Abstract
A randomized complete block design 2 × 4 experiment was designed and conducted for 15 weeks to characterize the relationships between production of total phenolics, flavonoid, anthocyanin, leaf gas exchange, total chlorophyll, phenylalanine ammonia-lyase (PAL) and malondialdehyde (MDA) activity in two varieties of Labisia pumila Benth, namely the var. alata and pumila, under four levels of evapotranspiration replacement (ER) (100%; well watered), (75%, moderate water stress), (50%; high water stress) and (25%; severe water stress). The production of total phenolics, flavonoids, anthocyanin, soluble sugar and relative leaf water content was affected by the interaction between varieties and SWC. As the ER levels decreased from 100% to 25%, the production of PAL and MDA activity increased steadily. At the highest (100%) ER L. pumila exhibited significantly higher net photosynthesis, apparent quantum yield, maximum efficiency of photosystem II (fv/fm) and lower dark respiration rates compared to the other treatment. The production of total phenolics, flavonoids and anthocyanin was also found to be higher under high water stress (50% ER replacement) compared to severe water stress (25% ER). From this study, it was observed that as net photosynthesis, apparent quantum yield and chlorophyll content were downregulated under high water stress the production of total phenolics, flavonoids and anthocyanin were upregulated implying that the imposition of high water stress can enhance the medicinal properties of L. pumila Benth.
Keywords: photosynthetic capacity, evapotranspiration replacements, total phenolics and flavonoids, anthocyanins, soluble sugar, lipid peroxidation activity
1. Introduction
Recently, there has been an increased interest in understanding the mechanism of plant acclimation to environmental stresses [1,2,3]. Plants respond to an adverse ecosystem by altering their morphology, physiology, and biochemistry [2]. Some of the adaptations to stress may include the changes in both the nature and levels of primary and secondary metabolites [4,5]. Recent advances in plant sciences have led to great interest in increasing the production of plant secondary metabolites for their medicinal and aromatic uses [6]. A better understanding of the environmental influences on the regulation of plant secondary metabolism is advantageous for the cultivation of medicinal plants such as Labisia pumila Benth [7].
Labisia pumila Benth., popularly known as kacip fatimah, is a sub-herbaceous plant with creeping stems from the family Myrsinaceae that is found widespread in Indochina and throughout the Malaysian forest [8]. Traditionally L. pumila has been used by Malay women to induce and facilitate childbirth as well as a post-partum medicine. The other uses of this herb are in treatments for dysentery, dysmenorrhea, flatulence, and gonorrhea [9]. This herb has received a lot of attention among scientists, herbalists and the pharmacy industry in Malaysia due to its therapeutic effects and high total contents of phenolic and flavonoid compounds. These polyphenolic compounds have received considerable interest because of their protective role against cancer and heart disease, attributed to their antioxidative activity against reactive oxygen species, which was reported to be higher than that of vitamins C and E [10].
Numerous studies have shown that environmental stress can enhance the production of several secondary metabolites in plants [11,12]. Hence, the production of high levels of secondary metabolites can be induced in plants by manipulating certain specific environmental stress conditions [13]. For instance, the accumulation of plant secondary metabolites can be induced with exposure to nutrient deficiency [14], UV [15], light intensity [16], CO2 [17] and temperature [18].
Water stress is one of the most important environmental stresses that can depress growth and alter the biochemical properties of plants [19]. According to Franz [20], Palevitch [21] and Marchese and Figueira [22] one of the most important factors affecting secondary metabolism is soil water capacity. Usually, limited availability of water has a negative effect on plant growth and development. However, an non-severe water deficit has sometimes proven beneficial for the accumulation of biologically-active compounds in medicinal and aromatic plants [21]. Under high water stress, there is a limit on the translocation of carbon to its sinks, with the remaining carbon accumulates as carbohydrates, which leads to an increase in the carbon pool that would be allocated for secondary metabolism, with little or no competition with growth and development [23]. Ghershenzon [4] demonstrated that, in herbaceous plants and shrubs, terpenes tend to increase under stress, mainly under severe water deficit conditions. This type of stress is known to increase the amount of secondary metabolites in a variety of medicinal plants, e.g., artemisinin in Artemisia annua L. [24], ajmalicine in Catharanthus roseus [25] and hyperforin in Hypericum perforatum [19].
There are numerous reports on the biochemical, primary, physiological and morphological responses of L. pumila to environmental stress [26,27,28], however fewer studies have been done on microenvironmental manipulation, especially the effects of soil water capacity on the enhancement of secondary metabolite production in L. pumila. The effects of soil water capacity on L. pumila is an important aspect for the establishment of this plant in field cultivation as recently, this herb has mostly been harvested directly from the forest. Domestication of this herb in greenhouses might provide a consistent supply of raw material and protect the plant from potential extinction due to overharvesting [29]. The study of the impact of water availability on the plant secondary metabolites of L. pumila might realize this possibility. The objective of the current study was thus to examine the effects of different soil water capacity levels on production of secondary metabolites (total phenolics, flavonoids and anthocyanin), soluble sugar, total chlorophyll content, leaf gas exchange, phenyll alanine lyase, (PAL) and maliondialdehyde (MDA) activity in two varieties of L. pumila (var. alata and var. pumila) under greenhouse conditions and the determination of the relationships between these parameters.
2. Results and Discussion
2.1. Total Phenolics and Flavonoids
The interaction effect between ER and varieties had a significant (p ≤ 0.05) impact on the production of total phenolics and flavonoids in L. pumila (Table 1). As the plant received less water (100% to 25% ER) the production of total phenolics and flavonoids was enhanced.
Table 1.
Interaction effects between varieties and evapotranspiration replacement on leaf total phenolics, flavonoids and anthocyanin production.
| Varieties | Evapotranspiration | Total Phenolics | Total Flavonoids | Anthocyanin |
|---|---|---|---|---|
| replacement | (mg gallic acid/g dry weight) | (mg rutin/g dry weight) | (mg petunidin/ g fresh weight) | |
| Alata | 100% | 0.81 ± 0.01 d | 0.52 ± 0.01 c | 0.72 ± 0.01 d |
| 75% | 1.23 ± 0.12 c | 0.67 ± 0.03 b | 0.81 ± 0.02 c | |
| 50% | 1.75 ± 0.02 b | 0.75 ± 0.13 a | 0.92 ± 0.01 a | |
| 25% | 1.72 ± 0.14 b | 0.64 ± 0.12 b | 0.90 ± 0.04 a | |
| Pumila | 100% | 0.79 ± 0.13 d | 0.57 ± 0.15 c | 0.69 ± 0.01 d |
| 75% | 1.27 ± 0.03 c | 0.69 ± 0.16 b | 0.79 ± 0.03 c | |
| 50% | 1.89 ± 0.03 a | 0.75 ± 0.11 a | 0.97 ± 0.05 a | |
| 25% | 1.83 ± 0.07 a | 0.71 ± 0.06 a | 0.88 ± 0.04 b |
All analyses are mean ± standard error of mean (SEM); N = 30 Means not sharing a common letter within a column were significantly different at p ≤ 0.05.
