Abstract
In this study, the antinociceptive properties of 3,4-dihydro-2,6-diaryl-4-oxo-pyrimidine-5-carbonitrile derivatives 5a–i at doses of 25 and 50 mg/kg were evaluated in mice, using the abdominal constriction test. Molecular modeling studies were also performed using density functional theory calculations. These data provided information about the electrostatic and ionization potentials and were used to compare the antinociceptive activity of the title compounds. The most active compounds were 3,4-dihydro-2-(4-chlorophenyl)-6-(4-methoxyphenyl)-4-oxo-pyrimidine-5-carbonitrile (5b) and 3,4-dihydro-2,6-diphenyl-4-oxo-pyrimidine-5-carbonitrile (5i), which inhibited the number of abdominal constrictions, at 50 mg/kg dose, in 88.6% and 88% of the sample, respectively. A preliminary SAR study demonstrated that halogen replacement in the phenyl rings of the compounds under study reduces the antinociceptive activity. DFT calculations showed that there is a high correlation between the ionization potentials and the analgesic properties of the compounds. It was found that compounds with a positive ionization potential (compounds 5b and 5i) were found to be the best analgesic drugs in this series.
Keywords: 4-(3H)-pyrimidinones, antinociceptive activity, molecular modeling, density functional theory
1. Introduction
Pyrimidinone derivatives are well-known for their pharmacological properties [1,2]. These compounds, structurally related to nucleic acids, have been reported to be anticancer [3,4,5,6,7], interferon inducer [8], antiviral [9,10], anti-hypertensive [11,12], hypoglycaemic [13,14], anticonvulsive [15], anti-histaminic [16], analgesic and antiinflammatory drugs [17,18,19,20,21,22,23].
Several methodologies are available for synthesizing this pharmacologically interesting class of heterocycles. Most of them make use of a condensation reaction between a Michael intermediate and amidines [24], guanidines [25], urea [26], thiourea [27], methylisourea and methylisothiourea [9,10], in the presence of organic bases as catalysts.
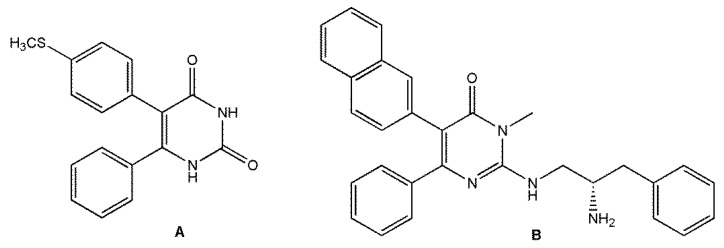
As cited above, the pyrimidinone scaffold is present in many analgesic and anti-inflammatory agents [17,18,19,20,21,22,23]. In fact, it has been reported that these substances can modulate the activity of important molecules involved in the inflammatory process, such as COX-2 or protein kinase (p38) modulators [17,18,19,28,29,30,31,32], which could support the pharmacological findings. It is well established that inflammation can stimulate many intracellular signalling pathways, including the p38 pathway, which is considered to be a central regulator of inflammation. For example, COX-2 is a mediator regulated by p38 protein kinase. Regulation of the p38 pathway thus leads to control of the inflammatory mediators and inhibition of the inflammation process [30,31,32]. Figure 1 shows the structures of two pyrimidinone derivatives capable of modulating the inflammatory response, where A is considered a COX-2 selective antagonist and B is a p38 pathway inhibitor [28,29,30,31,32].
Figure 1.
Pyrimidinone derivatives as anti-inflammatory drugs.
Because of the aforementioned pharmacological potential of this class of compounds, our group has been involved in synthesizing and testing the pharmacological activities of pyrimidinone and pyrimidine derivatives, for the purposes of drug discovery and development [17,18,19,24,27,33,34,35]. Recently, we have synthesized and evaluated the acute toxicity, and anti-edematogenic and antinociceptive activities of 3,4-dihydro-2-phenyl-6-para-fluorophenyl-4-oxo-pyrimidine-5-carbo-nitrile, a prototype for this class of compounds, which has shown promising pharmacological activities and low toxicity [19].
