Abstract
1,2,3-Triazoles have been extensively studied as compounds possessing important biological activities. In this work, we describe the synthesis of ten 2-(1-aryl-1H-1,2,3-triazol-4-yl)propan-2-ols via copper catalyzed azide alkyne cycloaddition (CuAAc or click chemistry). Next thein vitro antifungal activity of these ten compounds was evaluated using the microdilution broth method against 42 isolates of four different Candida species. Among all tested compounds, the halogen substituted triazole 2-[1-(4-chlorophenyl)-1H-(1,2,3)triazol-4-yl]propan-2-ol, revealed the best antifungal profile, showing that further modifications could be done in the structure to obtain a better drug candidate in the future.
Keywords: click chemistry; 1,2,3-triazoles; Candida spp.; antifungal activity
1. Introduction
Deep and superficial fungal infections have increased significantly over the past few decades. Control of fungal disease has proved to be difficult because of several risk factors. The number of patients at highest risk for these infections has been steadily increasing, especially among patients immunocompromised due to AIDS, organ transplantation, chemotherapy or other invasive procedures [1]. Because of this, there is a clear need for the development of effective antimycotic therapeutic agents for the treatment of fungal infections, since the major classes of antifungal drugs available have encountered resistance in clinical use [2,3]. Among these classes, azoles are the most used because of their broad spectrum, high potency and low toxicity [4].
Azoles are competitive inhibitors of lanosterol 14 α-demethylase (a cytochrome P-450 enzyme), leading to a decrease in the fungal biosynthesis of ergosterol, which is a key compound of fungal cell membranes, thereby preventing fungal growth [5,6]. Beyond the antifungal properties [7,8,9], triazoles possess a variety of interesting biological activities, forming part of the scaffolds of antibacterial and antituberculosis agents [10,11,12,13,14], neuraminidase inhibitors [15], anticancer compounds [16], antiviral agents [17], analgesic compounds [18], herbicides [19] and plant growth regulators [20].
Considering the above mentioned advantages of triazole-containing antifungal drugs and the increasing drug resistance mechanisms in these type of microorganisms, we decided to synthesize 2-(1-aryl-1H-1,2,3-triazol-4-yl)propan-2-ols capable of inhibiting cell growth of some Candida species with clinical relevance and testing their activity using the microdilution broth method.
2. Results and Discussion
2.1. Chemistry
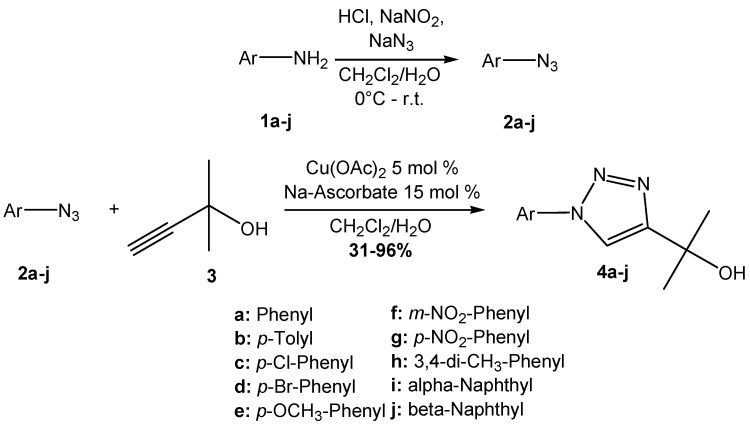
Prior to the synthesis of the 2-(1-aryl-1H-1,2,3-triazol-4-yl)propan-2-ols, the aromatic azides 2a–j were prepared from the corresponding anilines 1a–j following the Sandmeyer conditions [21]. The aromatic azides were then reacted with 2-methylbut-3-yn-2-ol (3) using Cu(OAc)2 and sodium ascorbate as catalyst in 1:1 dichloromethane:water [22,23] to give the products 4a–j in good yields (Scheme 1).
Scheme 1.
Synthesis of triazoles 4a–j.
For all synthesized molecules, only one regioisomer could be detected by 1H-NMR. Only one singlet was observed in the 1H-NMR spectrum for the triazole ring (δ 7.19–8.16 ppm), which can be attributed to the proton in the C-5 position of the triazole nucleus. According to earlier literature on copper catalyzed cycloadditions [24,25], it is believed that the obtained products are 1,4-regioisomers.
