Abstract
The synthesis of 3-methyl-1,4-diphenyl-7-thioxo-4,6,8,9-tetrahydropyrazolo[5,4-b] pyrimidino[5,4-e]pyridine-5-one (6) was achieved by two different one-pot multi-component synthesis (one-pot three-component and one-pot four component synthesis). Mono and dialkylation of 6 under different conditions gave compounds 7–11. The hydrazine 12 produced from reaction of 9 with N2H4 was subjected to reactions with some aromatic aldehydes, ethyl acetoacetate, acetyl acetone, ethyl cyanoacetate and triethyl orthoformate to give 13–17, respectively. Compound 12 upon reaction with CS2, nitrous acid, benzoin, chloroacetone and phenacyl bromide gave 18,20,21,22. Alkylation of 18 with ethyl iodide, ethyl chloroacetate and phenacyl bromide gave 19a–c. The antibacterial and antifungal activities of selected derivatives were evaluated.
Keywords: one-pot reactions; pyrazolo[5,4-b]pyrimidino[5,4-e]pyridines; synthesis; anti-microbial activity
1. Introduction
Multicomponent domino reactions (MDRs), particularly those performed in aqueous media, have become an increasingly useful tool for the synthesis of chemically and biologically important compounds because of their convergence, atom economy, and other suitable characteristics from the point of view of green chemistry [1,2,3,4,5,6,7,8,9,10]. Pyrazoles are excellent precursors for the synthesis of condensed polyfunctionally substituted heterocycles [11,12,13,14,15,16]. Pyrazolo-annulated heterocycles such as pyrazolopyridopyrimidines have attracted considerable interest because their derivatives display a wide range of pharmacological activities, e.g., as anticonvulsants [17], antiproliferative agents [18], anti-inflammatories and analgesic agents [19]. In addition these types of compounds are inhibitors of cyclic guanosine-3',5'-monophosphate phosphodiesterase (cGMP PDE), and are thereby agents against erectile dysfunction [20]. They also show miscellaneous biological properties such as virucidal, anticancer, fungicidal, bactericidal and vasodilatory activities [21]. For all the benefits mentioned above and as part of our program investigating syntheses using pyrazole and fused pyrazoles that have biological importance [22,23,24,25,26,27,28,29,30,31,32,33], we report herein the synthesis of pyrazolo[5,4-b]pyrimidino[5,4-e]-pyridinethiones in a one-pot four component environmental friendly method in light of recently reported methods [34,35,36,37].
2. Results and Discussion
2.1. Chemistry
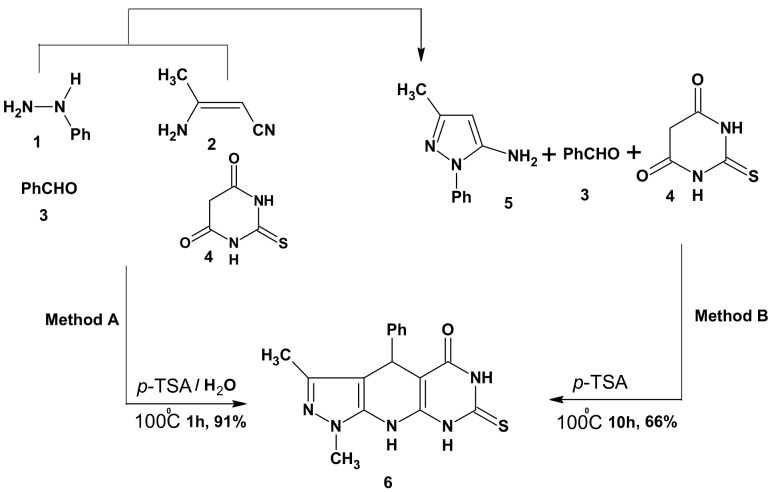
We first describe a comparison between two methods for the construction of pyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one (6), a one-pot four-component domino reaction of phenyl hydrazine (1), 3-aminocrotononitrile (2), benzaldehyde (3) and thiobarbituric acid (4) in water in the presence of one equivalent of p-toluenesulfonic acid (p-TSA) (Method A) and the three-component reaction method involving 5-amino-3-methyl-1-phenylpyrazole (5), benzaldehyde (3) and thiobarbituric acid (4) in presence of p-TSA under solvent-free conditions [35] (Method B).
The one-pot four-component method A showed some crucial advantages, such as short reaction time, excellent yield and high purity, which makes it more efficient and broadly applicable. The percentage yield and the reaction time of the one-pot four-components method in comparison with the one-pot three-component one to produce compound 6 was found to be 91/66% and 1 h/10 h, respectively (Scheme 1).
Scheme 1.
Synthetic pathways for compound 6.
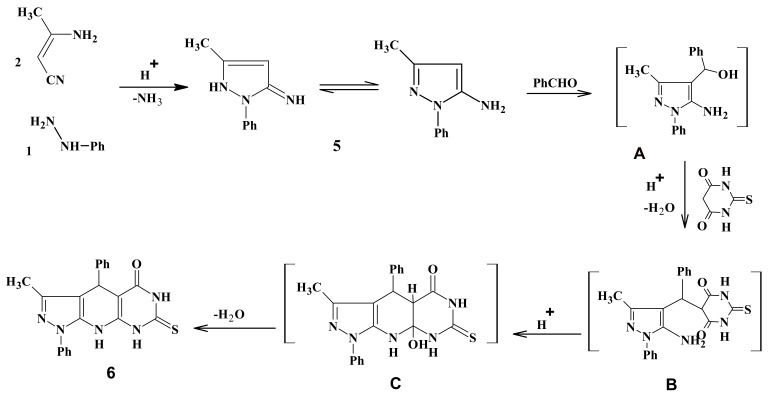
The plausible mechanism for the formation of compound 6 is proposed (Scheme 2) and it is in agreement with that proposed in the literature [38]. The domino sequence of reactions is presumably triggered by the formation of 5-amino-3-methyl-1-phenylpyrazole (5) from the acid-catalyzed reaction of phenylhydrazine (1) with 3-aminocrotononitrile (2). The readily formed 5 reacts in situ with benzaldehyde to afford intermediate A. The later reacted with thiobarbituric acid under the acidic conditions to presumably furnish the intermediate B, which subsequently undergoes annulations leading to the final intermediate C. This final intermediate gave compound 6 upon losing a molecule of water.
Scheme 2.
Mechanistic pathways for the formation of compound 6.
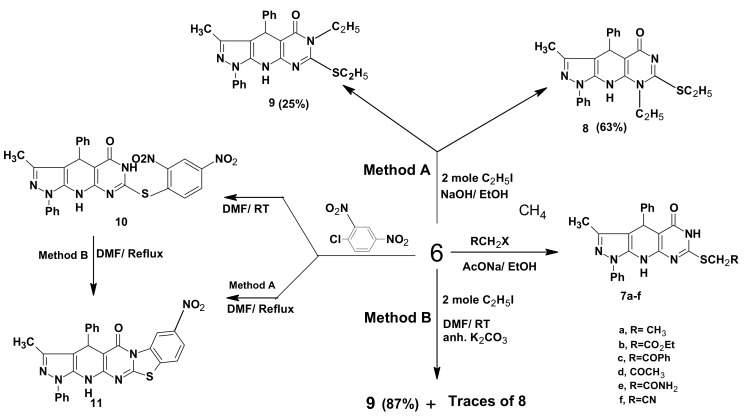
The structural features of compound 6 were elucidated from its spectral and analytical data. Thus, the IR spectrum revealed absorption bands at 3310, 3290, 3240, 1690, 1345 cm−1characteristic for three NH, C=O and C=S groups, respectively. The 1H-NMR (DMSO-d6) displayed three NH singlets (exchangeable with D2O) at 13.8, 12.2 and 9.4 ppm. A characteristic singlet peak for the pyridine CH-4 appeared at 5.57 ppm. Several alkylated derivatives were obtained from the versatile compound 6. Thus, upon treatment of 6 with ethyl iodide, ethyl chloroacetate, phenacyl bromide, chloroacetone, chloroacetamide and chloroacetonitrile in the presence of anhydrous sodium acetate in refluxing ethanol, the S-alkylated derivatives 7a–f were obtained. The IR spectral data of compounds 7a–f displayed no absorption band for C=S and showed bands at 1280–1345 cm−1 confirming the S-alkylation, as well as, compounds 7b–e revealed absorption bands for C=O at 1690–1735 cm−1. A characteristic absorption band at 2240 cm−1 has been observed for CN, confirming the structure of 7f. Regioselective dialkylation reactions of compound 6 using two equivalent of ethyl iodide indicate that, in the introduction of the second ethyl group into the pyrimidine ring there is a competition between N1 or N3 and revealed that it depends on the base conditions used in the reactions. Thus, refluxing in ethanolic NaOH for 4 h (Method A) afforded the two isomeric compounds 8 and 9 in 63% and 25% yield, respectively, whereas treatment of 6 with two equivalents of ethyl iodide in DMF in the presence of anhydrous K2CO3 at room temperature (Method B) afforded in high yield the isomer 9 and only traces of the isomer 8 (Scheme 2). The reaction was monitored by TLC. The reaction mixture was chromatographically work up over silica gel using Pet. ether (b.p. 60–80 °C) and ethyl acetate (1:1) as eluent, to afforded two products in pure form. A crystalline solid, m.p. 162–164 °C obtained in the first fraction was characterized as 6-ethyl-7-ethylthio-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e] pyridine-5-one (9). The second fraction afforded a solid, m.p. 190–192 °C, which was identified as 8-ethyl-7-ethylthio-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b] pyrimidino-[5,4-e]pyridin-5-one (8). By TLC comparison, data analysis and melting point determination, it was found that one of the products 9 was identical to that obtained from Method B. The chemical shift for the pyrimidinone carbonyl is markedly affected by the nature of the adjacent nitrogen [39,40,41,42,43]. The 13C-NMR spectral data of compound 9 showed that the δ values of the pyrimidine C=O at 165.82 ppm suggest that N-3 near to C=O is sp3-hybridized (pyrrole type) and different from the C=O adjacent to a sp2-hybridized nitrogen (pyridine type) in compound 8, which appears at 178.14 ppm [42,43]. Moreover, reaction of 6 with highly electron withdrawing aromatic halo compounds such as 2,4-dinitrochlorobenzene in DMF at room temperature yielded the S-aryl derivative 10, whereas, upon heating, benzothiazolo derivative 11 was obtained via ring closure reaction with the N3-pyrimidine ring [44]. As chemical evidence, the formation of compound 11 was achieved through heating of a sample of 10 in DMF. The structures of 10 and 11 were deduced from their satisfactory spectral and analytical data, for example, the mass spectrum of compound 11 showed its correct parent ion peak at m/z 506 (M+,100%) (Scheme 3).
