Abstract
In the present work, we report the synthesis and in vitro anticancer and antimicrobial activity evaluation of a new series of 1-substituted-β-carboline derivatives bearing a 4-benzylidene-4H-oxazol-5-one unity at C-3. The compound 2-[1-(4-methoxyphenyl)-9H-β-carbolin-3-yl]-4-(benzylidene)-4H-oxazol-5-one (11) was the most active derivative, exhibiting a potent cytotoxic activity against glioma (U251), prostate (PC-3) and ovarian (OVCAR-03) cancer cell lines with IC50 values of 0.48, 1.50 and 1.07 µM, respectively. An in silico study of the ADME properties of the novel synthesized β-carboline derivatives was also performed.
Keywords: β-carboline, 4H-oxazol-5-one, cytotoxic activity, antimicrobial activity
1. Introduction
Natural and synthetic tetrahydro-β-carbolines and β-carbolines are a class of alkaloids with a large spectrum of important pharmacological properties [1,2,3,4,5,6,7]. Among the activities presented, the antitumor activity has received special attention, and several studies on structure-activity relationship of β-carbolines have focused their anticancer activities [8−16]. The SAR studies have demonstrated that the introduction of appropriate substituents into the positions −1, −2, −3 and −9 of the β-carboline nucleus resulted in more potent antitumor β-carboline derivatives, with reduced toxicity.
The potential of β-carbolines and the importance of the search for new antitumor agents led our group to a continuing study of this class of compounds. In previous works, we reported the synthesis and in vitro antitumor activities, against a panel of human cancer cell lines, of a series of 1-substituted β-carboline derivatives bearing different substituents at C-3, such as 2-substituted-1,3,4-oxadiazole and 5-substituted-1,2,4-triazole rings [17], 3-alkylamino(methyl)-2-thioxo-1,3,4-oxadiazole groups [18] and N-(substituted-benzylidene)-carbohydrazide groups [19]. The anticancer assay results indicated several compounds with potent anticancer activity, with IC50 values lower than 1.0 μM for some of the human cancer cell lines tested [17,18,19]. In addition to the antitumor activity, β-carbolines containing the 2-thioxo-1,3,4-oxadiazole group at C-3 exhibited antimicrobial activity towards the fungus Candida albicans and the bacterium Bacillus subtilis [18]. Antimicrobial activity of β-carboline derivatives, mainly against Bacillus subtilis, Escherichia coli and Staphylococcus aureus bacteria and against Candida albicans fungi, was also reported in literature [20,21].
With the aim of evaluating the influence of different substituents at position-3 of 1-substituted-β-carbolines, in this work we propose the incorporation of a benzylidene-4H-oxazol-5-one unity at C-3, expecting an improvement of the antitumor and antimicrobial activities in relation to the most active β-carboline derivatives reported in our previous work [17,18,19]. The 4H-oxazol-5-one ring is found in natural and synthetic compounds possessing important biological activities, such as antimicrobial [22,23,24], antiviral [25], antiangiogenic [26], inhibitory of tyrosinase [27], cytotoxic and immunomodulatory properties [28]. Due their biological importance, several methods were reported for the synthesis of oxazolones [29,30,31].
Thus, in the present work, we report the synthesis and in vitro cytotoxic and antimicrobial activity evaluation of some novel 1-substituted β-carboline derivatives bearing a 4-(benzylidene)-4H-oxazol-5-one moiety at C-3. Additionally, an in silico study of the ADME properties of the novel synthesized β-carboline derivatives was carried out by investigating their Lipinski’s parameters, topological polar surface area (TPSA) and percentage of absorption (% ABS).
2. Results and Discussion
2.1. Chemistry
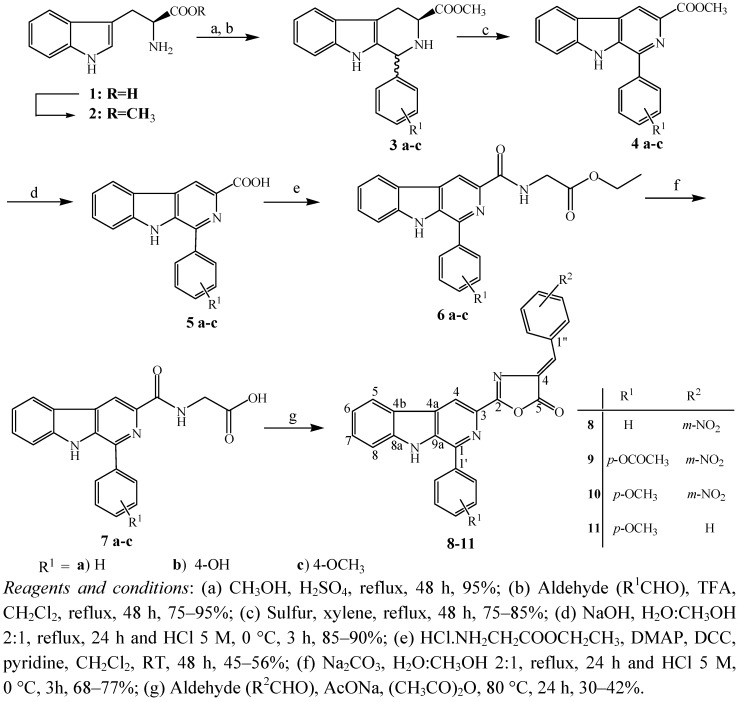
The synthetic route for the β-carboline-3-(4-benzylidene)-4H-oxazol-5-ones 8–11 is outlined in Scheme 1. The 1-substituded β-carboline-3-carboxylic acids 5a–c were prepared from commercial L-tryptophan (1), according to the synthetic protocol described by our group [17,18,19]. The Pictet-Spengler condensation of L-tryptophan methyl ester (2) with benzaldehyde, 4-hydroxybenzaldehyde and 4-methoxybenzaldehyde afforded the 1,2,3,4-tetrahydro-β-carbolines 3a–c. Oxidation of 3a–c with sulfur in refluxing xylene, led to the methyl β-carboline-3-carboxylates 4a–c, which were hydrolyzed under basic conditions to give the β-carboline-3-carboxylic acids 5a–c.
In order to synthesize the β-carboline-3-(4-benzylidene)-4H-oxazol-5-ones 8–11, the Erlenmeyer-Plöchl reaction, the most common route to oxazolones, was employed [31]. The derivatives 5a–c were converted to the respective N-(1-benzylidene-β-carboline-3-carbonyl)-glycine ethyl esters 6a–c by activation of the β-carboline-3-carboxylic acids 5a–c with N,N'-dicyclohexylcarbodiimide (DCC) and dimethylaminopyridine (DMAP), in pyridine, followed by treatment with glycine ethyl ester hydrochloride [32]. To prepare the β-carboline-3-carbonyl-amino acids 7a–c, the corresponding esters 6a–c were submitted to hydrolysis with sodium carbonate in refluxing methanol/water. Erlenmeyer-Plöchl reaction of 7a and 7cwith 3-nitrobenzaldehyde or benzaldehyde, afforded the corresponding β-carboline-3-oxazolones 8–11. The reaction of the derivative 7b, bearing the 4-hydroxyphenyl substituent at C-1, led to the oxazolone 9 with the hydroxyl group acetylated.
