Abstract
New coumarin derivatives, namely (2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-N-(4-oxo-2-phenylthiazolidin-3-yl)acetamide, N-(2-(3-methoxyphenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetamide, 2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-N-(4-oxo-2-(2,3,4trimethoxyphenyl)thiazolidin-3-yl)acetamide and N-(2-(4-bromophenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetamide) were synthesized starting from 4-methyl-7-hydroxycoumarin. The structures of the obtained compounds were confirmed by analytical IR and NMR spectra to elucidate the different positions of protons and carbons and as well as theoretical studies (DFT/B3LYP). The new compounds were screened for antibacterial activity. Most of them are more active against E. coli S. aureus and B. subtilis than standard references.
Keywords: coumarin, ethyl bromoacetate, thiazolidinones, DFT studies, antibacterial activities
1. Introduction
Small ring heterocycles containing nitrogen and sulfur have been under investigation for a long time because of their important medicinal properties. Among the wide range of heterocycles explored to develop pharmaceutically important molecules, thiazoles have played an important role in medicinal chemistry. A survey of literature has shown that compounds having a thiazole nucleus possess a broad range of biological activities such as anti-inflammatory [1], antibacterial [2] and antifungal properties [3]. Among these type of molecules 4-thiazolidinones have shown to have various important biological activities such as antibacterial, antifungal, antiviral, diuretic, antituberculostatic, anti-HIV, antihistaminic, anticancer, anticonvulsant, anti-inflammatory and analgesic properties [4,5,6,7,8,9,10,11]. Recently, a study reported the synthesis, chemical and wide rang biological properties of a series of 4-thiazolidinone molecules [12,13,14,15,16,17,18]. Some of these compounds showed moderate to good biological properties. The observed interesting biological properties of this class of compounds impelled us to synthesize new examples with possible improved biological properties with applicative possibilities.
In the current study we aimed to synthesize some new coumarins derived from umbelliferone (7-hydroxycoumarin), with predictable biological activities. The chemical structures of the synthesized compounds were proven by IR, NMR spectra and elemental analysis data.
2. Results and Discussion
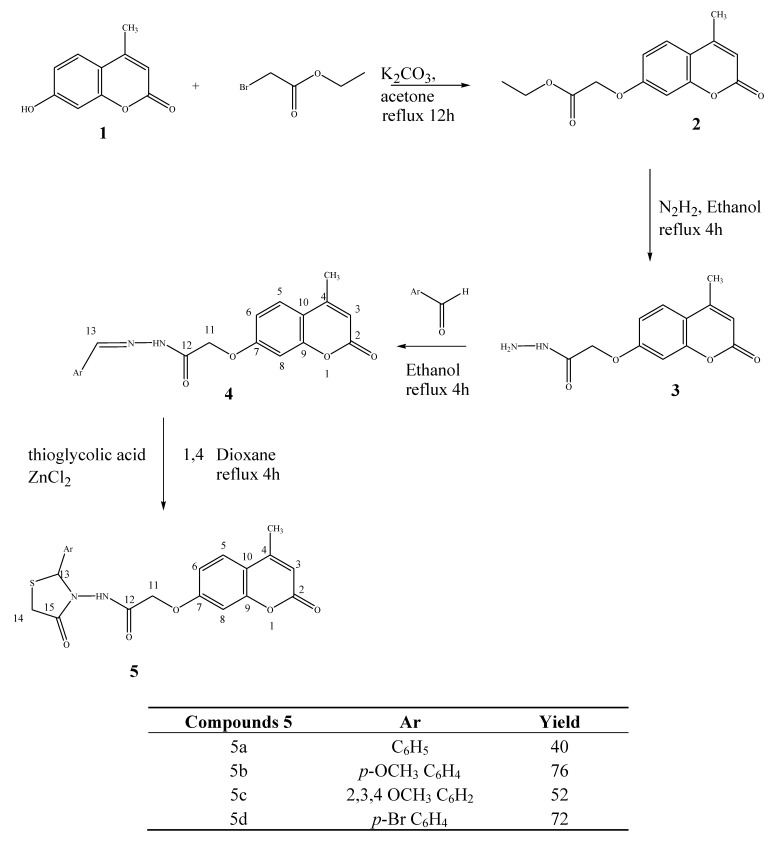
The starting compound (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid methyl ester (2) was prepared in 92% yield by esterification of 7-hydroxy-4-methylcoumarin (1). Hydrazinolysis of 2 with 86% hydrazine hydrate in ethanol at reflux for 4 h afforded hydrazide 3 in good yield. The FT-IR spectra of carbohydrazide 3 showed absorption bands at 3317 cm−1, possibly due to the hydrazide NH-NH2, 3269 cm−1 (aromatic CH), 1711 cm−1 (-C=O carbonyl stretching) and in the range 1621–1640 cm−1 (-CO-NH-NH2 groups). The 1H-NMR spectrum exhibited a singlet due to the -CO-NH-NH2 proton at δ 4.34 ppm. Methylene protons (–OCH2) resonate as singlets at 4.94 ppm.
Refluxing 3 and aromatic aldehydes with a catalytic amount of glacial acetic acid in absolute ethanol for 4 h afforded the new series of coumarin N′-benzylidene-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetohydrazide Schiff bases 4a–d. The structures of compounds 4a–d were inferred from their analytical and spectroscopic properties. Thus the IR spectrum of compound 4a showed characteristic bands in the range 1610 cm–1 (CONH), at 1681 cm–1 (C=O, lactone), and at 1258 cm–1 (-HC=N- azomethine). The 1H-NMR spectrum did not only show the absence of NH2 protons at 3.38, but also the presence of the N=CH proton at 8.24 ppm.
