Abstract
The increasing emergence especially of drug-resistant tuberculosis has led to a strong demand for new anti-tuberculosis drugs. Eighteen salicylanilide benzoates were evaluated for their inhibition potential against Mycobacterium tuberculosis, Mycobacterium avium and two strains of Mycobacterium kansasii; minimum inhibitory concentration values ranged from 0.5 to 16 μmol/L. The most active esters underwent additional biological assays. Four benzoates inhibited effectively the growth of five multidrug-resistant strains and one extensively drug-resistant strain of M. tuberculosis at low concentrations (0.25–2 μmol/L) regardless of the resistance patterns. The highest rate of multidrug-resistant mycobacteria inhibition expressed 4-chloro-2-[4-(trifluoromethyl)-phenylcarbamoyl]phenyl benzoate (0.25–1 μmol/L). Unfortunately, the most potent esters were still considerably cytotoxic, although mostly less than their parent salicylanilides.
Keywords: antimycobacterial activity, benzoic acid ester, cytotoxicity, in vitro activity, multidrug-resistant tuberculosis, salicylanilide, salicylanilide ester
1. Introduction
Tuberculosis (TB) represents a contagious infectious disease caused by Mycobacterium tuberculosis complex. It is still a harsh global public health problem, partly due to increasing emergence of multidrug-resistant tuberculosis [MDR-TB, which was defined as the infection that is resistant to at least isoniazid (INH) and rifampicin (RIF), the most effective first-line oral agents], and most recently the extensively drug-resistant tuberculosis (XDR-TB). XDR-TB consists in MDR-TB in combination with both resistance to any fluoroquinolone and at least one second-line injectable drug (kanamycin, amikacin, capreomycin). Every year almost 500,000 people are infected with MDR-TB and about 40,000 new XDR-TB cases are appraised annually, with an increasing trend expected in the future. While the standard therapeutic regimen for drug-sensitive TB lasts six months, the treatment of MDR-TB usually takes at least 18 months, and XDR-TB is often untreatable; the coincidence with HIV infection brings other serious problem [1,2]. Therefore the development of novel antimycobacterial agents is still challenging, and new structures with innovative mechanisms of action are especially needed [1,3,4,5]. Moreover, infections caused by nontuberculous (atypical) mycobacteria bring some challenges including those in the area of new drug discovery. Compounds with collateral anti-TB and anti-nontuberculosis mycobacteria activity may bring a satisfactory progress [6].
Salicylanilide (2-hydroxy-N-phenylbenzamide) derivatives may be such a promising group with a complex mechanism of action [7]. Their various esters have exhibited a significant antimycobacterial activity in micromolar or lower concentrations, including MDR-TB and atypical mycobacteria; they do not share any resistance with established antimycobacterial drugs. Esterification of salicylanilides may bring some both pharmacodynamic and pharmacokinetic advantages [7,8,9,10,11,12].
Some substituted esters of benzoic acid (BA) with substituted phenols have displayed antimycobacterial properties against typical and atypical species [13,14]. It was reported that M. tuberculosis is uniquely susceptible to weak acids compared to other mycobacteria. Some ester prodrugs of benzoic acid expressed a significant activity, especially at slightly acidic environment [15]. Recently, four salicylanilide benzoates were reported to block the growth of drug-sensitive M. tuberculosis strain with minimum inhibitory concentrations (MICs) ranging between 0.5–2 µmol/L and, moreover, it was found that they act as mild inhibitors of isocitrate lyase and methionine aminopeptidase, two enzymes essential for the maintenance of mycobacterial infection. These targets are different from those affected by clinically used drugs [16]. Salicylanilide benzoates [2-(phenylcarbamoyl)phenyl benzoates; Table 1] were synthesized and their MICs against eight bacterial and fungal strains were reported [17].
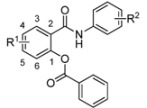
Table 1.
Antimycobacterial activity of salicylanilide benzoates 1.