2.2. Biology
For each experiment, inocula controls produced clearly detectable growth after the chosen incubation period, indicating that all isolates were viable and that the conditions used were suitable for fungal growth. The antifungal screening results by MIC measurements are summarized in Table 1. Most of the synthesized 1,2,3-triazoles showed weak (4a,b,d,e,f) or no activity (4g,h,i,j) against the Candida species used herein. However, for 2-[1-(4-chlorophenyl)-1H-(1,2,3)triazol-4-yl]propan-2-ol (4c) and for the reference drug (fluconazole), it was possible to determine a MIC for Candida growth. As it can be seen, fluconazole showed fungistatic activity in concentrations ranging from 0.5 to 64 μg·mL−1. Eight isolates (4987, 4986, 4984, 4970, 4790, 4608, 1059 and 109) had their growth inhibited in a dose-dependent pattern, presenting MIC values ranging from 16 to 32 μg·mL−1. In contrast to these findings, nine isolates (4990, 4802, 4388, 4263, 4261, 4124, 3719, 1150 and 934) were resistant to the reference azole showing MIC values above 64 μg·mL−1. It can be also observed that the triazole 4c showed good antifungal activity, presenting MIC values ranging from 64 to 256 μg·mL−1 against all the tested strains.
Table 1.
Antifungal activity of triazoles 4a–j and fluconazole against the studied Candida strains.
| Tested strain n° URM | Compounds (MICs in μg·mL−1) a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4a | 4b | 4c | 4d | 4e | 4f | 4g | 4h | 4i | 4j | Flu b | |
| 4990 | 2,048 | 1,024 | 256 | 2,048 | 1,024 | 2,048 | R | R | R | R | >64 |
| 4987 | 2,048 | 1,024 | 128 | 2,048 | 1,024 | 2,048 | R | R | R | R | 16 |
| 4986 | 2,048 | 1,024 | 128 | 2,048 | 1,024 | 2,048 | R | R | R | R | 16 |
| 4820 | 2,048 | 1,024 | 256 | 2,048 | 1,024 | 2,048 | R | R | R | R | 4 |
| 4819 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 4 |
| 4817 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 4 |
| 4609 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 4 |
| 4606 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 2 |
| 4388 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | >64 |
| 4387 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 2 |
| 4386 | 2,048 | 1,024 | 128 | 2,048 | 1,024 | 2,048 | R | R | R | R | 1 |
| 4385 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 2 |
| 4384 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 2 |
| 4260 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 2 |
| 4127 | R | 1,024 | 128 | 1,024 | 2,048 | 2,048 | R | R | R | R | 0.5 |
| 4126 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 0.5 |
| 4125 | 2,048 | 1,024 | 256 | 1,024 | 1,024 | 2,048 | R | R | R | R | 0.5 |
| 4124 | 2,048 | 1,024 | 256 | 2,048 | 1,024 | 2,048 | R | R | R | R | >64 |
| 3719 | R | 1,024 | 256 | 2,048 | 1,024 | 2,048 | R | R | R | R | 64 |
| 3716 | R | 1,024 | 256 | 2,048 | 1,024 | 2,048 | R | R | R | R | 0.5 |
| 4802 | 2,048 | 2,048 | 256 | 2,048 | 1,024 | R | R | R | R | R | 64 |
| 4263 | 2,048 | 2,048 | 256 | 2,048 | 1,024 | R | R | R | R | R | 64 |
| 1059 | 2,048 | 1,024 | 64 | 512 | 1,024 | 2,048 | R | R | R | R | 16 |
| 934 | 2,048 | 2,048 | 256 | 2,048 | 1,024 | R | R | R | R | R | 64 |
| 109 | 2,048 | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 16 |
| 4984 | R | 2,048 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 16 |
| 4970 | R | 2,048 | 128 | 1,024 | 512 | 2,048 | R | R | R | R | 16 |
| 4889 | R | 1,024 | 64 | 1,024 | 1,024 | 2,048 | R | R | R | R | 2 |
| 4818 | R | 2,048 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 4 |
| 4804 | R | 2,048 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 8 |
| 4608 | R | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 16 |
| 4607 | R | 1,024 | 128 | 1,024 | 1,024 | 2,048 | R | R | R | R | 4 |
| 4261 | R | 2,048 | 256 | 1,024 | 1,024 | R | R | R | R | R | >64 |
| 3627 | 2,048 | 1,024 | 64 | 1,024 | 1,024 | 2,048 | R | R | R | R | 0.5 |
| 3624 | 2,048 | 1,024 | 64 | 1,024 | 1,024 | 2,048 | R | R | R | R | 0.5 |
| 3621 | 2,048 | 1,024 | 64 | 1,024 | 1,024 | 2,048 | R | R | R | R | 0.5 |
| 22019 c | R | 2,048 | 256 | 2,048 | 1,024 | 2,048 | R | R | R | R | 8 |
| 4790 | R | 2,048 | 128 | 2,048 | 1,024 | 2,048 | R | R | R | R | 32 |
| 4262 | R | 1,024 | 128 | 2,048 | 1,024 | 2,048 | R | R | R | R | 4 |
| 1150 | R | 2,048 | 128 | 2,048 | 1,024 | 2,048 | R | R | R | R | >64 |
| 933 | R | 2,048 | 128 | 2,048 | 1,024 | 2,048 | R | R | R | R | 4 |
| 916 | R | 2,048 | 128 | 2,048 | 1,024 | 2,048 | R | R | R | R | 4 |
a The MIC value was defined as the lowest concentration of the antifungal agent and were read after two days at 37 °C. Inocula sizes contained approximately 2.5 × 103 cells·mL−1. Culture media tested were the RPMI 1640 (Sigma Chemical Co., St. Louis, MO, USA). The final concentration of triazoles was between 4–2,048 μg·mL−1 and 0.125–64 μg·mL−1 for fluconazole; b Fluconazole; c Candida parapsilosis ATCC 22019 was used as reference strain. R = Resistance.
