Abstract
Three new germacrane-type sesquiterpenoids, heishuixiecaoline A–C (compounds 1–3), were isolated along with ten known compounds 4–13 from fraction of Valeriana amurensis roots and rhizomes effective against Alzheimer’s disease (AD). The structures of 1–3 were elucidated on the basis of their spectroscopic data. We also investigated the protective effect of compounds 1–13 on the neurotoxicity of PC12 cells induced by amyloid-beta (Aβ25–25), respectively. As a result, germacrane-type sesquiterpenoids 1–4 and lignans 5–7 were seen to afford protection against Aβ-induced toxicity in PC 12 cells. This study will contribute to revealing the chemical basis for the therapeutic effect of V. amurensis against AD.
Keywords: Valeriana amurensis, germacrane-type sesquiterpenoids, heishuixiecaoline, lignans, PC 12 cell
1. Introduction
The genus Valeriana has been taxonomically placed in the family Valerianaceae, comprising ca. 30 species in China [1]. Valerian (Valerianacae) is a perennial herb and some species in this genus have been used widely as a mild sedative and sleep aid for centuries in Europe and North America [2,3,4]. Previous studies on genus Valeriana plants indicated anxiolytic, antidepressant, antispasmodic, sedative, antitumor, and anti-HIV activities [5,6,7,8,9]. As one species of Valeriana, Valeriana amurensis is abundantly distributed in northeast China, especially in the Great Xing’an Mountains area. However, there were no investigations about its pharmacology activity and chemical constituents except for the sedative and anti-hyperspasmia effects of V. amurensis volatile oil [10] until we reported its potential therapeutic effect in Alzheimer’s disease (AD) for the first time [11,12]. We have also screened and determined the AD-effective fraction (50% EtOH fraction from AB-8 macroporous resin column of 95% EtOH extract) from the previous studies [11,12], based on which a bioassay-guided isolation and phytochemical study of V. amurensis was performed and three new and ten known compounds were obtained from the effective fraction. The structures of known compounds 4–13 were determined by detailed 1D- and 2D-NMR analyses, ESI-MS and comparison of their spectral data with literature values. In this paper, the isolation and structural elucidation of the new germacrane-type sesquiterpenoids 1–3 is described. We also investigated the neuroprotective effects of compounds 1–15 in a PC12 neuronal cell line. The PC12 cell line, derived from rat pheochromocytoma, displays phenotypic characteristics of sympathetic neurons. The cells were grown in the presence of various toxins mimicking the conditions taking place in neurodegenerative diseases, including amyloid-beta (Aβ, the peptide composing the amyloid plaques in brains of AD patients).
2. Results and Discussion
Compound 1 was obtained as white amorphous powder, and assigned the molecular formula C17H24O3 from its HRESIMS (m/z 299.1619 [M+Na]+, calc. for C17H24O3Na, 299.1623) and NMR data (Table 1). Six degrees of unsaturation can be concluded for 1 according to the molecular formula C17H24O3. The IR spectrum displayed the presence of carbonyl (1736 cm−1), α,β-unsaturated aldehyde (1682 cm−1), and double-bond (1625 cm−1) absorptions.
Table 1.
The 1H-NMR data of 1–3 in CD3OD (δ in ppm, recorded at 400 MHz).