Scheme 3.
Synthetic pathways for compounds 7a–f, 8, 9, 10 and 11.
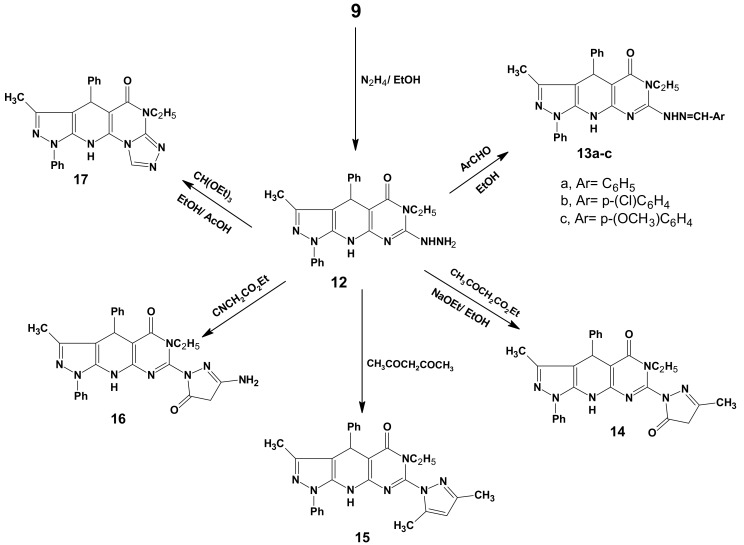
The sulfur-free compound 12, which was identified as 6-ethyl-7-hydrazino-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one, was obtained in excellent yield (85%) via nucleophilic displacement of the thioethyl group in compound 9 with hydrazine in boiling ethanol (Scheme 4).
Scheme 4.
Synthetic pathways for compounds 12–17.
The hydrazine derivative 12 was used as the key intermediate for the synthesis of some polyheterocyclic compounds. Thus, heating of compound 12 with some aromatic aldehydes namely, benzaldehyde, 4-chlorobenzaldeyde and 4-methoxybenzaldeyde in the presence of a few drops of acetic acid in ethanol resulted in the formation of the corresponding hydrazones 13a–c. The structure of compounds 13a–c was characterized by the disappearance of the NHNH2 group and revealed in each case two bands at 3425–3250 and 3170–3100 cm−1 assignable to two NH groups. Also, their 1H-NMR spectra showed the presence of the azomethine and two NH protons at 8.9–9.5 and 10.85–12.75 ppm, respectively. On the other hand, upon heating the hydrazino compound 12 with ethyl acetoacetate, acetylacetone and ethyl cyanoacetate in ethanolic sodium ethoxide solution, the N-pyrazolo derivatives 14–16 were produced. For example, the 1H-NMR spectrum for compound 15 revealed a singlet at 6.15 ppm due to the 4-H-pyrazole moiety. The 13C-NMR spectral data displayed two characteristic singlets at 151.12 and 152.5 ppm for C3 and C5 of the pyrazole nucleus, respectively, which was in agreement with the literature value [45]. The formation of the tetracyclic pyrazolo[5,4-b]1,2,4-triazolo[4',3'-2,1]pyrimidino[5,6-e]pyridine (17) was achieved upon reaction of 12 with triethyl orthoformate in ethanol in presence of a few drops of acetic acid (Scheme 4). The 1H-NMR spectrum of compound 17 revealed the disappearance of NHNH2 signals and appearance of a singlet signal at 8.55 ppm due to the triazole CH.
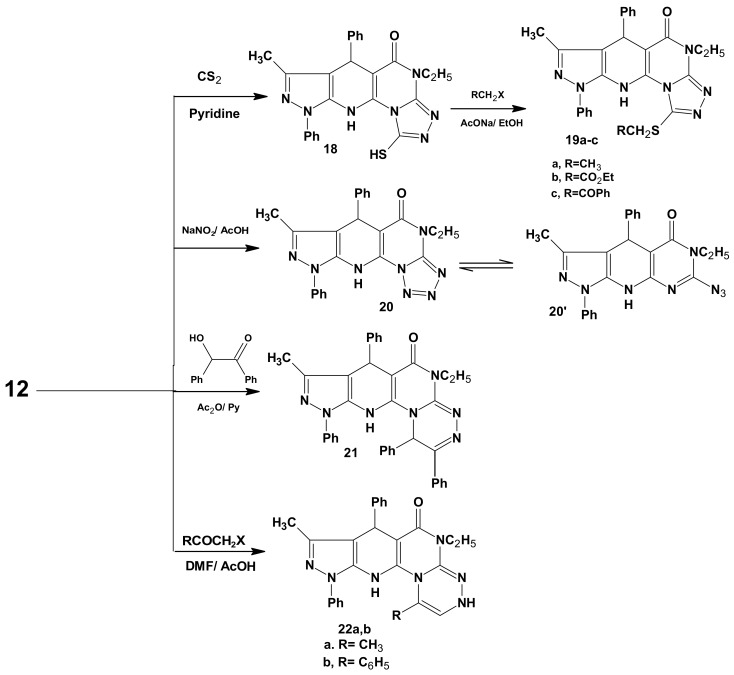
Furthermore, the interaction of the hydrazine 12 with CS2 in pyridine furnished the angular tetracyclic pyrazolo[5,4-b]1,2,4-triazolo[4',3'-2,1]pyrimidino[5,6-e]pyridin-3(2H)-thione (18) in good yield (Scheme 5).
Scheme 5.
Synthetic pathways for compounds 18–22a,b.
The thione 18 was easily converted into a series of S-alkylated derivatives 19a–c upon treatment with ethyl iodide, ethyl chloroacetate and phenacyl bromide in the presence of anhydrous sodium acetate in refluxing ethanol, respectively. As well, the reaction of 11 with sodium nitrite in acetic acid at 0 °C, led to the formation of the pyrazolotetrazolopyrimidinopyridine 20 in 75% yield. This new ring system is in equilibrium with the corresponding 2-azido tautomer 20' [46], which was confirmed by a characteristic absorption peak of the azido group at 2250 cm−1 in the IR. The tetracyclic pyrazolotriazinopyrimidinopyridine compounds 21 and 22a,b were obtained in good to high yield when compound 12 was allowed to react with benzoin in acetic anhydride- pyridine mixture and/or with some α-haloketones (chloroacetone and phenacyl bromide) in DMF/AcOH, respectively (Scheme 5). The structures of compounds 21 and 22a,b were confirmed by spectral data. For example, the 1H-NMR spectrum of compound 22b revealed a singlet at 9.3 due to CH-triazine and displayed two broad singlets (D2O-exchangable) at 10.75 and 11.25 due to NH groups. The mass spectra of compound 22b showed its correct parent ion peak at m/z 513 (M+, 100%).
2.2. Biological Results
Using the agar well-diffusion method [47], ten selected derivatives (compounds 6, 7a, 9, 11, 12, 13b, 17, 19c, 20, and 22a) were evaluated for their antibacterial and antifungal activities. Thus, these compounds were screened against Staphylococcus aureus, Bacillus cereus, Micrococcus luteus as a Gram positive bacteria and Escherichia coil, Pseudomonas aeruginosa and Serratia marcescens as Gram negative bacteria using chloramphenicol as control (Table 1). The MIC results indicated that three of the tested compounds (11, 19 and 22a) showed significant activity against Staphylococcus aureus (Table 1). Compounds 12 and 19c showed highly significant activity against Bacillus cereus and moderate activity against Micrococcus luteus (Table 1). Compound 22a revealed moderate activity against Staphylococcus aureus and Pseudomonas aeruginosa (Table 1). The rest of tested compounds were inactive against all bacterial strains used.
Table 1.
Antibacerial activity data [inhibition zone in mm/MICs (in mM).
| Compound No. | Diameter of the inihibition zone (mm) MIC (mM) |
|||||
|---|---|---|---|---|---|---|
|
S. aureus AUMC B.54 |
S. cereus AUMC B.52 |
M. luteus AUMC B.112 |
E. coli AUMC B.53 |
p. aeruginosa AUMC B.73 |
S. marcescens AUMC B.55 |
|
| 6 | - | - | - | - | - | - |
| 7a | - | - | - | - | - | - |
| 9 | - | - | - | - | - | - |
| 11 | 8 (2.5) | - | - | - | - | - |
| 12 | - | 10 (1.25) | 18 (20) | - | - | - |
| 13b | - | - | - | - | - | - |
| 17 | - | - | - | - | - | - |
| 19c | 8 (1.25 ) | 10 (0.15) | 10 (5) | - | - | - |
| 20 | - | - | - | - | - | - |
| 22a | 8 (5) | - | - | - | 11 (20) | - |
| CHL | 10 (0.08) | 12 (1.25) | 12 (2.5) | 10 (0.08) | 12 (0.3) | 13 (1.25) |
CHL = chloramphenicol as control.