Observing the drug structure, it can be noticed that 4c possesses a chlorine atom substituted in the para position of the phenyl ring present at the N-1 position of the triazole nucleus. Chlorine-substituted rings were found to be good antifungal tools, as reported by Wang and colleagues [26]. In their study, sixteen N-methyl-substituted phenoxybutan-1-amine chloro-substituted derivatives exhibited strong in vitro antifungal activity, being more active against the tested microorganisms than the used reference drug, voriconazole. Later, Wang et al. [27] decided to synthesize fourteen novel triazole-substituted compounds containing a phenoxyalkyl group. They also observed that the best antifungal drugs were those with halogen atoms as substituents in the phenyl rings.
The acute preliminary toxicological tests in rats showed that the oral administration of triazole 4c at the 2,000 mg·kg−1 dose did not produce any signs of toxicity or mortality, indicating that the lethal dose for 50% of the animal population in this study (LD50) is above 2,000 mg·kg−1. According to Lorke [28], substances presenting a LD50 higher than 2,000 mg·kg−1 can be considered low toxicity drugs. Since our pharmacological studies have shown that 4c is active in 64 to 256 μg·mL−1 concentrations, those toxicological findings demonstrate that this drug candidate is quite safe for further in vivo studies and can be considered an an antifungal lead for this class of compounds.
3. Experimental
3.1. General
All commercially available reagents were used without any further purification and the reactions were monitored by TLC analysis (TLC plates GF254 E. Merck). Melting points were determined on a Büchi apparatus and are uncorrected. Column chromatography was performed on Silica Gel 60 (70–230 mesh, Merck Chemicals International). NMR spectra were recorded with a Bruker AC-200 MHz spectrometer (Billerica, MA, USA) and referenced as follows: 1H (200 MHz), internal SiMe4 at δ = 0.00 ppm, 13C (50 MHz), internal standard at δ = 77.23 ppm. Exact mass measurements of the molecular ions were obtained on a Shimadzu LC/MS-IT-TOF Eletrospray.
3.2. Synthesis of the Aromatic Azides 2a–j
To a solution of the corresponding aniline 1a–j (4.1 mmol) dissolved in CH2Cl2 (30 mL), was added 6 N HCl (30 mL) at 0 °C. To this biphasic system was added dropwise a saturated aqueous solution of NaNO2 (10 mL). After stirring for 30 min at 0 °C, NaN3 (0.53 g, 8.2 mmol) was added at 0 °C. Stirring was maintained for 30 min, and the mixture was allowed to warm to room temperature. The two phases were separated, and the aqueous phase was extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were washed with aqueous solution of NaHCO3, then brine, dried (Na2SO4) and filtered from active charcoal. Evaporation of the solvent in vacuo gave the crude azides 2a–j that were used in the next step without further purification.
3.3. Synthesis of 2-(1-Aryl-1H-1,2,3triazol-4-yl)propan-2-ols 4a–j
2-Methylbut-3-yn-2-ol (3, 1,1 mmol) and the azido compound 2a–j (1 mmol) were suspended in a 1:1 mixture of CH2Cl2 and water (10 mL). To this solution was added a mixture of Cu(OAc)2 (36 mg, 0.2 mmol) and sodium ascorbate (79 mg, 0.4 mmol). The resulting mixture was stirred at room temperature until TLC analysis indicated complete consumption of the azide. The mixture was diluted with CH2Cl2 (5 mL) and water (5 mL). The organic layer was separated, and the water phase was extracted again with CH2Cl2 (5 mL). The combined organic layers were dried over Na2SO4. Removal of the solvent in vacuo gave a residue that was recrystallized from chloroform-hexanes to afford the corresponding triazoles 4a–j.