The increase in the production of plant secondary metabolites was found to be statistically higher in var. pumila than var. alata. The enhancement of total phenolics and flavonoids was optimized when L. pumila was exposed to high water stress (50% ER) compared to severe water stress (25% ER) and the control. The present result indicated the production of gallic acid and rutin can be increased with imposition of high water stress (50% ER replacement) compared to severe water stress (25% ER replacement) to L. pumila, thus increasing the plant’s medicinal properties. The enhancement of plant medicinal properties under water deficit has been observed by Fortier et al. [30] and Azhar et al. [31] in Oreochromis niloticus and Trachyspermum ammi, respectively. They reached a similar conclusion as in the present study, whereby they observed the imposition of high water stress had enhanced production of plant secondary metabolites compared to the imposition of severe water stress. Further, Ibrahim et al. [32] attributed the increase in total plant flavonoids and phenolics under high water stress to accumulation of soluble carbohydrates in plants as a result of reduced transportation of soluble sugar under water limitation. A positive significant relationship established between soluble carbohydrate and plant secondary metabolites (Total phenolics; R2 = 0.976; Total flavonoids; R2 = 0.986; p ≤ 0.05) justified these findings (Table 2).
The increase in total production of total phenolics and flavonoids under low soil field water capacity in the current study might be due to an increase in activity of phenylalanine ammonia-lyase (PAL) under low soil field water capacity. Oh et al. [33] found that the increase in phenolics and flavonoids compounds in lettuce was due to enhancement of PAL activity. From the correlations in Table 2, it was shown that total phenolics (R2 = 0.932; p ≤ 0.01) and flavonoids (R2 = 0.876; p ≤ 0.01) had a significant positive correlation with PAL activity, indicating the increase in production of total phenolics and flavonoids under low soil water capacity might be triggered by high PAL activity. Activation of phenylpropanoid pathways may be dependent on both the type and degree of soil water capacity and the genotype of Labisia pumila. According to Harrison and Were [34] the increase in production of secondary metabolites under low soil water capacity might be due to enhanced degradation of larger phenolics compounds into smaller ones as water deficit is enhanced, thus increasing the medicinal properties of the plants.
2.2. Anthocyanins
Anthocyanins are probably the largest group of phenolic compounds in the human diet, and their strong antioxidant activities suggest their importance in maintaining health. Anthocyanins are also important as antioxidants, which have roles in promoting good health and reducing the risk of chronic disease and also as anti-inflammatory agents. In the present study, anthocyanin content was found to be influenced by the interaction effects between field capacity and varieties (p ≤ 0.01; Table 1). The accumulation of anthocyanin exhibited similar patterns with total phenolics and flavonoids. In the leaves, the imposition of water stress at 50% SWC replacement had increased anthocyanin production to a maximum in both var. pumila (0.97 mg/g fresh weight) and var. alata (0.92 mg/g fresh weight); whilst the lowest values were recorded under non stress conditions (100% ER) at 0.69 mg/g fresh weight and 0.72 mg/g fresh weight, respectively. The same patterns that were observed in total phenolics and flavonoid accumulation suggest the enhancement of secondary metabolites production in L. pumila under high water stress (50% ER) conditions. Acute enhancement of anthocyanin content under high water stress condition was also observed by Pallioti et al. [35], where they observed the anthocyanin content was statistically 25% higher in grape fruit that were under high water stress compared to the plant under normal irrigation.
Table 2.
Correlations among the measured parameters in the experiments.
| Parameters | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.Total phenolics | 1.000 | |||||||||||
| 2.Total flavonoids | 0.987 * | 1.000 | ||||||||||
| 3.Anthocyanin | 0.876 * | 0.888 * | 1.000 | |||||||||
| 4.Photosynthesis | –0.879 * | –0.855 * | –0.871 * | 1.000 | ||||||||
| 5.AQY | –0.876 * | –0.901 * | –0.899 * | 0.978 * | 1.000 | |||||||
| 6.Dark respiration | 0.934 * | 0.876 * | 0.789 * | 0.234 | 0.113 | 1.000 | ||||||
| 7.fv/fm | –0.874 * | –0.786 * | –0.699 * | 0.766 * | 0.433 | –0.445 | 1.000 | |||||
| 8.RWC | –0.854 * | –0.921 * | –0.865 * | 0.897 * | 0.223 | 0.778 * | 0.899 * | 1.000 | ||||
| 9.Carbohydrate | 0.976 * | 0.986 * | 0.765 * | –0.876 * | 0.677 * | –0.765 * | –0.556 * | –0.876 * | 1.000 | |||
| 10.Chlorophyll | –0.766 * | –0.786 * | –0.865 * | -0.987 * | 0.332 | 0.344 | 0.776 * | –0.444 | –0.978 * | 1.000 | ||
| 11.PAL | 0.932 * | 0.876 * | 0.812 * | –0.781 * | 0.021 | 0.021 | 0.034 | 0.002 | 0.788 * | 0.234 | 1.000 | |
| 12.MDA | 0.911 * | 0.885 * | 0.934 * | –0.886 * | 0.032 | 0.043 | 0.001 * | 0.012 | 0.876 * | –0.002 | 0.987 * | 1.000 |
* significant at p ≤ 0.05 or p≤ 0.01; AQY = Apparent quantum yield; fv/fm = maximum efficiency of photosystem II; RWC = relative water content; PAL = phenylalanine ammonia lyase activity; MDA = maliondiadehyde.
Table 3.
Net photosynthesis, Apparent quantum yield, dark respiration and maximum efficiency of photosystem ii (fv/fm) under different evapotranspiration replacement status.
| Evapotranspiration | Net Photosynthesis | Apparent quantum yield | Dark respiration rate (Rd) | Maximum efficiency of photosystem II |
|---|---|---|---|---|
| replacement | (A) | (ɸ) | (µmol/m2/s) | (fv/fm) |
| (µmol/m2/s) | (µmol/m2/s) | |||
| 100% | 10.75 ± 0.01 a | 0.08 ± 0.01 a | 4.61 ± 0.21 d | 0.84 ± 0.01 a |
| 75% | 7.21 ± 0.22 b | 0.06 ± 0.02 b | 12.71 ± 0.23 c | 0.81 ± 0.02 b |
| 50% | 3.41 ± 0.12 c | 0.04 ± 0.07 c | 14.75 ± 0.13 b | 0.72 ± 0.06 c |
| 25% | 1.72 ± 0.13 d | 0.01 ± 0.04 d | 15.64 ± 0.14 a | 0.66 ± 0.04 d |
All analyses are mean ± standard error of mean (SEM); N = 60 Means not sharing a common letter within a column are significantly different at p ≤ 0.05.
Anthocyanins are the naturally occurring phenolic compounds responsible for the color of many flowers, fruits, and berries [36]. It is the most important group of water soluble pigments in plants and they have beneficial health effects as antioxidant and anti-inflammatory agents [37]. Tamura and Yamagami [38] reported that anthocyanins possess some positive therapeutic value, mainly associated with their antioxidant activities. Improvement in anthocyanin content in the current study with increasing water stress (50% ER) condition suggest a possible way to increase the quality of L. pumila by water stressing the plant.