N-(2-aryl-4-oxo-thiazolidin-3-yl)-2-(4-(2-aryl-4-oxo-thiazolidin-3-ylcarbamoyl)-methyl)-2-oxo-2H-chromen-7-yloxy)-acetamides 5a–d were obtained by reaction of the compounds 4a–d with thioglycolic acid in refluxing 1,4-dioxane for 6–8 h in the presence of anhydrous ZnCl2 (Scheme 1).
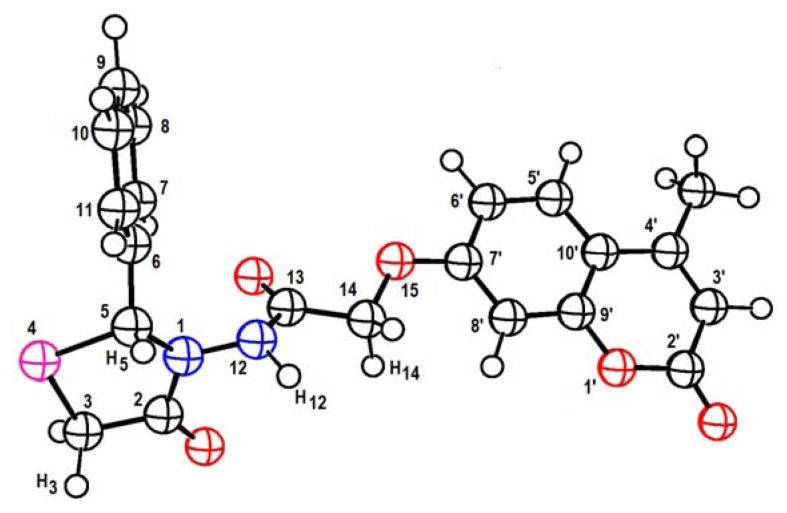
The IR spectrum of 5b showed a characteristic band at 1728 cm−1 that supports the presence in the molecule of a thiazolidinone C=O group. The 1H-NMR spectrum of 5b displayed signals between 7.30–7.60 δ ppm for aromatic protons and a doublet at 4.84 ppm ascribable to the thiazolidinones CH2 protons. The proposed 5a–d structures were evaluated by using a DFT/B3LYP approach implemented in the Gaussian 09 series programs [19]. The B3LYP hybrid functional has been used in describing potential energy surfaces (PES). The geometries of the compounds were fully optimized using analytic gradients. The harmonic vibrational frequencies of the stationary points of the PES have been calculated at the same level of theory in order to identify the local minima as well as to estimate the corresponding zero point vibrational energy (ZPE) [20]. For each atom no pseudopotential are used. A Def2-SVP EMSL Basis Set Exchange was employed for each atom [21]. Values of selected geometrical parameters are listed in Table 1 and optimized geometry for 5a is depicted in Figure 1.
Scheme 1.
Synthesis of 2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-N-(4-oxo-2-arylthiazolidin-3-yl) acetamide 5.
Table 1.
DFT/B3LYP optimized geometrical parameters a for 5a–d.
| Compounds | 5a | 5b | 5c | 5d |
|---|---|---|---|---|
| 1-2 | 1,385 | 1.384 | 1.383 | 1.386 |
| 1-12 | 1.374 | 1.374 | 1.372 | 1.374 |
| 1-5 | 1.463 | 1.465 | 1.462 | 1.462 |
| 2-3 | 1.520 | 1.521 | 1.520 | 1.520 |
| 3-4 | 1.829 | 1.829 | 1.826 | 1.829 |
| 4-5 | 1.863 | 1.864 | 1.866 | 1.863 |
| 5-6 | 1.510 | 1.506 | 1.509 | 1.510 |
| 12-13 | 1.394 | 1.393 | 1.389 | 1.394 |
| 13-14 | 1,530 | 1.530 | 1.531 | 1.529 |
| 14-15 | 1.401 | 1.401 | 1.402 | 1.401 |
| 15-7′ | 1.352 | 1.352 | 1.352 | 1.353 |
| 1′-2′ | 1.396 | 1.396 | 1.396 | 1.397 |
| 1′-9′ | 1.354 | 1.354 | 1.354 | 1.354 |
| 2′-3′ | 1.457 | 1.457 | 1.457 | 1.457 |
| 3′-4′ | 1.362 | 1.362 | 1.362 | 1.362 |
| 4′-10′ | 1.454 | 1.454 | 1.454 | 1.454 |
| 5-H5 | 1.105 | 1.106 | 1.101 | 1.105 |
| 12-H12 | 1.020 | 1.020 | 1.020 | 1.020 |
| 14-H14 | 1.107 | 1.108 | 1.108 | 1.108 |
| 1-2-3 | 111.1 | 111.0 | 110.7 | 111.1 |
| 2-3-4 | 107.8 | 107.8 | 107.6 | 107.8 |
| 3-4-5 | 92.9 | 92.8 | 93.0 | 92.9 |
| 4-5-1 | 103.4 | 103.2 | 103.4 | 103.5 |
| 1-5-6 | 115.5 | 115.6 | 115.6 | 115.5 |
| 1-5-H5 | 109.5 | 109.5 | 108.4 | 109.6 |
| 2-1-12 | 118.1 | 118.2 | 119.5 | 118.1 |
| 1-12-H12 | 114.9 | 114.9 | 114.7 | 114.8 |
| 1-12-13 | 119,0 | 118.9 | 121.2 | 119.0 |
| 12-13-14 | 111.9 | 111.9 | 111.2 | 112.0 |
| 13-14-15 | 108.3 | 108.3 | 108.9 | 108.4 |
| 1′-2′-3′ | 115.4 | 115.4 | 115.4 | 115.4 |
| 2′-3′-4′ | 123.3 | 123.3 | 123.3 | 123.3 |
| 3′-4′-10′ | 118.6 | 118.6 | 118.5 | 118.5 |
| 4′-10′-9′ | 118.0 | 118.0 | 117.9 | 118.0 |
| 1-2-3-4 | 6.1 | 7.5 | −13.9 | 6.5 |
| 2-3-4-5 | −15.4 | −17.0 | 18.6 | −15.8 |
| 3-4-5-1 | 19.9 | 21.1 | −17.9 | 20.0 |
| H5-5-6-11 | −12.6 | −11.8 | −0.7 | −12.8 |
| 3-4-5-6 | 144.3 | 145.8 | 109.4 | 144.5 |
| 1-5-6-7 | −136.1 | −135.1 | −122.1 | −136.4 |
| 2-3-5-12 | 1.0 | 1.1 | −0.9 | −11.6 |
| 2-5-7′-6′ | 113.0 | 114.1 | 109.1 | 118.2 |
| 12-13-14-15 | −158.1 | −157.1 | −161.7 | −155.2 |
| 13-14-15-7′ | −175.5 | −174.8 | −177.7 | −174.9 |
| 14-15-7′-6′ | 178.5 | 178.2 | 176.8 | 179.2 |
| 7′-8′-9′-10′ | 0.0 | 0.0 | 0.4 | 0.1 |
| 10′-9′-1′-2′ | 0.0 | 0.1 | 0.0 | 0.0 |
| 1′-2′-3′-4′ | 0.1 | 0.2 | 0.6 | 0.1 |
a Distances are in A˚ and angles in degrees.