To evaluate the effects of electron-donating groups at the R2 position of the benzylidene-4H-oxazol-5-one moiety on activity, compounds 7a and 7c were subjected to Erlenmeyer-Plöchl reaction with 4-N,N-dimethylaminobenzaldehyde and 4-methoxybenzaldehyde. However, these reactions failed to furnish the corresponding β-carboline-3-oxazolones, probably due to the relative low electrophilicity of the aldehydes employed.
Scheme 1.
The synthetic route to compounds 8–11.
All novel compounds were characterized by their spectroscopic data (IR, HR-ESIMS, 1H and 13C-NMR), which are described in the Experimental section. The (4-benzylidene)-4H-oxazol-5-one group at C-3 of 8–11 was evidenced by the presence of a singlet at δH 7.54–7.56 in the 1H-NMR spectra, corresponding to the benzylidene hydrogen, which showed a correlation with the carbon signals at δC 126.4–131.5, in the HSQC spectra. The signals at δC 164.0–164.5 and 168.0–166.9, in the 13C-NMR spectra, were assigned to the C-2 and C-5 carbons, respectively, of the 4H-oxazol-5-one ring. The IR spectra showed absorption bands characteristic for C=O stretching in the 1,780–1,816 cm−1 region.
The structures of 8–11 were also confirmed by their HR-ESI and EI mass spectra. The compounds showed the presence of the molecular ions [M+] consistent with the expected structures, and a base peak at m/z [M+−substituted-benzylidene oxazolone] corresponding to the cleavage between C-3 of β-carboline and C-2 of the 4H-oxazol-5-one ring.
2.2. Cytotoxic Activity
The IC50 values obtained for the synthesized compounds are shown in Table 1. Analysis of the IC50 values (Figure 1, Table 1) showed that compound 8 was active only against the melanoma (UACC-62) cell line, with IC50 of 7.52 μM. The same result was observed for the derivative 9, where the phenyl group at C-1 was changed for a 4-acetoxyphenyl group.
Table 1.
IC50 values (in μM) for compounds 8–11.
| Glioma U251 | Melanoma UACC-62 | Breast MCF7 | Prostate PC-3 | Ovarian OVCAR-03 | Colon HT-29 | VERO | |
|---|---|---|---|---|---|---|---|
| Doxorubicin a | 0.03 | 0.04 | 0.06 | 0.05 | 0.42 | 0.18 | 0.50 |
| 8 | 62.42 | 7.52 | >100 | >100 | 63.19 | >100 | >100 |
| 9 | 80.20 | 8.76 | >100 | >100 | >100 | >100 | >100 |
| 10 | 0.35 | 15.93 | 47.63 | 5.28 | 2.18 | >100 | >100 |
| 11 | 0.48 | 10.00 | 23.44 | 1.50 | 1.07 | 67.88 | 63.17 |
a Doxorubicin was the positive control.
On the other hand, the substitution of the phenyl group at C-1, in compound 8, for a 4-methoxy-phenyl group resulted in the more active compound 10, showing that a good electron-donating substituent at the phenyl ring is important for the cytotoxicity. A potent activity against glioma (U251) and ovarian (OVCAR-03) cell lines was observed for compound 10, which presented IC50 values of 0.35 and 2.18 μM, respectively. Keeping the 4-methoxyphenyl group at C-1 and in order to evaluate the effect on activity of other groups at the oxazolone moiety, the 3-nitrophenyl group was substituted for a phenyl group, resulting in compound 11, which displayed the better activity in comparison to all other synthesized compounds. Derivative 11 showed IC50 values ≤ 10.00 μM for four of the cells lines tested (Table 1). A potent activity was observed mainly against glioma (U251), prostate (PC-3) and ovarian (OVCAR-03) cell lines with IC50 values of 0.48, 1.50 and 1.07 μM, respectively.
In addition to the effective growth inhibition (IC50 values), compound 11 showed also cytostatic activity, with IC100 values of 15.50, 56.83, 64.17 and 112.42 μM for glioma (U251), ovarian (OVCAR-03), prostate (PC-3) and melanoma (UACC-62) human cell lines, respectively (Figure 1).
Figure 1.
Concentration (μM) versus cell growth (%) for compound 11.
The comparison of IC50 data of 11 (Table 1) with those of the most active β-carboline derivatives reported in our previous work [17,18,19] demonstrated, in general, a lower cytotoxic activity for this compound. However, specifically for the prostate (PC-3) and ovarian (OVCAR-03) cancer cell lines, the cytotoxic activity of 11 was similar to that of some active reported compounds, such as 1-(4-N,N-dimethylaminophenyl)-3-(5-thioxo-1,2,4-triazol-3-yl)-β-carboline (prostate: IC50 = 1.37 μM; ovarian: IC50 = 1.09 μM) [17] and N'-(2-chlorobenzylidene)-1-(4-hydroxyphenyl)-β-carboline-3-carbohydrazide (prostate: IC50 = 1.83 μM, ovarian: 1.65 μM) [19].
The IC50 data shown in Table 1 were also compared with those of previously reported β-carboline derivatives bearing the 4-methoxyphenyl group at position-1 and the 2-thioxo-1,3,4-oxadiazole, 2-methylthio-1,3,4-oxadiazole and 5-thioxo-1,2,4-triazole groups [17], and (substituted benzylidene)-carbohydrazide groups [19], at position-3. This comparison pointed of that, except for 1-(4-methoxyphenyl)-3-(2-methylthio-1,3,4-oxadiazol-5-yl) β-carboline (ovarian: IC50 = 2.13 μM) [17], the synthesized oxazoles 10 and 11 showed higher cytotoxic activity, particularly for prostate (PC-3) and ovarian (OVCAR-03) cancer cell lines, than all other 1-(4-methoxyphenyl)-3-substituted-β-carboline derivatives previously tested [17,19]. These data demonstrate the importance of the (substituted benzylidene)-4H-oxazol-5-one moiety on the cytotoxic activity of compounds 10 and 11.
2.3. Antimicrobial Activity
Compounds 8–11 were assayed against the bacteria Bacillus subtilis ATCC 2576, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 13388, Staphylococus aureus ATCC 6538, and against the fungi Candida albicans ATCC 10231, Candida parapsilosis ATCC-22019 and Candida tropicalis ATCC-28707. Contrarily to the expectation, the assay results showed no activity for compounds 8–11 (IC50 > 100 µM), indicating that the (substituted-benzylidene)-4H-oxazol-5-one group at C-3 did not contribute to the antimicrobial activity.