2.3. Leaf gas Exchange Properties
In this study, the net photosynthetic rate (A), apparent quantum yield (ɸ) and dark respiration rate (Rd) were determined by a portable infrared photosynthesis system LI-6400 (LI-COR, Lincoln, NE, USA) and Portable Plant Efficient Analyzer (Handy PEA; USA) for maximum efficiency of photosystem II (fv/fm). In general, the photosynthesis rate (A) was found to be higher in 100% ER (10.75 µmol/m2/s), followed by 75% ER (7.21 µmol/m2/s), 50% ER (3.41 µmol/m2/s) and lowest in 25% ER that just recorded 1.21 µmol/m2/s (Table 3). Similar pattern of decreasing values with increasing water stress was also observed with apparent quantum yield (ɸ). The latter (ɸ) was also found to have a significant positive correlation (Table 2) with net photosynthesis (R2 = 0.892; p ≤ 0.01), indicating the reduction in photosynthesis rate under high water stress might be followed by the reduction in ɸ that indicates that high water stress might reduce the efficiency of photosynthesis by reducing the efficiency of light harvesting of L. pumila [29]. However, it was found that as evapotranspiration replacement decreased from 100% to 75% > 50% and 25% the dark respiration rate increased significantly. For instance, as the ER reduced to 75% > 50% and 25% the dark respiration (Rd) rate enhanced by 175%, 220% and 240% respectively compared to plant under non stress (100% ER). From the correlation analysis (Table 2) was demonstrated that net photosynthesis had a significant negative relationship with total phenolics (R2 = −0.876; p ≤ 0.05), total flavonoids (R2 = −0.855; p ≤ 0.05) and anthocyanins content (R2 = −0.871; p ≤ 0.05) suggesting that the up-regulation of the shikimic acid pathway involved in production of secondary metabolites was under down-regulated photosynthesis [28]. The synthesis of basic skeleton for secondary metabolites is dependent on the carbon assimilated during photosynthesis [39]. In the current study, under low water field capacity the net photosynthesis decreased significantly. Under this condition photosynthesis was limited, probably due to low CO2 availability as a result of reduced stomatal and mesophyll conductance. These limited CO2 assimilation of the leaf tissues may result in increased allocation of photoassimilates to the production of secondary metabolites. The increase in production of secondary metabolites under low photosynthesis might be due to increased shikimic acid pathway activity under stressed conditions, especially under low water field capacity and low photosynthesis. The increase in production of secondary metabolites under low photosynthetic activity was also observed by Moghadam et al. [40] in Centalia asiatica. Furthermore, a significant positive correlation of dark respiration rate with total phenolics (R2 = 0.934; p ≤ 0.05), flavonoids (R2 = 0.876; p ≤ 0.05) and anthocyanins (R2 = 0.789; p ≤ 0.05) was observed; which indicates induction of high respiration and low photosynthetic rate can induce secondary metabolites synthesis in plants [29]. The reduction in maximum quantum yield (fv/fm) with increasing production of secondary metabolites as water field capacity was being reduced again demonstrated the possible production of L. pumila plant secondary metabolites under increasing plant water stress.
2.4. Relative Leaf Water Content
Relative water content was influenced by the interaction effect between variety and ER (p ≤ 0.05; Table 3). In both variety var. alata and var. pumila as field water capacity was reduced from 100% to 25% the relative water content decreased significantly. The reduction in relative water content in var. pumila was from 70.83%–94.79% and for var. alata was from 72.72%–89.31%. From the correlations in Table 2, it was shown that relative water content had a significant negative correlation with total phenolics (R2 = −0.854; p ≤ 0.05), flavonoids (R2 = −0.921; p ≤ 0.05) and anthocyanins content (R2 = −0.865; p ≤ 0.05). This data indicate the increased production of plant secondary metabolites under low relative water content in L. pumila. A similar obervation to that of the present study was also reported by Schreiner et al. [41], whereby they observed enhancement of 2-propenyl- and 3-indolylmethyl glucosinolate concentration in Brassica carinata when the relative water content was maintained below 80%. The significant negative correlation of relative water content with plant secondary metabolites was also observed by Xiao et al. [42] and Szabo et al. [43] in Populus cathogan and Papaver somniferum, respectively.
Table 4.
Interaction effects between varieties and soil water capacity replacement on relative water content and sucrose content.
| Varieties | Evapotranspiration | Relative leaf water | Sucrose content |
|---|---|---|---|
| replacement | content (%) | mg/g dry weight | |
| Alata | 100% | 89.31 ± 1.01 b | 27.52 ± 3.01 d |
| 75% | 87.32 ± 0.12 b | 32.41 ± 0.93 c | |
| 50% | 79.21 ± 0.32 c | 64.31 ± 0.83 b | |
| 25% | 72.72 ± 0.74 d | 63.64 ± 1.12 b | |
| Pumila | 100% | 94.79 ± 0.93 a | 27.57 ± 3.15 d |
| 75% | 85.27 ± 0.54 b | 35.69 ± 5.16 c | |
| 50% | 77.89 ± 1.02 c | 67.75 ± 2.11 a | |
| 25% | 70.83 ± 2.07 d | 66.32 ± 0.07 a |
All analyses are mean ± standard error of mean (SEM); N = 30 Means not sharing a common letter are significantly different at p ≤ 0.05.
2.5. Soluble Sugar (Sucrose) Content
The accumulation of soluble carbohydrates in L. pumila was influenced by the interaction between variety and ER status and followed a descending order of 50% soil water capacity >25% field capacity >75% field capacity >100% field capacity in both variety var. alata and var. pumila (Table 4). In the experiments, the soluble sugar contents at var. alata-100% ER, var. alata −75% ER, var. alata-50% ER, var. alata −25% ER, var. pumila-100% ER, var. pumila −75% ER and var. pumila −25% ER were 27.57, 32.41, 64.31, 63.64, 27.57, 35.69 and 66.32 mg sucrose/g dry weight, respectively, compared to 67.75 mg sucrose/g dry weight at var. pumila −50% ER. The present results suggest that at low field water capacity, especially at 50% ER, the production of soluble sugar was enhanced. Jaafar [44] regarded the accumulation of soluble carbohydrate as due to a reduction in soluble sugar transportation under water stress. Meanwhile, Ghasemzadeh et al. [45] described the accumulation of carbohydrates as a signal of an increase in production of secondary metabolites that enhances the medicinal quality of plants. The present finding was in agreement with Guo et al. [46] who found an increase in sucrose content corresponded with the enhanced production of ascorbic acid, glucosinolates, sulforaphane, anthocyanins, total phenolics and increased antioxidative activities in broccoli sprouts. The positive correlation between carbohydrate content and antioxidative properties in plants were also reported by other researchers [47,48]. The current results indicate the exposure of L. pumila to high water stress can enhance the health promoting effects of this plant with the increase in total phenolics, flavonoids and anthocyanin contents.
2.6. Total Chlorophyll Content
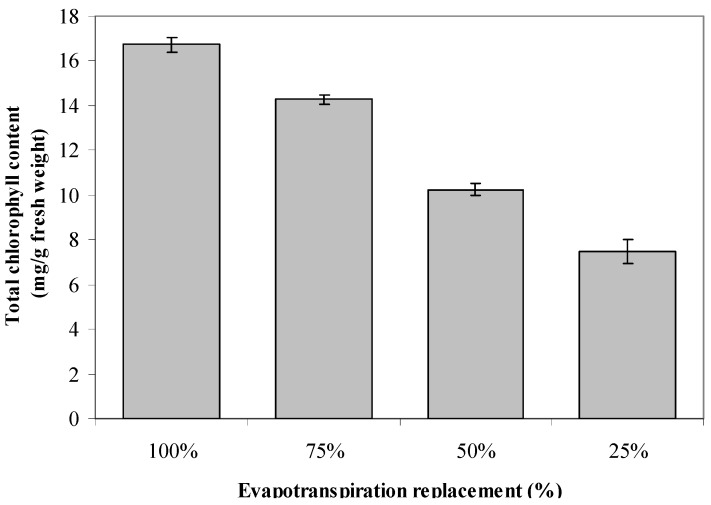
The production of total chlorophyll content was dramatically influenced by the SWC (p ≤ 0.01; Figure 1). As the levels of water stress became serious (going from 100% to 25%) with replacement of evapotranspiration, the production of total chlorophyll content was found to decrease. The increase in chlorophyll production with increasing water stress has been observed by Jaafar [44] in bell paper plants. It was found from the correlation analysis (Table 2) that total chlorophyll was significantly (p ≤ 0.01) and negatively related to the production of secondary metabolites. The negative relationship between chlorophyll content and secondary metabolite production fits well with the protein competition model (PCM) proposed by Jones and Hartley [49], whereby the secondary metabolites content is controlled by the competition between protein and secondary metabolites biosynthesis pathways and the metabolites regulation. The negative relationship between the secondary metabolites and chlorophyll is a sign of gradual switch-off of investment from protein to polyphenols production [50]. The present study indicated that when production of total chlorophyll was down-regulated under water stress condition it may be the signal for increased production of total phenolics, flavonoids and anthocyanins in L. pumila.