2-(1-Phenyl-1H-1,2,3 -triazol-4-yl)propan-2-ol (4a): White crystals; yield 86%; m.p.: 95–96 °C; Rf 0,60 (ethyl acetate-chloroform 9:1, v/v). 1H-NMR (CDCl3): δ 0.97 (6H, s); 2.92 (1H, bs); 6.73 (3H, m); 6.93 (1H, dd, J = 8 Hz, 2 Hz); 6.97 (1H, dd, J = 8 Hz, 2 Hz); 7.24 (1H, s). 13C-NMR (CDCl3): δ 30.3; 68.5; 117.7; 120.4; 128.5; 129.6; 136.9; 156.4. ESI–HRMS m/z: 226.0911 (calcd. for C11H13N3ONa [M+Na]+: 226.0956).
2-[1-(4-Tolyl)-1H-1,2,3 -triazol-4-yl]propan-2-ol (4b): White crystals; yield 78%; m.p.: 120–121 °C; Rf 0,65 (ethyl acetate-chloroform 9:1, v/v). 1H-NMR (CDCl3): δ 0.99 (6H, s); 1.68 (3H, s); 3.13 (1H, bs); 6.54 (2H, bd, J = 8.0 Hz); 6.85 (2H, bd, J = 8.0 Hz); 7.24 (1H, s). 13C-NMR (CDCl3): δ 20.9; 30.4; 68.4; 117.7; 120.2; 130.0; 134.6; 138.5; 156.2. ESI–HRMS m/z: 240.1049 (calcd. for C12H15N3ONa [M+Na]+: 240.1113).
2-[1-(4-Chlorophenyl)-1H-1,2,3 -triazol-4-yl]propan-2-ol (4c): White crystals; yield 67%; m.p.: 93–94 °C; Rf 0,67 (ethyl acetate-chloroform 9:1, v/v). 1H-NMR (CDCl3): δ 0.98 (6H, s); 2.90 (1H, bs); 6.75 (2H, dd, J = 8.0 Hz, 2 Hz); 6.93 (2H, dd, J = 8.0 Hz, 2.0 Hz); 7.24 (1H, s). 13C-NMR (CDCl3): δ 30.3; 68.5; 107.1; 117.6; 121.5; 129.7; 134.2; 135.4; 156.6. ESI–HRMS m/z: 260.0518 (calcd. for C11H12ClN3ONa [M+Na]+: 260,0567).
2-[1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl]propan-2-ol (4d): White crystals; yield 96%; m.p.: 95–96 °C; Rf 0,68 (ethyl acetate-chloroform 9:1, v/v). 1H-NMR (CDCl3): δ 1.70 (6H, s); 3.68 (1H, bs); 7.59 (4H, m); 7.97 (1H, s). 13C-NMR (CDCl3): δ 30.3; 68.4; 107.0; 121.7; 122.1; 132.7; 135.8; 156.7. ESI–HRMS m/z: 305.9989 (calcd. for C11H12BrN3ONa [M+Na]+: 304.0061).
2-[1-(4-Methoxyphenyl)-1H-1,2,3 -triazol-4-yl]propan-2-ol (4e): Red crystals; yield 69%; m.p.: 106–107 °C; Rf 0,65 (ethyl acetate-chloroform 9:1, v/v). 1H-NMR (CDCl3): δ 1.67 (6H, s); 3.00 (1H, bs); 3.82 (3H, s); 6.96 (2H, dd, J = 6.0 Hz, 4.0 Hz); 7.57 (2H, dd J = 6.0 Hz, 4.0 Hz); 7.81 (1H, s). 13C-NMR (CDCl3): δ 30.4; 55.5; 68.5; 114.6; 122.1; 130.5; 159.6. ESI–HRMS m/z: 238.0757 (calcd. for C12H13ClNO2 [M+H]+: 238.0635).
2-[1-(3-Nitrophenyl)-1H-1,2,3 -triazol-4-yl]propan-2-ol (4f): White crystals; yield 75%; m.p.: 98–100 °C; Rf 0,60 (ethyl acetate-chloroform 9:1, v/v). 1H-NMR (CDCl3): δ 1.63 (6H, s); 3.96 (1H, bs); 7.65 (1H, bd, J = 8.0 Hz);8.12 (2H, m); 8.16 (1H, s); 7.65 (1H, d, J = 2.1 Hz). 13C-NMR (CDCl3): δ 30.2; 68.4; 114.9; 122.9; 125.8; 130.8; 137.5; 148.6; 157.1. ESI–HRMS m/z: 271.0764 (calcd. for C11H12N4O3Na [M+Na]+: 271.0807).
