Abstract
Preliminary investigations on 14 plant extracts (obtained by ethanolic and aqueous extraction) identified those having high antioxidant and a significant total phenolic content. Antihyperglycemic, α-amylase and α-glucosidase inhibition activities were also observed. A correlation between the antihyperglycemic activity, total phenolic content and antioxidant (DPPH scavenging) activity was established. To further substantiate these findings, the possibility of tannins binding non-specifically to enzymes and thus contributing to the antihyperglycemic activity was also investigated. Our study clearly indicated that the antihyperglycemic activity observed in the plant extracts was indeed not due to non-specific tannin absorption.
Keywords: type 2 diabetes mellitus, α-glucosidase, α-amylase, antioxidant, total phenolic content
1. Introduction
Diabetes mellitus is a metabolic disease characterized by hyperglycemia resulting from defects in insulin action, insulin secretion or both. The most common type of diabetes mellitus is type 2 diabetes mellitus, which accounts for 85 to 95% of all cases and constitutes a major and growing public health problem [1]. Type 2 diabetes mellitus is generally managed through a stepwise program of intensive therapy that consists of lifestyle and sequential addition of oral antihyperglycemic agents (OHAs) and insulin as necessary. One therapeutic approach to decrease postprandial hyperglycemia in diabetic state is by retarding absorption of glucose through inhibition of carbohydrate hydrolyzing enzymes, like α-glucosidase and α-amylase, in the digestive tract [1,2]. These drugs however risk inducing hypoglycemia and over time, lose their efficacy, have prominent side effects and fail to significantly alter the course of diabetic complications.
Plants have been suggested as a rich, source of potentially useful antidiabetic drugs [3]. Recent scientific investigations have confirmed the efficacy of many of these preparations, some of which are remarkably effective [4,5]. Although there is widespread use of herbal dietary supplements that are believed to benefit type 2 diabetes mellitus, but still there is a great interest in the development of new drugs to prevent type 2 diabetes mellitus with minimal or without any side effects and to evaluate natural products in experimental studies [5,6,7]. The objective of this study was to investigate a number of tropical plant extracts for their antioxidant ability (DPPH radical scavenging activity), antihyperglycemic activity (as α-glucosidase and α-amylase inhibitors) and as well as determine the total phenolic content. We also studied the correlation between antioxidant ability and antihyperglycemic, α-glucosidase and α-amylase inhibition activity.
2. Results and Discussion
2.1. Antihyperglycemic and Antioxidant Ability of Tropical Plant Extracts
The details of the selected tropical plants used in this study are presented in Table 1. The antihyperglycemic activity, antioxidant activity and total phenolic content of selected tropical plants extracted under both ethanolic and aqueous conditions were studied and results are as shown in Table 2 and Table 3.
Table 1.
The selected tropical plants; scientific name, parts, local name and family.
| Scientific Name | Parts | Local Name | Family |
|---|---|---|---|
| Azadirachta indica | Leaf | Neem | Meliaceae |
| Artocarpus champeden | Leaf | Cempedak | Moraceae |
| Garcinia mangostana | Rind | Mangosteen | Clusiaceae |
| Nephelium lappaceum | Rind and leaf | Rambutan | Sapindaceae |
| Peltophorum pterocarpum | Leaf, bark, pod and flower | Yellow flame tree | Fabaceae |
| Syzygium aqueum | Leaf | Water jambu, water apple | Myrtaceae |
| Syzygium cumini | Leaf, bark and seed | Java plum, jambolana | Myrtaceae |
| Vitis vinifera | Seed | Grape | Vitaceae |
Table 2.
1/EC50 of α-glucosidase, α-amylase and DPPH activity, TPC and yield of ethanolic extraction of various plant extracts.