2.4. In Silico Study
An in silico computational study of the synthesized β-carboline-3-(4-benzylidene)-4H-oxazol-5-ones 8–11 was performed by determining the Lipinski’s parameters, topological polar surface area (TPSA) and percentage of absorption (% ABS) [33,34,35]. Calculations were performed using the Molinspiration Online Property Calculation Toolkit (www.molinspiration.com) [34] and OSIRIS Property Explorer (www.organic-chemistry.org/prog/peo) [35]. The percentage of absorption was estimated using the equation: % ABS = 109 − 0.345 × TPSA [33]. These data are shown in Table 2.
Table 2.
Lipinsk’s parameters and % ABS, TPSA, LogS for compounds 8–11.
| Comp. | Lipinsk’s parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| %ABS | TPSA a (A2) | nHBA (NO) | nHBD (OHNH) | logP a | MW | n violations | logS a | |
| 8 | 68.42 | 117.61 | 8 | 1 | 5.45 | 460.45 | 1 | −7.41 |
| 9 | 59.35 | 143.91 | 10 | 1 | 5.00 | 518.48 | 1 | −7.71 |
| 10 | 65.24 | 126.85 | 9 | 1 | 5.50 | 490.47 | 1 | −7.43 |
| 11 | 81.05 | 81.02 | 6 | 1 | 5.57 | 445.48 | 1 | −6.97 |
a www.molinspiration.com; b www.organic-chemistry.org/prog/peo; %ABS = 109 − 0.345 × TPSA; Number hydrogen bond acceptor(NO)= nHBA ≤ 10; Number hydrogen bond donors (OHNH) =nHBD ≤ 5; MW ≤ 500; Octanol-water partition coefficient = LogP < 5; Solubility = LogS > −4.
In vivo absorption of the new synthesized derivatives was tentatively assessed by means of theoretical calculations following Lipinski’s rule of five, which establishes that the absorption or permeation of an orally administered compound is more likely to be good if the drug satisfies the following criteria: (a) hydrogen bond donors ≤ 5 (OH and NH groups); (b) hydrogen bond acceptors ≤10 (N and O atoms); (c) molecular weight < 500; (d) calculated logP < 5 [33,34,35]. Compounds violating more than one of these rules may present bioavailability problems.
Our results (Table 2) revealed that the β-carboline-3-(4-benzylidene)-4H-oxazol-5-ones 8–11 violated only one of the Lipinski’s rules. Compound 9 has a molecular weight larger than 500 g/moL and the derivatives 8, 10 and 11 showed octanol-water partition coefficients (LogP) larger than 5.0.
All derivatives have number of hydrogen bond acceptors (n-ON = 6–10) and donors (n-OHNH = 1) in agreement with Lipinski’s rule. The calculated percent absorption (% ABS) of all derivatives ranged between 59.35 and 81.05%, indicating that these compounds should have good cellular plasmatic membrane permeability.
3. Experimental
3.1. Chemistry
Melting points were determined in a Micro-Química MQAPF-301 apparatus and are uncorrected. 1H- and 13C-NMR spectra were recorded in a Varian model Mercury plus 300 spectrometer (Palo Alto, CA, USA) at 300 MHz and 75.5 MHz, respectively, with DMSO-d6 as solvent and TMS as the internal standard. EI-MS spectra were recorded in a Thermoelectron Corporation Focus-DSQ II spectrometer (Austin, TX, USA). HR-ESI mass spectra were recorded on a Q-TOF (Micromass) spectrometer in positive ion mode (Manchester, UK). IR spectra were recorded on a BOMEM model MB‑100 spectrometer (Quebec, CA, USA). For TLC, Merck precoated plates (silica gel 60 G254) were used. Silica gel 60 Merck (230–400 mesh) was used in the column chromatography purification of some compounds. All reagents were purchased from commercial suppliers.
3.2. General Method for Synthesis of N-(1-benzylidene-β-carboline-3-carbonyl)-glycine Ethyl Esters 6a–c
To a solution of β-carboline-3-carboxylic acids 5a–c (0.7 mmol) in pyridine (5 mL) at 0 °C was added glycine ethyl ester hydrochloride (0.7 mmol), DMAP (0.07 mmol) in CH2Cl2 (5 mL). The mixture was stirred for 5 min and a solution of DCC (0.7 mmol) in CH2Cl2 (5 mL) was added, followed by stirring for 2 h, at 0 °C, and for 24 h at room temperature. This step was repeated with a new amount of DCC (0.7 mmol) in CH2Cl2 (5 mL), followed by evaporation of the solvent, addition of CH2Cl2 and filtration of the DCU precipitate formed. The solvent was removed from the filtrate and the residual DCU separated by precipitation with acetone and filtration. The solvent was removed and the residue obtained purified on a chromatographic column (silica gel, CHCl3/CH3OH 5%) to give the pure β-carbolines 6a–c in yields ranging from 45–56%.
N-(1-Phenyl-β-carboline-3-carbonyl)-glycine ethyl ester (6a). Yield: 55%, m.p. 194–195 °C. IR (KBr) νmax cm−1: 1737 (C=O); 1657 (C=O), 1622 (C=N); 1591–1494 (C=C). 1H-NMR (CDCl3): δ 8.80 (s, H-4), 8.18 (d, J = 7.8 Hz, H-5), 7.35 (td, J = 7.3 Hz and 1.5 Hz, H-6), 7.49–7.63 (m, H-7), 7.49–7.63 (m, H-8), 8.89 (s, NH-9), 7.99 (dd, J = 6.9 Hz and 1.5 Hz, H-2'), 7.51–7.64 (m, H-3'/5'), 7.49–7.64 (m, H-4'), 7.99 (dd, J = 6.9 Hz and 1.5 Hz, H-6'), 8.65 (t, J = 5.7 Hz, NH-2''), 4.31 (d, J = 5.7 Hz, NHCH2), 4.27 (q, J = 7.2 Hz, OCH2CH3), 1.33 (t, J = 7.2 Hz, CH3CH2O). 13C-NMR (CDCl3): δ 141.2 (C-1), 139.8 (C-3), 113.7 (C-4), 130.6 (C-4a), 122.4 (C-4b), 122.3 (C-5), 121.1 (C-6), 129.1 (C-7), 112.1 (C-8), 140.9 (C-8a), 137.9 (C-9a), 135.0 (C-1'), 128.4 (C-2'), 129.3 (C-3'/5'), 129.3 (C-4'), 128.4 (C-6'), 166.0 (C=O), 41.7 (NHCH2), 170.5 (C=O), 61.7 (OCH2CH3), 14.4 (CH3CH2O). EIMS, 70 eV, m/z (rel. int. %): 373 (40, M+∙), 243 (100), 271 (40).