The present study thus assesses the antinociceptive activity of a series of previously synthesized pyrimidinones using the abdominal constrictions test. Since the pyrimidinone ring exhibits good analgesic activity, our goal was to produce pharmacologically superior compounds by making changes in the scaffold. In total, nine molecules containing the pyrimidinone ring were tested. We also carried out DFT calculations in order to associate the antinociceptive activity of these compounds with their electronic surfaces through molecular modelling tools.
2. Results and Discussion
2.1. Antinociceptive Activity
The results regarding antinociceptive activity are displayed in Table 1. It can be seen that the most active compounds are 5b and 5i, at the dose of 50 mg/kg, and that the antinociceptive properties of some pyrimidinone derivatives (compounds 5b,d,i) are as good as or even better than the reference drug (indomethacin).
Table 1.
Antinociceptive activity of 5a–i and indomethacin in the acetic acid writhing test. Data are expressed as the mean ± SD for the dose-response profile of six animals.
| Compd. | Dose (mg/kg) | Antinociceptive activity (%) |
|---|---|---|
| 5a | 25 | - |
| 50 | 71.5 ± 6.9 | |
| 5b | 25 | 49.5 ± 9.9 |
| 50 | 88.6 ± 3.4 | |
| 5c | 25 | - |
| 50 | 75.1 ± 5.8 | |
| 5d | 25 | 68.8 ± 8.2 |
| 50 | 86.0 ± 4.1 | |
| 5e | 25 | 56.4 ± 11.9 |
| 50 | 73.9 ± 10.0 | |
| 5f | 25 | 22.1 ± 16.1 |
| 50 | 71.4 ± 8.5 | |
| 5g | 25 | 57.7 ± 9.9 |
| 50 | 70.0 ± 5.4 | |
| 5h | 25 | 47.4 ± 13.0 |
| 50 | 61.0 ± 13.7 | |
| 5i | 25 | 85.4 ± 4.6 |
| 50 | 88.0 ± 4.0 | |
| Indomethacin | 10 | 76.3 ± 4.8 |
In this study, antinociceptive activity was evaluated using acetic acid induced stimuli. The acetic acid writhing test is a peripheral and visceral nociception model, consisting of high intensity stimuli and rapid nociceptive response [36]. Acetic acid-induced effects can be eliminated using a wide range of analgesics, including non-steroidal anti-inflammatory drugs (such as indomethacin). Most non-steroidal anti-inflammatory drugs act as non-selective antagonists of the enzyme, cyclooxygenase, inhibiting both the cyclooxygenase-1 and cyclooxygenase-2 isoforms. These enzymes catalyze the production of inflammatory messengers from arachidonic acid and these mediators can play the role of messengers in the inflammation process.
Almost all drugs, at 25 and 50 mg/kg doses, significantly reduced the number of abdominal constrictions. It can also be seen, in all cases, that the analgesic effect was dose-dependent. Earlier studies have shown that this type of nucleus shows anti-inflammatory activity in animal models [17,18,19,20,21,22,23,28,29]. In view of these results and a comparison with the results found in earlier studies [17,18,19,28,29], we suggest that pyrimidinone compounds can interfere with the acute inflammatory response induced by acetic acid. Therefore, the title compounds might inhibit or even modulate the migration or production of chemical mediators in the inflammatory site [37]. The compounds described here may act as COX inhibitors, as described earlier in the literature for other pyrimidinone compounds [17,18,19,28,29]. However, from the data obtained in the present study, we are not able to determine the exact mechanism involved in the antinociceptive effects produced by these compounds. More specific and detailed studies therefore need to be carried out to better understand the pharmacological mechanisms involved.