The same compounds (6, 7a, 9, 11, 12, 13b, 17, 19c, 20 and 22a) were screened for their antifungal activities against six fungal strains: (Candida albicans AUMC No. 418, Trichophyton rubrum AUMC No. 1804, Aspergillus flavus AUMC No. 1276, Fusarium oxysporum AUMC No. 5119, Scopulariopsis brevicaulis AUMC No. 729, Geotrichum candidum AUMC No. 226) using clotrimazole as control. The results are listed in Table 2. The MIC values showed that compounds 22a, 12 and 17 exhibit moderate to low activity against Candida albicans AUMC No. 418. Compounds 19c, 12 and 11 showed moderate to low activity against Geotrichum candidum AUMC No. 226. Compounds 17, 19c, 12 and 10 revealed moderate to low activity against Trichophyton rubrum AUMC No. 1804. Compounds 19c, 12 and 17 showed moderate to poor activity against Aspergillus flavus AUMC No. 1276. Compounds 19c, 12 and 17 showed moderate to poor activity against Fusarium oxysporum AUMC No. 5119. Compounds 19c, 12, 17 and 11 showed moderate to poor activity against Scopulariopsis brevicaulis AUMC No. 729. The rest of tested compounds were inactive against all fungal strains used.
Table 2.
Antifungal activity data [inhibition zone in mm/ MICs (in mM).
| Compound No. | Diameter of the inihibition zone (mm) MIC (mM) |
|||||
|---|---|---|---|---|---|---|
|
C. albicans AUMC 418 |
G.candidum AUMC 226 |
T. rubrum AUMC 1804 |
A.flavus AUMC 3214 |
F. oxysporum AUMC 5119 |
S. brevicaylis AUMC 729 |
|
| 6 | - | - | - | - | - | - |
| 7a | - | - | - | - | - | - |
| 9 | - | - | - | - | - | - |
| 11 | - | 11 (20) | 8 (20) | - | - | 10 (20) |
| 12 | 9 (2.5) | 15 (5) | 10 (10) | 10 (2.5) | 16 (20) | 20 (5) |
| 13b | - | - | - | - | - | - |
| 17 | 10 (20) | - | 24p.i (20) | 10 (20) | 8 (20) | 12 (10) |
| 19c | 9 (2.5) | 16 (2.5) | 12 (5) | 14 (2.5) | 10 (10) | 28 (5) |
| 20 | - | - | - | - | - | - |
| 22a | 10 (0.6) | - | - | - | - | - |
| CLO | 12 (0.08) | 14 (0.08) | 35 (0.08) | 15 (0.15) | 14 (0.15) | 24 p.i (0.3) |
CLO = Clotrimazole as control; p.i = Partial inhibition.
3. Experimental
3.1. General
All melting points were determined on a Kofler melting point apparatus. IR spectra were recorded on a Pye Unicam SP3-100 spectrophotometer using the KBr wafer technique. The 1H-NMR spectra were recorded on a Bruker ARX 200 spectrometer (200 MHz for 1H and 50 MHz for 13C) at the Faculty of Science, University of King Saoud, Saudi Arabia, Riyadh and on a Jeol LA 400 MHz (400 MHz for 1H, 100 MHz for the 13C) at Assiut University, 1H and 13C NMR chemical shifts (δ) were reported in parts per million (ppm) and were referenced to the solvent peak; CDCl3 (7.26 ppm for 1H and 76.90 ppm for 13C) and DMSO-d6 (2.50 ppm for 1H and 39.70 ppm for 13C). Multiplicities are represented by s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). Coupling constants (J) are reported in Hertz (Hz). Mass spectra were taken on a JEOL JMS600 spectrometer at an ionizing potential of 70 eV (EI) at Assiut University. Elemental analyses were carried out using a Perkin-Elmer 240 C Micro analyzer at Assiut University and they were found to be within ± 0.4% of the theoretical values.
3.2. Synthesis of 3-Methyl-1,4-diphenyl-7-thioxo-4,6,8,9-tetrahydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one (6)
Method A: A mixture of thiobarbituric acid (1.44 g, 0.01 mol), 3-methyl-1-phenyl-1H-pyrazol-5-amine (1.73 g, 0.01 mol), benzaldehyde (1.1 g, 0.012 mol) and p-TSA (1 g, 0.05 mol) was heated at 100 °C for 10 h (monitored by TLC). After cooling, the reaction mixture was washed with water (20 mL) and residue recrystallized from EtOH to afford the pure product 6 as a yellow powder. Yield: 66%. Mp 202–204 °C. IR (KBr) cm−1: 3240, 3290, 3310 (3NH), 1690 (C=O), 1620 (C=N), 1345 (C=S). 1H-NMR (DMSO-d6): 2.75 (s, 3H, CH3), 5.57 (s, 1H, CH pyridine), 7.25–7.98 (m, 10H, Ar-H), 9.45 (s, 1H, NH), 12.20 (br s, 1H, NH), 13.80 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 11.93 (CH3), 33.8 (CH sp3), 102.11–160.12 (17C, sp2 carbon atoms), 161.73 (C=O), 174.45 (C=S). Anal. Calcd. For C21H17N5OS (387.45): C, 65.10; H, 4.42; N, 18.08; S, 8.28. Found: C, 65.15; H, 4.38; N, 18.04; S, 8.23.
Method B: A mixture of phenylhydrazine (1 mmol), 3-aminocrotononitrile (1mmol) and p-TSA (0.5 mmol) in water (10 mL) was added to benzaldehyde (1 mmol) and thiobarbituric acid (1 mmol) and the reaction mixture was heated under reflux at 100 °C for 1 h, after completion of the reaction (TLC), the reaction mixture cooled to room temperature, the precipitate filtered off and washed with water and recrystallized from EtOH to afford the pure product 6 as a yellow powder. Yield: 91%. All of spectral and physical data were in agreement with that described in method A.
3.3. General Procedure for the Preparation of 7a–f
A mixture of 6 (3.8 g, 0.01 mol), ethyl iodide and/or α-haloketone compound (0.01 mol) in ethanol (50 mL) was refluxed in the presence of anhydrous sodium acetate (0.9 g, 0.011 mol) for 4 h. The solid product separated from the hot mixture was filtered off, washed with water and recrystallized from the proper solvent.
7-Ethylthio-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one (7a). Yellow crystals. Yield: 72%. Mp. 135–137 °C (acetic acid). IR (KBr) cm−1: 3220, 3315 (2NH), 1690 (C=O), 1620 (C=N). 1H-NMR (DMSO-d6) δ (ppm): 1.55 (t, J = 7.4 Hz, 3H, CH3), 2.78 (s, 3H, CH3), 3.65 (q, J = 7.4 Hz, 2H, CH2), 5.55 (s, 1H, CH), 7.2–7.8 (m, 10H, Ar-H), 8.9 (s, 1H, NH), 10.2 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 12.03 (CH3), 15.34 (CH3), 24.12 (CH2), 33.5 (CH sp3), 103.15–160.12 (18C, sp2 carbon atoms), 161.73( C=O), Anal. Calcd. for C23H21N5OS (415.52): 66.48; H, 5.09; N, 16.85; S, 7.71. Found: C, 66.61; H, 5.13; N, 16.79; S, 7.68.
Ethyl-2-(3-methyl-5-oxo-1,4-diphenyl-4,6,9-trihydro-pyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-7-ylthio)acetate (7b). Yellow crystals. Yield: 67%. Mp. 95–97 °C (methanol). IR (KBr) cm−1: 3290, 3310 (2NH), 1735 (C=O), 1690 (C=O), 1620 (C=N). 1H-NMR (DMSO-d6) δ (pp): m1.27 (t, J = 7.4 Hz, 3H, CH3), 2.80 (s, 3H, CH3), 3.95 (s, 2H, SCH2), 4.25 (q, J = 7.4 Hz, 2H, CH2), 5.52 (s, 1H, CH), 7.2–7.9 (m, 10H, Ar-H), 8.7 (s, 1H, NH), 11.1 (s, 1H, NH). 13C-NMR (CDCl3) δ (ppm): 12.03 (CH3), 15.34 (CH3), 29.12 (CH2), 33.55 (CH sp3), 61.11 (CH2), 103.15–160.12 (18C, sp2 carbon atoms), 161.73 (C=O), 170.25 (C=O ester). Anal. Calcd. for: C25H23N5O3S (473.54): C, 63.41; H, 4.90; N, 14.79; S, 6.77. Found: C, 63.49; H, 5.01; N, 14.82; S, 6.86.
3-Methyl-7-(2-oxo-2-phenylethylthio)-1,4-diphenyl-4,6,9-trihydropyrazolo [5,4-b]pyrimidino[5,4-e]-pyridin-5-one (7c). Light yellow crystals.Yield: 71%. Mp. 170–172 °C (methanol). IR (KBr) cm−1: 3245, 3290 (2NH), 1,685 (C=O), 1690 (C=O), 1620 (C=N); 1H-NMR (DMSO-d6) δ ppm): 2.72 (s, 3H, CH3), 4.19 (s, 2H, SCH2CO), 5.57 (s, 1H, CH), 6.9–8.6 (m, 15H, Ar-H), 8.84 (s, 1H, NH), 10.73 (s, 1H, NH); 13C-NMR (DMSO-d6) δ (ppm): 12.93 (CH3), 34.42 (CH sp3), 35.60 (CH2), 102.75–160.30 (18C, sp2 carbon atoms), 162.03 (C=O), 195.30 (C=O) Anal. Calcd. for: C29H23N5O2S (505.58): C, 68.89; H, 4.59; N, 13.85; S, 6.34. Found: C, 68.97; H, 4.64; N, 13.91; S, 6.29.