| No. | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 5.31 (1H, dd, 4.4, 9.6) | 5.25 (1H, dd, 4.0, 9.2) | 3.59 (1H, dd, 6.8, 9.2) |
| 2a | 2.09 (1H, m) | 2.04 (1H, m) | 1.97 (2H, m) |
| 2b | 2.15 (1H, m) | 2.11 (1H, m) | |
| 3a | 2.09 (1H, m) | 2.03 (1H, m) | 1.78 (1H, m) |
| 3b | 2.69 (1H, m) | 2.69 (1H, dd, 4.0, 11.2) | 2.45 (1H, m) |
| 5 | 6.57 (1H, d, 9.8) | 6.56 (1H, d, 9.6) | 6.49 (1H, d, 6.8) |
| 6 | 1.85 (1H, t, 9.8) | 1.73 (1H, t, 9.6) | 1.46 (1H, dd, 6.8, 11.2) |
| 7 | 1.41 (1H, t, 9.8, 10.8) | 1.17 (1H, t, 10.8) | 0.86 (1H, dt, 2.4, 12.4) |
| 8 | 4.50 (1H, dt, 3.2, 10.8) | 3.35 (1H, dt, 4.4, 10.8) | 1.78 (1H, ddd, 4.0, 4.0, 14.4); 1.04 (1H, m) |
| 9 | 2.20 (1H, dd, 2.8, 11.2); 2.30 (1H, t, 11.2) | 2.23 (2H, m) | 2.13(1H, dt, 4.8, 12.8); 2.49(1H,m) |
| 12 | 1.18 (3H, s) | 1.19 (3H, s) | 1.12 (3H, s) |
| 13 | 1.20 (3H, s) | 1.34 (3H, s) | 1.14 (3H, s) |
| 14 | 9.30 (1H, s) | 9.24 (1H, s) | 9.35 (1H, s) |
| 15 | 1.34 (3H, s) | 1.28 (3H, s) | 5.07(1H, brs); 5.12(1H, brs) |
| 17 | 2.03 (3H, s) | -- | -- |
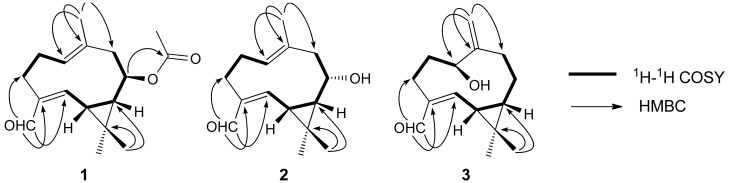
The 1H-NMR spectrum of compound 1 (Table 1) displayed four methyl singlets [δH 1.18 (H-12), 1.20 (H-13), 1.34 (H-15), 2.03 (H-17)], signals for two olefinic protons [δH 5.31 (H-1), 6.57 (H-5)], and an oxygenated methine proton [δH 4.50 (H-8)], and an aldehydic proton [δH 9.30 (H-14)]. Analysis of its 13C-NMR and DEPT spectra showed 17 carbon resonances, including four methyls, three methylenes, three methines (one oxygenated), two trisubstituted double bond [δC 145.1 (C-4), 155.8 (C-5), 128.7(C-1), 133.7 (C-10)], a carbonyl carbon [δC 172.1 (C-16)], and an aldehydic carbon [δC 196.6 (C-14)]. The acetate group was determined to be located at C-8 by the HMBC correlations from H-8 (δH 4.50) to C-16, C-11 (δC 23.7), C-7 (δC 40.8), and C-9 (δC 47.2). The other correlations in the HMBC and 1H-1H-COSY spectra as shown in Figure 1 confirmed the connectivities in compound 1.
Figure 1.
Key 1H-1H COSY and HMBC correlations of compounds 1–3.
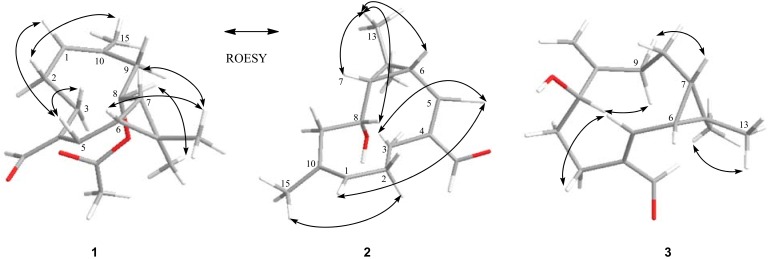
The relative configurations at C-6, C-7, and C-8 in 1 were deduced by a ROESY experiment (Figure 2) and 1H-NMR coupling constants [13].