| Plant Extracts | Part | Ethanol | ||||
|---|---|---|---|---|---|---|
| 1/EC50 (mg/mL) | TPC,GAE (mg/g) | % Yield of Extraction | ||||
| α-Glucosidase | α-Amylase | DPPH | ||||
| Azadirachta indica | Leaf | 39.8 ± 13.2 | 2.7 ± 0.9 | 1.2 ± 0.2 | 53.6 ± 12.5 | 14.4 ± 13.2 |
| Artocarpus champeden | Leaf | 91.7 ± 10.4 | ND * | 7.1 ± 1.1 | 556.2 ± 28.2 | 27.8 ± 9.6 |
| Garcinia mangostana | Rind | 200.0 ± 28.2 | 41.7 ± 3.2 | 11.9 ± 4.1 | 600.0 ± 34.9 | 22.0 ± 7.5 |
| Nephelium lappaceum | Rind | 370.4 ± 31.6 | 14.1 ± 8.5 | 15.7 ± 5.3 | 809.6 ± 53.2 | 33.2 ± 16.3 |
| Nephelium lappaceum | Leaf | 400.0 ± 45.7 | 31.2 ± 9.4 | 8.8 ± 1.2 | 321.0 ± 21.5 | 18.8 ± 7.3 |
| Peltophorum pterocarpum | Leaf | 292.3 ± 17.5 | 65.4 ±10.1 | 5.7 ± 13.2 | 256.2 ± 35.7 | 15.4 ± 2.4 |
| Peltophorum pterocarpum | Bark | 1,666.7 ± 596.4 | 400.0 ± 43.2 | 32.1 ± 18.6 | 797.6 ± 383.2 | 20.0 ± 11.2 |
| Peltophorum pterocarpum | Pod | 1,111.1 ± 857.3 | 49.3 ± 21.9 | 12.6 ± 10.2 | 372.6 ± 193.7 | 15.8 ± 7.5 |
| Peltophorum pterocarpum | Flower | 2,500.0 ± 966.8 | 123.5 ± 93.2 | 20.1 ± 17.3 | 594.0 ± 213.1 | 17.6 ± 12.4 |
| Syzygium aqueum | Leaf | 344.8 ± 175.3 | 23.6 ± 13.2 | 8.4 ± 5.6 | 265.4 ± 178.9 | 14.0 ± 12.5 |
| Syzygium cumini | Leaf | 769.2 ± 323.7 | 45.9 ± 28.2 | 10.9 ± 1.2 | 371.0 ± 197.6 | 1.0 ± 0.5 |
| Syzygium cumini | Bark | 400.0 ± 245.8 | 21.0 ± 17.3 | 6.6 ± 3.8 | 303.4 ± 153.8 | 2.8 ± 1.6 |
| Syzygium cumini | Seed | 625.0 ± 254.7 | 9.2 ± 75.8 | 9.1 ± 8.2 | 375.4 ± 268.1 | 8.0 ± 5.7 |
| Vitis vinifera (Grape) | Seed | 357.1 ±265.9 | 227.3 ± 145.9 | 34.8 ± 29.1 | 834.8 ± 475.5 | 2.6 ± 1.2 |
| Acarbose | 0.286 ± 0.08 | 83.3 ± 25.9 | ||||
* ND, Not detected.
Table 3.
1/EC50 of α-glucosidase, α-amylase and DPPH activity, TPC and yield of aqueous extraction of various plant extracts.
| Plant Extracts | Part | Aqueous | ||||
|---|---|---|---|---|---|---|
| 1/EC50 (mg/mL) | TPC GAE(mg/g) | % Yield of Extraction | ||||
| α-Glucosidase | α-Amylase | DPPH | ||||
| Azadirachta indica | Leaf | 3.8 ± 1.2 | ND | ND | 80.3 ± 34.5 | 19.0 ± 8.2 |
| Artocarpus champeden | Leaf | 44.8 ± 21.3 | 80.0 ± 27.8 | 7.6 ± 1.6 | 526.6 ± 115.7 | 9.4 ± 3.1 |
| Garcinia mangostana | Rind | 196.1 ± 65.5 | ND | 5.0 ± 1.5 | 282.2 ± 111.2 | 13.8 ± 7.2 |
| Nephelium lappaceum | Rind | 333.3 ± 192.5 | ND | 11.0 ± 8.2 | 460.6 ± 271.4 | 23.4 ± 15.7 |
| Nephelium lappaceum | Leaf | 322.6 ± 1.2 | ND | 6.3 ± 4.2 | 261.2 ± 91.7 | 9.2 ± 5.3 |
| Peltophorum pterocarpum | Leaf | 1,666.7 ± 986.1 | 55.9 ± 22.3 | 6.9 ± 1.7 | 333.2 ± 121.0 | 10.0 ± 4.3 |
| Peltophorum pterocarpum | Bark | 344.8 ± 226.4 | 370.4 ± 232.8 | 7.7 ± 2.5 | 306.8 ± 113.5 | 0.6 ± 0.04 |
| Peltophorum pterocarpum | Pod | ND * | ND | ND | 62.5 ± 10.1 | 1.4 ± 0.9 |
| Peltophorum pterocarpum | Flower | 434.8 ± 178.2 | ND | 6.7 ± 2.3 | 273.6 ± 173.1 | 10.2 ± 8.4 |
| Syzygium aqueum | Leaf | 370.4 ± 199.5 | 5.5 ± 2.8 | 3.4 ± 1.4 | 207.2 ± 96.5 | 4.8 ± 1.7 |
| Syzygium cumini | Leaf | 232.6 ± 191.3 | ND | 9.6 ± 2.5 | 301.6 ± 287.2 | 11.6 ± 9.7 |
| Syzygium cumini | Bark | 32.6 ± 11.1 | ND | 1.2 ± 0.5 | 152.7 ± 35.6 | |
| Syzygium cumini | Seed | 93.5 ± 23.5 | ND | 6.4 ± 1.9 | 267.4 ± 115.3 | |
| Vitis vinifera (Grape) | Seed | 256.4 ± 118.9 | ND | 2.9 ± 0.7 | 371.0 ± 224.5 | |
| Acarbose | 0.286 ± 0.03 | 83.3 ± 24.5 | ||||
* ND, Not detected.