N-[1-(4-Hydroxyphenyl)-β-carboline-3-carbonyl]-glycine ethyl ester (6b). Yield: 45%, m.p. 168–170 °C. IR (KBr) νmax cm−1: 1729 (C=O), 1626 (C=N), 1538–1464 (C=C). 1H-NMR (CDCl3): δ 8.72 (s, H-4), 8.27 (d, J = 7.8 Hz, H-5), 7.28 (t, J = 7.8 Hz, H-6), 7.54 (t, J = 7.8 Hz, H-7), 7.68 (d, J = 7.8 Hz, H-8), 11.69 (s, NH-9), 8.01 (d, J = 8.7 Hz, H-2'/6'), 7.04 (d, J = 8.7 Hz, H-3'/5'), 9.71 (brs, OH), 8.97 (t, J = 6.0 Hz, NH-2''), 4.17 (d, J = 6.0Hz, NHCH2), 4.18 (q, J = 7.2 Hz, OCH2CH3), 1.27 (t, J = 7.2 Hz, CH3CH2O). 13C-NMR (CDCl3): δ 141.3 (C-1), 138.6 (C-3), 111.9 (C-4), 127.9 (C-4a), 121.1 (C-4b), 121.3 (C-5), 119.7 (C-6), 128.2 (C-7), 112.4 (C-8), 141.0 (C-8a), 133.9 (C-9a), 129.3 (C-1'), 129.8 (C-2'/6'), 115.3 (C-3'/5'), 158.2 (C-4'), 165.2 (C=O), 40.9 (NHCH2), 169.8 (C=O), 60.3 (OCH2CH3), 13.9 (CH3CH2O). EIMS, 70 eV, m/z (rel. int. %): 389 (25, M+), 259 (100), 343 (10).
N-[1-(4-Methoxyphenyl-β-carboline-3-carbonyl]-glycine ethyl ester (6c). Yield: 56%, m.p. 184–186 °C. IR (KBr) νmax cm−1: 1742 (C=O); 1656 (C=O), 1609 (C=N), 1561–1464 (C=C). 1H-NMR (CDCl3): δ 8.79 (s, H-4), 8.19 (d, J = 7.8 Hz, H-5), 7.35 (td, J = 7.0 Hz; J = 1.5 Hz, H-6), 7.53–7.61 (m, H-7), 7.53–7.61 (m, H-8), 8.81 (s, NH-9), 7.95 (dd, J = 8.7 Hz and 1.8 Hz, H-2'/6'), 7.13 (dd, J = 8.7 Hz and 1.8 Hz, H-3'/5'), 3.92 (s, OCH3), 8.67 (t, J = 5.5 Hz, NH-2''), 4.32 (d, J = 5.5 Hz, NHCH2), 4.27 (q, J = 7.0 Hz, OCH2CH3), 1.33 (t, J = 7.0 Hz, CH3CH2O). 13C-NMR (CDCl3): δ 141.2 (C-1), 139.8 (C-3), 113.3 (C-4), 122.5 (C-4b), 122.3 (C-5), 121.1 (C-6), 128.9 (C-7), 112.0 (C-8), 140.8 (C-8a), 134.9 (C-9a), 130.5 (C-1'), 129.7 (C-2'/6'), 114.8 (C-3'/5'), 160.6 (C-4'), 55.6 (OCH3), 166.1 (C=O), 170.5 (C=O), 41.7 (NHCH2), 61.7 (OCH2CH3), 14.4 (CH3CH2O). EIMS, 70 eV, m/z (rel. int. %): 403 (60, M+), 301 (45), 273 (100), 258 (30).
3.3. General Method for the Synthesis of 1 N-(1-substituted-β-carboline-3-carbonyl)-glycine 7a–c
A suspension of N-(1-substituted-β-carboline-3-carbonyl)-glycine ethyl esters 6a–c (0.5 mmol) and Na2CO3 (1.5 mmol) in H2O:MeOH 2:1 (5 mL) was stirred for 24 h, at 80 °C. The mixture was cooled at 0 °C, and after stirring for 2 h, the solution was neutralized with a solution of 5 M HCl solution. The product was collected by filtration, dried and crystallized from ethanol, furnishing the compounds 7a–c in 68–77% yield.
N-(1-Phenyl-β-carboline-3-carbonyl)-glycine (7a). Yield: 77%, m.p. 236–238 °C. IR (KBr) νmax cm−1: 1731 (C=O); 1636 (C=N), 1593–1464 (C=C). 1H-NMR (DMSO-d6): δ 8.83 (s, H-4), 8.43 (d, J = 7.8 Hz, H-5), 7.32 (t, J = 7.8 Hz; H-6), 7.55–7.71 (m, H-7), 7.55–7.71 (m, H-8), 11.87 (s, NH-9), 8.14 (dd, J = 7.8 Hz and 1.2 Hz, H-2'/6'), 7.55–7.71 (m, H-3'/5'), 7.55–7.71 (m, H-4'), 8.87 (t, J = 5.1 Hz, NH-2''), 3.89 (d, J = 5.1 Hz, NHCH2). 13C-NMR (DMSO-d6): δ 141.6 (C-1), 139.5 (C-3), 112.9 (C-4), 129.9 (C-4a), 122.0 (C-4b), 121.2 (C-5), 120.2 (C-6), 128.9 (C-7), 112.7 (C-8), 140.6 (C-8a), 137.5 (C-9a), 134.2 (C-1'), 128.6 (C-2'/6'), 128.8 (C-3'/5'), 128.8 (C-4'), 164.7 (C=O), 41.9 (NHCH2), 171.4 (C=O). EIMS, 70 eV, m/z (rel. int. %): 345 (20, M+), 301 (20), 243 (95), 60 (100).
N-[1-(4-Hydroxyphenyl)-β-carboline-3-carbonyl]-glycine (7b). Yield: 74%, mp decomp. IR (KBr) νmax cm−1: 1729 (C=O), 1609 (C=N), 1540–1498 (C=C). 1H-NMR (DMSO-d6): δ 8.76 (s, H-4), 8.40 (d, J = 7.6 Hz, H-5), 7.31 (t, J = 7.6 Hz, H-6), 7.59 (t, J = 7.6 Hz, H-7), 7.70 (d, J = 7.6 Hz, H-8), 12.64 (s, NH-9), 8.04 (d, J = 8.7 Hz, H-2'/6'), 7.04 (d, J = 8.7 Hz, H-3'/5'), 9.86 (brs, OH), 8.95 (t, J = 6.0 Hz, NH-2''), 4.08 (d, J = 6.0 Hz, NHCH2), 11.76 (s, OH). 13C-NMR (DMSO-d6): δ 141.5 (C0-1), 139.2 (C-3), 112.3 (C-4), 128.4 (C-4a), 121.3 (C-4b), 121.9 (C-5), 120.1 (C-6), 128.3 (C-7), 112.7 (C-8), 141.0 (C-8a), 133.9 (C-9a), 129.6 (C-1’), 130.1 (C-2'/6'), 115.6 (C-3'/5'), 158.4 (C-4'), 165.2 (C=O), 41.2 (NHCH2), 171.6 (C=O). C20H15N3O4 EIMS, 70 eV, m/z (rel. int. %): 361 (20, M+), 260 (100), 229 (20), 60 (10).