 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC [μmol/L] | |||||||||||||
| R1 | R2 | M. tuberculosis 331/88 | M. avium 330/88 | M. kansasii 235/80 | M. kansasii 6509/96 | ||||||||
| 14 d | 21 d | 14 d | 21 d | 7 d | 14 d | 21 d | 7 d | 14 d | 21 d | ||||
| 1a | 4-Cl | 3-Cl | 2 | 2 | 4 | 8 | 2 | 4 | 8 | 4 | 4 | 8 | |
| 1b | 5-Cl | 3-Cl | 4 | 4 | 8 | 8 | 2 | 4 | 4 | 4 | 4 | 8 | |
| 1c | 4-Cl | 4-Cl | 4 | 4 | 8 | 16 | 4 | 8 | 8 | 4 | 8 | 8 | |
| 1d | 5-Cl | 4-Cl | 2 | 4 | 4 | 4 | 2 | 2 | 4 | 2 | 2 | 4 | |
| 1e | 4-Cl | 3-Br | 2 | 2 | 8 | 16 | 2 | 4 | 8 | 2 | 4 | 8 | |
| 1f | 5-Cl | 3-Br | 4 | 4 | 4 | 8 | 2 | 4 | 4 | 2 | 4 | 4 | |
| 1g | 4-Cl | 4-Br | 2 | 2 | 4 | 8 | 2 | 4 | 4 | 2 | 4 | 4 | |
| 1h | 5-Cl | 4-Br | 2 | 2 | 4 | 4 | 2 | 4 | 4 | 2 | 4 | 4 | |
| 1i | 4-Cl | 3-F | 4 | 4 | 8 | 16 | 2 | 4 | 8 | 2 | 4 | 4 | |
| 1j | 5-Cl | 3-F | 4 | 8 | 8 | 16 | 2 | 8 | 8 | 2 | 4 | 8 | |
| 1k | 4-Cl | 4-F | 4 | 4 | 8 | 16 | 2 | 8 | 8 | 2 | 4 | 4 | |
| 1l | 5-Cl | 4-F | 8 | 8 | 4 | 8 | 2 | 4 | 8 | 4 | 8 | 8 | |
| 1m | 4-Cl | 3,4-diCl | 1 | 1 | 8 | 8 | 2 | 4 | 4 | 1 | 2 | 2 | |
| 1n | 5-Cl | 3,4-diCl | 2 | 2 | 8 | 8 | 1 | 2 | 4 | 1 | 2 | 4 | |
| 1o | 4-Cl | 4-CF3 | 0.5 | 1 | 4 | 4 | 1 | 1 | 1 | 2 | 2 | 2 | |
| 1p | 5-Cl | 4-CF3 | 2 | 2 | 8 | 8 | 1 | 2 | 4 | 1 | 2 | 4 | |
| 1q | 4-Cl | 3-CF3 | 2 | 2 | 8 | 16 | 2 | 4 | 4 | 2 | 4 | 4 | |
| 1r | 4-Br | 4-CF3 | 1 | 1 | 4 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | |
| INH | - | - | 0.5-1 | 0.5-1 | >250 | >250 | >250 | >250 | >250 | 2 | 4 | 4-8 | |
| PAS | - | - | 62.5 | 62.5 | 32 | 125 | 125 | 1000 | >1000 | 32 | 125 | 500 | |
| BA | - | - | >1000 | >1000 | >1000 | >1000 | 1000 | >1000 | >1000 | 250 | 1000 | 1000 | |
One or two best MIC values for each strain are given in bold; INH: isoniazid; PAS: para-aminosalicylic acid; BA: benzoic acid. MICs of 1m, 1n, 1o and 1r against M. tuberculosis 331/88 were taken from reference [16].
This study brings a complex characteristic of antimycobacterial properties (including against atypical, MDR- and XDR-TB strains) of known salicylanilide benzoates. It is a part of our research effort concerned with a group of salicylanilide derivatives with improved activity and/or reduced toxicity in comparison to their parent molecules.