3-Methyl-7-(2-oxopropylthio)-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one (7d). Yellow crystals. Yield: 65%. Mp. 223–225 °C (ethanol); IR (KBr) cm−1: 3195, 3290 (2NH), 1685 (C=O), 1700 (C=O); 1H-NMR (CDCl3) δ (ppm): 2.75 (s, 3H, CH3), 3.35 (s, 3H, COCH3), 4.10 (s, 2H, SCH2), 5.60 (s, 1H, CH), 7.35–7.95 (m, 10H, Ar-H), 9.15 (s, 1H, NH), 10.2 (s, 1H, NH). 13C-NMR (CDCl3) δ (ppm): 11.93 (CH3), 29.32 (CH3), 33.82 (CH sp3), 38.22 (CH2), 103.15–160.12 (18C, sp2 carbon atoms), 161.53 (C=O), 202.11 (C=O). Anal. Calcd. for: C24H21N5O2S (443.52): C, 64.99; H, 4.77; N, 15.79; S, 7.23. Found: C, 65.13; H, 4.81; N, 15.83; S, 7.19.
2-(3-Methyl-5-oxo-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-7-ylthio)acetamide (7e). Orange crystals. Yield: 69%. Mp. 166–168 °C (DMF). IR (KBr) cm−1: 3420, 3400 (NH2), 3250, 3310 (2NH), 1685 (C=O), 1665 (C=O), 1690 (C=O). 1H-NMR (CDCl3) δ (ppm): 2.65 (s, 3H, CH3), 3.70 (s, 2H, SCH2), 5.30 (br.s, 2H, NH2), 6.15 (s, 1H, CH), 7.35–7.96 (m, 10H, Ar-H), 8.95 (s, 1H, NH), 10.78 (s, 1H, NH); 13C-NMR (CDCl3) δ (ppm): 12.11 (CH3), 30.34 (CH2), 34.52 (CH sp3), 104.85–159.90 (18C, sp2 carbon atoms), 162.30 (C=O), 171.45 (C=O) Anal. Calcd. for C23H20N6O2S (444.50): C, 62.15; H, 4.54; N, 18.91; S, 7.21. Found: C, 62.29; H, 4.61; N, 19.03; S, 7.15.
2-(3-Methyl-5-oxo-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-7-ylthio)ethanenitrile (7f). Orange crystals. Yield: 61%. Mp. 147–149 °C (ethanol). IR (KBr) cm−1: 3215, 3200 (2NH), 2,240 (C=N), 1665 (C=O); 1H-NMR (DMSO-d6) ) δ (ppm): 2.70 (s, 3H, CH3), 3.90 (s, 2H, SCH2CO), 6.12 (s, 1H, CH), 7.55–8.22 (m, 10H, Ar-H), 9.20 (s, 1H, NH), 10.6(s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 12.31 (CH3), 15.38 (CH2), 33.84 (CH sp3), 104.80–159.95 (18C, sp2 carbon atoms), 161.90 (C=O). Anal. Calcd. for C23H18N6OS (426.49): C, 64.77; H, 4.25; N, 19.70; S, 7.52. Found: C, 64.89; H, 4.31; N, 19.61; S, 7.61.
3.4. 8-Ethyl-7-ethylthio-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one (8) and 6-Ethyl-7-ethylthio-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one (9)
Method A: A mixture of 6 (3.8 g, 0.01 mol), ethyl iodide (3.1 g, 0.021 mol) and sodium hydroxide (0.8 g, 0.02 mol) in ethanol (50 mL) was refluxed for 4 h. Excess ethanol was distilled off and the residue obtained on cooling was found to be mixture of two products as evidenced by the TLC. The residue was subjected to column chromatography over silica gel using mixtures of pet-ether/ethyl acetate of increasing polarity of eluent to yield compound 9 as an orange solid and compound 8 as a yellow solid in the second fraction.
8-Ethyl-7-ethylthio-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one (8). Yield: 63%. Mp. 190–192 °C. IR (KBr) cm−1: 3190 (NH), 1700 (C=O), 1H-NMR (DMSO-d6) δ (ppm): 1.00 (t, J = 7.4 Hz, 3H, NCH2CH3), 1.35 (t, J = 7.4 Hz, 3H, SCH2CH3), 2.68 (s, 3H, CH3), 3.15 (q, J = 7.4 Hz, 2H, NCH2CH3), 3.95 (q, J = 7.4 Hz, 2H, SCH2CH3), 5.65 (s, 1H, CH), 7.40–7.80 (m, 10H, Ar-H), 10.45 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 12.50 (CH3), 12.88 (CH3), 15.09 (CH3), 26.13 (S-CH2), 37.04 (CH2), 39.25 (CH sp3), 105.77–162.15 (18C, sp2 carbon atoms), 178.14 (C=O).
6-Ethyl-7-ethylthio-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one (9). Yield: 25%. Mp. 162–164 °C. IR (KBr) cm−1: 3235 (NH), 1695 (C=O), 1H-NMR (DMSO-d6) δ (ppm): 1.32 (t, J = 7.4 Hz, 3H, NCH2CH3), 1.65 (t, J = 7.4 Hz, 3H, SCH2CH3), 2.78 (s, 3H, CH3), 3.45 (q, J = 7.4 Hz, 2H, NCH2CH3), 4.05 (q, J = 7.4 Hz, 2H, SCH2CH3), 5.75 (s, 1H, CH), 7.40–7.96 (m, 10H, Ar-H), 10.26 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 11.81 (CH3), 12.56 (CH3), 14.88 (CH3), 24.28 (S-CH2), 33.14 (CH2), 35.22 (CH sp3), 103.77–160.15 (18C, sp2 carbon atoms), 163.16 (C=O); Anal. Calcd. for: C25H25N5OS (443.56) C, 67.69; H, 5.68; N, 15.79; S, 7.23. Found: C, 67.81; H, 5.61; N, 15.81; S, 7.16.
Method B: A mixture of 6 (1.90 g, 0.005 mol) and ethyl iodide (1.55g, 0.01 mol) in DMF (30 mL) in the presence of anhydrous potassium carbonate (0.6 g, 0.002 mol) was stirred at room temperature for 10 h. The reaction mixture was cooled, poured onto ice cold water. The solid product separated was filtered off, washed with water and recrystallized from dioxane to yield compound 9. All of the physical and analytical data were in agreement with those of the product obtained using method A.
3.5. 7-(2,4-Dinitrophenylthio)-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e] pyridin-5-one (10)
A mixture of 6 (1.14 g, 0.003 mol) and 2,4-dinitrochlorobenzene (0.6 g, 0.003 mol) in DMF (40 mL) was stirred at room temperature for 5 h. The reaction mixture was cooled to 0 °C for 2 h, a yellow fine crystals was obtained, it was filtered off dried and recrystallized from dioxane. Yield: 68%. Mp. 251–253 °C. IR (KBr) cm−1: 3325, 3295 (2NH), 1690 (C=O), 1H-NMR (DMSO-d6) δ (ppm): 2.75 (s, 3H, CH3), 5.65 (s, 1H, CH), 7.14–8.92 (m, 13H, Ar-H), 10.26 (s, 1H, NH), 11.35 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 11.90 (CH3), 34.35 (CH sp3), 103.77–161.17 (25C, sp2 carbon atoms), 162.99 (C=O); Anal. Calcd. for: C27H19N7O5S (553.54) C, 58.58; H, 3.46; N, 17.71; S, 5.79. Found: C, 58.96; H, 3.69; N, 17.97; S, 6.14.
3.6. 3-Methyl-8-nitro-1,4-diphenyl-4,6,13-trihydrobenzothiazolo[3',2'-2,1]pyrimidino[5,4-e] pyrazolo-[5,4-b]pyridin-5-one (11)
Method A: A mixture of 6 (0.38 g, 0.001 mol) and 2,4-dinitrochlorobenzene (0.2 g, 0.001 mol) in DMF (20 mL) was refluxed for 2 h. The reaction mixture was concentrated for its half volume and allowed to cool, yellowish crystals was obtained, filtered off, dried and recrystallized from acetic acid. Yield: 59%. Mp. >300 °C. IR (KBr) cm−1: 3180 (NH), 1685 (C=O), 1H-NMR (DMSO-d6) δ (ppm): 2.79 (s, 3H, CH3), 4.95 (s, 1H, CH), 7.10–7.85 (m, 13H, Ar-H), 9.95 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 12.30 (CH3), 34.15 (CH sp3), 102.57–162.11 (25C, sp2 carbon atoms), 163.09 (C=O); Anal. Calcd. for: C27H18N6O3S (506.53) C, 64.02; H, 3.58; N, 16.59; S, 6.33. Found: C, 64.42; H, 3.99; N, 16.91; S, 6.64. MS m/z (%) 506.12 (M+, 100).
Method B: A sample of 10 (0.5 g, 0.001 mol) was heated under reflux in DMF (30 mL) for 2 h. The separated product 11 was obtained and purified as described in method A. All physical and analytical data of the two final products obtained from both methods A and B are identical.