Figure 1.
Optimized geometry for 5a.
Some conclusions can be extracted from the results obtained. The calculated values for length and angles are similar for compounds 5a–d. This fact suggests that the R group has no influence on interatomic distances and angles. Additionally the dihedral angles 1-2-3-4, 2-3-4-5 and 3-4-5-1 indicate that the thiazolidinone ring in the compounds is not in a plane and that fact is not dependent on the R group. The plane constituted by the thia-ring is perpendicular to that of the coumarin group (for exemple the angle 2-5-6′-7′ = 113.0 in 5a). It is important to point out that the 5c thia-ring displays a different orientation that those for the other calculated compounds. Important information on the chemical groups present in compounds 5a–d can be obtained from their calculated vibrational spectra. As example, intensities and their harmonic wave numbers calculated with the method DFT/B3LYP and the corresponding experimental values of the compound 5c are grouped in Table 2.
Table 2.
Theoretical and experimental vibrational frequencies (cm−1) and theoretical infrared intensities (km mol−1) of 5c.
| Experimental | Theoretical | |||
|---|---|---|---|---|
| Functional group | Frequencies | Intensities | Frequencies | Intensities |
| CO thiaz. | 1666 | strong | 1831 | 328 |
| CO lactone | 1682 | strong very | 1862 | 652 |
| CO amide | 1712 | mean | 1871 | 130 |
| NH | 3313 | weak | 3513 | 23 |
The values of the theoretical IR frequencies are in agreement with the experimental ones. The largest shifting is less than 10% that could be due to the approximations used in the DFT/B3LYP method; consequently the proposed structures were supported by theoretical calculations.
The NBO analysis allows the determination of the atomic natural charges. Table 3 reports the natural charges of the acid protons in compound 5c calculated at DFT/B3LYP level of theory. We find that proton H12 carried by the nitrogen was the most acidic.
Table 3.
NBO charge of the acidic protons of the compound 5c calculated at the DFT/B3LYP level of theory.
| Hi | Charge |
|---|---|
| H3 | 0.25 |
| H5 | 0.22 |
| H12 | 0.39 |
| H14 | 0.22 |
2.1. Antioxidant Activities
The imbalance between reactive oxygen species (ROS) and antioxidant defence mechanisms leads to oxidative modification in cellular membrane or intracellular molecules. Phytochemicals like phenolics, commonly found in plants, could constitute strong natural antioxidants and could play an important role in health care system.
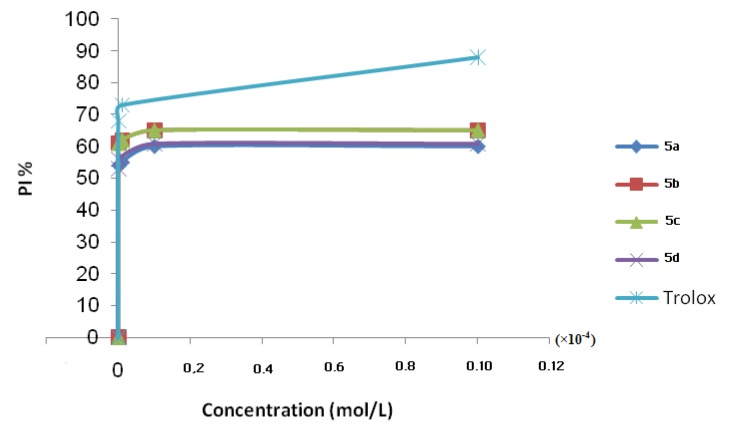
The effect of the different synthetic compounds on DPPH radical scavenging was compared to those of Trolox, using as positive control, and appreciated by the determination of the IC50 values. The results are shown in Figure 2 and listed in Table 4.
Figure 2.
Scavenging effect on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical of compound 5.
Table 4.
Values of IC50 exhibited by coumarinic derivatives 5a–d.
| Compounds 5 | IC50 (10−9 mol/L) |
|---|---|
| 5a | 92.60 |
| 5b | 82.00 |
| 5c | 8.62 |
| 5d | 9.43 |
| Trolox | 7.35 |
As shown in Figure 2, DPPH test revealed that increase in compounds concentration resulted in increase in free radical-scavenging activity in a dose dependent manner. Based on the IC50 values, the most active compound is 5c.