2. Results and Discussion
2.1. Chemistry
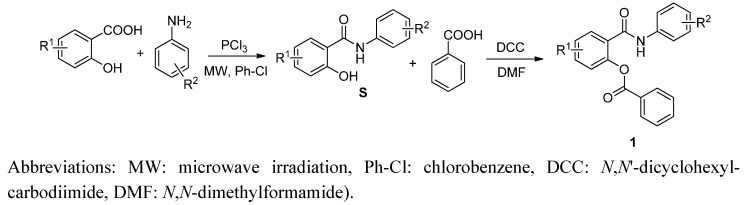
The synthesis and characterization (m.p., IR and NMR spectra, elemental analyses) of salicylanilide benzoates [2-(phenylcarbamoyl)phenyl benzoates; 1] were published recently [17]; their synthetic route is depicted in Scheme 1. Yield of esters 1 ranged from 44 up to 88% [17].
Scheme 1.
Synthesis of salicylanilides S and corresponding benzoates 1 (R1 for esters 1 = 4-Cl, 5-Cl, 4-Br; R2 = 3-Cl, 4-Cl, 3,4-diCl, 3-Br, 4-Br, 3-F, 4-F, 3-CF3, 4-CF3).
2.2. In Vitro Antimycobacterial Evaluation
Eighteen salicylanilide benzoates were evaluated against four mycobacterial strains—one tuberculous and three atypical ones (Mycobacterium avium and two strains of Mycobacterium kansasii). Results are summarized in Table 1.
All tested compounds exhibited a significant activity against drug-sensitive M. tuberculosis at micromolar concentrations (0.5–8 μmol/L) with 1m, 1o and 1r showing superiority (MICs ≤ 1 μmol/L). When we evaluated the isomers on the salicylic ring, 4-chloroderivatives were more beneficial than 5-chloro ones—only with 1c vs.1d being an exception and with no difference in the 1g vs.1h pair. The order of the moieties on the aniline ring is as follows (according to decreased potency): 4-CF3 ≥ 3,4-dichloro > 3-CF3 (limited data) ≈ 4-Br > 3-Cl = 3-Br > 4-Cl > 3-F > 4-F. The benzoylation of salicylanilides provided esters with noticeably improved activity when compared to the parent phenolic molecules S (Table 2) [18]—e.g., even eight-fold for 1m; no ester exhibited inferior activity than its “parent” salicylanilide, and just 1b, 1c, and 1r have identical MIC values.
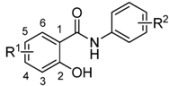
Table 2.
Antimycobacterial activity of parent salicylanilides S [18].
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| MIC [μmol/L] | ||||||||
| R1 | R2 | M. tuberculosis 331/88 | M. avium 330/88 | M. kansasii 235/80 | ||||
| 14 d | 21 d | 14 d | 21 d | 14 d | 21 d | |||
| S-a | 5-Cl | 3-Cl | 4 | 8 | 8 | 16 | 4 | 8 |
| S-b | 4-Cl | 3-Cl | 4 | 4 | 16 | 16 | 4 | 8 |
| S-c | 5-Cl | 4-Cl | 4 | 4 | 8 | 8 | 8 | 8 |
| S-d | 4-Cl | 4-Cl | 4 | 4 | 8 | 8 | 4 | 8 |
| S-e | 5-Cl | 3-Br | NT | NT | NT | NT | NT | NT |
| S-f | 4-Cl | 3-Br | NT | NT | NT | NT | NT | NT |
| S-g | 5-Cl | 4-Br | 8 | 16 | 8 | 8 | 4 | 4 |
| S-h | 4-Cl | 4-Br | 4 | 4 | 16 | 16 | 4 | 4 |
| S-i | 5-Cl | 3-F | 8 | 8 | 31 | 31 | 8 | 8 |
| S-j | 4-Cl | 3-F | 8 | 16 | 32 | 32 | 32 | 32 |
| S-k | 5-Cl | 4-F | 16 | 16 | 16 | 16 | 4 | 4 |
| S-l | 4-Cl | 4-F | 16 | 16 | 32 | 32 | 16 | 32 |
| S-m | 5-Cl | 3,4-diCl | 4 | 8 | 16 | 16 | 4 | 4 |
| S-n | 4-Cl | 3,4-diCl | 4 | 4 | 16 | 16 | 8 | 8 |
| S-o | 5-Cl | 4-CF3 | 2 | 2 | 8 | 8 | 1 | 1 |
| S-p | 4-Cl | 4-CF3 | 4 | 4 | 8 | 8 | 4 | 4 |
| S-q | 5-Cl | 3-CF3 | NT | NT | NT | NT | NT | NT |
| S-r | 4-Br | 4-CF3 | 1 | 1 | 1 | 1 | 2 | 4 |
NT: not tested. MICs of salicylanilides S were taken from reference [18].