It can be seen that the most active compounds are 5b and 5i, which exhibited more than 88% of antinociceptive activity in relation to the negative control group. These numbers are as significant as those observed for indomethacin (approximately 76%). In contrast to these findings, we found that compound 5h was the least active of this series. These results indicate that the replacement of hydrogen atoms in the phenyl rings by fluorine atoms decreases the biological activity (5i against 5h). This can be extended to 5f, which was less active than 5i, but better than 5h. This clearly demonstrates that the replacement of hydrogen atoms by fluorine atoms in both phenyl rings is even more prejudicial than introducing a fluorine atom into one ring. In the halogen-containing compounds, a chlorine substitution for a hydrogen atom seems to be less harmful than fluorine substitution in terms of antinociceptive activity (5e against 5f and 5b against 5g). The other interesting feature is that the electron-donating groups (EDG), such as the methoxy group at R1, do not alter these properties (5d against 5i and 5b against 5d). However, the compound becomes less active when R2 = OCH3 (5a and 5c against 5d).
2.2. Molecular Modeling Studies
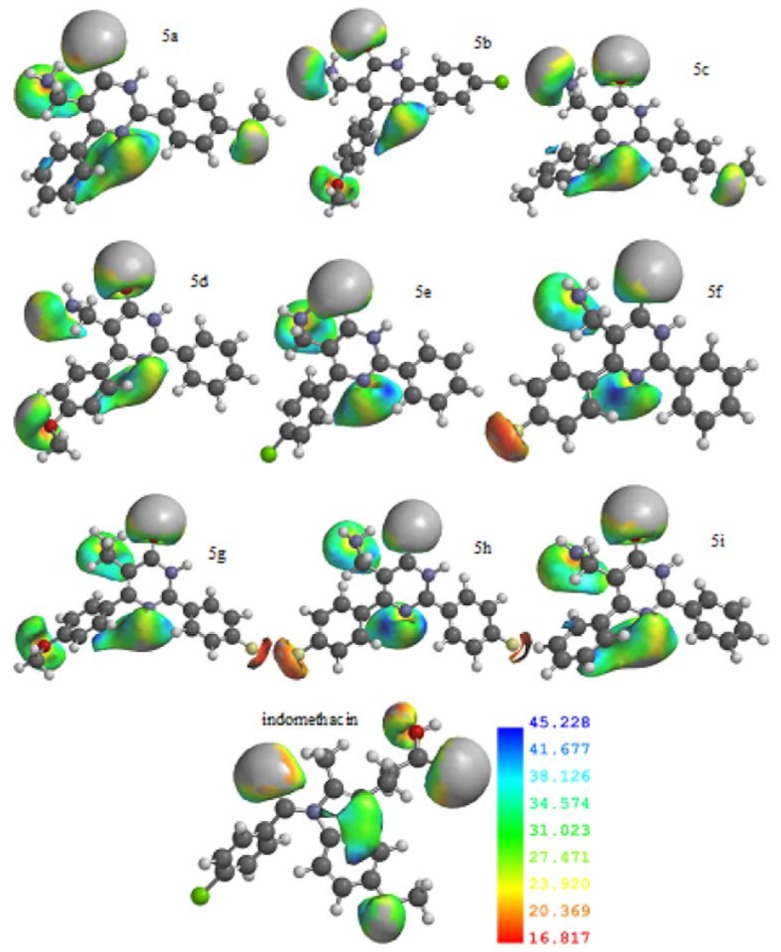
It was confirmed that the ionization potential is closely related to the antinociceptive activity exhibited by the pyrimidinone derivatives. Of the electronic surfaces calculated here using DFT, the ionization potential showed the greatest significant differences between the most and least active compounds. These calculations for the local ionization potentials of compounds 5a–i can be visualized in the tube model representation (Figure 2). The atoms are represented by colors: carbon (gray), nitrogen (blue), oxygen (red), chlorine (green), fluorine (yellow) and hydrogen (white).
Figure 2.
Comparative local ionization potentials of compounds 5a–i and indomethacin.
We found that compounds 5f, 5g and 5h show negative ionization potential, (orange-red regions), with 5h being the less active compound. On the other hand, good antinociceptive compounds, particularly 5b and 5i, have positive ionization potentials (blue-green regions), similar to the reference drug (indomethacin). This can be clearly seen in 5b, the most active compound.