2.2. ABTS Radical Cation Decolourization Assay
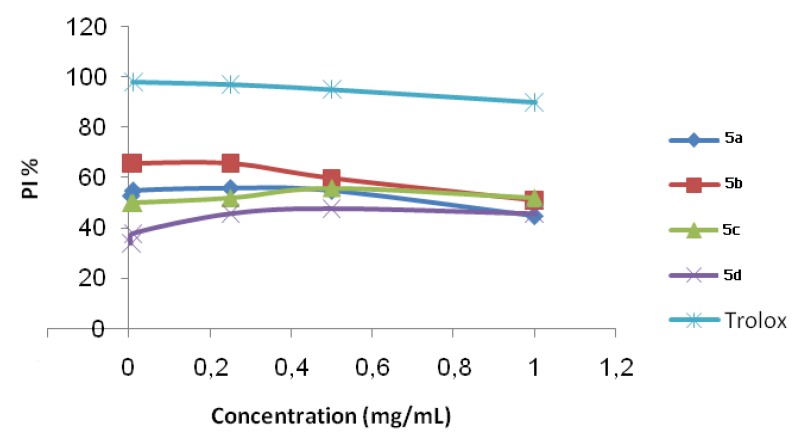
As shown for DPPH scavenging, the obtained results indicate the higher capacity of 5a–d to quench ABTS as compared to the synthetic antioxidant Trolox (Figure 3 and Table 5).
Figure 3.
Scavenging ability on ABTS radical of compounds 5.
Table 5.
Values of IC50 exhibited by coumarinic derivatives 5a–d.
| Compounds 5 | IC50 (10−9 mol/L) |
|---|---|
| 5a | 92.60 |
| 5b | 82.00 |
| 5c | 8.62 |
| 5d | 9.43 |
| Trolox | 7.35 |
2.3. Antibacterial Activity
The antibacterial activity of the tested compounds against a series of bacteria and fungi is shown in Table 6.
Table 6.
Antibacterial activity against bacteria of 5a–d, inhibition zone expressed in mm.
| Organism | Compounds | Standard-drug | |||
|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | ampicillin | |
| E. coli ATCC1225 | 19 | 19 | 19 | 19 | 16 |
| P. vulgaris | 20 | 20 | 20 | 20 | 22 |
| B. megaterium | 17 | 17 | 17 | 17 | 23 |
| S. aureus ATCC2353 | 18 | 18 | 18 | 18 | 25 |
All the compounds 5 displayed maximum activity against P. vulgaris. The compound 5b is highly active against E. coli. The compounds 5b and 5d also showed very good activity against B. megaterium, while compounds 5a and 5c showed good activity against S. aureus.
Our results also showed that the purified compounds had great potential for antibacterial activity against a panel of microorganisms. There is evidence in the literature that Gram-positive bacteria are more sensitive to plant extracts and essential oil than Gram-negative bacteria [22]. On the basis of inhibition zone diameters values P. vulgaris was more sensitive to the purified compounds than the other Gram positive bacteria. The observed differences in the inhibition zones within pathogenic bacteria could be probably due to cell membrane permeability or other genetic factors.
3. Experimental
3.1. General
All reagents were obtained from commercial sources. Solvents were either commercially obtained as analytical grade or freshly distilled prior to use. Analytical thin layer chromatography was carried out on precoated silica gel 60F254 plates using either UV absorption or iodine staining for visualization. Column chromatography was carried out using 100–200 mesh silica gel. 1H-NMR and 13C{1H}-NMR spectra were recorded on a Bruker DRX 300 MHz instrument (300/75 MHz); chemical shift values are reported relative to tetramethylsilane as internal standard. The IR spectra were recorded on a Thermo Mattson IR300 and Nicolet 510 PET spectrometers. Elemental microanalysis was carried out using a model 5500-Carlo Erba C.H.N.S.O elemental analyzer instrument.
General procedure for the preparation of ethyl 2-((4-methyl-2-oxo-2H-chromen-7-yl)oxy)acetate (2). A mixture of 7-hydroxy-4-methylcoumarin (5.6 mmol), anhydrous potassium carbonate (5.6 mmol) and ethyl bromoacetate (5.6 mmol) in dry acetone (10 mL) was refluxed with continuous stirring for 12 h. After filtration, the solution was concentrated under reduced pressure, vacuum dried and the solid product was recrystallized from ethanol. m.p. 185–186 °C, yield 92%; IR: νmax CO lactone: 1,714 cm−1, NH: 3,435 cm−1, CO (ketone): 1,606 cm−1; 1H-NMR: δ 7.76 (d, JH5.6 = 14.6 Hz, 1H, H-5), 7.04 (d, JH5.6 = 14.6 Hz, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.92 (s, 2H, -OCH2), 4.19 (q, JH2-H3 = 10.6 Hz, 2H, CH2, -CH2CH3), 4.02 (s, 2H, CH2), 1.22 (t, JH2-H3 = 10.6 Hz, 3H, CH3, -CH2CH3); 13C{1H}- NMR: δ 14.2 (CH2CH3), 34.8 (CH2CO), 61.3 (CH2CH3), 65.5 (COCH2O), 109.6 (C-8), 112.8 (C-6), 113.8 (C-3), 114.8 (C-10), 128.3 (C-5), 151.2 (C-9), 155.2 (C-4), 160.3 (C-7), 160.9 (C-2), 168.9 (CO-O), 169.3 (C-CO-C). Found, %: C, 64.12; H, 5.2; O, 30.40. C14H14O5. Calculated, %: C, 64.12; H, 5.38; O, 30.50.
2-(4-Methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazide (3). To a solution of ethanol (15 mL) and hydrazine hydrate (10 mmol), ethyl 2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetate (2, 10 mmol) was added, and the mixture was refluxed for 4 h. The product precipitated and was collected by suction filtration, washed with methanol and recrystallized from dil. Acetic acid. m.p. = 300 °C, yield 70%; IR: νmax CO lactone: 1,681 cm−1, CONH (amide): 1,612 cm−1, CN: 1,271 cm−1; 1H-NMR: δ 9.41 (s, 1H, NH), 7.76 (d, JH5.6 = 14.6 Hz, 1H, H-5), 7.04 (d, JH5.6 = 14.6 Hz, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.94 (s, 2H, -OCH2), 4.34 (s, 2H, NH2). 13C{1H}-NMR: δ 45.8 (CH2), 68.9 (CH2O-), 108.0 (C-8), 111.8 (C-6), 112.9 (C-3), 114.1 (C-10), 128.3 (C-5), 152.2 (C-9), 155.2 (C-4), 160.4 (C-7), 160.9 (C-2), 166.8 (COCH2O), 169.6 (COCH2). Found, %: C, 58.1; H, 4.82; N, 11.1; O, 25.70 C12H12O4N2. Calculated, %: C, 58.06; H, 4.87; N, 11.29; O, 25.78.