M. avium showed the lowest level of susceptibility among the investigated mycobacterial strains (MICs 4–16 μmol/L). Compounds 1d, 1h, 1o and 1r are the most active esters. In general, derivatives substituted in the aniline part by 3-chloro (1a, 1b), 4-bromo (1g, 1h), and 4-trifluoromethyl (1o, 1p, 1r) moieties exhibited better activity; on the other hand, 3-fluoroderivatives (1i, 1j) offered minimal benefit. With two exceptions, molecules derived from 5-chlorosalicylic acid showed a higher or equal activity than their 4-chloro isomers. The introduction of a benzoyl fragment into salicylanilide molecules resulted in an increased activity against M. avium—only three MIC values are higher than those of the parent salicylanilides (Table 2) [18], while others are equal or mostly lower in the case of benzoates, even four times in some cases.
Both clinically isolated and collection strains of M. kansasii were inhibited by salicylanilide benzoates 1 with MICs ≤ 8 μmol/L with clear 1o superiority. 4-CF3, 3-CF3, 3,4-diCl and 4-Br represent the more suitable aniline substitution patterns; no substituent of the aniline part was evaluated as being significantly less beneficial than others. The influence of the halogen position on the salicylic ring is ambiguous. When concentrated on MICs towards the strain 235/80, there is a surprising fact—when 5-chloro-2-hydroxy-N-phenylbenzamides are esterified, the activity against M. kansasii did not change (or was even diminished for 1k vs.S-k), whereas masking of the phenolic group of 4-chloro-2-hydroxy-N-phenylbenzamides resulted mostly in improved in vitro activity (up to four times); only 1h retained the same MIC values. Under our experimental conditions, benzoic acid itself revealed no activity against M. tuberculosis and M. avium and only a very weak inhibition potency for M. kansasii (MICs ≥ 250 μmol/L).
The most active derivatives proved a similar efficacy when compared to INH against drug-sensitive M. tuberculosis, and all derivatives exhibited substantially higher activity against M. avium and M. kansasii 235/80; the activities of INH and benzoates 1 against M. kansasii 6509/96 are almost identical. Second-line oral drug PAS seems to be significantly less active than the newly synthesized derivatives against all tested strains.
Salicylanilide benzoates expressed predominantly lower or equal MIC values in comparison to corresponding acetates [8] and benzenesulfonates [12]. Carbamates possessed a slightly higher in vitro inhibitory activity for M. tuberculosis, whereas MIC levels against atypical strains are approximately similar [9]. Salicylanilide N-acetyl-L-phenylalanine esters demonstrated a superior activity against M. tuberculosis and somewhat worse against M. avium [10]. Benzoates surpassed the antimycobacterial activity of salicylanilides esters with different N-benzyloxycarbonyl α-amino acids [11].