Generally, it was observed that the ethanolic extracts displayed far higher antihyperglycemic, antioxidant activity and total phenolic content than the aqueous ones. This trend was also observed in the percentage of yield of extraction in almost all the extracts. The plant Peltophorum pterocarpum, particularly its bark and flower parts, was seen to exhibit extremely high α-glucosidase, α-amylase, antioxidant as well as total phenolic content. This was followed by the extracts from Syzygium cumini, Nephelium lappaceum, Syzygium aqueum and Garcinia mangostana. Interestingly, it was observed that these extracts displayed activities comparable and at times higher than the commercial antihyperglycemic drug acarbose, and commercial grape seed extract.
It is noteworthy that Syzygium cumini has been extensively used in the empirical treatment of diabetes mellitus [8,9,10]. In this study, we observed that Peltophorum pterocarpum (bark, pod and flower) displayed far better α-glucosidase and α-amylase inhibition activity compared to the Syzygium cumini (leaf, bark and seed). Recently, Garcinia mangostana (mangosteen) rind juice has been marketed as a healthy beverage under the trade name of XANGO and it has been reported possessing several biological activities, in particular high blood pressure and blood glucose level reducing effects [11]. In this study, Peltophorum pterocarpum (leaf, bark, pod and flower), Nephelium lappaceum (rind and leaf) and Syzygium aqueum (leaf) showed prominent antioxidant ability and far better α-glucosidase and α-amylase inhibition activity compared to Garcinia mangostana (rind). It is therefore seemed essential that further studies to identify bioactive compounds and carry out in vivo studies involving these plants are required.
2.2. Correlation between Total Phenolic Content, Antioxidant and Antihyperglycemic Activity
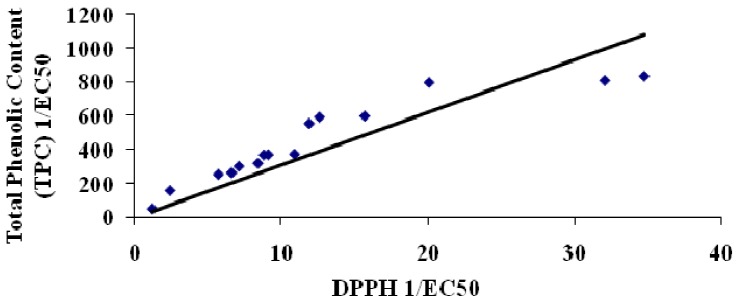
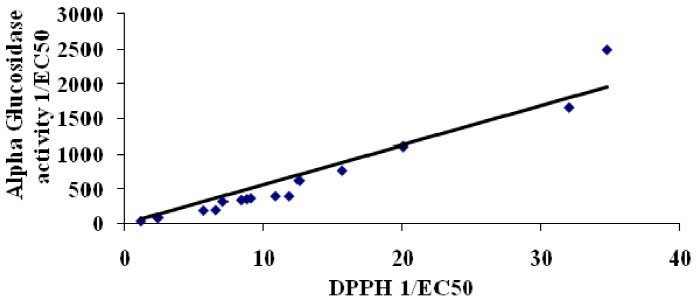
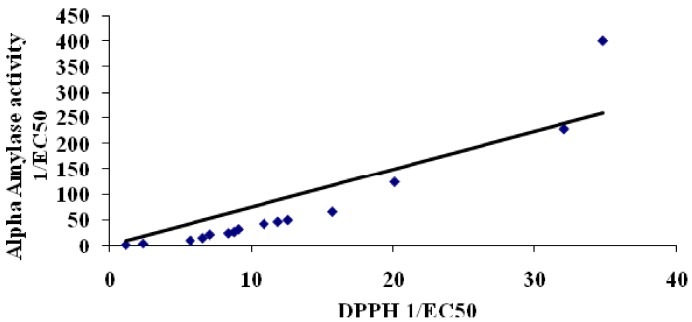
Correlations between total phenolic content and antioxidant activity of plant extracts have been established before [12,13]. In this study, we also established that the total phenolic content of the plant extracts displayed a highly significant correlation (R2 = 0.9218) with antioxidant ability (DPPH free radical scavenging activity, Figure 1). A similar study by Ling and coworkers [14] reported that selected Malaysian plant extracts displayed strong correlations between antioxidant ability and total phenolic content. In addition, the antihyperglycemic activity; both α-glucosidase (Figure 2) and α-amylase (Figure 3), also displayed a very strong correlation to antioxidant ability.