Figure 1.
The effects of different evapotranspiration replacement on chlorophyll content of L. pumila. N = 60. Bars represent standard error of differences between means (SEM).
2.7. Phenylalanine Ammonia-Lyase Activity
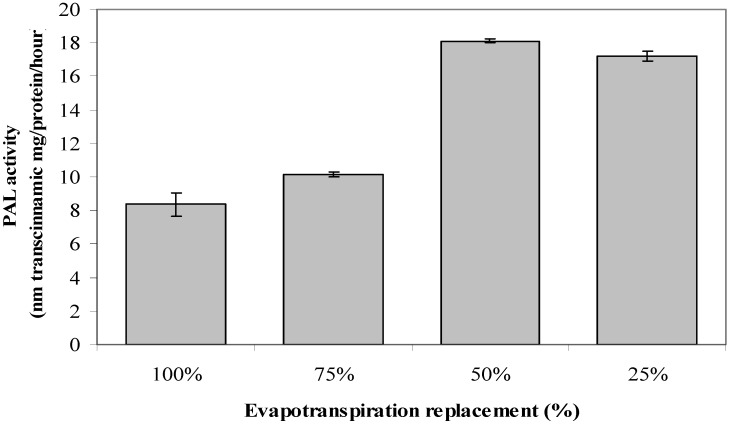
The PAL activity in L. pumila was influenced by ER (p ≥ 0.05; Figure 2). No varietal effects or any interaction with field capacity was observed. PAL activity was found to be the highest (18.11 nM trans-cinnamic mg/protein/h) when at 50% ER, and the lowest activity was demonstrated at 100% ER which registered a value of 8.35 nM trans-cinnamic mg/protein/h. The increase in the production of secondary metabolites in the present work could be related to the increase in PAL activities under low water field capacity replacement. Correlation analysis showed that PAL had established a significant (p ≤ 0.05) positive relationship with total phenolics (R2 = 0.932; p ≤ 0.05), flavonoids (R2 = 0.876; p ≤ 0.05) and anthocyanin (R2 = 0.812; p ≤ 0.05), suggesting an up-regulation of plant secondary metabolite production with increased PAL activity. This is basically due to the fact that PAL is an enzyme, which synthesizes a precursor for total phenolics and flavonoids biosynthesis. The high water stress in the present study might have increased the availability of the phenylalanine (Phe) pool as less protein was used for plant maintenance under high water stress hence, more Phe is available for the production of secondary metabolites [51,52]. These results suggested that an up-regulation of plant secondary metabolites production in L. pumila under high water stress might be due to increased in PAL activity due in turn to increased availability of Phe under stress conditions.
Figure 2.
The effects of different evapotranspiration replacement on PAL activity of L.pumila. N = 60. Bars represent standard error of differences between means (SEM).
2.8. Lipid Peroxidation Activity
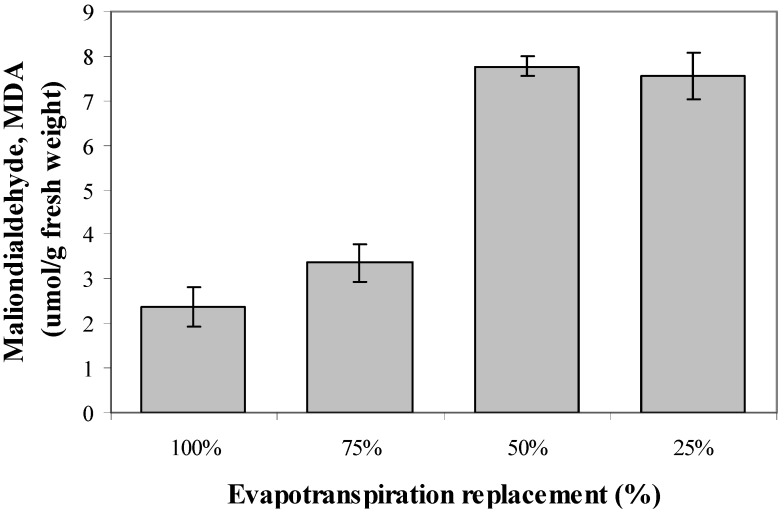
Malondialdehyde (MDA) is a highly reactive three carbon dialdehyde produced as a byproduct of polyunsaturated fatty acid peroxidation and arachidonic acid metabolism. The production of MDA was influenced by SWC imposed onto L. pumila seedlings (p ≤ 0.01; Figure 3). It was observed that as the levels of water stress was enhanced from 100% to 25% water field capacity replacement the production of MDA was also increased. This suggests that as water stress levels increased, the oxidative stress in L. pumila cells and tissues were enhanced, thus implying occurrence of lipid peroxidation in L. pumila under high water stress. From the correlations in Table 2, the MDA production has established significant positive correlations with total phenolics (R2 = 0.911; p ≤ 0.05) and total flavonoids (R2 = 0.885; p ≤ 0.05), indicating that an increase in MDA might be involved in the up-regulation of the secondary metabolite production under high water stress in L. pumila. The formation of MDA was considered as a measure of lipid peroxidation that was induced by a high water stress level [49]. MDA, a decomposition product of polyunsaturated fatty acid hydroperoxides, has been utilized very often as a suitable biomarker for oxidative stress. Usually the increase in lipid peroxidation was simultaneously accompanied by an increase in hydrogen peroxide levels, but in the present study hydrogen peroxide content was not measured. Hydrogen peroxide may function as a signal for the induction of plant defence systems and this could enhance secondary metabolite production [53]. The result suggests that the oxidative stress is a pre-requisite for secondary metabolite synthesis in L. pumila under high water stress.
Figure 3.
The effects of different evapotranspiration replacement on lipid peroxidation activity of L.pumila. N = 60. Bars represent standard error of differences between means (SEM).
