Abstract
A new 3,3′′-biflavanone, sikokianin D (1), was isolated from the roots of Wikstroemia indica, together with two known compounds. Their structures were elucidated by chemical evidence and spectral analyses, including HR-ESI-MS, and 1D- and 2D-NMR techniques.
Keywords: Wikstroemia indica, sikokianin D, C-3/C-3"-biflavanone
1. Introduction
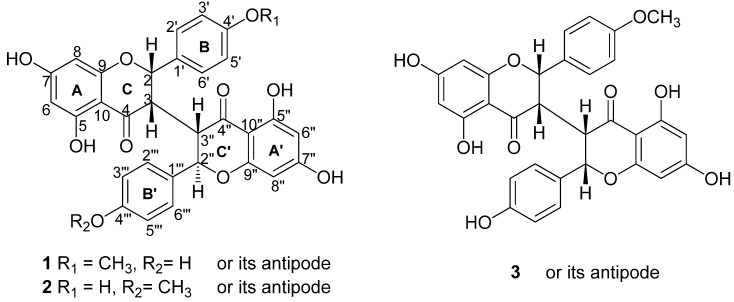
Wikstroemia indica (Linn.) C. A. Mey., a shrub of the Thymelaeaceae family, is wildely distributed in the southeast of China. Known as Liaogewang, it has long been used as a folk medicine in southern China for treating arthritis, tuberculosis, syphilis and pertussis [1]. Moreover, W. indica has antifungal, anti-inflammatory, anti-cancer, antiviral and antimalarial effects [2,3,4,5,6,7]. The chemical constituents of the roots have been investigated previously, leading to the identification of groups of flavonoid, coumarin and lignan compounds [2,3,4,5,6,7,8,9]. In previous paper [10], we have reported several C-3/C-3"-biflavanones from the roots of Stellera chamaejasme L. (Thymelaeaceae) collected in Yunnan. C-3/C-3"-Biflavanones have been shown to exhibit a wide range of pharmacological activities, such as antibacterial, anti-inflammatory, antimalarial, and antitumor activities [4,11,12,13,14]. In connection with these interesting biflavanones, we examined the chemical constituents of other Thymelaeaceae plants and one new C-3/C-3"-biflavanone, sikokianin D (1), together with two known compounds, namely sikokianin B (2) and sikokianin A (3) (Figure 1) was isolated from the roots of Wikstroemia indica. This paper describes the isolation and structure elucidation of these compounds.
Figure 1.
Chemical Structures of 1–3.
2. Results and Discussion
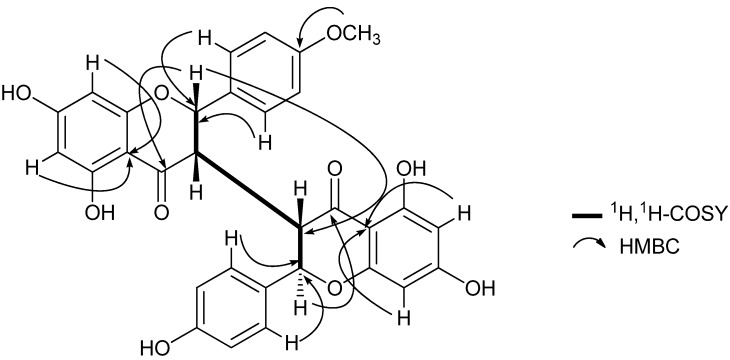
Compound 1 was obtained as a pale yellow amorphous powder with optical activity ([α] : + 231). The HR-ESI-MS of 1 exhibited a quasi-molecular-ion peak ([M+H]+) at m/z 557.1442 (calc. 557.1448), corresponding to the molecular formula C31H24O10. Moreover, this compound showed positive reaction with HCl-Mg reagent, indicating that it is a flavonoid. The 1H-NMR spectrum of 1 (Table 1) displayed signals of one methoxyl group (δH 3.79, s, 3H), two H-atoms corresponding to H-2 (δH 5.57, 1H, d, J = 5.0 Hz) and H-2" (δH 5.19, 1H, d, J = 9.5 Hz), and two H-atoms corresponding to H-3 (δH 3.19, 1H, br s) and H-3" (δH 3.26, 1H, dd, J = 9.5, 3.0 Hz) at the rings C and C' of the biflavanone. In the 1H- and 13C-NMR established by 1H-1H COSY and HMQC experiments (Table 1), the spectra showed its structural fragments to include two sets of typical 5,7-dioxygenated A rings (δH 5.74, 5.77, each 1H, d, J = 2.0 Hz; δH 5.78, 5.98, each 1H, d, J = 2.0 Hz), and two sets of para-oxygenated B rings (δH 7.22, 6.90, each 2H, d, J = 8.5 Hz; δH 6.93, 6.63, each 2H, d, J = 8.5 Hz). From the 13C-NMR data (Table 1), two carbonyl groups (δC 198.5, 196.1) were