2-[1-(4-Nitrophenyl)-1H-1,2,3 -triazol-4-yl]propan-2-ol (4g): Yellow crystals; yield 58%; m.p.: 123–124 °C; Rf 0,63 (ethyl acetate-chloroform 9:1, v/v). 1H-NMR (CDCl3): δ 1.69 (6H, s); 2.82 (1H, bs); 7.95 (2H, dd, J = 6.0 Hz, 4.0 Hz); 8.03 (1H, s); 8.37 (2H, dd, J = 6.0 Hz, 2.0 Hz). 13C-NMR (CDCl3): δ 30.4; 68.7; 107.1; 120.3; 125.5; 141.2; 147.0; 157.2. ESI–HRMS m/z: 265.1469 (calcd. for C11H13N4O4 [M+H2O−H]+: 265.2453).
2-[1-(3,4-Dimethylphenyl)-1H-1,2,3-triazol-4-yl]propan-2-ol (4h): yellow crystals; yield 31%; m.p.: 128–129 °C; Rf 0,80 (ethyl acetate:chloroform 9:1, v/v). 1H-NMR (CDCl3): δ 1.00 (6H, s); 1.60 (6H, s); 2.69 (1H, bs); 6.53 (1H, m);6.72 (2H, dd, J = 8.1 Hz, 2.0 Hz); 7.19 (1H, s). 13C-NMR (CDCl3): δ 19.3; 19.8; 30.4; 68.5; 107.1; 117.7; 121.5; 130.4; 134.9; 137.2; 138.1; 156.0. ESI–HRMS m/z: 254.1229 (Calcd for C13H17N3ONa [M+Na]+: 254.1269).
2-[1-( α -Naphthyl)-1H-1,2,3-triazol-4-yl]propan-2-ol (4i): Red crystals; yield 62%; m.p.: 152–153 °C; Rf 0,65 (ethyl acetate:chloroform 9:1, v/v). 1H-NMR (CDCl3): δ 1.75 (6H, s); 3.00 (1H, bs); 7.54 (4H, m); 7.82 (1H, s); 7.95 (3H, m). 13C-NMR (CDCl3): δ 30.5; 68.6; 122.3; 123.5; 127.0; 128.2; 128.5; 130.3; 133.7; 134.0; 155.5. ESI–HRMS m/z: 276.1070 (calcd. for C15H15N3ONa [M+Na]+: 276.1113).
2-[1-( β -Naphthyl)-1H-1,2,3-triazol-4-yl]propan-2-ol (4j): Yellow crystals; yield 60%; m.p.: 143–144 °C; Rf 0,68 (ethyl acetate:chloroform 9:1, v/v). 1H-NMR (CDCl3): δ 1.72 (6H, s); 2.97 (1H, bs); 7.53 (2H, d, J = 8.1 Hz); 7.89 (4H, m); 8.03 (1H, s); 8.12 (1H, d, J = 2.1 Hz). 13C-NMR (CDCl3): δ 30.4; 68.6; 118.3; 118.9; 126.9; 127.3; 127.8; 128.2; 129.9; 132.7; 133.1; 134.4; 155.6. ESI–HRMS m/z: 276.1071 (calcd. for C15H15N3ONa [M+Na]+: 276.1113).
3.4. Strains and Growth Cultures
Twenty strains of Candida albicans, five of Candida krusei, eleven of Candida parapsilosis and five of Candida tropicalis were supplied by the URM Culture Collection of the Department of Mycology, Biological Sciences Centre of the Federal University of Pernambuco, Recife, Brazil. Strains have been stocked in mineral oil at 18 °C [28]. Viability tests and subsequent taxonomic confirmation of their morphological, biochemical and physiological characteristics were carried out [29]. Species, accession numbers, stock time and isolation substratum are summarized in Table 2.
Table 2.
Samples of Candida species preserved in the Mycotheca Culture Collection—University of Recife Mycology (URM).