3.7. 6-Ethyl-7-hydrazino-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]-pyridin-5-one (12)
A mixture of 9 (1.76 g, 0.004 mol) and hydrazine hydrate (15 mL) in absolute ethanol (40 mL) was refluxed for 12 h. The reaction mixture was poured onto ice. The product was isolated and crystallized from acetic acid as white needles. Yield: 85%. Mp. 280–281 °C, IR (KBr) cm−1: 3440, 3335, 3210 (NH, NH2), 1695 (C=O); 1H-NMR (DMSO-d6) δ (ppm): 1.23 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.81 (s, 3H, CH3), 3.35 (q, J = 7.4 Hz, 2H, NCH2CH3), 4.95 (s, 2H, NH2, D2O exchangeable), 6.04 (s, 1H, CH), 7.55–8.22 (m, 10H, Ar-H), 9.82 (s, 1H, NH, D2O exchangeable), 10.55 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 12.21 (CH3), 13.01 (CH3), 30.58(CH2), 34.92 (CH sp3), 102.65–162.95 (18C, sp2 carbon atoms), 163.56 (C=O). Anal. Calcd. for: C23H23N7O (413.47): C, 66.81; H, 5.61; N, 23.71; Found: C, 66.95; H, 5.67; N, 23.79.
3.8. General Procedure for the Preparation of 13a–c
A mixture of compound 12 (0.413 g, 0.001 mol) and the appropriate aromatic aldehyde (0.001 mol) was stirred under reflux in ethanol (30 ml) in the presence of a few drops of glacial acetic acid for 5 h. The reaction mixture was allowed to cool to room temperature, poured into water, whereby a solid formed that was filtered off and crystallized from an appropriate solvent to produce 13a–c in good yields.
6-Ethyl-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one-7-benzaldehyde hydrazone (13a). Pale white crystals from acetic acid. Yield: 70%. Mp. 268–269 °C; IR (KBr) cm−1: 3425, 3295 (2NH), 1675 (C=O), 1625 (C=N), 1H-NMR (DMSO-d6) δ (ppm): 1.20 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.78 (s, 3H, CH3), 3.30 (q, J = 7.4 Hz, 2H, NCH2CH3), 6.10 (s, 1H, CH), 7.55–8.25 (m, 15H, Ar-H), 8.95 (s,1H, azomethine proton), 11.10 (brs, 1H, NH, D2O exchangeable), 12.75 (brs, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ (ppm): 11.91 (CH3), 12.99 (CH3), 30.81 (CH2), 35.20 (CH sp3), 102.65–162.95 (21C, sp2 carbon atoms), 162.66 (C=O), 164.11 (N=C). Anal. Calcd. for: C30H27N7O (501.58): C, 71.84; H, 5.43; N, 19.55; Found: C, 71.93; H, 5.59; N, 19.61.
6-Ethyl-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one-7-(p-chloro)benzaldehyde hydrazone (13b). Pale light yellow crystals, from dioxane. Yield: 72%. Mp. 310–311 °C (dec.); IR (KBr) cm−1: 3320, 3170 (2NH), 1685 (C=O), 1645 (C=N), 1H-NMR (DMSO-d6) δ (ppm): 1.25 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.88 (s, 3H, CH3), 3.35 (q, J = 7.4 Hz, 2H, NCH2CH3), 5.98 (s, 1H, CH), 7.25–8.15 (m, 14H, Ar-H), 9.15 (s,1H, azomethine proton), 10.85 (brs, 1H, NH, D2O exchangeable), 12.75 (brs, 1H, NH, D2O exchangeable); Anal. Calcd. for C30H26ClN7O (536.03): C, 67.22; H, 4.89; Cl, 6.61; N, 18.29; Found: C, 67.33; H, 4.94; Cl, 6.59; N, 18.41.
6-Ethyl-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridin-5-one-7-(p-methoxy)benzaldehyde hydrazone (13c). Pale white crystals from dioxane. Yield: 68%. Mp. 216–218 °C; IR (KBr)cm−1: 3330, 3100 (2NH), 1690 (C=O), 1635 (C=N), 1H-NMR (DMSO-d6) δ (ppm): 1.15 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.90 (s, 3H, CH3), 3.30(q, J = 7.4 Hz, 2H, NCH2CH3),3.94 (s, 3H, OCH3) 5.88 (s, 1H, CH), 7.20–7.95 (m, 14H, Ar-H), 9.50 (s,1H, methylenic proton), 10.77 (brs, 1H, NH, D2O exchangeable), 11.45(brs, 1H, NH, D2O exchangeable). Anal. Calcd. for C31H29N7O2 (531.61): C, 70.04; H, 5.50; N, 18.44; Found: C, 70.14; H, 5.59; N, 18.41.
3.9. 6-Ethyl-3-methyl-7-(3-methyl-5-oxo(2-pyrazolinyl))-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]-pyrimidino[5,4-e]pyridin-5-one (14)
A solution of compound 12 (0.413 g, 0.001 mol) and ethyl acetoacetate (0.130 g, 0.001 mol) in sodium ethoxide solution [prepared by dissolving sodium metal (0.023 g, 0.001 mol) in absolute ethanol (30 mL)] was heated under reflux with stirring for 4 h. The reaction mixture was allowed to cool and poured into cold water (100 mL) and neutralized by acetic acid, whereby a solid was precipitated, which was filtered off and crystallized from chloroform. Yield: 73%. Mp. 231–233 °C; IR (KBr) cm−1: 3315 (NH), 1698, 1676 (2C=O), 1550 (C=N), 1H-NMR (CDCl3) δ (ppm): 1.25 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.33 (s, 3H, CH3), 2.55 (s, 2H, CH2), 2.88 (s, 3H, CH3), 3.50 (q, J = 7.4 Hz, 2H, NCH2CH3), 5.88 (s, 1H, CH), 7.20–7.66 (m, 10H, Ar-H), 10.85(brs, 1H, NH, D2O exchangeable); 13C-NMR (CDCl3) δ (ppm): 11.33 (CH3), 12.79 (CH3), 24.37 (CH3), 30.65 (CH2), 34.78 (CH sp3), 44.65 (CH2 pyrazole), 103.65–162.95 (19C, sp2 carbon atoms), 163.66 (C=O), 164.815 (C=O pyrazole). Anal. Calcd. for: C27H25N7O2 (479.53): C, 67.63; H, 5.25; N, 20.45; Found: C, 67.71; H, 5.29; N, 20.41.
3.10. 7-(3,5-Dimethylpyrazolyl)-6-ethyl-3-methyl-1,4-diphenyl-4,6,9-trihydropyrazolo[5,4-b]-pyrimidine[5,4-e]pyridin-5-one (15)
A mixture of compound 12 (0.413 g, 0.001 mol) and acetylacetone (0.1 g, 0.001 mol) in absolute ethanol (30 mL) was stirred under reflux for 5 h. The reaction mixture was allowed to cool to 0 °C for 3 h. The precipitate was filtered off, dried and crystallized from ethanol as pale light crystals. Yield: 80%. Mp. 222–224 °C. IR (KBr) cm−1: 3225 (NH), 1700 (C=O), 1650 (C=N), 1H-NMR (CDCl3) δ (ppm): 1.27 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.41 (s, 3H, CH3), 2.43 (s, 3H, CH3), 2.68 (s, 3H, CH3), 3.63 (q, J = 7.4 Hz, 2H, NCH2CH3), 5.77 (s, 1H, CH), 6.15 (1H, CH pyrazole), 7.20–7.82 (m, 10H, Ar-H), 10.65(s, 1H, NH); 13C-NMR (CDCl3) δ (ppm): 11.33 (CH3), 12.79 (CH3),17.62 (CH3), 24.37 (CH3), 30.65 (CH2), 33.66 (CH sp3), 102.25–160.95 (21C, sp2 carbon atoms), 162.90 (C=O). Anal. Calcd. for: C28H27N7O (477.56): C, 70.42; H, 5.70; N, 20.53; Found: C, 70.52; H, 5.79; N, 20.61.
3.11. 7-(3-Amino-5-oxo(2-pyrazolinyl))-6-ethyl-3-methyl-1,4-diphenyl-4,6,9-tri-hydropyrazolo[5,4-b]-pyrimidino[5,4-e]pyridin-5-one (16)
To a warmed ethanolic sodium ethoxide solution [prepared by dissolving sodium metal (0.023 g, 0.001 mol) in absolute ethanol (30 mL)] was added compound 12 (0.413 g, 0.001 mol) and ethyl cyanoacetate (0.113 g, 0.001 mol). The mixture was stirred under reflux for 12 h, the reaction mixture was allowed to cool to room temperature, then poured into cold water (100 mL) and neutralized with acetic acid. The solid product was filtered off, washed with water, ethanol, dried and crystallized from ethanol as pale brown crystals. Yield: 85%. Mp. 310–312 °C. IR (KBr) cm−1: 3455, 3350 (NH2), 3220 (NH), 1695 (C=O), 1700 (C=O), 1640 (C=N), 1H-NMR (CDCl3) δ (ppm): 1.31 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.71 (s, 3H, CH3), 3.45 (s, 2H, CH2 pyrazole), 3.65 (q, J = 7.4 Hz, 2H, NCH2CH3), 6.01 (s, 1H, CH), 7.15–7.89 (m, 10H, Ar-H), 10.25 (s, 1H, NH), 12.11 (brs, NH2, D2O exchangeable). Anal. Calcd. for: C26H24N8O2 (480.52): C, 64.99; H, 5.03; N, 23.32; Found: C, 65.08; H, 5.10; N, 23.41.
3.12. 11-Ethyl-8-methyl-6,9-diphenyl-4,5,9,11-tetrahydropyrazolo[5,4-b]1,2,4-triazolo[4',3'-2,1]-pyrimidino[5,6-e]pyridin-10-one (17)
A mixture of compound 12 (0.413 g, 0.001 mol) and triethyl orthoformate (0.192 g, 0.0013 mol) in ethanol (30 mL) was refluxed in the presence of few drops of acetic acid for 3 h. The solid product that separated from the hot mixture was filtered off, and recrystallized from acetic acid as yellow crystals. Yield: 56%. Mp. >300 °C. IR (KBr)cm−1: 3315 (NH), 1695 (C=O), 1650 (C=N), 1H-NMR (DMSO-d6) δ (ppm): 1.24 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.71 (s, 3H, CH3), 3.45 (q, J = 7.4 Hz, 2H, NCH2CH3), 6.15 (s, 1H, CH), 7.15–7.92 (m, 10H, Ar-H), 8.55 (s, 1H, CH triazole), 11.05 (s, 1H, NH). Anal. Calcd. for C24H21N7O (423.47): C, 68.07; H, 5.01; N, 23.15; Found: C, 68.16; H, 4.96; N, 23.22. MS m/z (%) 423.21 (M+, 100).