This simple molecular modeling study showed how this calculation tool can be useful in predicting the particular activity of an investigative series of compounds. In conclusion, DFT calculations showed that there is a high correlation between the ionization potential and the biological activity exhibited by the title compounds.
3. Experimental
3.1. 3,4-Dihydro-2,6-diaryl-4-oxo-pyrimidine-5-carbonitriles
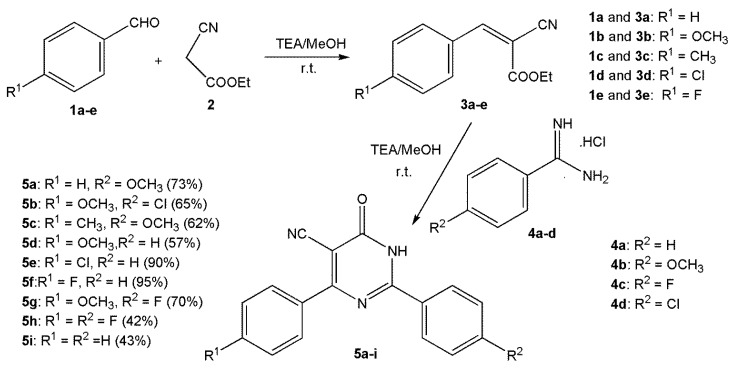
Compounds 5a–i used in this study were synthesized earlier by our research group [24]. A schema showing the synthesis of this series of compounds is shown below (Scheme 1). Indomethacin (reference drug) was purchased from Sigma-Aldrich.
Scheme 1.
Synthesis of compounds 5a–i.
3.2. Animals
Swiss male mice weighing 30 ± 5 g were used for pharmacological assays. They were kept under standard environmental conditions (22 ± 2 °C; 12:12 h dark/light cycle). Water and food (Labina®, Purina, Brazil) were provided ad libitum. The experimental protocols were approved by the Animal Experimentation Ethics Committee of the Universidade Federal de Pernambuco, UFPE (Process n° 015683/2005-34) and are in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
3.3. Antinociceptive Activity
The pharmacological tests were preceded by acute toxicity assays in mice in order to determine the doses to be used in this work [19]. The abdominal constrictions test was used to assess the antinociceptive activity of the synthesized compounds [38,39]. In this test, abdominal wall muscle constrictions and elongations, often followed by a characteristic hind-limb extension, are induced by acetic acid. The animals were kept without food overnight and divided in groups of six animals. The pyrimidinone derivatives were individually dissolved in a prepared 2.5% Tween 80 solution diluted in NaCl 0.9%. The drugs (compounds 5a–i) were given in the corresponding dose (25 or 50 mg/kg) intraperitoneally. After 15 minutes, the animals received the 0.8% acetic acid solution (1 mL/kg, intraperitoneally) and the writhes were counted for 30 minutes. The negative control group received the vehicle (2.5% Tween 80 in NaCl 0.9%), whereas the positive control group received indomethacin (10 mg/kg) before injection of acetic acid. The results were expressed as the reduction in the number of abdominal constrictions in relation to the negative control group.
3.4. Computational Methods
The Hyperchem v. 8.0 program [40] was used to draw the chemical structures of the compounds under study and their geometry optimized using an MM+ force field [41]. A new geometry optimization was then performed using the semi-empirical method AM1 (Austin Model 1) [42]. The optimized structures were subjected to conformational analysis using the random search method [43,44] and the options for 1,000 interactions and 100 cycles of optimization, and 10 was established as the number of conformers of lowest minimum energy. The selected dihedrals were evaluated in rotation in accordance with the standard conditions (default) of the program, i.e., number of simultaneous variations from 1 to 8; acyclic chains were submitted to rotations from 60 to 180° and ring torsions in the range of 30 to 120°.
The conformer of lowest minimum energy was selected, saved as .mol and exported to the Spartan 8 program [45]. The electrostatic potential, ionization potential and the HOMO and LUMO orbital surface of all compounds were obtained. To obtain and compare the surfaces of the ionization potential we adopted the range from 16,000 (red) to 45,000 (blue), and for comparison of the electrostatic potential maps, −284,000 (red) to 164,000 (blue).
