Abstract
A variety of novel 6-arylsubstituted benzo[j]phenanthridine- and benzo[g]-pyrimido[4,5-c]isoquinolinequinones were synthesized from 1,4-naphthoquinone, aryl-aldehydes and enaminones via a two-step synthetic approach. The cytotoxic activity of the aminoquinone derivatives was evaluated in vitro against one normal cell line (MRC-5 lung fibroblasts) and three human cancer cell lines (AGS human gastric adenocarcinoma; SK-MES-1 human lung cancer cells, and J82 human bladder carcinoma) in 72-h drug exposure assays using the MTT colorimetric method. Structure–activity relationships within the series of angular quinones reveal that the insertion of pyrrol-2-yl and furan-2-yl groups at the 6-position is more significant for the increase of the potency and selectivity index of the pharmacophores.
Keywords: N-heterocyclic quinones, enaminones, cytotoxicity, SAR analysis
1. Introduction
The quinone nucleus is common to many natural and synthetic products associated with anticancer and antibacterial activities [1]. Among these compounds the polycyclic members are typically DNA-intercalating agents due to the ability of their large and planar structure to bind strongly between the base pairs through hydrogen bonds and π-stacking interactions [2,3]. They usually have side chains or sugar substituents and basic nitrogens, which upon protonation further strengthen the DNA binding. Examples of quinone derivatives with antitumor activity include mitoxantrone [4], doxorubicin [4], mitomycin [5], streptonigrin [6] and actinomycin D (AMD) [2]. All known quinonic DNA intercalators have the potential to disrupt the normal function of DNA, leading to cell death [7]. This DNA damage can be caused either by the parent form or by its metabolic conversion to electrophilic or radical species [8].
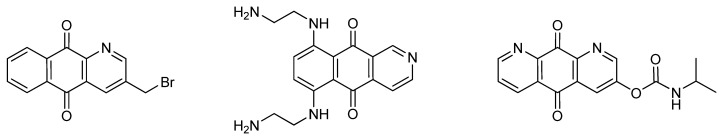
Aza- and diaza-anthraquinones such as those depicted in Figure 1, represent an important class of antitumor agents that exhibit promising in vitro and in vivo activity on tumor cell lines [9,10,11,12,13]. The antitumor activity of these agents seem to be mediated by DNA intercalation and redox cycling processes that are improved by the basic and electron-withdrawing properties of the N-heterocyclic ring [12].
Figure 1.
Examples of aza-anthraquinones with potent antitumor activity.
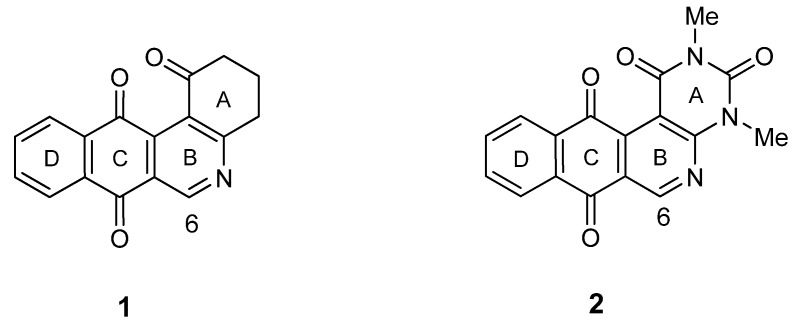
As part of a research program on the synthesis and antitumor evaluation of N-heterocyclic quinones we have described the synthesis and antitumor properties of benzo[j]phenanthridine- and benzo[g]-pyrimido[4,5-c]isoquinolinequinone derivatives [14]. Recent evidences demonstrate that some members of these series have potential anti-cancer activity through inhibition of TOP1 catalytic activity and induction of apoptosis [15]. The synthesis of these tetracyclic quinones, such as 1 and 2 (Figure 2), was accomplished by means a strategy involving the heterocyclization of acyl-1,4-benzoquinones with enaminones to construct the ABC-ring systems followed by a Diels-Alder reaction to assemble the D-benzene nucleus.
Figure 2.
Structure of angular N-heterocyclic quinones with antitumor activity.
In this context, we envisaged an alternative synthetic approach to the tetracyclic frameworks, in which the assembly of the ABCD-ring system would be accomplished through a one-step process by heterocyclization of 2-acyl-1,4-naphthoquinones with enaminones. Taking advantage of recently published preliminary results on the synthesis of heteroaroylnaphthohydroquinones by solar-chemical photo-Friedel–Crafts heteroacylation of 1,4-quinones [16], we decided to explore the mentioned strategy. Herein, we wish to report the synthesis and the in vitro antitumor evaluation of a variety of 6-aryl-substituted benzo[j]phenanthridine- and benzo[g]pyrimido[4,5-c]isoquinolinequinones. In the light of the results, structure-activity relationships (SARs) were examined, using 1 and 2 as lead compounds, assessing the effects on antitumor activity of structural changes made to the pyridine fused ring B by insertion of aryl- and heteroaryl groups.