3. Experimental
3.1. Plant Material and Maintenance
The experiments were carried out in glasshouse at Field 10, University Agriculture Park, Faculty of Agriculture Glasshouse Complex, Universiti Putra Malaysia (longitude 101° 44' N and latitude 2° 58' S, 68 m above sea level) with a mean atmospheric pressure of 1.013 kPa. Three-month old L. pumila seedlings were left for a month to acclimatize in a nursery until ready for the treatments. When the seedlings had reached 4 months of age they were fertilized with NPK blue at 15 g per plant. The seedlings were planted in soilless medium containing coco-peat, burnt paddy husk and well composted chicken manure in 5:5:1 (v/v) ratio in 25 cm diameter polyethylene bags. Day and night temperatures in the greenhouse were maintained at 27–30 °C and 18–21 °C, respectively, and relative humidity from 50 to 60%. The experiment was based on a factorial Randomized Complete Block Design (RCBD) with three replicates. The first factor was two varieties of L. pumila (var. alata and var. pumila) and the second factor was water stress treatment based on evapotranspiration replacement (ER). There were four levels of evapotranspiration replacement, i.e., (100% ER; well watered), (75% ER; moderate water stress), (50% ER; high water stress treatment) and (25% ER; severe water stress) [54]. All polybags initially receives equal volumes of water to maintain them to near to the predetermined polybag capacity (0.6 litre per 2 L of media) and moisture lost by ER was replaced on alternate days (100%, 75%, 50% and 25%). Each combination treatment consisted of 10 plants totaling a sum of 240 plants used in the experiments. Plants were harvested at 12 weeks after planting [55].
3.2. Total Phenolics and Flavonoids Quantification
The method of extraction and quantification for total phenolics and flavonoids contents followed after Ibrahim and Jaafar [56]. An amount of ground tissue samples (0.1 g) was extracted with 80% ethanol (10 mL) on an orbital shaker for 120 min at 50 °C. The mixture was subsequently filtered (Whatman™ No.1), and the filtrate was used for the quantification of total phenolics and total flavonoids. Folin-Ciocalteu reagent (diluted 10-fold) was used to determine the total phenolics content of the leaf samples. Two hundred µL of the sample extract was mixed with Follin–Ciocalteau reagent (1.5 mL) and allowed to stand at 22 °C for 5 min before adding NaNO3 solution (1.5 mL, 60 g L−1). After two hours at 22 °C, absorbance was measured at 725 nm. The results were expressed as mg g−1 gallic acid equivalent (mg GAE g−1 dry sample). For total flavonoids determination, a sample (1 mL) was mixed with NaNO3 (0.3 mL) in a test tube covered with aluminium foil, and left for 5 min. Then 10% AlCl3 (0.3 mL) was added followed by addition of 1 M NaOH (2 mL). Later, the absorbance was measured at 510 nm using a spectrophomtometer with rutin as a standard (results expressed as mg g−1 rutin dry sample).
3.3. Anthocyanins Content
Anthocyanin content was determined according to Bharti and Khurana [57]. Fresh leaves (1 g) were added in acidic methanol (10 mL, 1% v/v HCl) and incubated overnight. Anthocyanin was partitioned from chlorophyll with chloroform (10 mL), followed by double deionised water (9 mL). The test tubes containing the samples were shaken gently and allowed the mixture to settle. The absorbance was read at 505 nm. Petunidin was used as a standard. Anthocyanin content was recorded as mg/g petunidin fresh weight.
3.4. Leaf Gas Exchange Measurement
The measurement was obtained from a LICOR 6400 Portable Photosynthesis System closed infra-red gas analyzer (IRGA, Licor Inc. Nebraska, USA). Prior to use, the instrument was warmed for 30 min and calibrated with the ZERO IRGA mode. Two steps are required in the calibration process: first, the initial zeroing process for the built-in flow meter; and second, zeroing process for the infra-red gas analyzer. The measurements used optimal conditions set of 400 µmol mol−1 CO2 30 °C cuvette temperature, 60% relative humidity with air flow rate set at 500 cm3 min−1, and modified cuvette condition of 800 µmol m−2 s−1 photosynthetically photon flux density (PPFD). The measurements of gas exchange were carried out between 09:00 to 11:00 a.m. using fully expanded young leaves numbered three and four from plant apex to record net photosynthesis rate (A). The operation was automatic and the data were stored in the LI-6400 console and analyzed by the Photosyn Assistant software (Version 3, Lincoln Inc.). Several precautions were taken to avoid errors during measurements. Leaf surfaces were cleaned and dried using tissue paper before enclosed in the leaf cuvette [58]. The light response curve was produced followed procedures from Ibrahim and Jaafar [29] to generate the apparent quantum yield and dark respiration rate.
3.5. Chlorophyll Fluorescences Measurement
Measurements of chlorophyll fluorescence were taken from fully expanded leaf of the second leaves. Leaves were darkened for 15 min by attaching light-exclusion clips to the central region of the leaf surface. Chlorophyll fluorescence was measured using a portable chlorophyll fluorescence meter (Handy PEA, Hansatech Instruments Ltd, Kings Lynn, UK). Measurements were recorded up for 5 S [29]. The fluorescence responses were induced by emitting diodes. Measurement of fO (initial fluorescence), fM (maximum fluorescence) and fV (variable fluorescence) were obtained from this procedure. fV is derived as the differences between fM and fO.
3.6. Relative Leaf Water Content
Relative water content (RWC) was estimated by a modification of the method of Weatherley [59] and calculated as RWC = 100 × [FW − DM] / [TW − DM]. FW and DM denote fresh weight (g) and dry weight (g). Turgid weight (TW) was calculated after fully hydrating fresh leaves in darkness at 4 °C for 24 h. Results were expressed as percentages.
3.7. Sucrose Determination
Sucrose was measured spectrophotometrically using the method of Ibrahim and Jaafar [60]. Samples (0.5 g; 0.25 mm) were placed in 15 mL conical tubes, and distilled water added to make up the volume to 10 mL. The mixture was then vortexed and later incubated for 10 min. Anthrone reagent was prepared using anthrone (0.1 g) that was dissolved in 95% sulphuric acid (Fisher Scientific, USA, 50 mL). Sucrose was used as a standard stock solution to prepare a standard curve for the quantification of sucrose in the sample. The mixed sample of ground dry sample and distilled water was centrifuged at a speed of 3,400 rpm for 10 min and then filtered to get the supernatant. A sample (4 mL) was mixed with anthrone reagent (8 mL) and then placed in a water-bath set at 100 °C for 5 min before the sample was measured at an absorbance of 620 nm using a spectrophotometer model UV160U (Shimadzu Scientific, Kyoto, Japan). The soluble sugar in the sample was expressed as mg sucrose g−1 dry sample.
3.8. Chlorophyll Content
Total chlorophyll content was measured by method from Jaafar et al. [61] using fresh weight basis. Prior to each destructive harvest each seedling was analyzed for the leaf chlorophyll relative reading (SPAD meter 502, Minolta Inc., Alameda, CA, USA). The leaves of L. pumila with different greenness (yellow, light green and dark green) were selected for analysis and total leaf chlorophyll content was analyzed. For each type of leaf greenness, the relative SPAD value was recorded (five points/leaf) and the same leaves sampled for chlorophyll content determination. Leaf disk 3 mm in diameter was obtained from leaf sample using a hole puncher. For each seedling the measurement was conducted on the youngest fully expanded leaves on each plant, generally the second or third leaf from the tip of the stem was used. The leaf disks were immediately immersed in acetone (20 mL) in an aluminum foil-covered glass bottle for approximately 24 h at 0 °C until all the green colour had bleached out. Finally, the solution (3.5 mL) was transferred to measure absorbances at 664 and 647 nm using a spectrometer (UV-3101P, Labomed Inc., Palmer, Alaska, AK, USA). After that the least squares regression was used to develop predictive relation between SPAD meter readings and pigment concentrations (mg g−1 fresh weight) obtained from the chlorophyll destructive analysis.