Figure 2.
Key ROESY correlations of compounds 1–3.
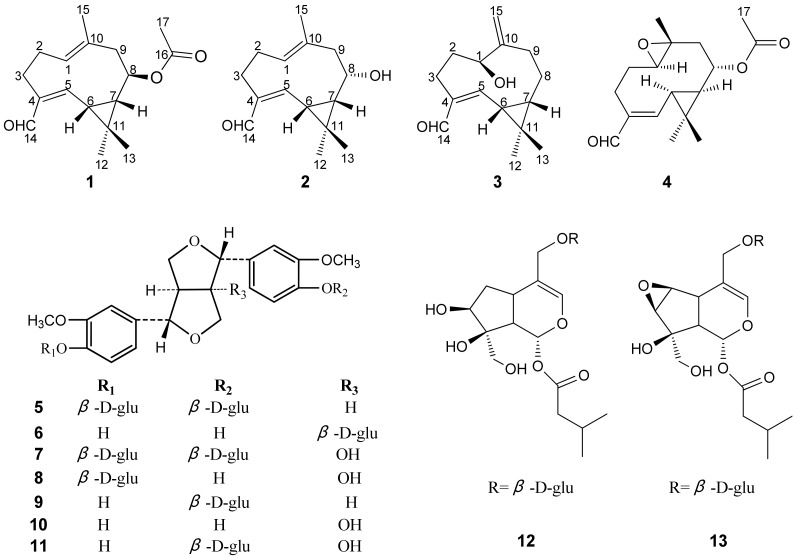
The coupling constant of 9.8 Hz and the NOE correlations between H-6 and H-7 suggested syn configuration of the cyclopropane moiety, the β-orientation of H-6 and H-7 were assigned by the correlations of H-7/CH3-13 and H-6/CH3-13. The α-orientation of H-8 was established by the correlations of H-8/CH3-12. The correlations of H-5/H-3a and H-2 (a, b)/CH3-15 indicated Δ4,5 and Δ1,10 to be Z- and E-configured, respectively, which was confirmed by a key NOE correlation of H-5 with H-1. Therefore, the structure of compound 1 was established as 10-methyl-6,7-dimethyl-methylene-8β-acetoxy-4-aldehyde-(4Z,10E)-dicyclodecadiene (Figure 3), and this compound was named heishuixiecaoline A.
Figure 3.
Structures of compounds 1–13.
Compound 2 was isolated as white amorphous powder, and its molecular formula was determined to be C15H22O2 by HRESIMS (m/z 257.1514 [M+Na]+, calc. for C15H22O2Na, 257.1517), requiring five degrees of unsaturation. The IR spectrum displayed the presence of hydroxyl (3429 cm−1), α,β-unsaturated aldehyde (1689 cm−1), and double-bond (1624 cm−1) absorptions. The 1H- and 13C-NMR spectra data (Table 1 and Table 2) of 2 were a little similar to those of 1, The differences found were the absence of the acetate group signals at δC 172.1 (C-16) and 21.3 (C-17) in compound 2, other differences such as the NMR data in C-7, C-8, and C-9 suggested hydroxy group is linked to C-8 in compound 2. The structure of 2 was further confirmed by the correlations of HMBC and 1H-1H COSY spectra as shown in Figure 1.
Table 2.