4. Conclusions
All pyrimidinone derivatives tested presented good antinociceptive activity, with a dose-dependent pattern. The most active compounds were 5b and 5i, at the dose of 50 mg/kg of body weight. Our results have shown that replacement of the hydrogen atom by fluorine in the phenyl rings of 3,4-dihydro-2,6-diaryl-4-oxo-pyrimidine-5-carbonitriles causes a reduction in analgesic activity. The comparative study of molecular modeling corroborated pharmacological trials, showing a high correlation between ionization potential and analgesic activity. The compounds that showed positive ionization potential (i.e., 5b and 5i) were also the most active in the antinociceptive activity tests.
Acknowledgments
We are indebted to the Brazilian National Research Council (CNPq) for financial support. One of us (L. Scotti) is thankful to this organization for a fellowship. The authors thank PRONEX/FACEPE and UEPB (Research and Post-Graduation Incentive Program/PRPGP). We also thank Rejane de Souza Silva for technical assistance during the pharmacological tests.
Conflict of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds 5a–i are available from the authors.
References and Notes
- 1.Brown D.J. Pyrimidines and their benzoderivatives. In: Katritzky A.R., Rees C.W., editors. Comprehensive Heterocyclic Chemistry. Volume 3. Pergamon Press; Oxford, UK: 1984. pp. 57–155. [Google Scholar]
- 2.Singh A.K. Analytical Reactions of Substituted Pyrimidines. Talanta. 1982;29:95–102. doi: 10.1016/0039-9140(82)80027-1. [DOI] [PubMed] [Google Scholar]
- 3.Stringfellow D.A. Antineoplastic properties of pyrimidinone interferon inducers. Adv. Enzyme Regul. 1981;19:335–348. doi: 10.1016/0065-2571(81)90023-6. [DOI] [PubMed] [Google Scholar]
- 4.Marquet R.L., Eggermont A.M.M., de Bruin R.W.F., Fiers W., Jeekel J. Combined treatment of colon adenocarcinoma in rats with tumor necrosis factor and the interferon inducer ABPP. J. Interferon Res. 1988;8:319–323. doi: 10.1089/jir.1988.8.319. [DOI] [PubMed] [Google Scholar]
- 5.Li L.H., Wallace T.L., Wierenga W., Skulnick H.I., DeKoning T.F. Antitumor activity of pyrimidinones, a class of small-molecule biological response modifiers. J. Biol. Response Mod. 1987;6:44–55. [PubMed] [Google Scholar]
- 6.Scheringa M., Ijzermans J.N., Jeekel J., Marquet R.L. The antitumor activity of the interferon inducer bropirimine is partially mediated by endogenous tumor necrosis factor α. Cancer Immunol. Immunother. 1990;32:251–255. doi: 10.1007/BF01741709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu M., Oh-Hashi F., Tsukagoshi S., Iwaguchi T., Kataoka T. In vitro and in vivo antitumor activity of the interferon inducer bropirimine. Anticancer Drugs. 1995;6:158–162. doi: 10.1097/00001813-199502000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Vroegop S.M., Chapman D.L., Decker D.E., Galinet L.A., Brideau R.J., Ready K.A., Dunn C.J., Buxser S.E. Pharmacokinetic properties, induction of interferon, and efficacy of selected 5-halo-6-phenyl pyrimidinones, bropirimine analogues, in a model of severe experimental autoimmune encephalomyelitis. Int. J. Immunopharmacol. 1999;21:647–662. doi: 10.1016/S0192-0561(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 9.Saladino R., Ciambecchini U., Maga G., Mastromarino P., Conti C., Botta M.A. A new and efficient synthesis of substituted 6-[(2′-dialkylamino)ethyl] pyrimidine and 4-N,N-dialkyl-6-vinyl-cytosine derivatives and evaluation of their anti-rubella activity. Bioorg. Med. Chem. Lett. 2002;10:2143–2153. doi: 10.1016/S0968-0896(02)00077-9. [DOI] [PubMed] [Google Scholar]