General procedure for the preparation of N′-benzylidene-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetohydrazide (4). A mixture of 2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazide (3, 3.06 g, 0.01 mol) and appropriate aromatic aldehyde (Ar/a-d, 0.01 mol) was refluxed in absolute ethanol (30 mL) in the presence of a catalytic amount of glacial acetic for 4 h. The reaction mixture was cooled; the solid separated was filtered and recrystallized from methanol to give compounds 4a–d.
N'-Benzylidene-2-((4-methyl-2-oxo-2H-chromen-7-yl)oxy)acetohydrazide (4a). m.p. 268–269 °C; yield 74%; IR: νmax CO lactone: 1681 cm−1, CONH (amide): 1610 cm−1, C=N: 1258 cm−1; 1H-NMR: δ 8.30 (s, 1H, HC=N-), 8.02 (s, 1H, NH), 7.76 (d, JH5.6 = 15.6 Hz, 1H, H-5), 7.72–7.31 (m, 10H, arom.), 7.04 (d, JH5.6 = 15.6 Hz, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.83 (s, 2H, -OCH2), 13C{1H}-NMR: δ 45.7 (CH2), 69.2 (CH2O-), 108.1 (C-8), 111.6 (C-6), 112.5 (C-3), 114.4 (C-10), 127.9 (C-5), 128.4 (C-3,5, Ar-), 129.0 (C-2,6, Ar-), 131.4 (C-4, Ar-), 133.9 (C-1, Ar-), 143.6 (N=CH-), 151.8 (C-9), 155.2 (C-4), 160.4 (C-7), 160.9 (C-2), 166.9 (COCH2O), 170.0 (CONH-). Found, %: C, 67.75; H, 4.8; N, 8.2; O, 19.1. C19H16O4N2. Calculated, %: C, 67.85; H, 4.79; N, 8.33; O, 19.03.
N′-(3-Methoxybenzylidene)-2-((4-methyl-2-oxo-2H-chromen-7-yl)oxy)acetohydrazide (4b). m.p. 225–226 °C, yield (76%); IR: νmax CO lactone:1,681 cm−1,CONH (amide): 1,614 cm−1, C=N: 1,255 cm−1; 1H-NMR: δ 8.73 (s, 1H, -HC=N-), 8.45 (s, 1H, NH), 7.72 (d, JH5.6 = 15.5 Hz, 1H, H-5), 7.60–7.30 (m, 8H, arom.), 3.65 (s, 3H, OCH3) ,7.04 (d, JH5.6 = 15.4 Hz ,1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.84 (s, 2H, -OCH2), 13C{1H} NMR: δ 45.6 (CH2), 68.9 (CH2O-), 107.8 (C-8), 111.5(C-6), 112.7 (C-3), 113.9 (C-10), 127.8 (C-5), 129.4 (C-3, Ar-), 130.6 (C-6, Ar-), 132.7 (C-4, Ar-),133.8 (C-1, Ar-), 134.3 (C-2, Ar-), 143.5 (N=CH-), 151.7 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2),166.4 (COCH2O), 169.9 (CONH-). Found, %: C, 65.5; H, 4.80; N, 7.6; O, 21.8. C20H18O5N2. Calculated, %: C, 65.57; H, 4.95; N, 7.65; O, 21.84.
N′-(2.3.4-Trimethoxybenzylidene)-2-((4-methyl-2-oxo-2H-chromen-7-yl)oxy)acetohydrazide (4c). m.p. 259–261 °C, yield 72%; IR: νmax CO lactone: 1,681 cm−1, CONH (amide): ,1618 cm−1, C=N: 1,281 cm−1. 1H-NMR: δ 8.31 (s, 1H, -HC=N-), 7.76 (d, JH5.6 = 15.5Hz, 1H, H-5), 7.67–7.30 (m, 8H, arom.), 7.04 (d, JH5.6 = 15.6 Hz ,1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 3.78 (s, 9H, 2,3,4 OCH3), 4.84 (s,2H, -OCH2), 13C{1H}-NMR: δ 45.5 (CH2), 69.1 (CH2O-), 107.9 (C-8), 111.4 (C-6),112.5 (C-3), 113.4 (C-10), 127.3 (C-6, Ar-), 127.9 (C-5), 129.3 (C-2, Ar-), 130.3 (C-5, Ar-), 131.2 (C- 4, Ar-), 135.2 (C-1, Ar-), 134.4 (C-3, Ar-), 143.4 (N=CH-), 151.6 (C-9), 155.2 (C-4), 160.6 (C-7), 160.9 (C-2), 166.7 (COCH2O), 169.8 (CONH-). Found, %: C, 61.9; H, 5.1; N, 6.5; O, 26.3. C22H22O7N2. Calculated, %: C, 61.97; H, 5.20; N, 6.57; O, 26.26.
