Abstract
A new clerodane-type diterpenoid, echinoclerodane A (1), was isolated from a Formosan gorgonian coral Echinomuricea sp. The structure of 1 was elucidated by spectroscopic methods. Echinoclerodane A (1) is the first clerodane-type compound obtained from the marine organisms belonging to the phylum Cnidaria. Echinoclerodane A (1) exhibited moderate cytotoxicity toward MOLT-4, HL-60, DLD-1 and LoVo tumor cells and inhibitory effects on the generation of superoxide anion and the release of elastase by human neutrophils.
Keywords: Echinomuricea, clerodane diterpene, echinoclerodane, cytotoxicity, superoxide anion, elastase
1. Introduction
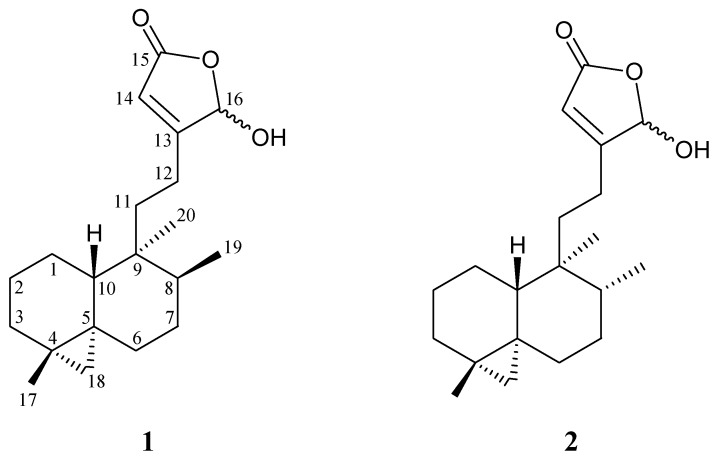
The search for bioactive natural products from marine organisms has been remarkably successful and gorgonian corals have proven to be rich sources of interesting natural terpenoid derivatives [1,2]. In previous studies, two bisabolane-type sesquiterpenoids, (7S,10R)-(+)-10,11-epoxycurcuphenol and (+)-curcuphenol; a labdane-type diterpenoid, echinolabdane A; and a steroid analogue, 6-epi-yonarasterol B, had been isolated from a Formosan gorgonian coral identified as Echinomuricea sp. (Plexauridae) [3,4]. In continuation of our search for new natural products from the marine invertebrates collected off the waters of Taiwan at the intersection of the Kuroshio current and the South China Sea surface current, we have further isolated a new clerodane-type diterpenoid, echinoclerodane A (1), from Echinomuricea sp. (Figure 1). In this paper, we describe the isolation, structure determination and biological activities of diterpenoid 1.
Figure 1.
Structures of echinoclerodane A (1) and dytesinin A (2).
2. Results and Discussion
Echinoclerodane A (1) was isolated as an oil and its molecular formula was determined to be C20H30O3 (m/z 341.2095 [M+Na]+) using HRESIMS. The IR spectrum of 1 showed bands at 3,318 and 1,741 cm–1, consistent with the presence of hydroxy and ester carbonyl groups. The 13C-NMR for 1 confirmed the presence of 20 carbon signals (Table 1), which were characterized by the DEPT spectrum as three methyls, eight sp3 methylenes, three sp3 methines, three sp3 quaternary carbons, one sp2 methine and two sp2 quaternary carbons. A suite of resonances at δC 171.8 (s, C-15), 171.2 (s, C-13), 116.9 (d, C-14) and 99.2 (s, C-16), could be assigned to an α,β-unsaturated-γ-hydroxy-γ-lactone moiety. Thus, from the reported data, the proposed skeleton of 1 was suggested to be a diterpenoid with four rings.
Table 1.