The 13C-NMR and DEPT data of 1–3 in CD3OD (δ in ppm, recorded at 100 MHz).
| No. | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 128.7 (CH) | 127.7 (CH) | 68.5 (CH) |
| 2 | 28.3 (CH2) | 28.3 (CH2) | 30.1 (CH2) |
| 3 | 24.5 (CH2) | 24.4 (CH2) | 22.8 (CH2) |
| 4 | 145.1 (C) | 144.1 (C) | 146.3 (C) |
| 5 | 155.8 (CH) | 157.4 (CH) | 155.4 (CH) |
| 6 | 31.9 (CH) | 32.1 (CH) | 28.5 (CH) |
| 7 | 40.8 (CH) | 44.1 (CH) | 36.3 (CH) |
| 8 | 73.6 (CH) | 69.9 (CH) | 23.2 (CH2) |
| 9 | 47.2 (CH2) | 50.8 (CH2) | 37.5 (CH2) |
| 10 | 133.7 (C) | 134.6 (C) | 149.0 (C) |
| 11 | 23.7 (C) | 23.5 (C) | 21.8 (C) |
| 12 | 28.3 (CH3) | 28.7 (CH3) | 28.1 (CH3) |
| 13 | 16.0 (CH3) | 16.0 (CH3) | 16.2 (CH3) |
| 14 | 196.6 (CH) | 196.6 (CH) | 196.7 (CH) |
| 15 | 18.2 (CH3) | 18.5 (CH3) | 113.4 (CH2) |
| 16 | 172.1 (C) | -- | -- |
| 17 | 21.3 (CH3) | -- | -- |
The relative configurations at C-6, C-7, and C-8 in 2 were deduced by a ROESY experiment (Figure 2). The syn configuration of H-7 and H-6 were determined as described in compound 1, and the β-orientation of H-7 and H-6 were assigned by the correlations of H-7/CH3-13 and H-6/CH3-13. The β-orientation of H-8 was established by the correlations of H-8/CH3-13, and the Z- and E-configuration of Δ4,5 and Δ1,10 were determined same to 1 by the correlations H-5/H-3a, H-2(a, b)/CH3-15, and H-5/H-1. Therefore, compound 2 was established as 10-methyl-6,7-dimethylmethylene-8α-hydroxy-4-aldehyde-(4Z,10E))-dicyclodecadiene (Figure 3), and this compound was named heishuixiecaoline B.
Compound 3 was obtained as white amorphous powder, which gave a molecular formula of C15H22O2, as deduced by HRESIMS (m/z 257.1510 [M+Na]+, calc. for C15H22O2Na, 257.1517), indicating five degrees of unsaturation. The IR spectrum showed the presence of hydroxyl (3433 cm−1), α,β-unsaturated aldehyde (1682 cm−1), and double-bond (1631 cm−1) absorptions.
Compound 3 could be assigned a similar structure to 2 by comparison of their 1H- and 13C-NMR spectra (Table 1 and Table 2). The major differences observed were the absence of the trisubstituted C=C double bond between C-1 and C-10 present in 3 and the appearance of an exocyclic double bond between C-10 (δC 149.0) and C-15 (δC 113.4) and the oxygenated methine signal at δC 68.5 (C-1) in 3. These were confirmed by the HMBC correlations from H-15 (δH 5.07, 5.12) to C-1, C-9 (δC 37.5), and C-10. The other correlations in the HMBC and 1H-1H COSY spectra as shown in Figure 1 confirmed the connectivities in compound 3.
On the basis of the ROESY correlations (Figure 2), the relative configuration of 3 was determined to be the same as that of 2, with H-1 assigned as α-oriented by the correlations of H-7/H-9a (β-H) and H-1/H-9b (α-H). The NOE correlations of H-3a/H-5 confirmed the Z-configuration of the double bond between C-4 and C-5. Thus, the structure of compound 3 (named heishuixiecaoline C) was assigned as 6,7-dimethylmethylene-4-aldehyde-1β-hydroxy-10(15)-ene-(4Z)-dicyclodecylene (Figure 3).