N-[1-(4-Methoxyphenyl-β-carboline-3-carbonyl]-glycine (7c). Yield: 68%, m.p. 247–249 °C. IR (KBr) νmax cm−1: 1741 (C=O), 1638 (C=N), 1545–1464 (C=C). 1H-NMR (DMSO-d6): δ 8.76 (s, H-4), 8.28 (d, J = 7.5 Hz, H-5), 7.30 (t, J = 7.5 Hz, H-6), 7.56 (t, J = 7.5 Hz, H-7), 7.69 (d, J = 7.5 Hz, H-8), 11.74 (s, NH-9), 8.11 (d, J = 8.7 Hz, H-2'/6'), 7.91 (d, J = 8.7 Hz, H-3'/5'), 3.91 (s, OCH3), 8.90 (t, J = 5.7 Hz, NH-2''), 4.13 (d, J = 5.7 Hz, NHCH2). 13C-NMR (DMSO-d6): δ 141.5 (C-1), 138.6 (C-3), 112.3 (C-4), 121.1 (C-4b), 121.4 (C-5), 119.9 (C-6), 128.1 (C-7), 112.5 (C-8), 140.4 (C-8a), 134.0 (C-9a), 129.5 (C-1'), 129.9 (C-2'/6'), 113.8 (C-3'/5'), 159.8 (C-4'), 55.0 (OCH3), 164.8 (C=O), 40.9 (NHCH2), 171.2 (C=O). EIMS, 70 eV, m/z (rel. int. %): 375 (25, M+∙), 274 (100), 229 (25), 60 (40).
3.4. General Method for Synthesis of 1-Substituted β-carboline-3-(4-benzylidene)-4H-oxazol-5-ones 8–11
To a solution of derivatives 7a–c (0.3 mmol), in acetic anhydride (5 mL), was added 4-nitro-benzaldehyde or benzaldehyde (0.75 mmol), followed by addition of sodium acetate (1.5 mmol). The solution was stirred for 24 h at 80 °C and then, for 1h at 0 °C. The solid formed was filtered under vacuum and washed with cold acetone to afford the compounds 8–11.
2-(1-Phenyl-9H-β-carbolin-3-yl)-4-(3-nitrobenzylidene)-4H-oxazol-5-one (8). Yield: 42%, m.p. 271–273 °C. IR (KBr) νmax cm−1: 3395 (N–H), 1810 (C=O); 1625 (C=N), 1547–1497 (C=C). 1H-NMR (DMSO-d6): δ 9.17 (s, H-4), 8.49 (d, J = 7.8 Hz, H-5), 7.41 (t, J = 7.8 Hz, H-6), 7.59–7.66 (m, H-7), 7.75 (d, J = 7.8 Hz, H-8), 12.17 (s, NH-9), 8.12 (d, J = 8.0 Hz, H-2'/6'), 7.68 (d, J = 8.0 Hz, H-3'/5'), 7.59–7.66 (m, H-4'), 7.54 (s, C=CH), 9.30 (s, H-2''), 8.34 (d, J = 7.5 Hz, H-4''), 7.85 (t, J = 7.5 Hz, H-5''), 8.80 (d, J = 7.5 Hz, H-6''). 13C-NMR (DMSO-d6): δ 143.1 (C-1), 137.2 (C-3), 116.9 (C-4), 129.1 (C-4a), 120.9 (C-4b), 122 (C-5), 120.8 (C-6), 129.3 (C-7), 113.0 (C-8), 141.5 (C-8a), 134.8 (C-9a), 132.3 (C-1'), 128.6 (C-2'/6'), 128.9 (C-3'/5'), 129.0 (C-4'), 167.0 (C=O), 164.4 (C=N), 135.7 (C=CH), 126.8 (C=CH), 135.1 (C-1''), 126.2 (C-2''), 148.2 (C-3''), 124.8 (C-4''), 130.4 (C-5''), 137.8 (C-6''). EIMS, 70 eV, m/z (rel. int. %): 460 (30, M+), 243 (100), 271 (30), 432 (5). HR-ESIMS: calcd for C27H17N4O4: 461.1250 [M+H]+; found: 461.1296.
2-[1-(4-Acetoxyphenyl)-9H-β-carbolin-3-yl]-4-(3-nitrobenzylidene)-4H-oxazol-5-one (9). Yield: 30%, m.p. 255–257 °C. IR (KBr) νmax cm−1: 1816 cm−1 (C=O), 1655 (C=N), 1553–1528 (C=C). 1H-NMR (DMSO-d6): δ 9.20 (s, H-4), 8.51 (d, J = 8.1 Hz, H-5), 7.41 (t, J = 8.1 Hz, H-6), 7.67 (d, J = 8.1 Hz, H-7), 7.75 (d, J = 8.1 Hz, H-8), 12.26 (s, NH-9), 8.20 (d, J = 8.4 Hz, H-2'/6'), 7.45 (d, J = 8.4 Hz, H-3'/5'), 2.37 (s, CH3), 7.56 (s, C=CH), 9.32 (s, H-2''), 8.34 (dd, J = 7.8 Hz and 2.1 Hz, H-4'), 7.87 (t, J = 7.8 Hz, H-5''), 8.83 (d, J = 7.8 Hz, H-6''). 13C-NMR (DMSO-d6): δ 142.3 (C-1), 141.5 (C-3), 117.0 (C-4), 129.1 (C-4a), 120.9 (C-4b), 122.1 (C-5), 121.0 (C-6), 129.4 (C-7), 113.0 (C-8), 144.1 (C-8a), 134.7 (C-9a), 132.3 (C-1'), 129.8 (C-2'/6'), 122.3 (C-3'/5'), 151.3 (C-4'), 20.9 (CH3), 169.2 (OCOCH3), 166.9 (C=O), 164.4 (C=N), 135.7 (C=CH), 126.4 (C=CH), 135.1 (C-1''), 126.2 (C-2''), 148.2 (C-3''), 124.8 (C-4''), 130.4 (C-5''), 137.8 (C-6''). HR-ESIMS: calcd for C29H19N4O6 [M+H]+: 519.1305; found: 519.1400.