also observed. These structural fragments were connected to form the given carbon framework of 1 as a dimer of flavanonol derivatives. The partial (-CH-CH-CH-CH-) structure inferred from the 1H-1H COSY spectrum (bold line in Figure 2) suggested that the linkage of the two flavanones was possible only at the C-3 and C-3" positions, which was supported by the comparison of the 1H- and 13C-NMR data of 1 with those of known 3,3"-biflavanones [4,6,8,10], and further confirmed by the HMBC correlations of H-2 (δH 5.57) with C-3" (δC 51.0). The B ring could be located at C-2, based on the observation of the clear cross-peaks of H-2' and H-6' (δH 7.22) with C-2 (δC 81.2). In the same way, linkage of the B' ring to C-2" of the C' ring was deduced by the correlations of H-2"'and H-6"'(δH 6.93) with C-2" (δC 83.3). The HMBC cross-peak between the methoxyl group and C-4' on the B ring indicated that the methoxyl group was connected to C-4'.
: + 231). The HR-ESI-MS of 1 exhibited a quasi-molecular-ion peak ([M+H]+) at m/z 557.1442 (calc. 557.1448), corresponding to the molecular formula C31H24O10. Moreover, this compound showed positive reaction with HCl-Mg reagent, indicating that it is a flavonoid. The 1H-NMR spectrum of 1 (Table 1) displayed signals of one methoxyl group (δH 3.79, s, 3H), two H-atoms corresponding to H-2 (δH 5.57, 1H, d, J = 5.0 Hz) and H-2" (δH 5.19, 1H, d, J = 9.5 Hz), and two H-atoms corresponding to H-3 (δH 3.19, 1H, br s) and H-3" (δH 3.26, 1H, dd, J = 9.5, 3.0 Hz) at the rings C and C' of the biflavanone. In the 1H- and 13C-NMR established by 1H-1H COSY and HMQC experiments (Table 1), the spectra showed its structural fragments to include two sets of typical 5,7-dioxygenated A rings (δH 5.74, 5.77, each 1H, d, J = 2.0 Hz; δH 5.78, 5.98, each 1H, d, J = 2.0 Hz), and two sets of para-oxygenated B rings (δH 7.22, 6.90, each 2H, d, J = 8.5 Hz; δH 6.93, 6.63, each 2H, d, J = 8.5 Hz). From the 13C-NMR data (Table 1), two carbonyl groups (δC 198.5, 196.1) were also observed. These structural fragments were connected to form the given carbon framework of 1 as a dimer of flavanonol derivatives. The partial (-CH-CH-CH-CH-) structure inferred from the 1H-1H COSY spectrum (bold line in Figure 2) suggested that the linkage of the two flavanones was possible only at the C-3 and C-3" positions, which was supported by the comparison of the 1H- and 13C-NMR data of 1 with those of known 3,3"-biflavanones [4,6,8,10], and further confirmed by the HMBC correlations of H-2 (δH 5.57) with C-3" (δC 51.0). The B ring could be located at C-2, based on the observation of the clear cross-peaks of H-2' and H-6' (δH 7.22) with C-2 (δC 81.2). In the same way, linkage of the B' ring to C-2" of the C' ring was deduced by the correlations of H-2"'and H-6"'(δH 6.93) with C-2" (δC 83.3). The HMBC cross-peak between the methoxyl group and C-4' on the B ring indicated that the methoxyl group was connected to C-4'.