- 10.de Lucca G.V., Liang J., de Lucca I. Stereospecific synthesis, structure-activity relationship, and oral bioavailability of tetrahydropyrimidin-2-one HIV protease inhibitors. J. Med. Chem. 1999;42:135–152. doi: 10.1021/jm9803626. [DOI] [PubMed] [Google Scholar]
- 11.Bernhart C.A., Haudricourt F.B., Assens J.L., Gougat J., Lacour C., Roccon A., Cazaubon C., Brelière J.C., Le Fur G., Nisato D. Cyclopentanespiro-3H-dihydro-pyrimidinones as Angiotensin II AT1 receptor antagonists. Bioorg. Med. Chem. Lett. 1994;4:157–162. doi: 10.1016/S0960-894X(01)81139-2. [DOI] [Google Scholar]
- 12.Salimbeni A., Canevotti R., Paleari F., Poma D., Caliari S., Fici F., Cirillo R., Renzetti A.R., Subissi A., Belvisi L., Bravi G., Scolastico C., Giachetti A. N-3-substituted pyrimidinones as potent, orally active, AT1 selective angiotensin II receptor antagonists. J. Med. Chem. 1995;38:4806–4820. doi: 10.1021/jm00024a008. [DOI] [PubMed] [Google Scholar]
- 13.Madhavan G.R., Chakrabarti R., Vikramadithyan R.K., Mamidi R.N., Balraju V., Rajesh B.M., Misra P., Kumar S.K., Lohray B.B., Lohray V.B., Rajagopalan R. Synthesis and biological activity of novel pyrimidinone containing thiazolidinedione derivatives. Bioorg. Med. Chem. 2002;10:2671–2680. doi: 10.1016/S0968-0896(02)00107-4. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi M., Wakasugi K., Saito R., Adachi Y., Yoshikawa Y., Sakurai H., Katoh A. Syntheses of vanadyl and zinc(II) complexes of 1-hydroxy-4,5,6-substituted 2(1H)-pyrimidinones and their insulin-mimetic activities. J. Inorg. Biochem. 2006;100:260–269. doi: 10.1016/j.jinorgbio.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 15.White D.C., Greenwood T.D., Downey A.L., Bloomquist J.R., Wolfe J.F. Synthesis and anticonvulsant evaluation of some new 2-substituted-3-arylpyrido[2,3-d]pyrimidinones. Bioorg. Med. Chem. 2004;12:5711–5717. doi: 10.1016/j.bmc.2004.07.068. [DOI] [PubMed] [Google Scholar]
- 16.Temple D.L., Yevich J.P., Covington R.R., Hanning C.A., Seidehamel R.J., Mackey H.K., Bartek M.J. Synthesis of 3,4-dihydro-4-oxothieno[2,3-d]pyrimidine-2-carboxylates, a new series of orally active antiallergy agents. J. Med. Chem. 1979;22:505–510. doi: 10.1021/jm00191a009. [DOI] [PubMed] [Google Scholar]
- 17.Ranise A., Bruno O., Bondavalli F., Schenone S., D’Amico M., Falciani M., Filippelli W., Rossi F. 5-Substituted 2,3-dihydro-6-mercapto-1,3-diphenyl-2-thioxo-4(3H)-pyrimidinones and their 6-(acylthio) derivatives with platelet antiaggregating, antiinflammatory, antiarrhythmic, antihyperlipidemic and other activities. Farmaco. 1994;49:551–558. doi: 10.1002/chin.199518174. [DOI] [PubMed] [Google Scholar]