2-[1-(4-Methoxyphenyl)-9H-β-carbolin-3-yl]-4-(3-nitrobenzylidene)-4H-oxazol-5-one (10). Yield: 32%, m.p. 201–203 °C. IR (KBr) νmax cm−1: 3330 (N–H), 1781 cm−1 (C=O), 1609 (C=N), 1547–1494 (C=C). 1H-NMR (DMSO-d6): δ 9.15 (s, H-4), 8.48 (d, J = 7.6 Hz, H-5), 7.40 (t, J = 7.6 Hz, H-6), 7.65 (t, J = 7.6 Hz, H-7), 7.71 (d, J = 7.6 Hz, H-8), 12.14 (s, NH-9), 8.12 (d, J = 8.7 Hz, H-2'/6'), 7.24 (d, J = 8.7 Hz, H-3'/5'), 3.91 (s, OCH3), 7.55 (s, C=CH), 9.32 (s, H-2''), 8.35 (d, J = 8.0 Hz, H-4''), 7.87 (t, J = 8.0 Hz, H-5”), 8.51 (d, J = 8.0 Hz, H-6''). 13C-NMR (DMSO-d6): δ 143.1 (C-1), 138.0 (C-3), 116.6 (C-4), 129.2 (C-4a), 120.0 (C-4b), 122.0 (C-5), 121.0 (C-6), 128.9 (C-7), 109.9 (C-8), 142.0 (C-8a), 134.7 (C-9a), 129.6 (C-1'), 130.1 (C-2'/6'), 114.3(C-3'/5'), 160.2 (C-4'), 55.4 (OCH3), 167.1 (C=O), 164.5 (C=N), 135.8 (C=CH), 126.7 (C=CH), 135.2(C-1''), 126.2 (C-2''), 148.2 (C-3''), 124.8 (C-4''), 131.0 (C-5''), 137.8 (C-6''). EIMS, 70 eV, m/z (rel. int. %): 490 (60, M+∙), 301 (30), 273 (100), 258 (35). HR-ESIMS: calcd for C28H19N4O5 [M+H]+: 491.1355; found: 491.1309.
2-[1-(4-Methoxyphenyl)-9H-β-carbolin-3-yl]-4-benzylidene-4H-oxazol-5-one (11). Yield: 40%, m.p. 245–247 °C. IR (KBr) νmax cm−1: 3330 (N–H), 1781 cm−1 (C=O), 1648 (C=N), 1573–1494 (C=C). 1H-NMR (DMSO-d6): δ 9.18 (s, H-4), 8.55 (d, J = 7.5 Hz, H-5), 7.41 (m, H-6), 7.64 (t, J = 7.5 Hz, H-7), 7.74 (d, J = 7.5 Hz, H-8), 12.00 (s, NH-9), 8.06 (d, J = 8.4 Hz, H- 2'/6'), 7.24 (d, J = 8.4 Hz, H-3'/5'), 3.90 (s, OCH3), 7.56 (s, C=CH), 8.40 (d, J = 7.2 Hz, H-2''/6''), 7.58 (d, J = 7.2 Hz, H-3''/H-5''), 7.37 (m, H-4''). 13C-NMR (DMSO-d6): δ 143.4 (C-1), 138.2 (C-3), 116.7 (C-4), 129.6 (C-4a) 121.5 (C-4b), 122.7 (C-5), 121.1 (C-6), 129.4 (C-7), 113.4 (C-8), 141.9 (C-8a), 135 (C-9a), 133.1 (C-1'), 130.5 (C-2'/6'), 114.7 (C-3'/5'), 160.6 (C0-4'), 55.5 (OCH3), 168.0 (C=O), 164.0 (C=N), 134.1 (C=CH), 131.5 (C=CH), 134 (C-1''), 132.7 (C-2''/6), 129.4 (C-3''/5''), 130.0 (C-4''). EIMS, 70 eV, m/z (rel. int. %): 445 (35, M+), 301 (30), 273 (100), 258 (35), 242 (15). HR-ESIMS: calcd for C28H20N3O3 [M+H]+: 446.1505; found: 446.1529.
3.5. Biological Assays
3.5.1. Cytotoxic Assay
The synthesized compounds were evaluated in vitro against six human cancer cell lines consisting of glioma (U251), melanoma (UACC-62), breast (MCF-7), prostate (PC-3), ovarian (OVCAR-03) and colon (HT-29). Cell lines were obtained from National Cancer Institute (Frederick, MD, USA). Normal cell line (VERO, renal, green monkey), from the Rio de Janeiro Cell Bank, was also used. Stock cultures were grown in medium containing 5 mL RPMI 1640 (GIBCO BRL) supplemented with 5% fetal bovine serum (FBS, GIBCO), at 37 °C with 5% CO2. Penicillin:streptomycin (1000 μg/L:1000 U/L, 1 mL/L) was added to the experimental cultures. Cells in 96-well plates (100 μL cells well−1) were exposed to compounds 8–11 in DMSO (concentrations of 0.25, 2.5, 25, and 250 μg·mL−1) at 37 °C, 5% of CO2 in air for 48 h. Final DMSO concentration did not affect the cell viability. Afterwards cells were fixed with 50% trichloroacetic acid and cell proliferation determined by spectrophotometric quantification (540 nm) of cellular protein content by using sulforhodamine B assay and doxorubicin as the positive control [36]. Three measurements were obtained: at time zero (To, at the beginning of incubation) and 48 h post-incubation for compound-free (C) and tested (T) cells. Cell proliferation was determined according to the equation 100 × [(T − T0)/C − T0], for T0 < T ≤ C, and 100 × [(T − T0)/T0], for T ≤ T0 and a concentration-response curve for each cell line was plotted using software ORIGIN 8.0® (OriginLab Corporation). Using the concentration–response curve for each cell line, the IC50 values (concentration that produces a 50% reduction in cellular growth when compared to untreated control cells) and IC100 values (concentration that promotes total growth inhibition) were determined through non-linear regression analysis using software ORIGIN 8.0® (OriginLab Corporation) [37]. Compounds with IC50 values < 100 μM were considered actives.