In conclusion, the benzoylation of salicylanilides S led to derivatives with predominantly higher in vitro activity against all four mycobacterial strains. The aim of improving the antimycobacterial potency was successfully achieved. The reason may lay in the increased lipophilicity of synthesized esters, which facilitates passage through biomembranes. With respect to the weak intrinsic activity of benzoic acid against M. kansasii, the possibility of synergistic action of released salicylanilides and benzoic acid could be included, as it has been previously observed.
2.3. In Vitro Activity against Drug-Resistant Tuberculosis Strains
Four esters with the lowest MICs (≤1 μmol/L against any mycobacterial strain) were selected for advanced biological tests. Benzoates 1m, 1n, 1o and 1r were evaluated for their in vitro activity against five MDR-TB strains and one XDR-TB strain (Table 3). All four derivatives exhibited very low MICs (0.25–2 μmol/L). Interestingly, in most cases MDR strains are even more sensitive than drug-sensitive M. tuberculosis. This susceptibility is independent on the resistance patterns indicating no cross-resistance with the conventionally used drugs.
Table 3.
Activity of selected benzoates against multidrug-resistant strains.
| MIC [μmol/L] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. tuberculosis 234/2005 | M. tuberculosis 53/2009 | M. tuberculosis Praha 1 | M. tuberculosis Praha 131 | M. tuberculosis 7357/1998 | M. tuberculosis 9449/2006 | ||||||||
| 14 d | 21 d | 14 d | 21 d | 14 d | 21 d | 14 d | 21 d | 14 d | 21 d | 14 d | 21 d | ||
| 1m | 0.25 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 1 | |
| 1n | 0.25 | 0.5 | 1 | 2 | 1 | 1 | 0.5 | 1 | 0.5 | 1 | 2 | 2 | |
| 1o | 0.25 | 0.25 | 0.5 | 1 | 0.5 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 0.5 | |
| 1r | 0.5 | 0.5 | 1 | 2 | 1 | 1 | 0.5 | 1 | 0.25 | 0.5 | 1 | 1 | |
| INH | 14.6 | 14.6 | 14.6 | 14.6 | 14.6 | 14.6 | 14.6 | 14.6 | 14.6 | 14.6 | 58.3 | 58.3 | |
The best MIC value for each strain is given in bold; INH: isoniazid. MDR-TB strains: 357/2005 and 7357/1998 (both resistant to INH, RIF, rifabutine, streptomycin, ethambutol and ofloxacin); 53/2009 (resistant to INH, RIF, rifabutine, streptomycin, ethambutol); Praha 1 (resistant to INH, RIF, rifabutine, streptomycin, ethambutol and clofazimine) and 9449/2006 (resistant to INH, RIF, rifabutine and streptomycin); XDR-TB strain: Praha 131 (resistant to INH, RIF, rifabutine, streptomycin, ethambutol, ofloxacin, gentamicin and amikacin).
4-Trifluoromethyl derivative 1o was assayed as the most active compound. Based on the pair 1m vs. 1n, the preferable location of the chlorine is the position 4 of the salicylic ring (compound 1m). Compound 1o, the derivative of 5-chlorosalicylic acid, exhibited a better in vitro activity than 1r, which was synthesized from 5-bromosalicylic acid. The salicylanilide benzoates 1m, 1n, 1o, and 1r exhibited a higher activity against drug-resistant strains (expressed as MICs) than salicylanilide esters with N-acetyl-L-phenylalanine [10] and similar or slightly better MIC values when compared to salicylanilide carbamates [9].
2.4. Cytotoxicity Evaluation
Salicylanilides and their esters were referred to share some toxic effects on eukaryotic cells [7,8,9,10,16,19]. Therefore we examined the in vitro cytotoxicity of three most anti-MDR-TB active benzoates (compounds 1m, 1o, 1r) and their parent salicylanilides (S-m, S-o, S-r) in the liver Hep G2 model. The cytotoxicity values, which are expressed as IC50, i.e., concentration which decreases the viability of the cells to 50% from the maximal viability, was taken over from reference [16] (Table 4).