3.13. 11-Ethyl-8-methyl-6,9-diphenyl-10-oxo-4,5,9,11-tetrahydropyrazolo[5,4-b]1,2,4-triazolo[4',3'-2,1]pyrimidino[5,6-e]pyridin-3(2H)-thione (18)
A mixture of compound 12 (2.06g, 0.005 mol) and carbon disulfide (0.95 g, 0.005 mol) in ethanolic sodium hydroxide (10 mL, 10%) was heated on a water bath for 2 h. The solvent was evaporated under reduced pressure, the residue was diluted with water (30 mL), acidified with HCl. The solid product was filtered off and recrystallized from acetic acid as orange crystals. Yield: 69% Mp.285–287 °C. IR (KBr) cm−1: 3295 (NH), 1705 (C=O), 1620 (C=N), 1H-NMR (DMSO-d6). δ (ppm): 0.99 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.45 (s, 3H, CH3), 3.15 (q, J = 7.4 Hz, 2H, NCH2CH3), 5.65 (s, 1H, CH), 7.30–7.99 (m, 10H, Ar-H), 10.75 (s, 1H, NH). Anal. Calcd. for: C24H21N7OS (455.53): C, 63.28; H, 4.65; N, 21.52; S, 7.04; Found: C, 63.36; H, 4.72; N, 21.62.; S, 6.98. MS m/z (%) 455.17 (M+, 100).
3.14. General Procedure for the Preparation of 19a–c
These compounds were synthesized following a procedure analogous to that for compounds 7a–f using a mixture of 18 (0.44 g, 0.001 mol), ethyl iodide and/or α-haloketone (0.001 mol). The solid product separated from the hot mixture was filtered off, washed with water and recrystallized from ethanol.
3-Ethylthio-11-ethyl-8-methyl-6,9-diphenyl-4,5,9,11-tetrahydropyrazolo[5,4-b]1,2,4-triazolo[4',3'-2,1]pyrimidino[5,6-e]pyridin-10-one (19a). Yellow crystals. Yields: 63%. Mp. 245–247 °C. IR (KBr) cm−1: 3305 (NH), 1705 (C=O), 1630 (C=N), 1H-NMR (DMSO-d6) ppm δ: 1.30 (t, J = 7.4 Hz, 3H, NCH2CH3), 1.58 (t, J = 7.4 Hz, 3H, SCH2CH3), 2.69 (s, 3H, CH3), 3.11 (q, J = 7.4 Hz, 2H, NCH2CH3), 3.55( q, J = 7.4 Hz, 2H, SCH2CH3), 5.95 (s, 1H, CH), 7.40–7.85 (m, 10H, Ar-H), 10.30 (s, 1H, NH, D2O exchangeable). 13C-NMR (DMSO-d6) δ (ppm): 11.13 (CH3), 12.50 (CH3),14.65 (CH3), 29.75 (CH2), 35.60 (CH sp3), 42.80 (CH2), 103.15–160.95 (19C, sp2 carbon atoms), 162.20 (C=O). Anal. Calcd. for: C26H25N7OS (483.59): C, 64.58; H, 5.21; N, 20.27; S, 6.63; Found: C, 64.69; H, 5.30; N, 20.32; S, 6.78.
Ethyl-3-(11-ethyl-8-methyl-6,9-dipheny-10-oxo--4,5,9,11-tetrahydro-pyrazolo[5,4-b]1,2,4-triazolo[4',3'-2,1]pyrimidino[5,6-e]pyridine)acetate (19b). Yellow crystals. Yield: 79%. Mp. 189–191 °C; IR (KBr) cm−1: 3280 (NH), 1705 (C=O), 1735 (C=O), 1600 (C=N), 1H-NMR (DMSO-d6) δ (ppm): 1.25 (t, J = 7.4 Hz, 3H, NCH2CH3), 1.45 (t, J = 7.4 Hz, 3H, SCH2CH3), 2.70 (s, 3H, CH3), 3.10 (q, J = 7.4 Hz, 2H, NCH2CH3), 3.80 (s, 2H, SCH2), 4.15 (q, J = 7.4 Hz, 2H, SCH2CH3), 6.15 (s, 1H, CH), 7.40–7.85 (m, 10H, Ar-H), 11.03 (s, 1H, NH, D2O exchangeable). Anal. Calcd. for: C28H27N7O3S (541.49): C, 62.09; H, 5.02; N, 18.10; S, 5.92; Found: C, 62.23; H, 4.87; N, 18.19; S, 5.99.
11-Ethyl-3-(2-oxo-2-phenylethylthio)--8-methyl-6,9-dipheny-10-oxo-4,5,9,11-tetrahydro-pyrazolo[5,4-b]1,2,4-triazolo[4',3'-2,1]pyrimidino[5,6-e]pyridine-10-one (19c). Pale yellow crystals. Yield: 68%. Mp. 272–274 °C. IR (KBr) cm−1: 3290 (NH), 1705 (C=O), 1690 (br, C=O), 1600 (C=N), 1H-NMR (DMSO-d6) δ (ppm): 1.18 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.77 (s, 3H, CH3), 3.32 (q, J = 7.4 Hz, 2H, NCH2CH3), 4.35 (s, 2H, SCH2), 5.95 (s, 1H, CH), 7.20–8.25 (m, 15H, Ar-H), 10.80 (s, 1H, NH, D2O exchangeable). Anal. Calcd. for C32H27N7O2S (573.67): C, 67.01; H, 4.74; N, 17.09; S, 5.59; Found: C, 67.21; H, 4.83; N, 17.23.; S, 5.49. MS m/z (%) 573.57 (M+, 100).
3.15. 4-Ethyl-7-methyl-6,9-diphenyl-4,6,10,11-tetrahydropyrazolo[5,4-b]1,2,3,4-tetraazolo[1',5'-1,2]-pyrimidino[5,6-e]pyridin-5-one (20)
A solution of sodium nitrite (0.07 g, 0.001 mol) in the least amount of water was added dropwise to an ice-cold solution of compound 12 (0.413 g, 0.001 mol) in acetic acid (10 mL) kept in an ice bath at −5 °C. The reaction mixture was allowed to stand overnight at room temperature, then it was poured into water (100 mL). The precipitate that formed was filtered off and crystallized from dioxane. It separated as pale yellow needles. Yield: 75%. Mp. > 300 °C; IR (KBr) cm−1: 3350 (NH), 2250 (N3), 1695 (C=O), 1620 (C=N). 1H-NMR (DMSO-d6) δ (ppm): 1.33 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.60 (s, 3H, CH3), 3.54 (q, J = 7.4 Hz, 2H, NCH2CH3), 5.85 (s, 1H, CH), 7.20–7.90 (m, 10H, Ar-H), 11.25 (s, 1H, NH, D2O exchangeable). 13C-NMR (DMSO-d6) δ (ppm ): 11.03 (CH3), 12.35 (CH3), 33.45 (CH sp3), 40.65 (CH2), 101.75–160.15 (18C, sp2 carbon atoms), 163.30 (C=O). Anal. Calcd. for C23H20N8O (424.46): C, 65.08; H, 4.75; N, 26.40; Found: C, 65.19; H, 4.83; N, 26.55.
3.16. 5-Ethyl-8-methyl-1,2,7,10-tetraphenyl-5,7,11,12-tetrahydro-1H-pyrazolo[5,4-b]1,2,4-triazino[4',3'-2,1]-pyrimidino[5,6-e]pyridin-6-one (21)
A mixture of compound 12 (0.413g, 0.001 mol) and benzoin (0.21 g, 0.001 mol) was heated under reflux in a mixture of pyridine and acetic anhydride (20 mL) (1:1) for 5 h. The reaction mixture was allowed to cool, poured onto ice cold water and neutralized with dilute HCl, The solid product was filtered off and recrystallized from acetic as pale gray needles. Yield: 55%. Mp. > 300 °C; IR (KBr) cm−1: 3270 (NH), 1690 (C=O), 1640(C=N). 1H-NMR (DMSO-d6) δ (ppm): 1.35 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.40 (s, 3H, CH3), 3.15 (s, 1H, CH), 3.35 (q, J = 7.4 Hz, 2H, NCH2CH3), 6.05 (s, 1H, CH), 7.20–7.90 (m, 20H, Ar-H), 10.40 (s, 1H, NH, D2O exchangeable). 13C-NMR (DMSO-d6) δ (ppm): 11.03 (CH3), 12.55 (CH3), 32.35 (CH sp3), 38.70 (CH2), 44.55 (CH sp3), 105.80–161.85 (31C, sp2 carbon atoms), 162.90 (C=O). Anal. Calcd. for C37H31N7O (589.66): C, 75.35; H, 5.82; N, 16.19; Found: C, 75.73; H, 6.12; N, 16.53.
3.17. General Procedure for the Preparation of 22a,b
A mixture of compound 12 (0.001 mol) with chloroacetone or phenacyl bromide (0.001 mol) in DMF (30 mL) and drops of glacial acid (0.2 mL) was heated in 90 °C for 10 h. The solid that precipitated upon cooling was filtered off and crystallized from ethanol.