| Species | Accession nº (URM) | Storage (years) | Substratum |
|---|---|---|---|
| C. albicans | 4990 | 01 | Vaginal secretion |
| C. albicans | 4987 | 01 | Vaginal secretion |
| C. albicans | 4986 | 01 | Vaginal secretion |
| C. albicans | 4820 | 02 | Ungual scrap |
| C. albicans | 4819 | 02 | Ungual scrap |
| C. albicans | 4817 | 02 | Ungual scrap |
| C. albicans | 4609 | 03 | Blood |
| C. albicans | 4606 | 03 | Blood |
| C. albicans | 4388 | 05 | Oropharyngeal secretion |
| C. albicans | 4387 | 05 | Oropharyngeal secretion |
| C. albicans | 4386 | 05 | Oropharyngeal secretion |
| C. albicans | 4385 | 05 | Oropharyngeal secretion |
| C. albicans | 4384 | 05 | Oropharyngeal secretion |
| C. albicans | 4260 | 05 | Oropharyngeal secretion |
| C. albicans | 4127 | 07 | Inguinal area |
| C. albicans | 4126 | 07 | Urine |
| C. albicans | 4125 | 07 | Spittle |
| C. albicans | 4124 | 07 | Oropharyngeal secretion |
| C. albicans | 3719 | 10 | Tooth scrap |
| C. albicans | 3716 | 10 | Tooth scrap |
| C. krusei | 4802 | 02 | * |
| C. krusei | 4263 | 05 | Oropharyngeal secretion |
| C. krusei | 1059 | 48 | * |
| C. krusei | 934 | 49 | Appendix biopsy |
| C. krusei | 109 | 52 | * |
| C. parapsilosis | 4984 | 01 | Vaginal secretion |
| C. parapsilosis | 4970 | 01 | Vaginal secretion |
| C. parapsilosis | 4889 | 02 | Blood |
| C. parapsilosis | 4818 | 02 | Ungual scrap |
| C. parapsilosis | 4804 | 02 | IFM |
| C. parapsilosis | 4608 | 03 | Blood |
| C. parapsilosis | 4607 | 03 | Blood |
| C. parapsilosis | 4261 | 05 | Oropharyngeal secretion |
| C. parapsilosis | 3627 | 12 | Spittle |
| C. parapsilosis | 3624 | 12 | Spittle |
| C. parapsilosis | 3621 | 12 | Spittle |
| C. parapsilosis | ATCC22019 | - | - |
| C. tropicalis | 4790 | 02 | Cassava powdery |
| C. tropicalis | 4262 | 06 | Oropharyngeal secretion |
| C. tropicalis | 1150 | 46 | Tongue |
| C. tropicalis | 933 | 49 | Vaginal secretion |
| C. tropicalis | 916 | 49 | Feces |
* Substratum not identified.
3.5. In vitro Antifungal Susceptibility
Reference microdilution trays, containing serial drug dilutions were prepared by following the CLSI M27-A3 guidelines [30]. The triazoles were dissolved in dimethylsulfoxide (DMSO) and then these stock solutions were stored at −80 °C. The concentrations tested ranged from 2 to 2,048 μg·mL−1. Fluconazole was used as reference drug at concentrations from 0.125 to 64 μg·mL−1. In order to obtain a fungal inoculum containing 1–5 × 106 CFU·mL−1, each strain was cultured on a tube containing 20 mL of Sabouraud Dextrose Agar (SDA) plus yeast extract at 35 °C for two days. After this time, yeast suspensions were prepared in sterile physiological solution (0.85%) and maintained at 28 ± 2 °C and then were adjusted to 90% transmittance at 530 nm. Two serial dilutions from 1:100 and 1:20 sequentially were made to obtain a final inoculum containing 1.0 × 103 and 5 × 103 CFU·mL−1. The microdilution wells containing 100 µL of the twofold serial dilutions of the test and reference drugs in standard RPMI 1640 medium (Sigma Chemical Co., St. Louis, MO, USA), buffered to pH 7.0 with 0.165 mol·L−1 of morpholinopropanesulphonic acid (MOPS, Sigma), were inoculated with 100 µL of inoculum. After inoculation, the microplates were incubated at 35 °C in a non CO2 incubator and were read visually 48 h after the incubation. MICs corresponded to the lowest drug dilution that showed growth inhibition compared to untreated yeasts. C. parapsilosis ATCC 22019 was used as reference strain. All tests wereperformed in triplicate.
3.6. Animals and Preliminary Toxicological Tests
Adult male Wistar rats (Rattus norvegicus), aged 2–3 months, weighing 220–260 g, were obtained from the Pound of the Department of Physiology and Pharmacology at the Federal University of Pernambuco. They were kept under standard environmental conditions (23 ± 2 °C; 12:12 h dark/light cycle) and water and animal food (Labina®, Purina, Brazil) were made available ad libitum. The animals were randomly divided into two groups (n = 3–4/group) and deprived of feed for 12 h with access to water ad libitum. Further, group 1 received vehicle (solution of 2.5% tween 80) and group 2 received 2-[1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl]propan-2-ol (4c) in a single oral dose of 2,000 mg·kg−1. The observations were performed at 30, 60, 120, 180 and 240 min after the oral treatments and then, daily for 14 days. Behavioral changes, weight, consumption of food and water, clinical signs of toxicity, and mortality were recorded daily [31]. The experimental protocol was approved by the Federal University of Pernambuco’s Ethics Committee for Animal Experimentation (Process no 23076.003830). Studies of acute toxicity were performed according to “Up and down” method with slight modifications, as described by OECD 425 [32].
