient and selective synthesis of quinoline derivatives. Tetrahedron. 2008;64:10068–10074. doi: 10.1016/j.tet.2008.08.018. [DOI] [Google Scholar]
- 26.Boudet N., Lachs J.R., Knochel P. Multiple regioselective functionalizations of quinolines via magnesiations. Org. Lett. 2007;9:5525–5528. doi: 10.1021/ol702494k. [DOI] [PubMed] [Google Scholar]
- 27.Kappaun S., Sovic T., Stelzer F., Pogantsch A., Zojer E., Slugovc C. Molecular fluorescent pH-probes based on 8-hydroxyquinoline. Org. Biomol. Chem. 2006;4:1503–1511. doi: 10.1039/b600912c. [DOI] [PubMed] [Google Scholar]
- 28.Amatore C., Jutand A. Mechanistic and kinetic studies of palladium catalytic systems. J. Organomet. Chem. 1999;576:254–278. doi: 10.1016/S0022-328X(98)01063-8. [DOI] [Google Scholar]
- 29.Haman B.C., Hartwig J.F. Tandem ring-closing metathesis transannular cyclization as a route to hydroxylated pyrrolizidines. Asymmetric synthesis of (+)-Australine. J. Am. Chem. Soc. 1998;120:7369–7370. [Google Scholar]
- 30.Reddy E.A., Islam A., Mukkanti K., Bandameedi V., Bhowmik D.R., Pal M. Regioselective alkynylation followed by Suzuki coupling of 2,4-dichloroquinoline: Synthesis of 2-alkynyl-4-arylquinolines. Beil. J. Org. Chem. 2009;5:1–6. doi: 10.3762/bjoc.5.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maguire M.P., Sheets K.R., McVety K., Spada A.P., Zilberstein A. A new series of PDGF receptor tyrosine kinase inhibitors: 3-substituted quinoline derivatives. J. Med. Chem. 1994;37:2129–2137. doi: 10.1021/jm00040a003. [DOI] [PubMed] [Google Scholar]
- 32.Zamboni R., Belley M., Champion E., Charette L., DeHaven R., Frenette R., Gauthier J.Y., Jones T.R., Leger S., Masson P., et al. Development of a novel series of styrylquinoline compounds as high-affinity leukotriene D4 receptor antagonists: Synthetic and structure-activity studies leading to the discovery of (±)-3-[[[3-[2-(7-chloro-2-quinolinyl)-(E)-ethenyl]phenyl][[3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]propionic acid. J. Med. Chem. 1992;35:3832–3844. doi: 10.1021/jm00099a011. [DOI] [PubMed] [Google Scholar]
- 33.Nolt M.B., Zhao Z., Wolkenberg S.E. Controlled derivatization of polyhalogenated quinolines utilizing selective cross-coupling reactions. Tetrahedron Lett. 2008;49:3137–3141. and references cited therein. [Google Scholar]
- 34.Rossiter S., Péron J.-M., Whitfield P.J., Jones K. Synthesis and anthelmintic properties of arylquinolines with activity against drug-resistant nematodes. Bioorg. Med. Chem. Lett. 2005;15:4806–4808. doi: 10.1016/j.bmcl.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 35.Jégou G., Jenekhe S.A. Highly fluorescent poly(arylene ethynylene)s containing quinoline and 3-alkyl thiophene. Macromolecules. 2001;34:7926–7928. doi: 10.1021/ma0111562. [DOI] [Google Scholar]
- 36.Chen G.S., Talekar R.S., Wong K.-T., Chi L.-C., Chern J.-W. Physical properties of 8-substituted 5,7-dichloro-2-styrylquinolines as potential light emitting materials. J. Chi. Chem. Soc. 2007;54:1387–1394. [Google Scholar]
- 37.Bratzel M.P., Aaron J.J., Winefordner J.D. Investigation of excited singlet state properties of 8-hydroxyquinoline and its derivatives by fluorescence spectrometry. Anal. Chem. 1972;44:1240–1245. doi: 10.1021/ac60315a020. [DOI] [Google Scholar]
- 38.Maluleka M.M., Mphahlele M.J. 6,8-Dibromo-4-chloroquinoline-3-carbaldehyde as a synthon in the development of novel 1,6,8-triaryl-1H-pyrazolo[4,3-c]quinolines. Tetrahedron. 2012 doi: 10.1016/j.tet.2012.10.103. [DOI] [Google Scholar]
- 38.Bruker APEX2. Version 2009.1–0. Bruker AXS Inc.; Madison, WI, USA: 2005A. [Google Scholar]
- 39.Bruker SAINT+. Version 7.60A. (includes XPREP and SADABS) Bruker AXS Inc.; Madison, WI, USA: 2005B. [Google Scholar]
- 40.Bruker SHELXTL. Version 5.1. (includes XS, XL, XP, XSHELL) Bruker AXS Inc.; Madison, WI, USA: 1999. [Google Scholar]
- 41.Farrugia L.J. XRDIFF: Simulation of x-ray diffraction patterns. J. Appl. Cryst. 1997;30:565–566. doi: 10.1107/S0021889897003117. [DOI] [Google Scholar]
- 42.Spek A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003;36:7–13. doi: 10.1107/S0021889802022112. [DOI] [Google Scholar]
- 43.Lipunova G.N., Nosova E.V., Trashakhova T.V., Charushin V.N. Azinylarylethenes: Synthesis and photophysical and photochemical properties. Russ. Chem. Rev. 2011;80:1115–1133. doi: 10.1070/RC2011v080n11ABEH004234. [DOI] [Google Scholar]