N′-(4-Bromobenzylidene)-2-((4-methyl-2-oxo-2H-chromen-7-yl)oxy)acetohydrazide (4d). m.p. 273–275 °C, yield 52%; IR: νmax CO lactone: 1,681 cm−1, CONH (amide): 1,612 cm−1, 1H-NMR: 8.30 (s, 1H, -HC=N-), 8.23 (s, 1H, NH), 7.76 (d, JH5.6 = 15.5 Hz, 1H, H-5), 7.61–7.30 (m, 6H, arom.), 7.04 (d, JH5.6 = 15.5 Hz, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.82 (s, 2H, -OCH2), 13C{1H}- NMR: δ 45.6 (CH2), 69.2 (CH2O-), 103.8 (C-3, Ar-), 107.6 (C-8), 108.7 (C-5, Ar-),111.3 (C-6), 112.7 (C-3), 113.4 (C-10), 127.2 (C-6, Ar-), 127.9 (C-5), 135.2 (C-1, Ar-), 143.0 (N=CH-), 151.2 (C-9), 155.1 (C-4), 160.5 (C-7), 160.9 (C-2), 162.4 (C-2, Ar-), 162.6 (C-4, Ar-), 166.5 (COCH2O), 169.4 (CONH-). Found, %: C, 54.8; H, 3.6; Br, 19.1; N, 6.7; O, 15.4. C19H15O4N2. Calculated, %: C, 54.96; H, 3.64; Br, 19.24; N, 6.75; O, 15.41.
General procedure for the preparation of 2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-N-(4-oxo-2 arylthiazolidin-3-yl) acetamide (5). A mixture of N′-benzylidene-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetohydrazide (4, 0.01 mol) and mercaptoacetic acid (1.82 g, 0.02 mol) in 1,4-dioxane (30 mL) containing a pinch of anhydrous ZnCl2 was refluxed for 6–8 h. The reaction mixture was cooled and poured onto crushed ice. The solid thus obtained was filtered, washed with water and recrystallized from DMF yielding 5a–d.
2-(4-Methyl-2-oxo-2H-chromen-7-yloxy)-N-(4-oxo-2-phenylthiazolidin-3-yl)acetamide (5a). m.p. 202–204 °C, yield 40%; IR: νmax CO lactone: 1,681 cm−1, CONH (amide): 1,615 cm−1; 1H-NMR: 2.15 (s, 3H), 8.12 (s, 1H, -NH), 7.76 (d, JH5.6 = 10.6 Hz, 1H, H-5), 7.71–7.23 (m, 10H, arom.), 7.04 (d, JH5.6 = 10.6 Hz, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, -SCHN-), 4.83 (s, 2H, ‑OCH2), 3.38 (s, 2H, COCH2S-); 13C{1H}-NMR: δ 35.8 (COCH2S), 45.5 (CH2), 57.4 (NCHS), 69.1 (CH2O-) 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.2 (C-4, Ar-), 127.8 (C-5), 128.7 (C-3,5, Ar-),128.8 (C-2,6 Ar-), 139.2 (C-1, Ar-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (COCH2O), 168.8 (SCH2CO-N), 173.3 (CONH-). Found, %: C, 61.3; H, 4.4; N, 6.8; O, 19.5; S, 7.7. C21H18O5N2S. Calculated, %: C, 61.45; H, 4.42; N, 6.83; O, 19.49; S, 7.81.
N-(2-(3-Methoxyphenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetamide (5b). m.p. 184 °C, yield 76%; IR: νmax CO lactone: 1,681 cm−1, CONH (amide): 1,614 cm−1; 1H-NMR: 8.62 (s, 1H, NH-), 2.20 (s, 3H), 7.76 (d, JH5.6 = 10.5 Hz, 1H, H-5), 7.60–7.30 (m, 8H, arom.), 3.67 (s, 3H, OCH3), 7.04 (d, JH5.6 = 10.5 Hz, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.84 (s, 2H, -OCH2), 3.38 (s, 2H, COCH2S); 13C{1H}-NMR: δ 35.7 (COCH2S), 45.5 (CH2), 57.4(NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.0 (C-5), 129.0 (C-3, Ar-), 130.6 (C-6, Ar-), 132.5 (C-4, Ar-), 133.4 (C-1, Ar-), 134.0 (C-2, Ar-), 143.0 (N=CH-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (COCH2O), 173.0 (CONH-). Found, %: C, 59.9; H, 4.5; N, 6.4; O, 21.8; S, 7.2. C22H20O6N2S. Calculated, %: C, 59.99; H, 4.58; N, 6.36; O, 21.79; S, 7.28.
2-(4-Methyl-2-oxo-2H-chromen-7-yloxy)-N-(4-oxo-2-(2,3,4-trimethoxyphenyl)thiazolidin-3-yl)acetamide (5c). m.p. 239–241 °C, yield 52%; IR: νmax 1,712 (C=O, lactone), 1,624 (C=O, amide) cm−1; 1H-NMR: 2.12 (s, 3H), 8.30 (s, 1H, NH-), 7.76 (d, JH5.6 = 10.4 Hz, 1H, H-5), 7.61–7.30 (m, 6H, arom.), 7.04 (d, JH5.6 = 10.4 Hz, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.82 (s, 2H, ‑OCH2), 3.75 (s, 9H, 2,3,4 OCH3), 4.28 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C{1H}-NMR: δ 35.7 (COCH2S), 45.5 (CH2), 47.4 (NCHS), 69.10 (CH2O-), 103.7 (C-3, Ar-), 107.6 (C-8), 108.4 (C-5, Ar-), 110.1 (C-1, Ar-), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.8 (C-5),131.3 (C-6, Ar-), 151.2 (C-9), 157.2 (C-2, Ar-), 158.2 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH),168.8 (NCOCH2), 173.3 (CH2CONH). Found, %: C, 57.4; H, 4.75; N, 5.7; O, 25.6; S, 6.5. C24H24O8N2S. Calculated, %: C, 57.59; H, 4.83; N, 5.60; O, 25.57; S, 6.41.