5-Ethyl-1,8-dimethyl-7,10-diphenyl-5,7,11,12-tetrahydro-3H-pyrazolo[5,4-b]1,2,4-triazino[4',3'-2,1]-pyrimidino[5,6-e]pyridin-6-one (22a). Pale white crystals. Yield: 51%. Mp. 241–243 °C (dec.). IR (KBr) cm−1: 3310, 3290 (2NH), 1685 (C=O) 1630 (C=N), 1H-NMR (DMSO-d6) δ (ppm): 1.20 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.33 (s, 3H, CH3), 2.65 (s, 3H, CH3), 3.54 (q, J = 7.4 Hz, 2H, NCH2CH3), 5.70 (s, 1H, CH), 7.15–7.95 (m, 10H, Ar-H), 9.25 (s, 1H, triazine), 10.80 (s, 1H, NH, D2O exchangeable), 10.95 (s, 1H, NH, D2O exchangeable). 13C-NMR (DMSO-d6) δ (ppm ): 11.90 (CH3), 12.45 (CH3), 16.55 (CH3), 30.98 (CH2), 34.95 (CH sp3), 36.15 (CH sp3), 104.90–161.85 (19C, sp2 carbon atoms), 162.90 (C=O). Anal. Calcd. for C26H25N7O (451.52): C, 69.16; H, 5.58; N, 21.71; Found: C, 69.24; H, 5.73; N, 21.81.
5-Ethyl-8-methyl-1,7,10-triphenyl-5,7,11,12-tetrahydro-3H-pyrazolo[5,4-b]1,2,4-triazino[4',3'-2,1]-pyrimidino[5,6-e]pyridin-6-one (22b). Pale white crystals. Yield: 63%. Mp. >300 °C, IR (KBr) cm−1: 3400, 3390 (2NH), 1685 (C=O) 1630 (C=N), 1H-NMR (DMSO-d6) δ (ppm): 1.19 (t, J = 7.4 Hz, 3H, NCH2CH3), 2.55 (s, 3H, CH3), 4.04 (q, J = 7.4 Hz, 2H, NCH2CH3), 6.10 (s, 1H, CH), 7.15–8.15 (m, 15H, Ar-H), 9.30 (s, 1H, triazine), 10.75 (s, 1H, NH, D2O exchangeable), 11.25 (s, 1H, NH, D2O exchangeable). Anal. Calcd. for: C31H27N7O (513.59): C, 72.50; H, 5.30; N, 19.09; Found: C, 72.64; H, 5.41; N, 19.16. MS m/z (%) 513.48 (M+, 100).
3.18. Antimicrobial Activity
The antimicrobial activity of 10 new chemical compounds was tested in vitro against six bacterial species obtained from contaminated soil, water and food substances (Staphylococcus aureus [AUMC No. B-54], Bacillus cereus [AUMC No. B-52], Micrococcus luteus (+ve) [AUMC NoB-112], Escherichia coli [AUMC No. B-53], Pseudomonas aeruginosa [AUMC No. B-73] and Serratia marcescens [AUMC No. B-55]. They were also tested against six fungal species which are involved in human and animal diseases (Trichophyton rubrum [AUMC No. 1804], Candida albicans [AUMC No. 418], Geotrichum candidum [AUMC No. 226], Scopulariopsis brevicaulis[AUMC No. 729] and Aspergillus flavus[AUMC No. 3214] or plant diseases (Fusarium oxysporum [AUMC No. 5119]. These strains are common contaminants of the environment in Egypt and some of all microbial strains were kindly provided by the Assiut University Mycological Centre (AUMC). To prepare inocula for bioassay, bacterial strains were individually cultured for 48 h in 100 mL conical flasks containing 30 mL nutrient broth medium. Fungi were grown for 7 days in 100 mL conicals containing 30 mL Sabouraud's dextrose broth. Bioassay was done in 10 cm sterile plastic Petri plates in which microbial suspension (1 mL/plate) and 15 mL appropriate agar medium (15 mL/plate) were poured. Nutrient agar and Sabouraud's dextrose agar were respectively used for bacteria and fungi. After solidification of the media, 5 mm diameter cavities were cut in the solidified agar (4 cavities/plate) using sterile cork borer. Chemical compounds dissolved in DMSO at 2%w/v (=20 mg/mL) were pipetted in the cavities (20 µL/cavity). Cultures were then incubated at 28 °C for 48 h in case of bacteria and up to 7 days in case of fungi. Results were read as the diameter (in mm) of inhibition zone around cavities [29]. To determine the minimum inhibitory concentrations (MICs), chemical compounds giving positive results were diluted with DMSO to prepare a series of descending concentrations down to 0.02 mg/mL. Diluted chemicals were similarly assayed as mentioned before and the least concentration (below which no activity) was recorded as the MIC.
4. Conclusions
In conclusion, we have reported the synthesis of some novel heterocyclic pyrazolo-pyrimidinopyridines and related compounds. Ten of the newly synthesized compounds have been screened for their biological activities against three Gram positive, three Gram negative bacteria, as well as six fungal strains. Most of the tested compounds showed activities against the strains used. Compound 19c proved to be the most potent compound of all those used.
Acknowledgements
The authors would like to express their gratitude to R. M. Mahfouz, Professor of Inorganic Chemistry at Assiut Univerisity for running the 13C-NMR analysis at King Saoud University, Saudi Arabia.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/17/12/14464/s1.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Ganem B. Strategies for innovation in multicomponent reaction design. Acc. Chem. 2009;42:463–472. doi: 10.1021/ar800214s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padwa A. Domino reactions of rhodium (II) carbenoids for alkaloid synthesis. Chem. Soc. Rev. 2009;38:3072–3081. doi: 10.1039/b816701j. [DOI] [PubMed] [Google Scholar]
- 3.Ali R., Amir T.M., Morteza R., Aram R. Novel three-component reaction of a secondary amine and a 2-hydroxybenzaldehyde derivative with an isocyanide in the presence of silica gel: an efficient one-pot synthesis of benzo[b]furan derivatives. Tetrahedron Lett. 2009;50:5625–5627. [Google Scholar]
- 4.Zeinab Z., Mehdi K., Ali R., Alireza F., Ali S., Katarzyna Ś., Tadeusz Lis A.S. Synthesis of functionalized furo[3,2-c]coumarins via a one-pot oxidative pseudo three-component reaction in poly(ethylene glycol. Tetrahedron. 2012;68:6721–6726. [Google Scholar]
- 5.Domling A. Multicomponent reactions. Chem. Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 6.D'Souza D.M., Muller T.J. Multi-component syntheses of heterocycles by transition-metal catalysis. J. Chem. Soc. Rev. 2007;36:1095–1108. doi: 10.1039/B608235C. [DOI] [PubMed] [Google Scholar]
- 7.Ali R., Ali S. Iminophosphorane-mediated one-pot synthesis of 1,3,4-oxadiazole Derivatives. ARKIVOC. 2008;xvi:235–242. [Google Scholar]
- 8.Ali S., Ali R., Nouri B., Richard W. The reaction of (N-isocyanimino)triphenylphosphorane with dialkyl acetylenedicarboxylates in the presence of 1,3-diphenyl-1,3-propanedione: A novel three-component reaction for the stereoselective synthesis of dialkyl (Z)-2-(5,7-diphenyl-1,3,4-oxadiazepin-2-yl)-2-butenedioates. Tetrahedron Lett. 2007;48:2617–2620. [Google Scholar]
- 9.Ali S., Ali R. The reaction of (N-isocyanimino)triphenylphosphorane with benzoic acid derivatives: A novel synthesis of 2-aryl-1,3,4-oxadiazole derivatives. Tetrahedron Lett. 2007;48:1549–1551. [Google Scholar]
- 10.Shore G., Yoo W.J., Li C.J., Organ M. Propargyl amine synthesis catalysed by gold and copper thin films using microwave assistant, continuous flow organic synthesis. Chem. Eur. J. 2010;16:126–133. doi: 10.1002/chem.200902396. [DOI] [PubMed] [Google Scholar]
- 11.Hardy C.R. The chemistry of pyrazolopyridine. Adv. Heterocyl. Chem. 1984;36:343–409. [Google Scholar]
- 12.Elnagdi M.H., Elgemeie G.H., El-Moghayar R.M.H. Chemistry of pyrazolopyrimidines. Adv. Heterocycl. Chem. 1987;41:319–376. [Google Scholar]
- 13.Jiaro Q., Jaime P., Silva C., Rodrigo A., Braulio I., Manuel N., Justo C., Mike H. Solvent free synthesis of fused pyrazolo [1,5-a]pyrimidines by reaction of 5-amino-1-H-pyrazoles and β-triketones. Open Org. Chem. J. 2008;2:92–99. [Google Scholar]
- 14.Jiaro Q., Jaime P., Rodrigo A., Manuel N., Justo C., Mike H. Regioselective synthesis of novel substituted pyrazolo[1,5-a]pyrimidines under solvent-free conditions. Tetrahedron Lett. 2008;49:6254–6256. [Google Scholar]
- 15.Jiaro Q., Jaime P., Hugo S., Rodrigo A., Braulio I., Manuel N., Justo C. Regioselective synthesis of fused benzopyrazolo[3,4-b]quinolines under solvent-free conditions. Tetrahedron Lett. 2007;48:1987–1990. [Google Scholar]