3.9. Phenylalanine-Ammonia-Lyase (PAL) Activity Determination
Phenylalanine-ammonia-lyase (PAL) activity was measured using the method described by Martinez and Lafuente [62]. The enzyme activity was determined by measuring spectrophotometrically the production of trans-cinnamic acid from L-phenylalanine. Enzyme extract (10 µL) was incubated at 40 °C with 12.1 mM L-phenylalanine (90 µL, Sigma) that were prepared in 50 mM Tris-HCl, (pH 8.5). After 15 min of reaction, trans-cinnamic acid yield was estimated by measuring increase in the absorbance at 290 nm. Standard curve was prepared by using a trans-cinnamic acid standard (Sigma) and the PAL activity was expressed as nM trans-cinnamic acid µg−1 protein h−1.
3.10. Malondialdehyde (MDA) Content Determination
Lipid peroxidation of plant parts was estimated by the level of malondialdehyde (MDA) production using the thiobarbituric acid (TBA) method as described by Ibrahim and Jaafar [63]. One gram of ground (0.25 mm) plant sample was homogenized with a mortar and pestle in 0.5% trichloracetic acid (TCA, 1 mL). The homogenate was centrifuged at 9,000 rpm for 20 min. The supernatant (0.5 mL) was mixed with 20% TCA (2.5 mL) containing 0.5% TBA and heated in a boiling water bath for 30 min and allowed to cool in an ice bath quickly. The supernatant was centrifuged at 9,000 rpm for 10 min, and resulting supernatant was used for determination of MDA content. Absorbance at 532 nm was recorded.
3.11. Statistical Analysis
Data were analyzed using analysis of variance by SAS version 17. Mean separation test between treatments was performed using Duncan multiple range test and standard error of differences between means was calculated with the assumption that data were normally distributed and equally replicated [64,65,66].
4. Conclusions
Our results indicate that the manipulation of soil water capacity may be an effective method to increase the expression of secondary metabolites compounds in L. pumila. Higher total flavonoids, phenolics, and anthocyanin levels were demonstrated in L. pumila when soil water capacity was at 50% (high water stress). The significant negative correlations of production of total flavonoids, phenolics and anthocyanins content with photosynthesis, apparent quantum yield and maximum efficiency of photosystem II (fv/fm) indicate the occurrence of the up-regulation of production of CBSM under reduced photosynthetic capacity under high water stress. The increase in the production of L. pumila secondary metabolites under low water field capacity might be due to enhancement of PAL and MDA activity that were shown to have significant positive correlations with plant secondary metabolites. Under high and severe water stress it was also noted that the production of chlorophyll was reduced significantly, indicating the increased production of plant secondary metabolites.
Acknowledgements
The authors are grateful to the Ministry of Higher Education Malaysia for financing this work under the Research University Grant Scheme No. 91007.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References and Notes
- 1.Harborne J.B. Introduction to Ecological Biochemistry,3rd ed. Academic Press; London, UK: 1993. [Google Scholar]
- 2.Heath M.C., Boller T. Biotic interactions levels of complexity in plant interactions with herbivores, pathogens and mutualists. Curr. Opin. Plant Biol. 2002;5:277–278. doi: 10.1016/S1369-5266(02)00278-9. [DOI] [Google Scholar]
- 3.Jain D.C., Mathur A.K., Gupta M.M., Singh A.K., Verma R.K., Gupta A.P., Kumar S. Isolation of high artemisinin-yielding clones of Artemisia annua. Phytochemistry. 1996;43:993–901. doi: 10.1016/S0031-9422(96)00369-X. [DOI] [Google Scholar]
- 4.Ghershenzon J. Changes in levels of plant secondary metabolites under water and nutrient stress. In: Timmermann B.N., Steelin C., Loewus F.A., editors. Recent Advances in Phytochemistry-Phytochemical Adaptations to Stress. Plenum Press; New York, NY, USA: 1984. pp. 273–220. [Google Scholar]
- 5.Hansen U., Seufert G. Terpenoid emission from Citrus sinensis (L.) OSBECK under drought stress. Phys. Chem. Earth. 1999;24:681–687. [Google Scholar]
- 6.Marchese J.A., Rehder V.L.G. The influence of temperature in the production of artemisinin in Artemisia annua L. Braz. J. Med. Plant. 2001;4:89–93. [Google Scholar]
- 7.Magiero E.C., Assmann J.M., Marchese J.A., Capelin D., Paladini M.V., Trezzi M.M. Allelopathic effect of Artemisia annua L. on the germination and initial development of lettuce (Lactuca sativa L.) and wild poinsettia (Euphorbia heterophylla L.) seedlings. Braz. J. Med. Plant. 2009;11:317–324. [Google Scholar]
- 8.Jaafar H.Z.E., Mohamed H.N.B., Rahmat A. Accumulation and partitioning of total phenols in two varieties of Labisia pumila Benth. under manipulation of greenhouse irradiance. Acta Hort. 2008;797:387–392. [Google Scholar]
- 9.Sulaiman B., Mansor M., Jaafar A. Some medicinal plants in mount Bubu Peral. In: Shaari K., Abdul K.A., Ali M., editors. Proceeding in Medicinal Products from Tropical Rain Forest; Kepong, Malaysia: Forest Research Institute of Malaysia; 1992. pp. 37–43. [Google Scholar]
- 10.Jamia A.J., Ibrahim J., Khairana H., Juriyati H. Perkembangan Penyelidikan dan Pembangunan Kacip Fatimah. New Dimension in Complementary Health Care; Kuala Lumpur, Malaysia: 2004. pp. 13–19. [Google Scholar]
- 11.Ferreira J.F.S. Nutrient deficiency in the production of artemisinin, dihydroartemisinic acid, and artemisinic acid in Artemisia annua L. J. Agric. Food Chem. 2007;55:1686–1694. doi: 10.1021/jf063017v. [DOI] [PubMed] [Google Scholar]
- 12.Marchese J.A., Rehder V.L.G., Casiraghi V., Tedesco A.C., Lira R. Flowering in plants of Artemisia annua L. standed to different conditions of photoperiod and temperature. Acta Hort. 2002;569:275–280. [Google Scholar]
- 13.Putalun W., Luealon W., De-Eknamkul W., Tanaka H., Shoyama Y. Improvement of artemisinin production by chitosan in hairy root cultures of Artemisia annua L. Biotechnol. Lett. 2007;29:1143–1146. doi: 10.1007/s10529-007-9368-8. [DOI] [PubMed] [Google Scholar]
- 14.Stewart A.J., Chapman W., Jenkins G.I., Graham I., Martin T., Crozier A. The effect of nitrogen and phosphorous deficiency on flavonol accumulation in plant tissues. Plant Cell Environ. 2001;24:1189–1197. doi: 10.1046/j.1365-3040.2001.00768.x. [DOI] [Google Scholar]
- 15.Winkel S.B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002;5:218–223. doi: 10.1016/S1369-5266(02)00256-X. [DOI] [PubMed] [Google Scholar]
- 16.Mosaleeyanon K., Zobayed S.M.A., Afreen F., Kozai T. Relationships between net photosynthetic rate and secondary metabolite contents in St. John’s wort. Plant Sci. 2005;169:523–531. [Google Scholar]