Known compounds were identified as volvalerenal C (4) [13], (+) pinoresinol-4,4'-di-O-β-D-glucopyranoside (5) [14], (+) pinoresinol-8-O-β-D-glucopyranoside (6) [15], 8-hydroxypinoresinol-4,4'-di-O-β-D-glucopyranoside (7) [16], (+) 8-hydroxypinoresinol-4'-O-β-D-glucopyranoside (8) [14], (+) pinoresinol-4-O-β-D-glucopyranoside (9) [17], (+) 8-hydroxypinoresinol (10) [14], (+) 8-hydroxypinoresinol-4-O-β-D-glucopyranoside (11) [14], patrinoside (12) [18], and kanokoside A (13) [19] by comparing their NMR spectroscopic data with the literature values. The structures of compounds 1-13 are shown in Figure 3.
The neuroprotective effects of compounds 1–13 against Aβ25–35 induced cell death in PC12 cells were assessed using an established MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) assay. Aβ25–35 induced cytotoxicity (56.94 ± 1.30% viability) in the cells when it was added at a concentration of 20 μM for 24 h. When PC12 cells were pre-incubated with vitamin E or compounds 1–15, the toxicity of Aβ25–35 was significantly alleviated by vitamin E and compounds 1–7 in a dose-dependent manner (Table 3). While compounds 8–13 showed negligible protective effects on the cell viability (data not shown).
Table 3.
Neuroprotective effects of Compounds 1–7 against Aβ25–35-induced PC12 cells death.
| Compound | Cell viability (%) | ||
|---|---|---|---|
| 5 μM | ** 12 μM | * 25 μM | |
| 1 | 64.43 ± 3.02 | 69.77 ± 2.45 | 77.24 ± 2.14 |
| 2 | 65.16 ± 4.20 | 70.31 ± 3.38 | 78.33 ± 3.29 |
| 3 | 65.07 ± 3.26 | 72.97 ± 3.47 | 77.84 ± 2.18 |
| 4 | 64.85 ± 4.14 | 70.83 ± 3.12 | 80.38 ± 4.46 |
| 5 | 68.59 ± 2.63 | 75.81 ± 4.79 | 84.75 ± 2.66 |
| 6 | 71.52 ± 3.34 | 78.78 ± 4.22 | 89.54 ± 3.27 |
| 7 | 67.79 ± 2.26 | 75.80 ± 2.19 | 85.04 ± 3.23 |
| Vitamin E | 61.76 ± 1.48 | 70.47 ± 2.56 | 79.80 ± 2.72 |
Effects of the tested compounds on Aβ-induced PC12 cell death. Cell viability was measured by MTT assay. Results are expressed as mean ± SD (n = 8) of three independent experiments. The 100% value was obtained from untreated control cells. * Significant difference compared 25 μM with 5 and 12 μM of compounds 1–7 (* p < 0.01), respectively. ** Significant difference compared 12 μM with 5 of compounds 1–7 (** p < 0.05), respectively.
3. Experimental
3.1. General
The NMR spectra were recorded on a Bruker DPX 400 (400 MHz for 1H-NMR and 100 MHz for 13C-NMR, respectively, instrument (Bruker SpectroSpin, Karlsruhe, Germany). Chemical shifts are given as δ values with reference to tetramethylsilane (TMS) as an internal standard, and coupling constants are given in Hz. The HRESIMS analyses were conducted on Xero Q Tof MS spectrometer (Waters, Milford, MA, USA); Preparative HPLC was carried out on a Waters 600 instrument equipped with a Waters UV-2487 detector. A Waters Sunfire prep C18 OBD (19 × 250 mm i.d.) column was used for preparative purpose. IR Spectra (Shimadzu FTIR-8400S, Kyoto, Japan); Anal. TLC (silica gel 60 F254, Merck, Darmstadt, Germany). Column chromatography (CC): silica gel (200–300 mesh, Haiyang Chemical Group Co. Ltd, Qingdao, China); ODS-A (120A, 50 mm; YMC, Kyoto, Japan). Macroporous absorption resin (AB-8 Crosslinked Polystyrene, Nan Kai, Tianjin, China) was employed for column chromatography. PC12 cells obtained from Institute of biochemistry and cell biology (Shanghai, China) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, NRH0020), supplemented with 5% fetal bovine serum and 1% antibiotic mixture comprising penicillin-streptomycin, in a humidified atmosphere at 37 °C with 5% CO2. Microplate reader (Safire2, Tecan Group Ltd., Maennedorf, Switzerland) was used to determine the cell viability.