Silva Pinto and co-workers [15], have also shown a similar correlation of α-glucosidase activity and antioxidant in Gingko bilibo L. leaves extract. This is the first time a correlation between α-amylase activity and antioxidant ability is described.
Figure 1.
Correlation between Total Phenolic Content and DPPH assay (antioxidant). Correlation = 0.9218.
Figure 2.
Correlation between α-glucosidase assay (antihyperglycemic) and DPPH assay (antioxidant). Correlation = 0.9734.
Figure 3.
Correlation between α-amylase assay (antihyperglycemic) and DPPH assay (antioxidant). Correlation = 0.9469.
2.3. Tannins Interference in Antihyperglycemic Activity
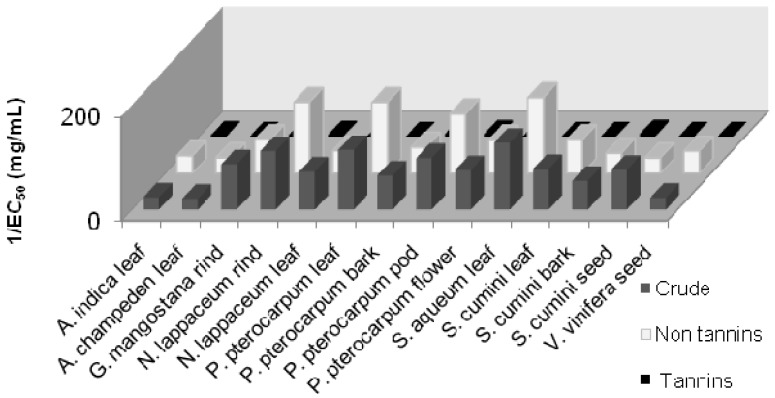
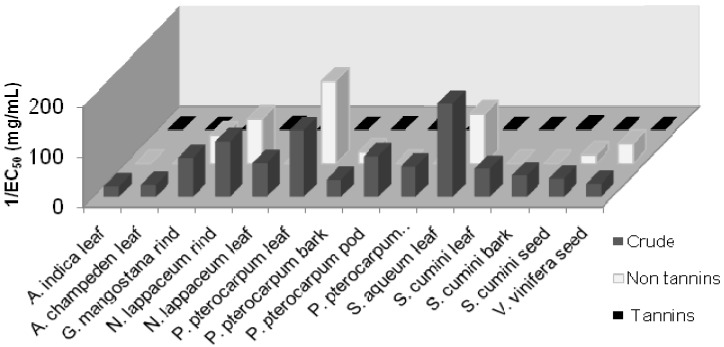
In our efforts to ensure that the α-glucosidase and α-amylase activity in the plant extracts are not due to non-specific absorption of tannins present in the extract, we removed the tannins present in the ethanolic extracts and re-assayed for its α-glucosidase and α-amylase inhibition activities. Figure 4 and Figure 5 show that the tannin extracts do not have any α-glucosidase and α-amylase inhibition activity while both the crude and non-tannin extracts still display significant α-glucosidase and α-amylase inhibition activity.
Figure 4.
α-Glucosidase inhibition activity in the crude, non-tannin, and tannin extracts of various plants.