- 17.Ibrahim M.H., Jaafar H.Z.E., Rahmat A., Abdul Rahman Z. The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in Labisia pumila Benth.under high CO2 and nitrogen fertilization. Molecules. 2011;16:162–174. doi: 10.3390/molecules16010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couceiro M.A., Afreen F., Zobayed S.M.A., Kozai T. Variation in concentrations of major bioactive compounds of St. John’s wort: Effects of harvesting time, temperature and germoplasm. Plant Sci. 2006;170:128–134. [Google Scholar]
- 19.Zobayed S.M.A., Afreen F., Kozai T. Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St. John’s wort. Plant Physiol. Bioch. . 2005;43:977–984. doi: 10.1016/j.plaphy.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Franz C. Nutrient and water management for medicinal and aromatic plants. Acta Hort. 1983;132:203–215. [Google Scholar]
- 21.Palevitch D. Recent advances in the cultivation of medicinal plants. Acta Hort. 1987;208:29–35. [Google Scholar]
- 22.Marchese J.A., Figueira G.M. The use of pre and post-harvest technologies and good agricultural practices in the production of medicinal and aromatic plants. Braz. J. Med. Plant. 2005;7:86–96. [Google Scholar]
- 23.Herms D.A., Mattson W.J. The dilemma of plants: to grow or defend. Quart. Rev. Biol. 1992;67:283–235. [Google Scholar]
- 24.Charles D.I., Simon J.E., Shock C.C., Feibert E.B.G., Smith R.M. Effect of water stress and post-harvest handling on artemisinin content in the leaves of Artemisia annua L. In: Janick J. Simon J.E., editor. Proceedings of the Second International Symposium: New Crops, Exploration, Research and Commercialization; Indianapolis, USA. October 1991; New York, NY, USA: John Wiley and Sons Inc.; 1993. pp. 640–643. [Google Scholar]
- 25.Jaleel C.A., Sankar B., Murali P.V., Gomathinayagam M., Lakshmanan G.M.A., Panneerselvam R. Water deficit stress effects on reactive oxygen metabolism in Catharanthus roseus; impacts on ajmalicine accumulation. Colloid. Surfaces B. 2008;62:105–111. doi: 10.1016/j.colsurfb.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Lee S.C., Norliza A.L., Sze Y.L., Chew T.L., Mohd R.S., Ramlan A.Z. Flavonols and phenolics from Labisia pumila (Kacip Fatimah) Food Chem. 2011;127:1186–1192. doi: 10.1016/j.foodchem.2011.01.122. [DOI] [PubMed] [Google Scholar]
- 27.Zaizuhana S., Puteri J.N.M.B., Noral’ashikin Y., Muhammad H., Rohana A.B., Zakiah I. The in vivo rodent micronucleus assay of Kacip Fatimah (Labisia pumila) extract. Trop. Biomed. 2006;23:214–219. [PubMed] [Google Scholar]
- 28.Jaafar H.Z.E., Mohamed H.N.B., Rahmat A. Accumulation and partitioning of total phenols in two varieties of Labisia pumila Benth. under manipulation of greenhouse irradiance. Acta Hort. 2008;797:387–392. [Google Scholar]
- 29.Ibrahim M.H., Jaafar H.Z.E. Photosynthetic capacity, photochemical efficiency and chlorophyll content of three varieties of Labisia pumila benth. Exposed to open field and greenhouse growing conditions. Acta Physiol. Plant. 2011;33:2179–2185. doi: 10.1007/s11738-011-0757-1. [DOI] [Google Scholar]
- 30.Fortier E., Desjardins Y., Tremblay N., Bélec C., Côté M. Effects of exposure to soluble fraction of industrial solid waste on lipid peroxidation and DNA methylation in erythrocytes of Oreochromis niloticus, as assessed by quantification of MDA and m5dC rates. Acta Hort. 2010;856:55–62. [Google Scholar]
- 31.Azhar N., Hussain B., Ashraf M.Y., Abbasi K.Y. Water stress mediated changes in growth, physiology and secondary metabolites of desi ajwain (Trachyspermum Ammi L.) Pak. J. Bot. 2011;43:15–19. [Google Scholar]
- 32.Ibrahim M.H., Jaafar H.Z.E., Rahmat A., Zaharah A.R. Effects of nitrogen fertilization on synthesis of primary and secondary metabolites in three varieties of Kacip Fatimah (Labisia pumila Blume) Int. J. Mol. Sci. 2011;12:5238–5254. doi: 10.3390/ijms12085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh M.M., Trick H.N., Rajashekar C.B. Secondary metabolism and antioxidant are involved in environmental adaptation and stress tolerance in lettuce. J. Plant Physiol. 2009;166:180–199. doi: 10.1016/j.jplph.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Harrison K., Were L.M. Effect of gamma irradiation on total phenolics content, yield and antioxidant capacity of almond skin extract. Food Chem. 2007;102:932–937. doi: 10.1016/j.foodchem.2006.06.034. [DOI] [Google Scholar]
- 35.Palliotti A., Poni S., Silvestroni O., Tombesi S., Bernizzoni F. Morpho-structural and physiological performance of Sangiovese and Montepulciano cvv. (Vitis vinifera) under non-limiting water supply conditions. Funct. Plant Biol. 2010;38:888–898. doi: 10.1071/FP11093. [DOI] [PubMed] [Google Scholar]
- 36.Wang S.Y., Jiao H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals and singlet oxygen. J. Agric. Food Chem. 2000;48:5677–5684. doi: 10.1021/jf000766i. [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Nair M.G., Strasburg G.M., Chang Y.C., Booren A.M., Gray J.I., DeWitt D.L. Antioxidant and anti-inflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999;62:294–296. doi: 10.1021/np980501m. [DOI] [PubMed] [Google Scholar]
- 38.Tamura H., Yamagami A. Antioxidative activity of monoacylated anthocyanins isolated from Muscat bailey A grape. J. Agric. Food Chem. 1994;42:1612–1615. doi: 10.1021/jf00044a005. [DOI] [Google Scholar]
- 39.Asada K. Production and action of active oxygen species in photosynthetic tissues. In: Foyer C.H., Mullineaux P.M., editors. Causes of Photooxidative Stress and Amelioration of Defense System in Plants. CRS Press; Boca Raton, FL, USA: 1994. pp. 77–103. [Google Scholar]
- 40.Moghaddam S.S., Jaafar H.B., Aziz M.A., Ibrahim R., Rahmat A.B., Philip E. Flavonoid and leaf gas exchange responses of Centella asiatica to acute gamma irradiation and carbon dioxide enrichment under controlled environment conditions. Molecules. 2011;16:8930–8944. doi: 10.3390/molecules16118930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schreiner M., Beyene B., Krumbein A., Stützel H. Ontogenetic changes of 2-propenyl and 3-Lndolylmethyl glucosinolates in Brassica carinata leaves as affected by water supply. J. Agric. Food Chem. 2009;57:7259–7263. doi: 10.1021/jf901076h. [DOI] [PubMed] [Google Scholar]
- 42.Xiao X., Yang F., Zhang S., Korpelainen H., Li C. Physiological and proteomic responses of two contrasting Populus cathayana populations to drought stress. Physiol. Plant. 2009;136:150–168. doi: 10.1111/j.1399-3054.2009.01222.x. [DOI] [PubMed] [Google Scholar]