3.2. Plant Material
The roots and rhizomes of V. amurensis were collected from the Great Xing’an Mountains area in 2010. The original plant was identified by Xiaowei Du of Heilongjiang University of Chinese Medicine. A voucher specimen (No. 20100806) was deposited at the Herbarium of Heilongjiang University of Chinese Medicine, China.
3.3. Extraction and Isolation
The dried roots and rhizomes of V. amurensis (7.0 kg) was extracted with 95% EtOH (56 L) for 2 h × 3 times under reflux conditions to give a residue (578.2 g) after removal of solvent under reduced pressure. The EtOH extract was suspended in H2O and then partitioned with petroleum ether (5 × 4 L). The remaining water extract (450.6 g) was fractioned by AB-8 macroporous resin column (10 × 60 cm) with H2O, 50% and 95% EtOH. The obtained 50% EtOH fraction (153.0 g) possesses potential therapeutic effect towards AD. The 50% EtOH fraction (100.0 g) was subjected to silica gel (200–300 mesh) column chromatography, eluted with CHCl3-CH3OH (from 50:1 to 1:1, v/v) to afford fractions I–VI. Fraction I (19.5 g) was subjected to column chromatography over silica gel, eluted with petroleum ether-EtOAc (from 40:1 to 1:1, v/v), to give six fractions, I1–I6. Fraction I3 (5.1 g) and I4 (3.4 g) was chromatographed over silica gel, eluted with petroleum ether-EtOAc (from 40:1 to 1:1, v/v), to afford fractions I3a-I3f and I4a–I4f. Compound 1 (25 mg) and 3 (32 mg) were isolated from fraction I3b by repeated column chromatography over silica gel, eluted with ether-EtOAc (from 40:1 to 15:1, v/v). Fraction I4a was subjected to column chromatography over silica gel eluted with petroleum ether-EtOAc (from 20:1 to 5:1, v/v) to obtain Compound 2 (35 mg).
Heishuixiecaoline A (1): white amorphous powder; +93.9 (c 0.102, MeOH); UV (MeOH) λmax (logε) 257.5 nm; IR (KBr): 1736, 1682, 1625, 1460, 1450, 1373, 1244, 1139, 1015cm−1; 1H-NMR (CD3OD) and 13C-NMR (CD3OD) data are shown in Table 1 and Table 2; HRESIMS m/z 299.1619 [M+Na]+ (calcd for C17H24O3Na, 299.1623).
Heishuixiecaoline B (2): white amorphous powder; +100.2 (c 0.104, MeOH); UV (MeOH) λmax (logε) 261 nm; IR (KBr): 3429, 2938, 2813, 2706, 1689, 1624, 1452, 1370, 1184, 1002 cm−1; 1H-NMR (CD3OD) and 13C-NMR (CD3OD) data are shown in Table 1 and Table 2; HRESIMS m/z 257.1514 [M+Na]+ (calcd for C15H22O2Na, 257.1517).
Heishuixiecaoline C (3): white amorphous powder; −22.3 (c 0.112, MeOH); UV (MeOH) λmax (logε) 266.9 nm; IR (KBr): 3433, 1682, 1631, 1443, 1381, 1264, 1190 cm−1; 1H-NMR (CD3OD) and 13C-NMR (CD3OD) data are shown in Table 1 and Table 2; HRESIMS m/z 257.1510 [M+Na]+ (calcd for C15H22O2Na, 257.1517).