Figure 5.
α-Amylase inhibition activity in the crude, non-tannin, and tannin extracts of various plants.
These results clearly indicate that the antihyperglycemic activity seen in the plant extracts is not due to the tannins.
3. Experimental
3.1. Plant Collection and Extraction
The 14 local plant parts (rind, bark, leaf, seed, pod, and flower) were obtained from different places in Malaysia. The plants were authenticated by the Herbarium of the Forest Research Institute of Malaysia (FRIM). The plant extracts were standardized using a HPLC method. The method used to prepare the extracts was effectively the same as previously described by [2].
3.2. α-Glucosidase and α-Amylase Inhibition Assays
The α-glucosidase and α-amylase inhibition assays was performed using the modified method of [16]. Pancreatic α-amylase (porcine based) and intestinal α-glucosidase (from Saccharomyces cerevisiae) were the enzymes used in this study. These enzymes are mainly involves in the digestion of carbohydrate. Inhibition of these enzymes will slower down absorption of glucose into blood thus will reduce postprandial hyperglycemia. Inhibition activity of these enzymes was performed using α-amylase and α-glucosidase colorimetric assays [2]. The absorbance of the inhibition activity of these enzymes was measured using microplate reader-end point reading (BioRad) at appropriate wavelengths; 400 nm (α-glucosidase) and 540 nm (α-amylase). The percentage inhibition was calculated as follows:
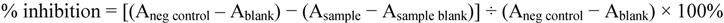 |
where A = absorbance.
The activity of the plant extracts were assessed by plotting percentage inhibition against a range of plant extracts concentrations. EC50 value (effective concentration with 50% inhibition) was thus determined and expressed as means ± SEM of the triplicate measurements.
3.3. Antioxidant Assay (DPPH Scavenging Activity)
The DPPH free radicals scavenging activity was assessed according to the modified method of [17]. The percentage inhibition and EC50 value was determined as described above.
3.4. Determination of Total Phenolic Content (TPC)
The total phenolic content was determined by using the Folin-Ciocalteu method modified according to [18]. The percentage inhibition and EC50 value was determined as described above.The content of phenolic compounds in a respective sample was expressed in mg/g of extract, Gallic acid equivalent (GAE).
3.5. Precipitation of Tannins by Hide Powder
The method used to remove tannins from plant extracts are based on the adsorption of these compounds onto proteins [19]. Hide powder is used in this study, where 0.06 g is added into 2 mL of crude extract and stirred for 2 h. The tannin adsorbed hide powder is filtered using a membrane funnel (0.02 µm) and filtrate evaporated to evaluate its concentration, the solution is a tannin-free ethanol extract.
4. Conclusions
It was concluded that ethanolic extracts of a variety of Malaysian plant parts displayed far better antihyperglycemic (α-glucosidase and α-amylase), antioxidant (DPPH scavenging ability) and total phenolic content than the corresponding aqueous extracts. Among the 14 plant extracts tested, Peltophorum pterocarpum (bark and flower parts) exhibited extremely high α-glucosidase, α-amylase and antioxidant, as well as total phenolic content. The antihyperglycemic activity of this plant extract was seen to be far better than that of the antidiabetic drug acarbose. In addition, there was a strong correlation between the antihyperglycemic (α-glucosidase and α-amylase inhibition activity) and antioxidant ability. This is the first study showing a correlation between α-amylase inhibition activity and antioxidant ability of these plant extracts. Finally, we confirmed that the antihyperglycemic activity of the plant extracts was not due to the non-specific absorption of tannins.
Acknowledgements
This research work was supported in part by Research Grants from the Ministry of Science, Technology and Innovation. Special thanks to Thavamanithevi Subramaniam (SIRIM Berhad), Theanmalar Masilamani (SIRIM Berhad) and Lim Chuan Gee (SIRIM Bhd).
Footnotes
Sample Availability: Samples are available from the authors T. Manaharan and U. Devi Palanisamy.