- 18.Abignente E., Sacchi A., Laneri S., Rossi F., D’Amico M., Berrino L., Calderaro V., Parrillo C. Research on heterocyclic compounds. XXXII. Synthesis and cyclooxygenase-independent antiinflammatory and analgesic activity of imidazo[1,2-a]pyrimidine derivatives. Eur. J. Med. Chem. 1994;29:279–286. doi: 10.1016/0223-5234(94)90097-3. [DOI] [Google Scholar]
- 19.dos Anjos J.V., Mendonça F.J.B., Jr., Costa-Silva J.H., de Souza I.A., de Melo S.J. Estudo Preliminar da Toxicidade e das Atividades Anti-edematogênica e Anti-nociceptiva da 3,4-diidro-2-fenil-6-para-flúor-fenil-4-oxo-pirimidina-5-carbonitrila. Lat. Am. J. Pharm. 2008;27:343. [Google Scholar]
- 20.Skulnick H.I., Ludens J.H., Wendling M.G., Glenn E.M., Rohloff N.A., Smith R.J., Wierenga W. Pyrimidinones. 3. N-substituted 6-phenylpyrimidinones and pyrimidinediones with diuretic/hypotensive and antiinflammatory activity. J. Med. Chem. 1986;29:1499–1504. doi: 10.1021/jm00158a030. [DOI] [PubMed] [Google Scholar]
- 21.Ranise A., Bruno O., Schenone S., Bondavalli F., Falcone G., Filippelli W., Sorrentino S. Synthesis of 6-thiosubstituted 5-ethoxycarbonyl-1,3-diphenyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-ones, 6-substituted 5-hydroxy-1,3-diphenyl-2,3-dihydrothieno[2,3-d]pyrimidin-4(1H)-ones and their esters with local anesthetic, antiarrhythmic, antiinflammatory and analgesic activities. Farmaco. 1997;52:547–555. [PubMed] [Google Scholar]
- 22.Modica M., Santagati M., Santagati A., Cutuli V., Mangano N., Caruso A. Synthesis of new [1,3,4]thiadiazolo[3,2-a]thieno[2,3-d]pyrimidinone derivatives with antiinflammatory activity. Pharmazie. 2000;55:500–502. doi: 10.1002/chin.200042143. [DOI] [PubMed] [Google Scholar]
- 23.Amr A.E.G.E., Sabry N.M., Abdulla M.M. Synthesis, Reactions, and Anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon. Monatsh. Chem. 2007;138:699–707. doi: 10.1007/s00706-007-0651-0. [DOI] [Google Scholar]
- 24.Mendonça F.J.B., Jr., dos Anjos J.V., Falcão E.P.S., Yamamoto A.P., de Melo S.J., Srivastava R.M. A simple approach for the synthesis of 2,6-diaryl-4-oxo-3,4-dihydropyrimidine-5-carbonitriles. Heterocycl. Commun. 2005;11:479–484. doi: 10.1515/HC.2005.11.6.479. [DOI] [Google Scholar]
- 25.Wierenga W. Antiviral and other bioactivities of pyrimidinones. Pharmacol. Ther. 1985;30:67–89. doi: 10.1016/0163-7258(85)90048-8. [DOI] [PubMed] [Google Scholar]
- 26.Kappe C.O. Biologically active dihydropyrimidones of the Biginelli-type—A literature survey. Eur. J. Med. Chem. 2000;35:1043–1052. doi: 10.1016/S0223-5234(00)01189-2. [DOI] [PubMed] [Google Scholar]
- 27.de Melo S.J., Luu-Duc C., Thomasson F., Narcisse G., Gaultier C. 5-Fluoro (3H) pyrimidine-4-ones: synthesis, reactivity and pharmacological properties. Ann. Pharm. Fr. 1992;50:39–51. [PubMed] [Google Scholar]
- 28.Agarwal S.K., Tadiparthi R., Aggarwal P., Shivakumar S., Dey D., Nag B. New diaryl pyrimidinone derivatives are tumor necrosis factor alpha inhibitors, useful for the treatement of rheumatoid arthritis, osteoporosis, multiple myeloma and ischemic heart disease. WO/2003/084935. International Patent. 2003 Apr 10;
- 29.Devadas B., Hartmann S.J., Heier R.F., Jerome K.D., Kolodziej S.A., Mathias J.P., Norton M.B., Promo M.A., Rucker P.V., Selness S.R. New substituted pyridinone pyrazole urea and pyrimidinone pyrazole urea compounds useful for treating e.g. astma, inflammation and rheumatoid arthritis. WO/2007/091176. International Patent. 2007 Feb 5;