3.5.2. Antimicrobial Activity Assay
Antibacterial and antifungal assays for compounds 8–11 were carried out according to previously reported experimental protocols [38,39]. The bacterial strains were grown overnight at 36 °C in Nutrient Agar (Merck, Darmstadt, Germany), and the strains were grown in Saboraud Dextrose Agar. Inoculum for the assays was prepared by diluting scraped cell mass in 0.85% NaCl solution, adjusted to McFarland scale 0.5 and confirmed by spectrophotometrical reading at 580 nm. Cell suspensions were finally diluted to 104 UFC·mL−1 for use in the activity assays. Minimal Inhibitory Concentration (MIC) tests were carried out using Müller-Hinton broth (bacteria) or RPMI-1640 (yeasts) on a tissue culture test plate (96 wells). Each compound was tested in duplicate. The stock solutions of compounds firstly in DMSO and subsequently in Tween 80 water solution (0.1%) were diluted and transferred into the first well, and serial dilutions were performed so that concentrations in the range of 250–1.6 μg·mL−1 were obtained. Chloramphenicol and nystatin (Merck) were used as the reference antibiotic control. The inoculum was added to all wells and the plates were incubated at 36 °C for 24 h. Antibacterial activity was detected by adding 0.5% aqueous solution of ntriphenyltetrazolium chloride (TTC, 20 μL, Merck). MIC was defined as the lowest concentration of the compounds that inhibited visible growth, as indicated by TTC staining (dead cells are not stained by TTC). For antifungal activity evaluation, after the incubation period changes in the RPMI-1640 medium color were verified from pink (original color) to yellow. The change indicates an acidification from medium by the microorganisms’ growth.
4. Conclusions
In conclusion, four 1-substituted β-carboline-3-(4-benzylidene)-4H-oxazol-5-ones 8–11 were prepared and assayed for their antitumor and antimicrobial activities. The compound 2-[1-(4-methoxyphenyl)-9H-β-carbolin-3-yl]-4-(benzylidene)-4H-oxazol-5-one (11) was the most active derivative, exhibiting potent cytotoxic activity against glioma (U251), prostate (PC-3) and ovarian (OVCAR-03) cancer cell lines with IC50 values of 0.48, 1.50 and 1.07 μM, respectively. In silico studies indicate that compounds of this class are potential new anticancer drug candidates.
Acknowledgments
This work was supported by Fundação Araucária (Brazil, PR), Fundação de Amparo a Pesquisa de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil). We thank Fundação Araucária, CAPES and CNPq for fellowships (FCS, MAF, JEC, MCTD, MFR, MHS).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/17/3/6099/s1.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 8–11 are available from the authors.
References and Notes
- 1.Cao R., Peng W., Wang Z., Xu A. β-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007;14:479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- 2.Yao K., Zhao M., Zhang X., Wang Y., Li L., Zheng M., Peng S. A class of oral N-[(1S,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carbonyl]-N-(amino-acid-acyl) hydrazine: Discovery, synthesis, in vitro anti-platelet aggregation/in vivo anti-thrombotic evaluation and 3D QSAR analysis. Eur. J. Med. Chem. 2011;46:3237–3249. doi: 10.1016/j.ejmech.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 3.Bi W., Bi Y., Xue P., Zhang Y., Gao X., Wang Z., Li M., Baudy-Floc’h M., Ngerebara N., Gibson M.K., et al. A new class of β-carboline alkaloid-peptide conjugates with therapeutic efficacy in acute limb ischemia/reperfusion injury. Eur. J. Med. Chem. 2011;46:1453–1462. doi: 10.1016/j.ejmech.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Liu J., Jiang X., Zhao M., Zhang X., Zheng M., Peng L., Peng S. A class of 3S-2-aminoacyltetrahydro-β-carboline-3-carboxylic acids: Their facile synthesis, inhibition for platelet activation, and high in vivo anti-thrombotic potency. J. Med. Chem. 2010;53:3106–3116. doi: 10.1021/jm901816j. [DOI] [PubMed] [Google Scholar]
- 5.Costa E.V., Pinheiro M.L.B., de Souza A.D.L., Barison A., Campos F.R., Valdez R.H., Ueda-Nakamura T., Dias B.P., Nakamura C.V. Trypanocidal activity of oxoaporphine and pyrimidine-β-carboline alkaloids from the branches of Annona foetida Mart. (Annonaceae) Molecules. 2011;16:9714–9720. doi: 10.3390/molecules16119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polanski W., Reichmann H., Gille G. Stimulation, protection and regeneration of dopaminergic neurons by 9-methyl-β-carboline: A new anti-Parkinson drug? Expert Rev. Neurother. 2011;11:845–860. doi: 10.1586/ern.11.1. [DOI] [PubMed] [Google Scholar]
- 7.Valdez R.H., Tonin L.T.D., Ueda-Nakamura T., Silva S.O., Dias B.P., Kaneshima E.N., Yamada-Ogatta S.F., Yamauchi L.M., Sarragiotto M.H., Nakamura C.V. In vitro and in vivo trypanocidal synergistic activity of N-butyl-1-(4-dimethylamino)phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxamide) associated with benznidazole. Antimicrob. Agents Chemother. 2012;56:507–512. doi: 10.1128/AAC.05575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda R., Kurosawa M., Okabayashi T., Takei A., Yoshiwara M., Kumakura T., Sakai N., Funatsu O., Morita A., Ikekita M., et al. 3-(3-phenoxybenzyl)amino-β-carboline: A novel antitumor drug targeting α-tubulin. Bioorg. Med. Chem. Lett. 2011;21:4784–4787. doi: 10.1016/j.bmcl.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 9.Shen L., Park E.-J., Kondratyuk P., Guendisch D., Marler L., Pezzuto J.M., Wright A.D., Sun D. Design, synthesis, and biological evaluation of callophycin A and analogues as potential chemopreventive and anticancer agents. Bioorg. Med. Chem. 2011;19:6182–6195. doi: 10.1016/j.bmc.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z., Cao R., Shi B., Guo L., Sun J., Ma Q., Fan W., Song H. Synthesis and biological evaluation of 1,9-disubstituted β-carbolines as potent DNA intercalating and cytotoxic agents. Eur. J. Med. Chem. 2011;46:5127–5137. doi: 10.1016/j.ejmech.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Yang Y., Zhao M., Liu L., Zheng M., Wang Y., Wu J., Peng S. A class of Trp-Trp-AA-OBzl: Synthesis, in vitro anti-proliferation/in vivo anti-tumor evaluation, intercalation-mechanism investigation and 3D QSAR analysis. Eur. J. Med. Chem. 2011;46:3410–3419. doi: 10.1016/j.ejmech.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z., Cao R., Shi B., Yi W., Yu L., Song H., Ren Z. Synthesis and biological evaluation of novel β-carbolines as potent cytotoxic and DNA intercalating agents. Chem. Pharm. Bull. 2010;58:901–907. doi: 10.1248/cpb.58.901. [DOI] [PubMed] [Google Scholar]