- 43.Szabó B., Lakatos Á., Koszegi T., Botz L. Investigation of abiogenic stress-induced alterations in the level of secondary metabolites in poppy plants (Papaver somniferum L.) Acta Biol. Hung. 2008;59:425–438. doi: 10.1556/ABiol.59.2008.4.4. [DOI] [PubMed] [Google Scholar]
- 44.Jaafar H.Z.E. Ph.D. Thesis. University of Nottingham; Nottingham, UK: 1995. Impact of Environmental Stress on Reproductive Development in Sweet pepper (Capsicum anuum) [Google Scholar]
- 45.Ghasemzadeh A., Jaafar H.Z.E., Asmah R. Antioxidant activities, total phenolics and flavonoids Content in two varieties of malaysia young ginger (Zingiber officinale Roscoe) Molecules. 2010;15:4324–4333. doi: 10.3390/molecules15064324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo R., Yuan G., Wang Q. Effects of sucrose and mannitol accumulation of health promoting component and activity of metabolic enzyme in brocolli sprout. Sci. Hort. 2011;128:159–165. doi: 10.1016/j.scienta.2011.01.014. [DOI] [Google Scholar]
- 47.Kubula J., Sriamoopunns S., Meeso N. Phytochemical, vitamin C and sugar content of thai wild fruits. Food Chem. 2011;126:972–981. doi: 10.1016/j.foodchem.2010.11.104. [DOI] [Google Scholar]
- 48.Veenashi B.R., Muralikrishna G. In vitro antioxidant activity of xylo-oligosharides derived from cereal and millet brans - a competitive study. Food Chem. 2011;126:1475–1481. doi: 10.1016/j.foodchem.2010.11.163. [DOI] [Google Scholar]
- 49.Jones C.G., Hartley S.E. A protein competition model of phenolic allocation. Oikos. 1999;86:27–44. doi: 10.2307/3546567. [DOI] [Google Scholar]
- 50.De-souza A.P., Gaspar M., Da-silva E.A., Ulian E.C., Waclawovsky A.J., Nishiyama M.Y., Dos M.R.V., Bucjkeridge M.S. Elevated CO2 increases photosynthesis, biomass and productivity and modifies gene expression in sugarcane. Plant Cell Environ. 2008;31:1116. doi: 10.1111/j.1365-3040.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 51.Ibrahim M.H., Jaafar H.Z.E. The influence of carbohydrate, protein and phenylanine ammonia lyase on up-regulation of production of secondary metabolites (total phenolics and flavonoid) in Labisia pumila (Blume) Fern-Vill (Kacip Fatimah) under high CO2 and different nitrogen levels. Molecules. 2011;16:4172–4190. doi: 10.3390/molecules16054172. [DOI] [Google Scholar]
- 52.Ibrahim M.H., Hawa Z.E.J. Carbon dioxide fertilization enhanced antioxidant compounds in Malaysian Kacip Fatimah (Labisia pumila Blume) Molecules. 2011;16:6068–6081. doi: 10.3390/molecules16076068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flohr L., Fuzinatto C.F., Melegari S.P., Matias W.G. Effects of exposure to soluble fraction of industrial solid waste on lipid peroxidation and DNA methylation in erythrocytes of Oreochromis niloticus, as assessed by quantification of MDA and m5dC rates. Ecotoxicol. Environ. Saf. 2012;76:63–70. doi: 10.1016/j.ecoenv.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Klapwijk D., De Lint P.J.A.L. Fresh weight and flowering of tomato plants as influenced by container type and watering condition. Acta Hort. 1974;39:237–247. [Google Scholar]
- 55.Ibrahim M.H., Jaafar H.Z. Reduced Photoinhibition under Low Irradiance Enhanced Kacip Fatimah (Labisia pumila Benth) Secondary Metabolites, Phenyl Alanine Lyase and Antioxidant Activity. Int. J. Mol. Sci. 2012;13:5290–5306. doi: 10.3390/ijms13055290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim M.H., Jaafar H.Z.E. Enhancement of leaf gas exchange and primary metabolites, up-regulate the production of secondary metabolites of Labisia Pumila Blume seedlings under carbon dioxide enrichment. Molecules. 2011;16:3761–3777. doi: 10.3390/molecules16053761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bharti A.K., Khurana J.P. Molecular characterization of transparent testa (tt) mutants of Arabidopsis thaliana (ecotype Estland) impaired in flavonoid biosynthesic pathway. Plant Sci. 2003;165:1321–1332. doi: 10.1016/S0168-9452(03)00344-3. [DOI] [Google Scholar]
- 58.Ibrahim M.H., Jaafar H.Z.E., Haniff M.H., Raffi M.Y. Changes in growth and photosynthetic patterns of oil palm seedling exposed to short term CO2 enrichment in a closed top chamber. Acta Physiol. Plant. 2010;32:305–313. doi: 10.1007/s11738-009-0408-y. [DOI] [Google Scholar]
- 59.Weatherley P.E. Studies in the water relations of the cotton plant. I. The field measurements of water deficits in leaves. New Phytol. 1950;49:81–87. doi: 10.1111/j.1469-8137.1950.tb05146.x. [DOI] [Google Scholar]
- 60.Ibrahim M.H., Jaafar H.Z.E. The relationship of nitrogen and C/N on secondary metabolites and antioxidant activities in three varieties of Malaysia Kacip Fatimah (Labisia pumila Blume) Molecules. 2011;16:5514–5526. doi: 10.3390/molecules16075514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaafar H.Z.E., Ibrahim M.H., Por L.S. Effects of CO2 enrichment on accumulation of total phenols, flavonoid and chlorophyll content in two varieties of Labisia pumila Benth. exposed to different shade levels; Proceedings of International Conference on Balanced Nutrient Management for Tropical Agriculture; Kuantan, Pahang, Maalysia. February 2010; Kuala Lumpur, Malaysia: UPM; 2010. pp. 112–114. [Google Scholar]
- 62.Martinez-Tellez M.A., Lafuente M.T. Effects of high temperature conditioning on ethylene, phenylalanine ammonia lyase, peroxidase and polyphenol oxidase in flavedo of chilled “Fortune” mandarin fruit. J. Plant Physiol. 1997;150:674–678. doi: 10.1016/S0176-1617(97)80282-9. [DOI] [Google Scholar]
- 63.Ibrahim M.H., Jaafar H.Z.E., Asmah R., Zaharah A.R. Involvement of Nitrogen on Flavonoids, Glutathione, Anthocyanin, Ascorbic Acid and Antioxidant Activities of Malaysian Medicinal Plant Labisia pumila Blume (Kacip Fatimah) Int. J. Mol. Sci. 2012;13:393–408. doi: 10.3390/ijms13010393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibrahim M.H., Jaafar H.Z.E. Primary, Secondary Metabolites, H2O2, Malondialdehyde and Photosynthetic Responses of Orthosiphon stimaneus Benth. to Different Irradiance Levels. Molecules. 2012;17:1159–1176. doi: 10.3390/molecules17021159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ibrahim M.H., Jaafar H.Z. Impact of Elevated Carbon Dioxide on Primary, Secondary Metabolites and Antioxidant Responses of Eleais guineensis Jacq. (Oil Palm) Seedlings. Molecules. 2012;17:5195–5211. doi: 10.3390/molecules17055195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaafar H.Z., Ibrahim M.H., Karimi E. Phenolics and Flavonoids Compounds, Phenylanine Ammonia Lyase and Antioxidant Activity Responses to Elevated CO2 in Labisia pumila (Myrisinaceae) Molecules. 2012;17:6331–6347. doi: 10.3390/molecules17066331. [DOI] [PMC free article] [PubMed] [Google Scholar]