- 30.Liang C., Koenig M. New pyrimidinones derivatives for treating disordes related to abnormal protein kinase activities e.g., cancer and inflammation. WO/2007/081901. International Patent. 2007 Jan 5;
- 31.Lu Y., Xiang T., Bartberger M.D., Bernard C., Bostick T., Huang L., Liu L., Siegmund A., Sukay G., Guo G., et al. An efficient one-pot construction of substituted pyrimidinones. Tetrahedron. 2006;62:11714–11723. doi: 10.1016/j.tet.2006.09.047. [DOI] [Google Scholar]
- 32.Zhang J., Shen B., Lin A. Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol. Sci. 2007;28:286–295. doi: 10.1016/j.tips.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 33.de Melo S.J., dos Santos L.C., Falcão E.P.S., Srivastava R.M., Luu-Duc C. Synthesis of new 4-amino-2,6-diarylpyrimidine-5-carbonitriles. J. Chem. Res. 2002;5:216–217. doi: 10.3184/030823402103171906. [DOI] [Google Scholar]
- 34.Falcão E.P.S., de Melo S.J., Srivastava R.M., Catanho M.T.J.A., do Nascimento S.C. Synthesis and antiinflammatory activity of 4-amino-2-aryl-5-cyano-6-{3- and 4-(N-phthalimidophenyl)} pyrimidines. Eur. J. Med. Chem. 2006;41:276–282. doi: 10.1016/j.ejmech.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 35.da Silva J.B.P., Ramos M.N., Barros Neto B., de Melo S.J., Falcão E.P.S., Catanho M.T.J.A. Quantitative Structure Activity Relationships (QSAR) of 4-Amino-2,6-Diarylpyrimidine-5-Carbonitriles Having Anti-inflammatory Activity. J. Braz. Chem. Soc. 2008;19:337. doi: 10.1590/S0103-50532008000200021. [DOI] [Google Scholar]
- 36.Braggio M.M., Lima M.E.L., Veasey E.A., Haraguchi M. Atividades farmacológicas das folhas de Sesbania virgata (CAV.) PERS. Arq. Inst. Biol. (Sao Paulo) 2002;69:49–53. [Google Scholar]
- 37.Dannhardt G., Kiefer W. Cyclooxygenase inhibitors-current status and future prospects. Eur. J. Med. Chem. 2001;36:109–126. doi: 10.1016/S0223-5234(01)01197-7. [DOI] [PubMed] [Google Scholar]
- 38.Koster R., Anderson M., de Beer E.J. Acetic acid for analgesic screening. Fed. Proc. 1959;18:412–416. [Google Scholar]
- 39.Kruger L. Methods in Pain Research. CRC Press; Los Angeles, CA, USA: 2001. pp. 11–39. [Google Scholar]
- 40.Hyperchem Program Release 8.0 for Windows. Hybercube, Inc.; Gainesville, FL, USA: 2009. [Google Scholar]
- 41.Allinger N.L. A hydrocarbon force-field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977;99:8127–8134. doi: 10.1021/ja00467a001. [DOI] [Google Scholar]
- 42.Dewar M.J.S.E., Zoebisch G., Healy E.F., Stewart J.J.P. AM1: A new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985;107:3902–3909. doi: 10.1021/ja00299a024. [DOI] [Google Scholar]
- 43.Cohen N.C. Guidebook on Molecular Modeling in Drug Design. Academic Press; San Diego, CA, USA: 1996. [Google Scholar]
- 44.Leach A.R. Molecular Modeling: Principles and Applications. Prentice Hall; London, UK: 2001. [Google Scholar]
- 45.SpartanModel. Wavefunction, Inc.; Irvine, CA, USA: 2009. [(accessed on 10 May 2011)]. Available online: http://www.wavefun.com/products/windows/SpartanModel/win_model.html. [Google Scholar]