4. Conclusions
In conclusion, a series of analogs of 1,2,3-triazoles with ten distinct substituents at the N-1 of the triazole ring were synthesized and assessed for their antifungal activity. All compounds were tested against 42 pathogenic strains of four different Candida species. Modification of substituents has a great impact on the minimal inhibitory concentration values, since we could obtain triazole derivatives showing no antimycotic activity, with moderate antifungal activity and one compound with promising activity. The antifungal tests data show that the chloro-substituted triazole derivative exhibited, in particular, good fungal growth inhibition, showing that further modifications in the 2-(1-aryl-1H-1,2,3-triazol-4-yl) series can be done in order to obtain more potent prototypes.
Acknowledgments
The authors gratefully acknowledge CAPES, CNPq, PPP/FACEPE and PRONEX/FACEPE for fellowships and financial support.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Samples Availability: Samples of the compounds 4a–j are available from the authors.
References and Notes
- 1.Lopez-Martinez R. Candidosis, a new challenge. Clin. Dermatol. 2010;6:178–184. doi: 10.1016/j.clindermatol.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Chen A., Sobel J.D. Emerging azole antifungals. Expert Opin. Emerg. Drugs. 2005;10:21–33. doi: 10.1517/14728214.10.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Rybak M.J., Akins R.L. Emergence of methicillin-resistant Staphylococcus aureus with intermediate lycopeptide resistance: clinical significance and treatment options. Drugs. 2001;6:1–7. doi: 10.2165/00003495-200161010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Sheehan D.J., Hitchcock C.A., Sibley C.M. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei Q.L., Zhang S.S., Gao J., Li W.H., Xu L.Z., Yu Z.G. Synthesis and QSAR studies of novel triazole compounds containing thioamide as potential antifungal agents. Bioorg. Med. Chem. 2006;14:7146–7153. doi: 10.1016/j.bmc.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 6.Warn P.A., Sharp A., Parmar A., Majithiya J., Denning D.W., Hope W.W. Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: Mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect. Antimicrob. Agents Chemother. 2009;53:3453–3461. doi: 10.1128/AAC.01601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou Y., Zhao Q., Liao J., Hua H., Yu S., Chai X., Xu M., Wua Q. New triazole derivatives as antifungal agents: Synthesis via click reaction, in vitro evaluation and molecular docking studies. Bioorg. Med. Chem. Lett. 2012;22:2959–2962. doi: 10.1016/j.bmcl.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 8.Ulusoy N., Gursoy A., Otuk G. Synthesis and antimicrobial activity of some 1,2,4-triazole-3-mercaptoacetic acid derivatives. Farmaco. 2001;56:947–952. doi: 10.1016/S0014-827X(01)01128-4. [DOI] [PubMed] [Google Scholar]
- 9.Demirbas N., Demirbas A., Karaoglu S.A. Synthesis and biological activities of new 1,2,4-triazol-3-one derivatives. Bioorg. Khim. 2005;31:430–440. doi: 10.1007/s11171-005-0054-0. [DOI] [PubMed] [Google Scholar]
- 10.Bagihalli G.B., Avaji P.G., Patil S.A., Badami P.S. Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activities of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur. J. Med. Chem. 2008;43:2639–2649. doi: 10.1016/j.ejmech.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Karthikeyan M.S., Prasad D.J., Poojary B., Bhat K.S., Holla B.S., Kumari N.S. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg. Med. Chem. 2006;14:7482–7489. doi: 10.1016/j.bmc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Dabak K., Sezer O., Akar A., Anac O. Synthesis and investigation of tuberculosis inhibition activities of some 1,2,3-triazole derivatives. Eur. J. Med. Chem. 2003;38:215–218. doi: 10.1016/S0223-5234(02)01445-9. [DOI] [PubMed] [Google Scholar]