The stereochemistry at the C-2/C-3 and C-2"/C-3" positions in 1 was determined as cis-trans by comparison of the J values (JH-2 = 5.0 Hz and JH-2" = 9.5 Hz) with those of the known 3,3"-biflavanones. The key NOESY correlations between H-2" (δH 5.19) with H-2'(H-6') (δH 7.22) further confirmed the conclusion above. The relative stereochemistry of compound 1 was confirmed as shown in Figure 1 and the compound named sikokianin D.
Compound 2 was first reported as sikokianin B of which the location of MeO group was unsettled [8], and the exact configuration was elucidated by Nunome [4]. Sikokianin B and sikokianin C were determined by comparing their 1H- and 13C-NMR and MS data with published values.
Table 1.
NMR data of sikokianin D (1) in CD3OD (500 MHz for 1H, 125 MHz for 13C).
| No. | δH Mult (J = Hz) | δC |
|---|---|---|
| 2 | 5.57 d (5.0) | 81.2 d |
| 3 | 3.19 br s | 49.3 d |
| 4 | - | 198.5 s |
| 5 | - | 165.0 s |
| 6 | 5.74 d (2.0) | 96.0 d |
| 7 | - | 168.1 s |
| 8 | 5.77 d (2.0) | 97.0 d |
| 9 | - | 165.0 s |
| 10 | - | 103.6 s |
| 1' | - | 130.0 s |
| 2' | 7.22 d (8.5) | 128.4 d |
| 3' | 6.90 d (8.5) | 114.9 d |
| 4' | - | 160.9 s |
| 5' | 6.90 d (8.5) | 114.9 d |
| 6' | 7.22 d (8.5) | 128.4 d |
| 2'' | 5.19 d (9.5) | 83.3 d |
| 3'' | 3.26 dd (9.5, 3.0) | 51.0 d |
| 4'' | - | 196.1 s |
| 5'' | - | 165.3 s |
| 6'' | 5.78 d (2.0) | 97.0 d |
| 7'' | - | 167.9 s |
| 8'' | 5.98 d (2.0) | 96.4 d |
| 9'' | - | 163.9 s |
| 10'' | - | 105.1 s |
| 1''' | - | 128.9 s |
| 2''' | 6.93 d (8.5) | 130.3 d |
| 3''' | 6.63 d (8.5) | 116.1 d |
| 4''' | - | 158.9 s |
| 5''' | 6.63 d (8.5) | 116.1 d |
| 6''' | 6.93 d (8.5) | 130.3 d |
| 4'-OCH3 | 3.79 s | 55.7 q |
Figure 2.
Key 1H-1H COSY and HMBC correlations of 1.
3. Experimental
3.1. General
Melting points were measured on a Thermal Values analytical microscope and are uncorrected. Optical rotations were recorded on a Perkin-Elmer 341 polarimeter. IR spectra were recorded on a Nicolet FI-IR 200SXY spectrophotomer. The spectra of high resolution-electrospray ionization-mass spectrometry (HR-ESI-MS) were acquired with a Micromass Q-TOF mass spectrometer (Waters Corporation USA). 1H- and 13C-NMR spectra were measured in CD3OD with TMS as the internal standard on a Bruker DMX-500 NMR instrument. Silica gel G254 and H (Qingdao Sea Chemical Factory, China) were used for TLC and column chromatography, respectively.
3.2. Plant Material
The roots of Wikstroemia indica were purchased from a Chinese medicine pharmacy in Guangzhou, China, in September, 2011. The authentication process was carried out by Le Cai (Yunnan University). A voucher specimen was deposited in the Zhejiang University City College.