1H- (400 MHz, CDCl3) and 13C- (100 MHz, CDCl3) NMR data, 1H–1H COSY and HMBC correlations for diterpenoid 1.
| Position | δH (J in Hz) | δC, Mult. | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1a/b | 0.75 dd (8.4, 2.8); 1.42 m | 19.9, CH2 | H2-2, H-10 | C-9, -10 |
| 2a/b | 1.19 m; 1.49 m | 23.2, CH2 | H2-1, H2-3 | C-1, -3, -4 |
| 3 | 1.58 m | 32.1, CH2 | H2-2 | C-4, -18 |
| 4 | 17.4, C | |||
| 5 | 26.3, C | |||
| 6a/b | 1.02 m; 1.80 td (14.0, 2.8) | 27.6, CH2 | H2-7 | C-5, -7, -8, -10, -18 |
| 7a/b | 1.35 m; 1.92 tt (14.0, 4.0) | 27.9, CH2 | H2-6, H-8 | C-6, -8, -19 |
| 8 | 1.68 m | 35.6, CH | H2-7, H3-19 | C-6, -7, -9, -19 |
| 9 | 39.1, C | |||
| 10 | 1.64 dd (12.4, 4.0) | 40.9, CH | H2-1 | C-8, -9, -10, -20 |
| 11 | 1.39 m; 1.59 m | 35.5, CH2 | H2-12 | C-8, -9, -12 |
| 12 | 2.35 dd (8.8, 7.2) | 21.5, CH2 | H2-11, H-14 | C-11, -13, -14, -16 |
| 13 | 171.2, C | |||
| 14 | 5.84 br s | 116.9, CH | H2-12 | C-12, -13,-15 |
| 15 | 171.8, C | |||
| 16 | 6.01 s | 99.2, CH | C-13, -15 | |
| 17 | 1.04 s | 22.4, CH3 | C-3, -4, -5 | |
| 18a/b | 0.13 d (4.4); 0.52 d (4.4) | 24.5, CH2 | C-3, -4, -5, -6, -10, -17 | |
| 19 | 0.97 d (7.2) | 14.2, CH3 | H-8 | C-7, -8, -9 |
| 20 | 1.00 s | 19.8, CH3 | C-8, -9 |
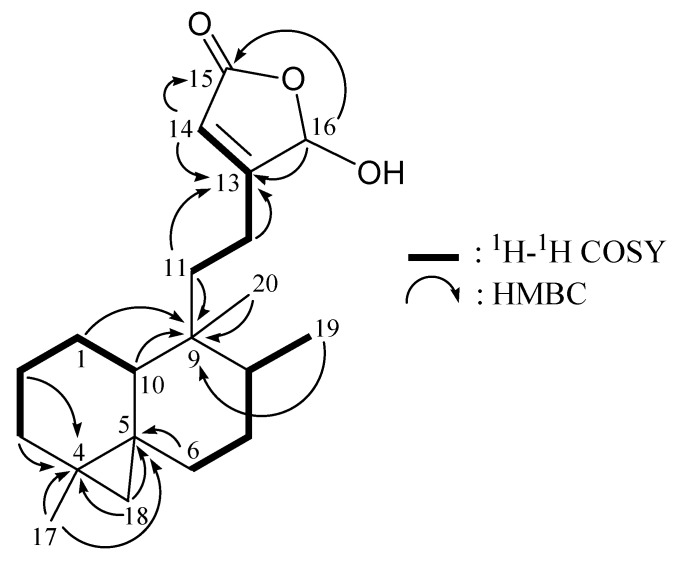
From a 1H–1H COSY experiment (Table 1 and Figure 2), it was possible to establish the spin systems that map out the proton sequences from H-10/H2-1/H2-2/H2-3, H2-6/H2-7/H-8/H3-19, H2-11/H2-12 and H2-12/H-14 (by allylic coupling), which was accomplished with the assistance of an HMBC experiment (Table 1 and Figure 2).
Figure 2.
The 1H–1H COSY and selective key HMBC (protons→quaternary carbons) correlations for 1.
The key HMBC correlations between the protons and quaternary carbons of 1, including H2-2, H2-3, H3-17, H2-18/C-4; H2-6, H3-17, H2-18/C-5; H2-1, H-8, H-10, H2-11, H3-19, H3-20/C-9; H2-12, H-14, H-16/C-13; and H-14, H-16/C-15, permitted elucidation of the carbon skeleton. The tertiary methyls at C-4 and C-9 were confirmed by the HMBC correlations between H3-17/C-3, -4, -5 and H3-20/C-8, -9. The methine unit at δC 99.2 (d, C-16) was more shielded than expected for an oxygenated C-atom and correlated with a methine proton at δH 6.01 (H-16) in the HMQC spectrum, and this proton showed a 2J-correlation and a 3J-correlation with C-13 and C-15, respectively, in the HMBC spectrum and concluded to be a part of a hemiketal constellation.