N-(2-(4-Bromophenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetamide (5d). m.p. 240–241 °C, yield 72%; IR: νmax 1,727 (CO, lactone), 1,616 (C=O, amide) cm−1; 1H-NMR: 2.14 (s, 3H), 8.22 (s, 1H, NH-), 7.76 (d, JH5.6 = 10.5 Hz, 1H, H-5), 7.67–7.30 (m, 8H, arom.), 7.04 (d, JH5.6 = 10.5 Hz, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.84 (s, 2H, -OCH2), 3.38 (s, 2H, COCH2S); 13C{1H}-NMR: δ 35.7 (COCH2S), 45.5 (CH2), 57.4 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.3 (C-6, Ar-), 127.8 (C-5), 129.3 (C-2, Ar-), 130.3 (C-5, Ar-), 131.2 (C-4, Ar-), 135.2 (C-1, Ar-), 134.4 (C-3, Ar-), 143.0 (N=CH-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 173.0 (COCH2O), 173.0 (CONH-). Found, %: C, 51.6; H, 3.4; Br, 16.4; N, 5.75; O, 16.4; S, 6.7. C21H17O5N2BrS. Calculated, %: C, 51.54; H, 3.50; Br, 16.33; N, 5.72; O, 16.35; S, 6.55.
3.2. DPPH Test
The DPPH test aims to measure the capacity of the compounds for scavenging the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH.) by donation of hydrogen atom or an electron [23] If the compounds have the capacity to scavenge the DPPH free radical, the initial blue/purple solution will change to a yellow colour due to the formation of diphenylpicrylhydrazine. Scavenging capacity was read spectrophotometrically by monitoring the decrease of the absorbance at 517 nm. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity.
The percent DPPH scavenging effect was calculated using the following equation:
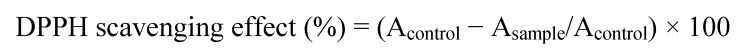 |
where Acontrol is the absorbance of the control reaction were the sample is replaced by 500 µL ethanol. Tests were carried out in triplicate.
3.3. ABTS Radical Cation Decolourization Assay
The potential of N-(2-aryl-4-oxo-thiazolidin-3-yl)-2-(4-(2-aryl-4-oxo-thiazolidin-3-ylcarbamoyl)-methyl)-2-oxo-2H-chromen-7-yloxy)-acetamides 5a–d to scavenge free radicals was also assessed by checking their ability to quench ABTS. depicted by the concentration-dependent decolourization of ABTS.. ABTS radical-scavenging activity of 5a–d was determined according to Güven et al. [24]. The ABTS. cation radical was produced by the reaction between 5 mL of 14 mM ABTS solution and 5 mL of 4.9 mM potassium persulfate (K2S2O8) solution, stored in the dark at room temperature for 16 h. Before use, this solution was diluted with ethanol to get an absorbance of 0.700 ± 0.020 at 734 nm. In a final volume of 1 mL, the reaction mixture comprised 950 µL of ABTS. solution and 50 µL of 5a–d at various concentrations. The reaction mixture was homogenized and its absorbance was recorded at 734 nm. Ethanol blanks were run in each assay, and all measurements were done after at least 6 min. Similarly, the reaction mixture of standard group was obtained by mixing 950 µL of ABTS. solution and 50 µL of Trolox. As for the antiradical activity, ABTS scavenging ability was expressed as IC50 (µg/mL). The inhibition percentage of ABTS radical was calculated using the following formula:
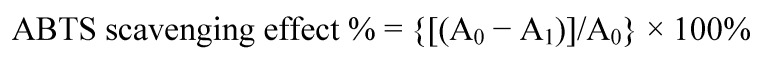 |
where A0 is the absorbance of the control at 30 min, and A1 is the absorbance of the sample at 30 min. All samples were analyzed in triplicate.
3.4. Antibacterial Activity
Antimicrobial activity of the purified compounds was evaluated by means of agar-well diffusion assay according to Güven et al. [25] with some modifications. The nutrient agar broth prepared by the usual method, was inoculated aseptically with 0.5 mL of 24 h old subculture of S. aureus ATCC 2353, B. megaterium, P. vulgaris, and E. coli ATCC1225 in separate conical flasks at 40–50 °C and mixed well by gentle shaking. About 25 mL of the contents of the flask were poured and evenly spread in petridish (90 mm in diameter) and allowed to set for two h. The cups (10 mm in diameter) were formed by the help of borer in agar medium and filled with 0.04 mL (40 μg/mL) solution of sample in DMF. The plates were incubated at 37 °C for 24 h and the control was also maintained with 0.04 mL of DMF in similar manner and the zones of inhibition of the bacterial growth were measured in millimetre
4. Conclusions
A series of new coumarin-based thiazolidinone compounds were successfully synthesized, characterized and tested for their antibacterial and antioxidant activities. Most of the synthesized compounds are more active against E. coli and S. aureus than standard references. Some of the compounds were found equipotent to ampicillin, but less active than other used standard drugs
Acknowledgements
This work was carried out with financial aid of the Qassim University in KSA through project No. 1303.
Footnotes
Sample Availability: Samples of the compounds 2-5 are available from the authors.