- 16.Aly M.F., El-Naggar G.M., El-Emary T.I., Girgg R., Metwally S.A., Sivagnanam S. X=Y-ZH Compounds as potential 1,3-Dipoles part 41. Azomethine Ylide Formation from the reaction of -Amino acids and esters wth Alloxan (Strecker Degradation) and with 1-phenyl-3-methyl pyrazoline 4,5-dione. Tetrahedron. 1994;50:895–906. [Google Scholar]
- 17.Selleri S., Burni F., Castanzo A., Gueririni G., Malmbeg-Aiello P., Lavarone G., Martini C. Synthesis and preliminary evaluation of pyrazolo[1,5-a]pyrido[3,4-e]pyrimidin-6-(7H)-ones and related comounds as benzodiazepine receptor ligands and anticonvulsant agents. Eur. J. Med. Chem. 1992;27:985–990. doi: 10.1016/0223-5234(92)90033-W. [DOI] [Google Scholar]
- 18.Poreba K., Wietrzyk J., Opolski A. Synthesis and antiproliferative activity in vitro of new 2,3 or 4 substituted pyrido[2',3':3,4]pyrazolo[1,5-a]pyrimidines. Acta Pol. Pharm. 2006;63:189–194. [PubMed] [Google Scholar]
- 19.Ismail M.M.F., Ammar Y.A., El-Zahaby H.S.A., Eisa S.I., Barakat S.E. Synthesis of Novel-1-pyrazolylpyridinopyrimidin2-ones as potential Anti-inflammatory and Analgesic Agent. Arch. Pharm. Chem. Life Sci. 2007;340:476–481. doi: 10.1002/ardp.200600197. [DOI] [PubMed] [Google Scholar]
- 20.Bi Y., Stoy P., He B., Adam L., Krupinski J., Normandin D., Pongrac R., Seliger L., Waston A., Macor J.E. The discovery of novel, potent and selective PDE inhibitors. Bioorg. Med. Chem. Lett. 2001;11:2461–2464. doi: 10.1016/S0960-894X(01)00466-8. [DOI] [PubMed] [Google Scholar]
- 21.Ahluwalia V.K., Dahiya A., Garg V. Reaction of 5-amino-4-formyl-3-methyl(or phenyl)-1-phenyl-1H-pyrazoles with active methylene compounds: Synthesis of fused heterocyclic rings. 1997;36B:88–90. doi: 10.1002/chin.199740182. and references sited therein. [DOI] [Google Scholar]
- 22.Metwally S.A., El-Naggar G.M., El-Emary T.I. Reactions of 4-(Dicyanomethylene)-3-methyl-1-phenyl-2-pyrazolin-5-one towards methylene comoponds. Liebgs Ann. Chem. 1991;62:961–962. doi: 10.1002/jlac.1991199101164. [DOI] [Google Scholar]
- 23.Metwally S.A., El-Naggar G.M., Younis M.I., Elnagdi M.H., El-Emary T.I. Reactions of 4-(Dicyanomethylene)-3-methyl-1-phenyl-2-pyrazolin-5- one towards Amines and Phenols. Liebgs Ann. Chem. 1989;40:1037–1040. doi: 10.1002/jlac.198919890262. [DOI] [Google Scholar]
- 24.El-Emary T.I., El-Dean A.M., El-Kashef H.S. Facile synthesis of some new pyrazolo [3,4-b] pyrazines and their antifungal activity. II Farmaco. 1998;53:383–388. doi: 10.1016/S0014-827X(98)00014-7. [DOI] [Google Scholar]
- 25.El-Kashef H.S., El-Emary T.I., Gasquet M., Timon-David M.J., Vanele P. New pyrazolo [3,4-b] pyrazines: Synthesis and biological activity. Pharmazie. 2000;55:572–577. doi: 10.1002/chin.200047151. [DOI] [PubMed] [Google Scholar]
- 26.El-Emary T.I., El-kashef H.S., Hussein A.M. New Polycyclic Azines Derived from Pyrazolo [3,4-b] pyridine. Pharmazie. 2000;55:356–358. [PubMed] [Google Scholar]
- 27.Hussein A.M., El-Emary T.I. Polycyclic Pyrazoles: Routes to New Pyrazoloazines. J. Chem. Res. 1998:228–236. doi: 10.1039/a703809g. [DOI] [Google Scholar]
- 28.El-Emary T.I., Bakhite E.A. Synthesis and biological screening of new 1,3- diphenylpyrazoles with different moieties at position-4. Pharmazie. 1999;2:106–111. [PubMed] [Google Scholar]
- 29.El-Emary T.I., Abdel-Mohsen Sh.A. Synthesis and antimicrobial activity of some new 1,3-diphenyl pyrazoles bearing pyrimidine, pyrimidinethione, thiazolopyrimidine, triazolopyrimidine, thio and alkyl-thiotriazolpyrimidinone moieties at 4- position. Phosphorous Sulfur Silicon. 2006;181:2459–2474. doi: 10.1080/10426500600754695. [DOI] [Google Scholar]
- 30.El-Emary T.I., Khalil A., Ali G.A., El-Adasy A.A. A facile synthesis of some new Thiazolo[3,2-a]pyridines containing pyrazolyl moiety and their antimicrobial activity. Phosphorous Sulfur Silicon. 2005;180:19–30. doi: 10.1080/10426500490494778. [DOI] [Google Scholar]
- 31.El-Emary T.I. Synthesis of newly substituted pyrazoles and substituted pyrazolo[3,4-b]pyridines based on 5-amino-3-methyl-1-phenylpyrazole. J. Chin. Chem. Soc. 2007;54:507–518. doi: 10.1002/jccs.200700072. [DOI] [Google Scholar]
- 32.El-Emary T.I. Synthesis, reactions and biological activity of some new pyrazolo [3,4-b] pyrazines. J. Chin. Chem. Soc. 2006;53:391–401. doi: 10.1002/jccs.200600050. [DOI] [Google Scholar]
- 33.El-Emary T.I. Synthesis of some newly condensed and uncondensed pyrazolo[3,4-b]pyridines. Assiut Univ. J. Chem. 2006;35:45–63. [Google Scholar]
- 34.Jairo Q., Diana M., Braulio I., Ridrigo A., Manuel N., Adolfo S., Justo C., John N. Regioselective synthesis of 4,7,8,9-tetrahydro-2H-pyrazolo[3,4-b]quinolin-5(6H)-ones. Mechanism and structural analysis. Tetrahedron. 2001;57:6947–6953. [Google Scholar]
- 35.Bazgir A, Khanaposhanti M., Sooki A. One-pot synthesis and antibacterial activities of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-dione derivatives. Bioorg. Med. Chem. Lett. 2008;18:5800–5803. doi: 10.1016/j.bmcl.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 36.Chebanov V.A., Sakhno Y.I., Desenk S.M., Chernenko V.N., Musatov V.I., Shishkina S.V., Shishkin O.V., Kappe O. Cyclocondensation reactions of 5-aminopyrazoles, pyruvic acids and aldehydes. Multicomponent approaches to pyrazolopyridines and related products. Tetrahedron. 2007:1229–1242. [Google Scholar]
- 37.Shaabani A., Seyyedhamez M., Maleki A., Behnan M., Rezazdeh F. Synthesis of fully substituted pyrazolo[3,4-b]pyridine-5-carboxamide derivatives via a one-pot four-component reaction. Tetrahedron Lett. 2009;50:2911–2913. doi: 10.1016/j.tetlet.2009.03.200. [DOI] [Google Scholar]
- 38.Balamurugan K., Perumal S., Menedez J.C. New four-component reaction in water: a convergent approach to the metal-free synthesis of spiro[indoline/ acenaphthylene-3,4'-pyrazolo[3,4-b]pyridine derivatives. Tetrahedron. 2011;67:3201–3208. doi: 10.1016/j.tet.2011.03.020. [DOI] [Google Scholar]
- 39.Shawali A.S., Elghandour A.H., Sayed A.R. A novel one-pot synthesis of 3-arylazo[1,2,4]triazolo[4,3-a]pyrimidin-5-(1H)-ones. Synth. Commun. 2001;31:731–740. doi: 10.1081/SCC-100103263. [DOI] [Google Scholar]
- 40.Bedford G.R., Taylor P.J., Webb G.A. 15N-NMR studies of guanidines. II—The fused-in guanidine unit of some oxoheterocycles: A combined 15N-NMR, 13C-NMR and IR study. Magn. Res. Chem. 1995;33:389–394. [Google Scholar]
- 41.Elguero J., Goya P., Martinez A., Rozas I. On the Tautomerism of 2-Phenacyl-4-pyrimidinones and Related Compounds. Chem. Ber. 1989;122:919–924. doi: 10.1002/cber.19891220521. [DOI] [Google Scholar]
- 42.Greenhill J.V., Ismail M.J., Bedford G.R., Edwards P.N., Taylor P.J. Conformational and tautmeric studies of acyl guanidines Part 2. Vibrations and C-13 nuclear magnetic resonance spectroscopy. J. Chem. Soc. Perkin Trans. 1985;2:1265–1274. doi: 10.1039/p29850001265. [DOI] [Google Scholar]
- 43.Reiter J., Bongo L., Dyortsok P. On triazoles XI. structure elucidation of isomeric 1,2,4-triazolopyrimidinones. Tetrahedron. 1987;43:2497–2504. [Google Scholar]
- 44.Rami V.J. One-Pot Synthesis of Mono- and Dinitro-1,2,4-triazino[3,2-b]benzothiazoles. Liebigs Ann. Chem. 1988;11:1089–1090. doi: 10.1002/jlac.198819881113. [DOI] [Google Scholar]
- 45.Heinisch G., Holzer W. Pyrazoles 3. N-1 Protected 4-Substituted Pyrazoles—Synthesis and Nmr Investigation. Heterocycles. 1988;27:2443–2457. [Google Scholar]
- 46.Khalil Z.H., Geies A.A. Synthesis and reactions of some thieno[2,3-d]pyrimidine derivatives. Phosphorus Sulfur Silicon. 1991;60:223–231. doi: 10.1080/10426509108036785. [DOI] [Google Scholar]
- 47.Kwon-Chung K.J., Bennett J.W. Principles of antifungal and antibacterial therapy. Med. Mycol. Lea Febiger. Philadel. 1992:81–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.