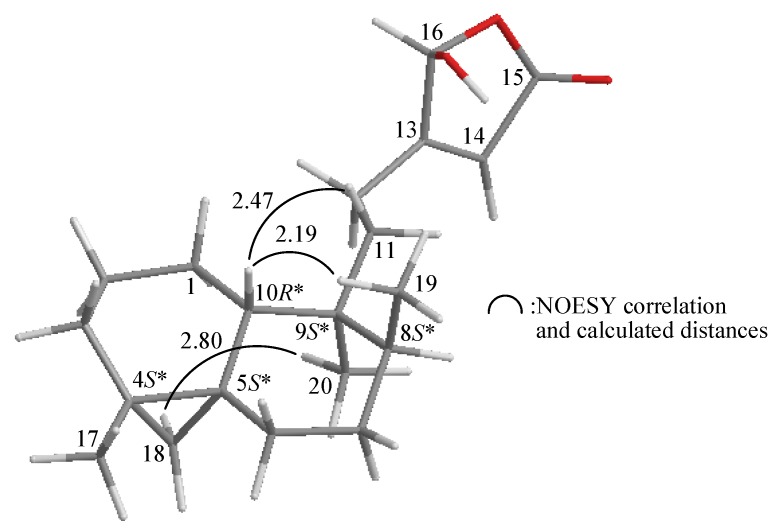
The relative configuration of 1 was elucidated mainly from a NOESY spectrum as being compatible with that of 1 offered by computer modeling [5], in which the close contacts of atoms in space calculated were consistent with the NOESY correlations (Figure 3). In the NOESY spectrum of 1, the correlations of H-10 with H2-11 and H3-19, indicated that these protons (H-10, H2-11 and H3-19) were situated on the same face and these were assigned as β protons, since the C-20 methyl is an α-substituent at C-9. An endo H-C18 proton exhibited a correlation with Me-20, suggesting that the cyclopropane moiety between C-4/5 was α-oriented. Based on the above findings, the main structure of 1 was elucidated unambiguously, and the chiral carbons for 1 were assigned as 4S*, 5S*, 8S*, 9S*, 10R* although the relative configuration for 16-hydroxy group could not be determined at this stage by this method. By comparison of the spectral data, echinoclerodane A (1) was found to be the 8-epimer of a known marine-derived clerodane-type diterpenoid, dytesinin A (2) (Figure 1), isolated from an Okinawa tunicate Cystodytes sp. [6].
Figure 3.
The computer-generated model of 1 using MM2 force field calculations and the calculated distances (Å) between selected protons with key NOESY correlations.
The cytotoxicity of diterpenoid 1 against the K562 (human erythromyeloblastoid leukemia), MOLT-4 (human acute lymphoblastic leukemia), HL-60 (human acute promyelocytic leukemia), DLD-1 (human colorectal adenocarcinoma), LoVo (human colorectal adenocarcinoma) and DU-145 (human prostate carcinoma) cells was studied, and the results were shown in Table 2. These data showed that echinoclerodane A exhibited moderate cytotoxicity against MOLT-4, HL-60, DLD-1 and LoVo cells. The in vitro anti-inflammatory effects of diterpenoid 1 were also tested. Echinoclerodane A (1) displayed a significant inhibition effect on the generation of superoxide anion (inhibition rate 68.6%) and this compound showed a moderately inhibition effect (inhibition rate 35.4%) on the release of elastase by human neutrophils at a concentration of 10 μg/mL, respectively [7].
Table 2.
Cytotoxic activity of diterpenoid 1.
| Compounds | Cell lines IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| K562 | MOLT-4 | HL-60 | DLD-1 | LoVo | DU-145 | |
| 1 | 37.05 | 13.18 | 14.89 | 23.44 | 21.69 | 53.93 |
| Doxorubicin a | 0.29 | 0.001 | 0.08 | 4.00 | 1.65 | 0.01 |
a Doxorubicin was used as positive control.