- 13.Shanmugavelan P., Nagarajan S., Sathishkumar M., Ponnuswamy A., Yogeeswari P., Sriram D. Efficient synthesis and in vitro antitubercular activity of 1,2,3-triazolesas inhibitors of Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2011;21:7273–7276. doi: 10.1016/j.bmcl.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 14.Joshi S., Khosla N., Tiwari P. In vitro study of some medicinally important Mannich bases derived from antitubercular agent. Bioorg. Med. Chem. 2004;12:571–576. doi: 10.1016/j.bmc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Kai H., Hinou H., Nishimura S.-I. Aglycone-focused randomization of 2-difluoromethylphenyl-type sialoside suicide substrates for neuraminidases. Bioorg. Med. Chem. 2012;20:2739–2746. doi: 10.1016/j.bmc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Holla B.S., Veerendra B., Shivananda M.K., Poojary B. Synthesis, characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur. J. Med. Chem. 2003;38:759–767. doi: 10.1016/S0223-5234(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 17.Tiew K.-C., Dou D., Teramoto T., Lai H., Alliston K.R., Lushington G.H., Padmanabhan R., Groutas W.C. Inhibition of Dengue virus and West Nile virus proteases by click chemistry-derived benz[d]isothiazol-3(2H)-one derivatives. Bioorg. Med. Chem. 2012;20:1213–1221. doi: 10.1016/j.bmc.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turan-Zitouni G., Kaplancikli Z.A., Erol K., Kilic F.S. Synthesis and analgesic activity of some triazoles and triazolo-thiadiazines. Farmaco. 1999;54:218–223. doi: 10.1016/S0014-827X(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 19.He J., Feng L., Li J., Tao R., Wang F., Liao X., Sun Q., Long Q., Ren Y., Wan J., He H. Design, synthesis and biological evaluation of novel 2-methylpyrimidine-4-ylamine derivatives as inhibitors of Escherichia coli pyruvatedehydrogenase complex E1. Bioorg. Med. Chem. 2012;20:1665–1670. doi: 10.1016/j.bmc.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Oh K., Yamada K., Asami T., Yoshizawa Y. Synthesis of novel brassinosteroid biosynthesis inhibitors based on the ketoconazole scaffold. Bioorg. Med. Chem. Lett. 2012;22:1625–1628. doi: 10.1016/j.bmcl.2011.12.120. [DOI] [PubMed] [Google Scholar]
- 21.Malolanarasimhan K., Lai C.C., Kelley J.A., Iaccarino L., Reynolds D., Young H.A., Marquez V.E. Synthesis and biological study of a flavone acetic acid analogue containing an azido reporting group designed as a multifunctional binding site probe. Bioorg. Med. Chem. 2005;13:2717–2722. doi: 10.1016/j.bmc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 22.Lee B.Y., Park S.R., Jeon H.B., Kim K.S. A new solvent system for efficient synthesis of 1,2,3-triazoles. Tetrahedron Lett. 2006;47:5105–5109. doi: 10.1016/j.tetlet.2006.05.079. [DOI] [Google Scholar]
- 23.dos Anjos J.V., Sinou D., de Melo S.J., Srivastava R.M. Synthesis of glycosyl-triazole linked 1,2,4-oxadiazoles. Carbohydr. Res. 2007;342:2440–2449. doi: 10.1016/j.carres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Himo F., Lovell T., Hilgraf H., Rostovtsev V.V., Noodleman L., Sharpless K.B., Fokin V.V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005;127:210–216. doi: 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]
- 25.dos Anjos J.V., Neves Filho R.A.W., Nascimento S.C., Srivastava R.M., Melo S.J., Sinou D. Synthesis and cytotoxic profile of glycosyltriazole linked to 1,2,4-oxadiazole moiety at C-5 through a straight-chain carbon and oxygen atoms. Eur. J. Med. Chem. 2009;44:3571–3576. doi: 10.1016/j.ejmech.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Wang W., Sheng C., Che X., Ji H., Cao Y., Miao Z., Yao J., Zhang W. Discovery of highly potent novel antifungal azoles by structure-based rational design. Bioorg. Med. Chem. Lett. 2009;19:5965–5969. doi: 10.1016/j.bmcl.2009.07.144. [DOI] [PubMed] [Google Scholar]
- 27.Wang W., Wang S., Liu Y., Dong G., Cao Y., Miao Z., Yao J., Zhang W., Sheng C. Novel conformationally restricted triazole derivatives with potent antifungal activity. Eur. J. Med. Chem. 2010;45:6020–6026. doi: 10.1016/j.ejmech.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 28.Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 29.Sherf A.F. A method for maintaining Phytomonas sepedomica for long periods without transfer. Phytopathology. 1943;31:30–32. [Google Scholar]
- 30.de Hoog G.S., Guarro J., Gene J., Figueras M.J. Atlas of Clinical Fungi. 2nd. Centraalbureau voor Schimmelcultures; Utrecht, The Netherlands and Universitat Rovira i Virgili: Reus, Spain: 2000. p. 1124. [Google Scholar]
- 30. Clinical Laboratory Standards Institute (CLSI) Reference Method for Broth Dilution Testing of Yeasts. Approved standard-third edition M27-A3 CLSI; Wayne, PA, USA: 2008. [Google Scholar]
- 31.Malone M.H. Pharmacological Approaches to Natural Products Screening and Evaluation. In: Wagner H., Wolf P., editors. New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity. Springer-Verlag; Berlin, Germany: 1977. pp. 23–53. [Google Scholar]
- 32.Organisation for Economic Co-operation and Development (OECD). OECD Guideline for Testing of Chemicals: Acute Oral Toxicity–Up-and-Down Procedure; n° 425; OECD Publishing: Paris, France, 2001.