3.3. Extraction and Isolation
Air-dried powder roots (2.6 kg) of W. indica were extracted exhaustively with 95% aq. EtOH (9 L × 3) at r. t. After concentration in vacuo, a crude extract (270 g) was obtained, which was suspended in 1 L H2O, and the suspension was extracted successively with petroleum ether (PE, 1 L × 3), EtOAc (1 L × 3), and BuOH (1 L × 3) to yield 34, 110, 89 g fractions, resp. The EtOAc extract was subjected to CC with PE/EtOAc gradient system of increasing polarity (9/1→5/5, 3600 mL) to give five fractions (Fraction 1–5). Fraction 3 was chromatographed repeatedly over SiO2 column with MeOH/H2O (7/3→9/1, 1,200 mL) to afford 3 (15 mg). Fraction 4 was subjected to MPLC on octadecyl silica gel (3.5 × 30 cm) eluting by gradient elution with MeOH-H2O (5 mL/min, linear gradient, 50:50→90:10) to yield compounds 1 (28 mg) and 2 (36 mg).
Sikokianin D (1). Yellow amorphous powder, mp 213–215 °C; ([α] : +231 (c = 0.48, MeOH); IR (KBr, cm−1): 3362, 1643; 1H-NMR and 13C-NMR data, see Table 1; HR-ESI-MS: m/z 557.1442 [M+H]+, calcd for C31H25O10, 557.1448.
: +231 (c = 0.48, MeOH); IR (KBr, cm−1): 3362, 1643; 1H-NMR and 13C-NMR data, see Table 1; HR-ESI-MS: m/z 557.1442 [M+H]+, calcd for C31H25O10, 557.1448.
Sikokianin B (2). Yellow amorphous powder. 1H-NMR: δH 3.23 (1H, t, J = 3.5 Hz, H-3), 3.33 (1H, dd, J = 9.5, 3.0 Hz, H-3"), 3.76 (3H, s, OCH3), 5.17 (1H, d, J = 9 Hz, H-2"), 5.53 (1H, d, J = 4.5 Hz, H-2), 5.75 (1H, d, J = 2.0 Hz, H-6), 5.84 (1H, d, J = 2.0 Hz, H-8), 5.86 (1H, d, J = 2.0 Hz, H-6"), 5.97 (1H, d, J = 2.0 Hz, H-8"), 6.74~7.16 (8H, m, H-Ar). HR-ESI-MS: m/z 557.1446 [M+H]+. Spectral data were in accordance with those reported in the literature [4,8], which confirmed that the isolated compound 2 was sikokianin B.
Sikokianin A (3). Yellow amorphous powder. 1H-NMR: δH 2.91 (1H, d, J = 2.0 Hz, H-3), 2.98 (1H, d, J = 2.0 Hz, H-3"), 3.82 (3H, s, OCH3), 5.32 (1H, d, J = 2.0 Hz, H-2), 5.37 (1H, d, J = 2.0 Hz, H-2"), 5.75 (2H, d, J = 0.5 Hz, H-6, H-6"), 5.88 (2H, d, J = 0.5 Hz, H-8, H-8"), 6.63~7.04 (8H, m, H-Ar). HR-ESI-MS: m/z 557.1448 [M+H]+. Spectral data were in accordance with those reported in the literature [8], which confirmed that the isolated compound 3 was sikokianin A.
4. Conclusions
In conclusion, one new biflavanone, 5,5',7,7'-tetrahydroxy-2-(4-hydroxyphenyl)-2'-(4-methoxy-phenyl)-[3,3'-bichroman]-4,4'-dione (1), together with two known compounds, sikokianin B (2) and sikokianin A (3) was isolated from the EtOH extract of the roots of Wikstroemia indica.
Acknowledgements
This material is based upon work funded by Zhejiang Provincial Natural Science Foundation of China (LQ12H30003, LQ12H31001), Foundation of Qianjiang Talents (QJD1002012), Science Research Foundation of Zhejiang Health Bureau (2012KYA068).
Footnotes
Sample Availability: Contact the authors.