3. Experimental
3.1. General Experimental Procedures
Optical rotation values were measured with a Jasco-P1010 digital polarimeter. Infrared spectra were obtained on a Varian Diglab FTS 1000 FT-IR spectrophotometer. NMR spectra were recorded on a Varian Mercury Plus 400 FT-NMR at 400 MHz for 1H and 100 MHz for 13C in CDCl3 at 25 °C. Proton chemical shifts were referenced to the residual CHCl3 signal (δH 7.26 ppm). 13C-NMR spectra were referenced to the center peak of CDCl3 at δC 77.1 ppm. ESIMS and HRESIMS data were recorded on Bruker APEX II mass spectrometer. Column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck) and spots were visualized by spraying with 10% H2SO4 solution followed by heating. HPLC was performed using a system comprised of a Hitachi L-7100 pump, a Hitachi L-7455 photodiode array detector and a Rheodyne 7725 injection port. A normal phase column (Hibar 250 × 10 mm, Merck, silica gel 60, 5 μm) was used for HPLC.
3.2. Animal Material
Specimens of the gorgonian coral Echinomuricea sp. were collected by hand using scuba equipment off the coast of the southern Taiwan and stored in a freezer until extraction. This organism was identified by comparison with previous descriptions [8,9]. A voucher specimen (NMMBA-TW-GC-127) was deposited in the National Museum of Marine Biology and Aquarium, Taiwan.
3.3. Extraction and Isolation
The freeze-dried and minced material of Echinomuricea sp. (wet weight 1.68 kg, dry weight 428 g) was extracted with a 1:1 mixture of methanol (MeOH) and dichloromethane (CH2Cl2). The residue was partitioned with ethyl acetate (EtOAc) and H2O. The EtOAc phase was further partitioned between MeOH and n-hexane. The n-hexane phase was separated by silica gel and eluted using n-hexane/EtOAc/MeOH to yield 21 fractions A–U. Fraction N was separated on Sephadex LH-20 and eluted using a 1:1 mixture of MeOH/CH2Cl2 to yield 13 fractions. Fraction N3 was purified by NP-HPLC using a mixture of n-hexane and EtOAc (8:1, flow rate 5 mL/min) as the mobile phase to afford compound echinoclerodane A (1) (8.3 mg); oil;  −43 (c 0.07, CHCl3); IR (neat) νmax 3,318, 1,741 cm–1; 1H- (CDCl3, 400 MHz) and 13C- (CDCl3, 100 MHz) NMR data, see Table 1; ESIMS m/z 341 [M+Na]+; HRESIMS: m/z 341.2095 (calcd. for C20H30O3Na, 341.2093).
−43 (c 0.07, CHCl3); IR (neat) νmax 3,318, 1,741 cm–1; 1H- (CDCl3, 400 MHz) and 13C- (CDCl3, 100 MHz) NMR data, see Table 1; ESIMS m/z 341 [M+Na]+; HRESIMS: m/z 341.2095 (calcd. for C20H30O3Na, 341.2093).
3.4. Molecular Mechanics Calculations
The implementation of the MM2 force field [5] in the CHEM3D PRO software from CambridgeSoft Corporation (Cambridge, MA, USA; ver. 9.0, 2005) was used to calculate the molecular models.
3.5. Cytotoxicity Testing
The cytotoxicity of diterpenoid 1 was assayed with a modification of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric method according to previously described procedures [10,11].
3.6. Superoxide Anion Generation and Elastase Release by Human Neutrophils
Human neutrophils were obtained by means of dextran sedimentation and Ficoll centrifugation. Superoxide generation and elastase release were carried out according to the procedures described previously [12,13]. Briefly, superoxide anion production was assayed by monitoring the superoxide dismutase-inhibitable reduction of ferricytochrome c. Elastase release experiments were performed using MeO-Suc-Ala-Ala-Pro-Valp-nitroanilide as the elastase substrate.
4. Conclusions
Clerodane-type diterpenoids are extensively present in terrestrial plants [14], and compounds of this type were also obtained from tunicates [6]. Octocorals have been proven to be rich sources of natural terpenoid derivatives and terpenoid analogues are often found in large amounts in marine invertebrates [15]. It is worth noting that the new clerodane metabolite 1 (echinoclerodane A) is the first clerodane-type derivative isolated from the marine organisms belonging to the phylum Cnidaria and this compound exhibited cytotoxicity and anti-inflammatory activity. The study material Echinomuricea sp. has begun to be transplanted to culturing tanks with a flow-through sea water system located in the National Museum of Marine Biology and Aquarium, Taiwan for the extraction of additional natural products in order to establish a stable supply of bioactive material.