References and Notes
- 1.Cheplick S., Kwon Y.I., Bhowmik P., Shetty K. Phenolic linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresour. Technol. 2010;101:404–413. doi: 10.1016/j.biortech.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 2.Manaharan T., Ling L.T., Appleton D., Cheng H.M., Masilamani T., Palanisamy U. Antioxidant and antihyperglycemic potential of Peltophorum pterocarpum plant parts. Food Chem. 2011;129:1355–1361. doi: 10.1016/j.foodchem.2011.05.041. [DOI] [Google Scholar]
- 3.Mukherjee P.K., Maiti K., Mukherjee K., Houghton P.J. Leads from Indian medicinal plants with hypoglycemic potentials. J. Ethnopharmacol. 2006;106:1–28. doi: 10.1016/j.jep.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Ali H., Houghton P.J., Soumyanath A. Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006;107:449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Saha S., Verma R. Inhibitory potential of traditional herbs on α-amylase activity. Pharm. Biol. 2012;50:326–331. doi: 10.3109/13880209.2011.608075. [DOI] [PubMed] [Google Scholar]
- 6.Gowri P.M., Tiwari A.K., Ali A.Z., Rao J.M. Inhibition of alpha-glucosidase and amylase by bartogenic acid isolated from Barringtonia racemosa Roxb. Seeds. Phytother. Res. 2007;21:796–799. doi: 10.1002/ptr.2176. [DOI] [PubMed] [Google Scholar]
- 7.Funke I., Melzig M.F. Effect of different phenolic compounds on alpha-amylase activity: Screening by microplate-reader based kinetic assay. Pharmazie. 2005;60:796–797. [PubMed] [Google Scholar]
- 8.Kumar A., Illavarasan R., Jayachandran T., Decaraman M., Aravindan P., Padmanabhan N., Krishan M.R.V. Antidiabetic activity of Syzygium cumini and its isolated compound against streptozotocin-induced diabetic rats. J. Med. Plants Res. 2008;2:246–249. [Google Scholar]
- 9.Helmstadter A. Syzygium cumini (L.) skeels (Mrytaceae) against diabetes-125 years of research. Pharmazie. 2008;63:91–101. [PubMed] [Google Scholar]
- 10.Karthic K., Kirthiram K.S., Sadasivam S., Thayumanavan B. Identification of alpha amylase inhibitors from Syzygium cumini Linn seeds. Indian J. Exp. Biol. 2008;46:677–680. [PubMed] [Google Scholar]
- 11.Wong L.P., Klemmer P.J. Severe lactic acidosis associated with juice of the mangosteen fruit Garcinia mangostana. Am. J. Kidney Dis. 2008;51:829–833. doi: 10.1053/j.ajkd.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 12.Gao X., Bjork L., Trajkovski V., Uggla M. Evaluation of antioxidant activities of rosehip ethanol extracts in different test systems. J. Sci. Food Agric. 2000;80:2021–2027. doi: 10.1002/1097-0010(200011)80:14<2021::AID-JSFA745>3.0.CO;2-2. [DOI] [Google Scholar]
- 13.Ling L.T., Yap S.A., Radhakrishnan A.K., Subramaniam T., Cheng H.M., Palanisamy U.D. Standardised Mangifera indica extract is an ideal antioxidant. Food Chem. 2009;113:1154–1159. doi: 10.1016/j.foodchem.2008.09.004. [DOI] [Google Scholar]
- 14.Ling L.T., Radhakrishnan A.K., Subramaniam T., Cheng H.M., Palanisamy U.D. Assessment of antioxidant capacity and cytotoxicity of selected Malaysian plants. Molecules. 2010;15:2139–2151. doi: 10.3390/molecules15042139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva Pinto M.D., Kwon Y.I., Apostolidis E., Lajolo F.M., Genovese M.I., Shetty K. Potential of Gingko biloba L. leaves in the management of hyperglycemia and hypertension using in vitro models. Bioresour. Technol. 2009;100:6599–6609. doi: 10.1016/j.biortech.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Liu J.P., Zhang M., Wang W.Y., Grinsgard S. Chinese herbal medicines for type-2-diabetes mellitus. J. Ethnopharmacol. 2004;115:173–183. [Google Scholar]
- 17.Choi C.W., Kim S.C., Hwang S.S., Choi B.K., Ann H.J., Lee M.Y. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay guided comparison. Plant Sci. 2002;63:1161–1168. [Google Scholar]
- 18.Miliauskas G., Venskutonis P.R., van Beek T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2005;85:231–237. [Google Scholar]
- 19.Mekhfi H., Elhaouari M., Bnouham M., Aziz M., Ziyyat A., Legssyer A. Effects of extracts and tannins from Arbutus unedo leaves on rat platelet aggregation. Phytother. Res. 2006;20:135–139. doi: 10.1002/ptr.1822. [DOI] [PubMed] [Google Scholar]