Table 4.
Cytotoxicity and selectivity indexes of selected salicylanilides S and their benzoates 1.
| IC50 [µmol/L] Hep G2 | SI for M. tuberculosis 331/88 | SI for MDR-TB strains | SI for XDR-TB strain | ||||
|---|---|---|---|---|---|---|---|
| 14 d | 21 d | 14 d | 21 d | 14 d | 14 d | ||
| 2m | 2.54 | 2.54 | 2.54 | 5.08–10.16 | 2.54–5.08 | 5.08 | 2.54 |
| 2o | 2.40 | 4.80 | 2.40 | 4.80–9.60 | 2.40–9.60 | 9.60 | 4.80 |
| 2r | 2.34 | 2.34 | 2.34 | 2.34–9.36 | 1.17–4.68 | 4.68 | 2.34 |
| S-m | 0.84 | 0.21 | 0.10 | - | - | - | - |
| S-o | 0.36 | 0.18 | 0.18 | - | - | - | - |
| S-r | 2.71 | 2.71 | 2.71 | - | - | - | - |
IC50 and MIC values of salicylanilides against M. tuberculosis were taken from reference [16]. SI = IC50/MIC100.
The esterification of 5-chloro-N-(3,4-dichlorophenyl)-2-hydroxybenzamide (S-m) and 5-chloro-2-hydroxy-N-[4-(trifluoromethyl)phenyl]benzamide (S-o) by benzoic acid led to the derivatives with significantly decreased toxicity (approximately three and seven times, respectively); contrarily, the benzoylation of S-r has resulted in a slightly higher cytotoxicity (2.71 vs. 2.34 µmol/L).
Based on the comparison of MIC and IC50, it is possible to predict that the antimycobacterial activity of salicylanilide benzoates is not only the result of a general cytotoxic impact, but that they probably should have additional specific effect(s) against M. tuberculosis—e.g., recently reported inhibition of isocitrate lyase and methionine aminopeptidase [16].
However, benzoates still exhibited IC50 values in micromolar range similar to the activities against atypical mycobacteria. Selectivity indexes (SI) for M. tuberculosis ranges from 2.34 to 4.80. The situation for MDR-TB and XDR-TB strains is quite advantageous with SI values of 1.17–10.16; the ratios are more favourable for 1o. Generally, only SI values about the break point of 10 could be considered to be still border sufficient, others are poor.
Although benzoic acid possesses only a very mild cytotoxicity (IC50 of 2,881 µmol/L in our assay), unfortunately the benzoylation did not fill up our expectation about the significant toxicity reduction of parent salicylanilides, in contrast to improved antimycobacterial efficacy. Otherwise, esterification of salicylanilides may be a perspective way to reduce undesired cytotoxicity; it is necessary to search new acids, because benzoic acid brings a certain, but insufficient benefit.
3. Experimental
3.1. Chemistry
Synthesis and characterization of the presented 2-(phenylcarbamoyl)phenyl benzoates 1a–r was published by Krátký et al. [17].
3.2. In Vitro Antimycobacterial Susceptibility Testing
All compounds were tested against Mycobacterium tuberculosis 331/88 (H37Rv) (dilution of the strain was 10−3) and three nontuberculous strains: Mycobacterium avium 330/88 (resistant to INH, RIF, ofloxacin and ethambutol; dilution 10−5) and two strains of Mycobacterium kansasii—235/80 (dilution 10−4) and clinically isolated strain 6509/96 (dilution 10−5). The description of the used method can be found in [12]. The following concentrations of esters were used: 1,000, 500, 250, 125, 62.5, 32, 16, 8, 4, 2, 1, 0.5, 0.25 and 0.125 µmol/L. MIC (µmol/L) is the lowest concentration at which the complete inhibition of mycobacterial growth was occurred. Isoniazid (INH) and structurally similar para-aminosalicylic acid (PAS) were chosen as reference drugs for the comparison. The most active compounds were evaluated in the similar conditions and concentrations against six MDR-TB strains (dilution 10−3) with different resistance patterns: 7357/1998, 234/2005, 53/2009, 9449/2006, Praha 1 and Praha 131 (XDR-TB strain). All these strains were resistant to INH, rifamycines, and streptomycin; in some cases additional resistance was present.