Sample Availability: Not Available.
Acknowledgments
This work was supported by grants from the National Dong Hwa University; the National Museum of Marine Biology and Aquarium (Grant No. 10120022); the Division of Marine Biotechnology, Asia-Pacific Ocean Research Center, National Sun Yat-sen University, (Grant No. 00C-0302-05); and the National Science Council (Grant No. NSC 101-2325-B-291-001, 100-2325-B-291-001, 101-2320-B-291-001-MY3 and 98-2320-B-291-001-MY3), Taiwan, awarded to P.-J.S.
References
- 1.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2012;29:144–222. doi: 10.1039/c2np00090c. [DOI] [PubMed] [Google Scholar]
- 2.Berrue F., Kerr R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009;26:681–710. doi: 10.1039/b821918b. [DOI] [PubMed] [Google Scholar]
- 3.Chung H.-M., Hwang T.-L., Wang W.-H., Fang L.-S., Sung P.-J. Curcuphenol derivatives from the gorgonian Echinomuricea sp. Heterocycles. 2009;78:2595–2600. doi: 10.3987/COM-09-11771. [DOI] [Google Scholar]
- 4.Chung H.-M., Hong P.-H., Su J.-H., Hwang T.-L., Lu M.-C., Fang L.-S., Wu Y.-C., Li J.-J., Chen J.-J., Wang W.-H., et al. Bioactive compounds from a gorgonian coral Echinomuricea sp. (Plexauridae) 2012;10:1169–1179. doi: 10.3390/md10051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allinger N.L. Conformation analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977;99:8127–8134. doi: 10.1021/ja00467a001. [DOI] [Google Scholar]
- 6.Shimbo K., Tsuda M., Fukushi E., Kawabata J., Kobayashi J. Dytesinins A and B, new clerodane-type diterpenes with a cyclopropane ring from the tunicate Cystodytes sp. Tetrahedron. 2000;56:7923–7926. doi: 10.1016/S0040-4020(00)00711-0. [DOI] [Google Scholar]
- 7.In the in vitro anti-inflammatory bioassay, the inhibitory effect on the generation of superoxide anion and the release of elastase by activated neutrophils were used as indicators. To indicate significant activity of pure compounds, an inhibition rate > 40% is required (inhibition rate < 10%, not active, 20% > inhibition rate > 10%, weakly anti-inflammatory; 40% > inhibition rate > 20%, modestly anti-inflammatory). Diphenyl indonium (DPI) and elastatinal were used as reference compounds in anti-inflammatory activity testing. DPI display an inhibitory effect on the generation of superoxide anion (IC50 = 0.9 μg/mL), and elastatinal exhibited an inhibitory effect on the release of elastase (IC50 = 30.1 μg/mL) by human neutrophils, respectively
- 8.Bayer F.M. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981;94:902–947. [Google Scholar]
- 9.Fabricius K., Alderslade P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed. Australian Institute of Marine Science; Queensland, Australia: 2001. pp. 59–60.pp. 194–195. [Google Scholar]
- 10.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 11.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., Currens M.J., Seniff D., Boyd M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 12.Yu H.-P., Hsieh P.-W., Chang Y.-J., Chung P.-J., Kuo L.-M., Hwang T.-L. 2-(2-Fluorobenz-amido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radical Biol. Med. 2011;50:1737–1748. doi: 10.1016/j.freeradbiomed.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Hwang T.-L., Wang C.-C., Kuo Y.-H., Huang H.-C., Wu Y.-C., Kuo L.-M., Wu Y.-H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010;80:1190–1200. doi: 10.1016/j.bcp.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Hanson J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2011;28:1755–1772. doi: 10.1039/c1np90021h. [DOI] [PubMed] [Google Scholar]
- 15.Harper M.K., Bugni T.S., Copp B.R., James R.D., Lindsay B.S., Richardson A.D., Schnabel P.C., Tasdemir D., van Wagoner R.M., Verbitski S.M., et al. Introduction to the Chemical Ecology of Marine Natural Products. In: McClintock J.B., Baker B.J., editors. Marine Chemical Ecology. CRC Press; Washington, DC, USA: 2001. pp. 3–69. [Google Scholar]