- 13.Ma C., Cao R., Shi B., Zhou X., Ma Q., Sun J., Guo L., Yi W., Chen Z., Song H. Synthesis and cytotoxic evaluation of 1-carboxamide and 1-amino side chain substituted β-carbolines. Eur. J. Med. Chem. 2010;45:5513–5519. doi: 10.1016/j.ejmech.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z., Cao R., Yu L., Shi B., Sun J., Guo L., Ma Q., Yi W., Song X., Song H. Synthesis, cytotoxic activities and DNA binding properties of β-carboline derivatives. Eur. J. Med. Chem. 2010;45:4740–4745. doi: 10.1016/j.ejmech.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 15.Cao R., Guan X., Shi B., Chen Z., Ren Z., Peng W., Song H. Design, synthesis and 3D-QSAR of β-carboline derivatives as potent antitumor agents. Eur. J. Med. Chem. 2010;45:2503–2515. doi: 10.1016/j.ejmech.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 16.Wu J., Li C., Zhao M., Wang W., Wang Y., Peng S. A class of novel carboline intercalators: Their synthesis, in vitro anti-proliferation, in vivo anti-tumor action, and 3D QSAR analysis. Bioorg. Med. Chem. 2010;18:6220–6229. doi: 10.1016/j.bmc.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 17.Formagio A.S.N., Tonin L.T.D., Foglio M.A., Madjarof C., de Carvalho J.E., da Costa W.F., Cardoso F.P., Sarragiotto M.H. Synthesis and antitumoral activity of novel 3-(2-substituted-1,3,4-oxadiazol-5-yl) and 3-(5-substituted-1,2,4-triazol-3-yl) β-carboline derivatives. Bioorg. Med. Chem. 2008;16:9660–9667. doi: 10.1016/j.bmc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Savariz F.C., Formagio A.S.N., Barbosa V.A., Foglio M.A., de Carvalho J.E., Duarte M.C.T., Filho B.P.D., Sarragiotto M.H. Synthesis, antitumor and antimicrobial activity of novel 1-substituted phenyl-3-[3-alkylamino(methyl)-2-thioxo-1,3,4-oxadiazol-5-yl] β-carboline derivatives. J. Braz. Chem. Soc. 2010;21:288–298. doi: 10.1590/S0103-50532010000200014. [DOI] [Google Scholar]
- 19.Barbosa V.A., Formagio A.S.N., Savariz F.C., Foglio M.A., Spindola H.M., de Carvalho J.E., Meyer E., Sarragiotto M.H. Synthesis and antitumor activity of β-carboline 3-(substituted-carbohydrazide) derivatives. Bioorg. Med. Chem. 2011;19:6400–6408. doi: 10.1016/j.bmc.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 20.Wu S., Fu Y., Yan R., Wu Y., Lei X., Ye S. Synthesis of neamine-carboline conjugates for RNA binding and their antibacterial activities. Tetrahedron. 2010;66:3433–3440. doi: 10.1016/j.tet.2010.03.034. [DOI] [Google Scholar]
- 21.Schupp P., Poehner T., Edrada R., Ebel R., Berg A., Wray V., Proksch P. Eudistomins W and X, two new β-carbolines from the Micronesian Tunicate Eudistoma sp. J. Nat. Prod. 2003;66:272–275. doi: 10.1021/np020315n. [DOI] [PubMed] [Google Scholar]
- 22.Desai N.C., Bhavsar A.M., Baldaniya B.B. Synthesis and antimicrobial activity of 5-imidazolinone derivatives. Indian J. Pharm. Sci. 2009;71:90–94. doi: 10.4103/0250-474X.51953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Argade N.D., Kalrale B.K., Gill C.H. Microwave assisted improved method for the synthesis of pyrazole containing 2,4,-disubstitute oxazole-5-one and their antimicrobial activity. Eur. J. Chem. 2008;5:120–129. [Google Scholar]
- 24.Salgin-Goken U., Gokhan-Kelekçi N., Goktas O., Koysal Y., Kilic E., Isik S., Aktay G., Ozalp M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic, anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007;15:5738–5751. doi: 10.1016/j.bmc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui I.R., Singh P.K., Srivastava V., Singh J. Facile synthesis of acyclic analogues of carbocyclic nucleoside as potential anti-HIV pro-drug. Indian J. Chem. 2010;49B:512–520. [Google Scholar]
- 26.Perron-Sierra F.M., Pierré A., Burbridge M., Guilbaud N. Novel bicyclic oxazolone derivatives as anti-angiogenic agents. Bioorg. Med. Chem. Lett. 2002;12:1463–1466. doi: 10.1016/S0960-894X(02)00197-X. [DOI] [PubMed] [Google Scholar]
- 27.Khan K.M., Mughal U.R., Khan M.T.H., Zia-Ullah, Perveen S., Choudhary M.I. Oxazolones: New tyrosinase inhibitors; synthesis and their structure-activity relationships. Bioorg. Med. Chem. 2006;14:6027–6033. doi: 10.1016/j.bmc.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Mesaik M.A., Rahat S., Khan K.M., Zia-Ullah, Choudhary M.I., Murad S., Ismail Z., Atta-ur-Rahman, Ahmad A. Synthesis and immunomodulatory properties of selected oxazolone derivatives. Bioorg. Med. Chem. 2004;12:2049–2057. doi: 10.1016/j.bmc.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Bala S., Saini M., Kamboj S. Methods for synthesis of Oxazolones: A Review. Int. J. ChemTech Res. 2011;3:1102–1118. [Google Scholar]
- 30.Tikdari A.M., Fozooni S., Hamidian H. Dodecatungstophosphoric acid (H3PW12O40), samarium and ruthenium (III) chloride catalyzed synthesis of unsaturated 2-phenyl-5(4H)-oxazolone derivatives under solvent-free conditions. Molecules. 2008;13:3246–3252. doi: 10.3390/molecules13123246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleary T., Rawalpally T., Kennedy N., Chavez F. Catalyzing the Erlenmeyer Plöchl reaction: Organic bases versus sodium acetate. Tetrahedron Lett. 2010;51:1533–1536. doi: 10.1016/j.tetlet.2009.11.125. [DOI] [Google Scholar]
- 32.Ma C., Cao R., Shi B., Li S., Chen Z., Yi W., Peng W., Ren Z., Song H. Synthesis and cytotoxic evaluation of N2-benzylated quaternary β-carboline amino acid ester conjugates. Eur. J. Med. Chem. 2010;45:1515–1523. doi: 10.1016/j.ejmech.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 33.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23:3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- 34.Ertl P. Calculation of Molecular Properties and Bioactivity Score. [(accessed on 31 January 2012)]. Available online: http://www.molinspiration.com.
- 35.Sander T. Molecular Property Explorer. [(accessed on 31 January 2012)]. Available online: http://www.organic-chemistry.org/prog/peo.
- 36.Monks A., Scudiero D., Skehan P., Shoemaker R., Paull K., Vistica D., Hose C., Langley J., Cronise P., Vaigro-Wolff A., et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 37.Shoemaker R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 38.NCCLS. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Approved Standard. 6th. NCCLS document M7-A6. NCCLS; Wayne, MI, USA: 2003. [Google Scholar]
- 39.NCCLS. Methods for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved standard. 2nd. NCCLS document M27-A. NCCLS; Wayne, MI, USA: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.