3.4. Determination of Cell Viability
Cell viability was measured by quantitative colorimetric assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method as described previously [20]. Briefly, the cells were cultured at a density 5 × 104 cells per well in growth medium for 24 h in 96-well plates, and then preincubated without or with various concentrations (5, 12, 25 μM) of compounds 1–13, which was followed 24 h later by exposure to 20 μM aggregated Aβ25–35 (Sigma, St. Louis, MO, USA) prepared as described previously [21]. Vitamin E was used as a reference compound [22]. 25 μL/well of MTT solution (5 mg/mL) was added and cells were incubated at 37 °C for 4h. Supernatants were then aspirated off and formazan crystals were dissolved with DMSO. The optical density of each well was determined at 490 nm using a microplate reader. Results were expressed as the percentages of reduced MTT, assuming the absorbance of control cells as 100%. For comparing the results of MTT between the study groups, the t-test was used.
4. Conclusions
We investigated the chemical constituents of V. amurensis based on its activity towards AD for the first time and three new germacrane-type sesquiterpenoids were obtained. Their structures were identified as 10-methyl-6,7-dimethylmethylene-8β-acetoxy-4-aldehyde-(4Z,10E)-dicyclodecadiene (1), 10-methyl-6,7-dimethylmethylene-8α-hydroxy-4-aldehyde-(4Z,10E))-dicyclodecadiene (2), and 6,7-dimethyl-methylene-4-aldehyde-1β-hydroxy-10(15)-ene-(4Z)-dicyclodecylene (3), respectively. Seven known cpompounds were also isolated and identified. All these compounds were screened and cell tests showed that germacrane-type sesquiterpenoids 1–4 and lignans 5–7 were the active components of Valeriana amurensis against AD.
Acknowledgments
This research was supported by the Program of International S&T Cooperation of China (No. 2010DFA32440).
Footnotes
Sample Availability: Samples of heishuixiecaoline A, B, and volvalerenal C are available from the authors.
References
- 1.Huang B.K., Zheng H.C., Qin L.P., Zheng Q.M., Xin H.L. Investigation on resource of genus Valeriana in China. J. Chin. Med. Mater. 2004;27:632–634. [PubMed] [Google Scholar]
- 2.Houghton P.J. The biological activity of valerian and related plants. J. Ethnopharmacol. 1988;22:121–142. doi: 10.1016/0378-8741(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 3.Houghton P.J. The scientific basis for the reputed activity of Valerian. J. Pharm. Pharmacol. 1999;51:505–512. doi: 10.1211/0022357991772772. [DOI] [PubMed] [Google Scholar]
- 4.Yager J., Siegfreid S.L., DiMattero T.L. Use of alternative remedies by psychiatric patients: Illustrative vignettes and a discussion of the issues. Am. J. Psychiatry. 1999;156:1432–1438. doi: 10.1176/ajp.156.9.1432. [DOI] [PubMed] [Google Scholar]
- 5.Bounthanh C., Bergmann C., Beck J.P., Haag-Berrurier M., Anton R. Valepotrictes, a new class of cytotoxic and antitumor agents. Planta Med. 1981;41:21–28. doi: 10.1055/s-2007-971668. [DOI] [PubMed] [Google Scholar]
- 6.Tortarolo M., Braun R., Hübner G.E., Maurer H.R. In vitro effects of epoxide-bearing valepotriates on mouse early hematopoietec progenitor cells and human T-lymphocytes. Arch. Toxicol. 1982;51:37–42. doi: 10.1007/BF00279319. [DOI] [Google Scholar]