4. Conclusions
In summary, salicylanilide benzoates revealed a significant antimycobacterial activity; their mechanism of action is still not fully elucidated and seems to be multiple. The masking of salicylanilide phenolic group by lipophilic aromatic acid resulted in the derivatives with improved antimycobacterial potency in the micromolar range (0.25–16 μmol/L). Additionally, the most active esters stopped the growth of MDR-TB strains with MIC values from 0.25 μmol/L. Salicylanilide benzoates represent a group with a promising in vitro antimycobacterial activity. Nevertheless, the expectancy of the reduced cytotoxicity was accomplished only partly—two esters of three tested ones exhibited a significantly lower toxicity when compared to parent salicylanilides, but these molecules are unfortunately still relatively toxic. Thus, the next search for new highly active and less cytotoxic derivatives still remains a topic of interest.
Acknowledgments
This work was financially supported by GAUK 27610/2010 and IGA NT 13346 (2012). This publication is a result of the project implementation: “Support of establishment, development, and mobility of quality research teams at the Charles University”, project number CZ.1.07/2.3.00/30.0022, supported by The Education for Competitiveness Operational Programme (ECOP) and co-financed by the European Social Fund and the state budget of the Czech Republic. We thank J. Urbanová, M.A., for language assistance.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds S-a–S-r and 1a-r are available from the authors.
References
- 1.Krátký M., Vinšová J. Advances in the Development of Antituberculotics Acting on Multidrug-Resistant Strains. Chem. Listy. 2010;104:998–1005. [Google Scholar]
- 2.Sturdy A., Goodman A., Jose R.J., Loyse A., O’Donoghue M., Kon O.M., Dedicoat M.J., Harrison T.S., John L., Lipman M., et al. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: A study of injectable use and toxicity in practice. J. Antimicrob. Chemother. 2011;66:1815–1820. doi: 10.1093/jac/dkr221. [DOI] [PubMed] [Google Scholar]
- 3.La Rosa V., Poce G., Canseco J.O., Buroni S., Pasca M.R., Biava M., Raju R.M., Porretta G.C., Alfonso S., Battilocchio C., et al. MmpL3 Is the Cellular Target of the Antitubercular Pyrrole Derivative BM212. Antimicrob. Agents Chemother. 2012;56:324–331. doi: 10.1128/AAC.05270-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grzegorzewicz A.E., Pham H., Gundi V.A.K.B., Scherman M.S., North E.J., Hess T., Jones V., Gruppo V., Born S.E.M., Kordulakova J., et al. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat. Chem. Biol. 2012;8:334–341. doi: 10.1038/nchembio.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batt S.M., Jabeen T., Bhowruth V., Quill L., Lund P.A., Eggeling L., Alderwick L.J., Futterer K., Besra G.S. Structural basis of inhibition of Mycobacterium tuberculosis DprE1 by benzothiazinone inhibitors. Proc. Natl. Acad. Sci. USA. 2012;109:11354–11359. doi: 10.1073/pnas.1205735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook J.L. Nontuberculous mycobacteria: Opportunistic environmental pathogens for predisposed hosts. Br. Med. Bull. 2010;96:45–59. doi: 10.1093/bmb/ldq035. [DOI] [PubMed] [Google Scholar]
- 7.Krátký M., Vinšová J. Salicylanilide Ester Prodrugs as Potential Antimicrobial Agents—A Review. Curr. Pharm. Des. 2011;17:3494–3505. doi: 10.2174/138161211798194521. [DOI] [PubMed] [Google Scholar]