References
- 1.Geronikaki A., Hadjiparlon-Litina D., Chatzioponlos C., Soloupis G. Synthesis and biological evolution of new 4, 5-disubstituted-thiazoylamides derivatives of 4-hydroxy-Piperidine or 4-N-methyl-Piperadine. Molecules. 2003;8:472–479. doi: 10.3390/80600472. [DOI] [Google Scholar]
- 2.Sup R.C., Sup R.Y., Bang C.W. Synthesis and antiinflammatory activity of [2-(benzothiazol-2-ylimino)-4-oxo-3-pheylthiazolidin-5-yl]-acetic acid derivatives. J. Korean Chem. Soc. 1995;93:237–240. [Google Scholar]
- 3.Sonwane S.K., Srivastava S.D. Synthesis and biological significance of 2-amino-4-phenyl-1, 3-thiazole derivatives. Proc. Natl. Acad. Sci. India. 2008;78:129–136. [Google Scholar]
- 4.Capan G., Ulusoy N., Ergenc N., Kiraz M. New 6-phenylimidazo[2,1-b]thiazole derivatives: Synthesis and antifungal activity. Monatsh. Chem. 1999;130:1399–1407. [Google Scholar]
- 5.Vigorita M., Ottana R., Monforte F., Maccari R., Trovato A., Monforte M.T., Ftaviano M. Synthesis and antiinflammatory, analgesic activity of 3,3′-(1,2-Ethanediyl)-bis[2-aryl-4-thiazolidinone] chiral compounds Part 10. Bioorg. Med. Chem. Lett. 2001;11:2791–2794. doi: 10.1016/s0960-894x(01)00476-0. [DOI] [PubMed] [Google Scholar]
- 6.Kavitha C., Basappa S., Nanjunda S., Mantelingu K., Doreswamy S., Sridhar M.A., Sprasad J., Rangappa K. Synthesis of new bioactive venlafaxine analogs: Novel thiazolidin-4-ones as antimicrobials. Bioorg. Med. Chem. 2006;14:2290–2299. doi: 10.1016/j.bmc.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Ottana R., Maccari R., Barreca M., Rotondo G., Rossi A., Chiricosta A., Paola G., Sautebin R., Cuzzocrea L., Vigorita S. Arylidene-2-imino-4-thiazolidinones: Design and synthesis of novel anti-inflammatory agents. Bioorg. Med. Chem. 2005;13:4243–4252. doi: 10.1016/j.bmc.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 8.Kucukguzel G., Kocatepe A., Clercq E., Sahin F., Gulluce M. Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem. 2006;41:353–359. doi: 10.1016/j.ejmech.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Al-Amiery A.A., Musa A.Y., Kadhum A.H., Mohamad A. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules. 2011;16:6833–6843. doi: 10.3390/molecules16086833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Amiery A., Kadhum A., Mohamad A. Antifungal activities of new coumarins. Molecules. 2012;17:5713–5723. doi: 10.3390/molecules17055713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Amiery A.A., Musa A.Y., Kadhum A.H., Mohamad A.B. The antioxidant activity of new coumarin derivatives. Int. J. Mol. Sci. 2011;12:5757–5761. doi: 10.3390/ijms12095747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ocal N., Yolacan C., Kaban S., Leonor Y., Vargas M., Kouznetsov V.J. Transformations of schiff bases derived from quinoline-8-carbaldehyde. Synthesis of C-8 substituted quinolines. J. Heterocycl. Chem. 2001;38:233–236. [Google Scholar]
- 13.Mendez L., Kouznetsov V., Poveda J., Yolaçan C., Ocal N., Aydogan F. Transformations of 4-N-arylamino-4-(8-quinolinyl)-1-butenes and 3-aryl-2-(8-quinolinyl)-4-thiazolidinones. Heterocycl. Commun. 2001;7:129–134. [Google Scholar]
- 14.Aydogan F., Ocal N., Turgut Z., Yolacan C. Synthesis of thiazolidino-fused compounds. Bull. Korean Chem. Soc. 2001;22:476–480. [Google Scholar]
- 15.Shashikant R., Prajact K., Nachiket S., Sunil A., Deepak S., Smita K., Daithankar A. Synthesis and biological evaluation of some 1, 3, 4-thiadiazoles. J. Chem. Pharm. Res. 2009;1:1191–1198. [Google Scholar]
- 16.Srivastava S.D. Synthesis and Antimicrobial Activity of Some 2-[(4-Substituted-Phenyl-3-Chloro- Azetidin-2-One)-5-(2′-Methylamino-4-Phenyl-1′,3′-Thiazolyl-]-1,3,4-Thiadiazoles. J. Sci. Islam. Repub. Iran. 2009;20:227–232. [Google Scholar]
- 17.Sharma S.C. Synthesis of new fungicides. 2-(4′-arylthiazolyl-2′-imino)-3-aryl-4-thiazolidones. Bull. Chem. Soc. Jpn. 1967;40:2422–2424. doi: 10.1246/bcsj.40.2422. [DOI] [PubMed] [Google Scholar]
- 18.Akerblom E.B. Synthesis and structureactivity relations of a series of antibacterially active 5-(5-nitro-2-furfurylidene)thiazolones, 5-(5-nitro-2-urylpropenylidene)thiazolones, and 6-(5-nitro-2-furyl)-4H-1,3-thiazinones. J. Med. Chem. 1974;17:609–615. doi: 10.1021/jm00252a008. [DOI] [PubMed] [Google Scholar]
- 19.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09 series programs-Revision A.1. Gaussian, Inc.; Wallingford, CT, USA: 2009. [Google Scholar]
- 20.Lee C., Yang W., Parr R. Synthesis and studying the antitumor activity of novel 5-(2-methylbenzimidazol-5-yl)-1,3,4-oxadiazole-2(3H)-thiones. Phys. Rev. B. 1988;37:785–803. [Google Scholar]
- 21.Weigend F., Ahlrichs R. Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005;7:3297–3304. doi: 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- 22.Şahin F., Karaman I., Güllüce M., Öğütçü H., Şengül M., Adigüzel A., Öztürk S., Kotan R. Evaluation of antimicrobial activities of Satureja hortensis L. J. Ethnopharmacol. 2003;85:231–238. doi: 10.1016/S0378-8741(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 23.Soares J., Dins T., Cunha A., Ameida L. Antioxidant activities of some extracts of thymus zygis. Free Radical Res. 1997;26:469–476. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- 24.Güven K., Yücel E., Çetintaş F. Antimicrobial activities of fruits of crataegus and pyrus species. Pharm. Biol. 2006;44:79–86. doi: 10.1080/13880200600591253. [DOI] [Google Scholar]
- 25.Tepe B., Daferera D., Sokmen A., Sokmen M., Polissiou M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae) Food Chem. 2005;90:333–339. doi: 10.1016/j.foodchem.2003.09.013. [DOI] [Google Scholar]