- 7.Morazzoni P., Bombardelli E. Valeriana officinalis: Traditional use and recent evaluation of activity. Fitoterapia. 1995;66:99–112. [Google Scholar]
- 8.Murakami N., Ye Y., Kawanishi M., Aoki S., Kudo N., Yoshida M., Nakayama E., Shiodac T., Kobayashi M. New rev-transport inhibitor with anti-HIV activity from Valerianae Radix. Bioorg. Med. Chem. Lett. 2002;12:2807–2810. doi: 10.1016/S0960-894X(02)00624-8. [DOI] [PubMed] [Google Scholar]
- 9.Hattesohl M., Feistel B., Sievers H., Lehnfeld R., Hegger M., Winterhoff H. Extracts of Valeriana officinalis L. s.l. show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties. Phytomedicine. 2008;15:2–15. doi: 10.1016/j.phymed.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Wu J.K., Huo J.H., Du X.W. Pharmacological Effects of volatile oil of Valeriana amurensis on CNS. J. Chin. Med. Mater. 2007;30:977–980. [PubMed] [Google Scholar]
- 11.Zuo Y.M., Zhang Z.L., Wang Q.H., Xie N., Kuang H.X. Effects of Valeriana amurensis on the expressions of β-APP, Aβ1–40 and Caspase-3 in Alzheimer’s disease model rat’s brain. J. Chin. Med. Mater. 2010;33:233–236. [PubMed] [Google Scholar]
- 12.Zhang Z.L., Zuo Y.M., Wang Q.H., Xiao H.B., Kuang H.X. Effects of Valeriana amurensis on the expressions of iNOS, COX-2 and IκB-α in Alzheimer’s disease model rat’s brain. J. Chin. Med. Mater. 2010;33:581–583. [PubMed] [Google Scholar]
- 13.Wang P.C., Ran X.H., Chen R., Luo H.R., Liu Y.Q., Zhou J., Zhao Y.X. Germacrane-type sesquiterpenoids from the roots of Valeriana officinalis var. latifolia. J. Nat. Prod. 2010;73:1563–1567. doi: 10.1021/np100452a. [DOI] [PubMed] [Google Scholar]
- 14.Britta S., Silke S., Josef H., Nasser K., Sonja H., Christa M. Lignans Isolated from Valerian: Identification and Characterization of a New Olivil Derivative with Partial Agonistic Activity at A1 Adenosine Receptors. J. Nat. Prod. 2002;65:1479–1485. doi: 10.1021/np010464q. [DOI] [PubMed] [Google Scholar]
- 15.Wu L.J. Natural Pharmaceutical Chemistry. 5th ed. People’s Medicinal Publishing House; Beijing, China: 2004. pp. 228–232. [Google Scholar]
- 16.Yu D.Q., Yang J.S. Handbook of Analytical Chemistry. 2nd ed. Chemical Industry Press; Beijing, China: 2002. pp. 862–866. [Google Scholar]
- 17.Hu X.J., Jin H.Z., Su J., Zhang W., Xu W.Z., Yan S.K., Liu R.H., Lü H.Z., Zhang W.D. Chemical Constituents from Daphne koreana Nakai. Chin. J. Nat. Med. 2008;6:411–414. doi: 10.3724/SP.J.1009.2008.00411. [DOI] [Google Scholar]
- 18.Tomassini L., Brkic D., Foddai S., Nicoletti M. Iridoid glucosides from Viburnum rhytidophyllum. Phytochemistry. 1997;44:751–753. doi: 10.1016/S0031-9422(96)00600-0. [DOI] [Google Scholar]
- 19.Ayse K.U., Zuhal Güvenalp L., Ömür D., Isabelle B., Karsten S., Axel Z. 4'-Deoxy iridoid glycosides from Centranthus longiflorus. Phytochemistry. 2002;61:937–941. doi: 10.1016/s0031-9422(02)00476-4. [DOI] [PubMed] [Google Scholar]
- 20.Hansen M.B., Nielsen S.E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 21.Pike C.J., Burdick D., Walencewicz A.J., Glabe C.G., Cotman C.W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J. Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Q.L., Buz’Zard A.R., Lau B.H. Pycnogenol protects neurons from amyloid-beta peptide-induced apoptosis. Brain Res. Mol. Brain Res. 2002;104:55–65. doi: 10.1016/S0169-328X(02)00263-2. [DOI] [PubMed] [Google Scholar]