- 8.Vinsova J., Imramovsky A., Buchta V., Ceckova M., Dolezal M., Staud F., Jampilek J., Kaustova J. Salicylanilide Acetates: Synthesis and Antibacterial Evaluation. Molecules. 2007;12:1–12. doi: 10.3390/12010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Férriz J.M., Vávrová K., Kunc F., Imramovský A., Stolaříková J., Vavříková E., Vinšová J. Salicylanilide carbamates: Antitubercular agents active against multidrug-resistant Mycobacterium tuberculosis strains. Bioorg. Med. Chem. 2010;18:1054–1061. doi: 10.1016/j.bmc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 10.Krátký M., Vinšová J., Buchta V., Horvati K., Bösze S., Stolaříková J. New amino acid esters ofsalicylanilides active against MDR-TB and other microbes. Eur. J. Med. Chem. 2010;45:6106–6113. doi: 10.1016/j.ejmech.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 11.Imramovský A., Vinšová J., Férriz J.M., Doležal R., Jampílek J., Kaustová J., Kunc F. New antituberculotics originated from salicylanilides with promising in vitro activity against atypical mycobacterial strains. Bioorg. Med. Chem. 2009;17:3572–3579. doi: 10.1016/j.bmc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Krátký M., Vinšová J., Rodriguez N.G., Stolaříková J. Antimycobacterial Activity of Salicylanilide Benzenesulfonates. Molecules. 2012;17:492–503. doi: 10.3390/molecules17010492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew R., Kruthiventi A.K., Prasad J.V., Kumar S.P., Srinu G., Chatterji D. Inhibition of Mycobacterial Growth by Plumbagin Derivatives. Chem. Biol. Drug Des. 2010;76:34–42. doi: 10.1111/j.1747-0285.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 14.Muddassar M., Jang J.W., Gon H.S., Cho Y.S., Kim E.E., Keum K.C., Oh T., Cho S.N., Pae A.N. Identification of novel antitubercular compounds through hybrid virtual screening approach. Bioorg. Med. Chem. 2010;18:6914–6921. doi: 10.1016/j.bmc.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Gu P., Constantino L., Zhang Y. Enhancement of the antituberculosis activity of weak acids by inhibitors of energy metabolism but not by anaerobiosis suggests that weak acids act differently from the front-line tuberculosis drug pyrazinamide. J. Med. Microbiol. 2008;57:1129–1134. doi: 10.1099/jmm.0.2008/000786-0. [DOI] [PubMed] [Google Scholar]
- 16.Krátký M., Vinšová J., Novotná E., Mandíková J., Wsól V., Trejtnar F., Ulmann V., Stolaříková J., Fernandes S., Bhat S., Liu J.O. Salicylanilide derivatives block Mycobacterium tuberculosis through inhibition of isocitrate lyase and methionine aminopeptidase. Tuberculosis. 2012;92:434–439. doi: 10.1016/j.tube.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Krátký M., Vinšová J., Buchta V. In Vitro Antibacterial and Antifungal Activity of Salicylanilide Benzoates. ScientificWorldJournal. 2012;2012:290628. doi: 10.1100/2012/290628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waisser K., Bureš O., Holý P., Kuneš J., Oswald R., Jirásková L., Pour M., Klimešová V., Kubicová L., Kaustová J. Relationship between the Structure and Antimycobacterial Activity of Substituted Salicylanilides. Arch. Pharm. Pharm. Med. Chem. 2003;336:53–71. doi: 10.1002/ardp.200390004. [DOI] [PubMed] [Google Scholar]
- 19.Hilliard J.J., Goldschmidt R.M., Licata L., Baum E.Z., Bush K. Multiple Mechanisms of Action for Inhibitors of Histidine Protein Kinases from Bacterial Two-Component Systems. Antimicrob. Agents Chemother. 1999;43:1693–1699. doi: 10.1128/aac.43.7.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]