References and Notes
- 1.Ko F.N., Chang Y.L., Kuo Y.H., Lin Y.L., Teng C.M. Daphnoretin, a new protein kinase C activator isolated from Wikstroemia indica C. A. Mey. Biochem. J. 1993;295:321–327. doi: 10.1042/bj2950321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee K.H., Tagahara K., Suzuki H., Wu R.Y., Haruna H., Hall I.H., Huang H.C., Ito K., Iida T., Lai J.S. Antitumor agents. 49 tricin, kaempferol-3-beta-D-glucopyranoside and (+)-nortrachelogenin, antileukemic principles from Wikstroemia indica. J. Nat. Prod. . 1981;44:530–535. doi: 10.1021/np50017a003. [DOI] [PubMed] [Google Scholar]
- 3.Hu K., Kobayashi H., Dong A., Iwasaki S., Yao X. Antifungal, antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica. Planta Med. 2000;66:564–567. doi: 10.1055/s-2000-8601. [DOI] [PubMed] [Google Scholar]
- 4.Nunome S., Ishiyama A., Kobayashi M., Otoguro K., Kiyohara H., Yamada H., Omura S. In vitro antimalarial activity of biflavonoids from Wikstroemia indica. Planta Med. 2004;70:76–78. doi: 10.1055/s-2004-815462. [DOI] [PubMed] [Google Scholar]
- 5.Wang L.Y., Unehara T., Kitanaka S. Anti-inflammatory activity of new guaiane type sesquiterpene from Wikstroemia indica. Chem. Pharm. Bull. 2005;53:137–139. doi: 10.1248/cpb.53.137. [DOI] [PubMed] [Google Scholar]
- 6.Huang W.H., Zhang X.L., Wang Y.F., Ye W.C., Ooi V.E.C., Chung H.Y., Li Y.L. Antiviral biflavonoids from radix Wikstroemiae (Liaogewanggen) Chin. Med. 2010;5:23. doi: 10.1186/1749-8546-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho W.S., Xue J.Y., Sun S.S.M., Ooi V.E.C., Li Y.L. Antiviral activity of daphnoretin isolated from Wikstroemia indica. Phytother. Res. 2010;24:657–661. doi: 10.1002/ptr.2935. [DOI] [PubMed] [Google Scholar]
- 8.Niwa M., Jiang P.F., Hirata Y. Two new C-3/C-3"-biflavanones from Wikstroemia sikokiana. Chem. Pharm. Bull. 1986;34:3631–3634. doi: 10.1248/cpb.34.3631. [DOI] [Google Scholar]
- 9.Chen Y., Fu W.W., Sun L.X., Wang Q., Qi W., Yu H. A new coumarin from Wikstroemia indica (L.) C. A. Mey. Chin. Chem. Lett. . 2009;20:592–594. doi: 10.1016/j.cclet.2009.01.002. [DOI] [Google Scholar]
- 10.Li J., Zhao W., Hu J.L., Cao X., Yang J., Li X.R. A new C-3/C-3"-biflavanone from the roots of Stellera chamaejasme L. Molecules. 2011;16:6465–6469. doi: 10.3390/molecules16086465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro O., Lopez J., Vergara A. Isoflavans and a Stilbene from Wood of the Decay-Resistant Tropical Tree Diphysa robinioides. J. Nat. Prod. 1986;49:680–683. doi: 10.1021/np50046a022. [DOI] [Google Scholar]
- 12.Zeng Y.Q., Recio M.C., Máñez S., Giner R.M., Cerdá-Nicolás M., Ríos J.L. Isolation of two triterpenoids and a biflavanone with anti-inflammatory activity from Schinus molle fruits. Planta Med. 2003;69:893–898. doi: 10.1055/s-2003-45096. [DOI] [PubMed] [Google Scholar]
- 13.Liu W.K., Cheung F.W.K., Liu B.P.L., Li C.M., Ye W.C., Che C.T. Involvement of p21 and FasL in Induction of Cell Cycle Arrest and Apoptosis by Neochamaejasmin A in Human Prostate LNCaP Cancer Cells. J. Nat. Prod. 2008;71:842–846. doi: 10.1021/np8001223. [DOI] [PubMed] [Google Scholar]
- 14.Tian Q.H., Li J., Xie X., Sun M.L., Sang H.R., Zhou C.H., An T.Y., Hu L.H., Ye R.D., Wang M.W. Stereospecific Induction of Nuclear Factor-κB Activation by Isochamaejasmin. Mol. Pharmacol. 2005;68:1534–1542. doi: 10.1124/mol.105.014720. [DOI] [PubMed] [Google Scholar]