2. Results and Discussion
2.1. Chemistry
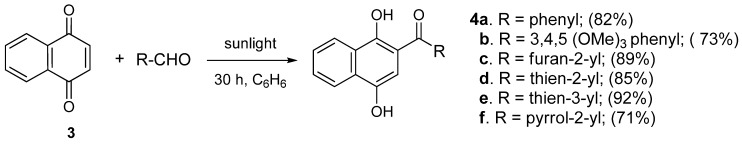
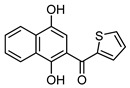
Acylnaphthohydroquinones 4a–f together the enaminones 3-aminocyclohex-2-en-1-one (5) and 5-amino-1,3-dimethyluracil (6) were selected as precursors to explore the synthesis of the designed N-heterocyclic quinones. The synthesis of the required hydroquinones 4a–f was performed by solar photo-Friedel-Crafts reaction of 1,4-naphthoquinone (3) with the corresponding aryl- and heteroarylcarbaldehydes, according to our recently reported procedure [16]. In all the examined cases acylhydroquinones 4 were obtained in good to excellent yields (Scheme 1). The structure of compounds 4a and 4c–f were confirmed by comparison of their spectral data to those of authentic samples [16]. In the case of the previously unreported compound 4b, its structure was established by 1H-, 13C-NMR, and HRMS data.
Scheme 1.
Preparation of acylnaphthohydroquinones 4a–f.
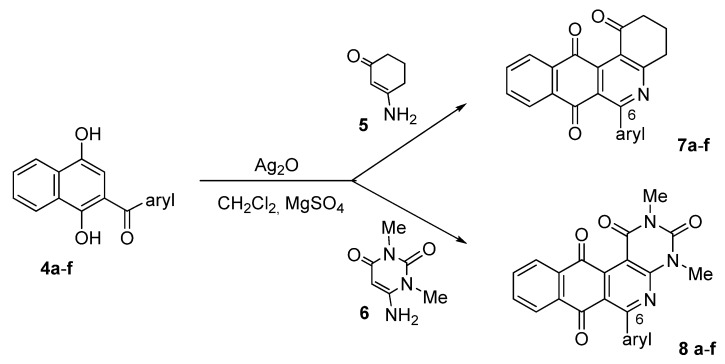
The synthesis of the target members of the series of benzo[j]phenanthridine- and benzo[g]pyrimido[4,5-c]isoquinolinequinones 7 and 8, containing phenyl and heteroaryl substituents at 6-position, was accomplished by reaction of acylnaphthohydroquinones 4, enaminones 5/6 and silver (I) oxide in dichloromethane (Scheme 2) using a previously reported one-pot procedure [14]. The broad application of this synthetic pathway allowed us to synthesize derivatives with different aryl-substituents in position 6 of the heterocyclic ring systems. The results are summarized in Table 1.
Scheme 2.
Synthesis of 6-substituted angular quinones 7 and 8.
Table 1.
Preparation of benzo[j]phenanthridine- and benzo[g]pyrimido[4,5-c]isoquinolinequinone derivatives.
| Acylnaphthohydroquinone | Enaminone | Product | N° | Yield (%) |
|---|---|---|---|---|
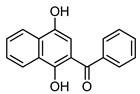 4a |
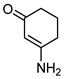 5 |
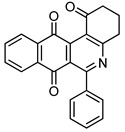 |
7a | 45 |
 4b |
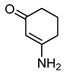 5 |
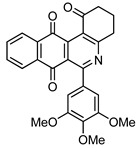 |
7b | 93 |
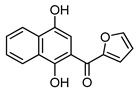 4c |
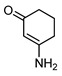 5 |
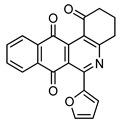 |
7c | 26 |
 4d |
 5 |
 |
7d | 95 |
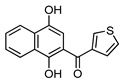 4e |
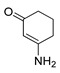 5 |
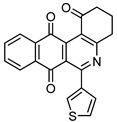 |
7e | 16 |
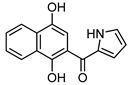 4f |
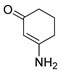 5 |
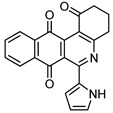 |
7f | 11 |
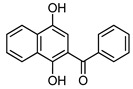 4a |
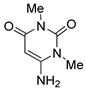 6 |
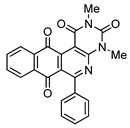 |
8a | 81 |
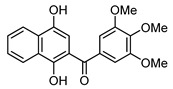 4b |
 6 |
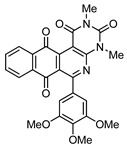 |
8b | 68 |
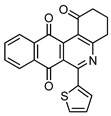
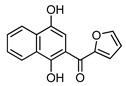 4c |
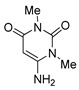 6 |
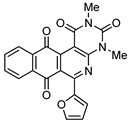 |
8c | 73 |
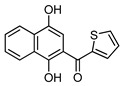 4d |
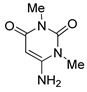 6 |
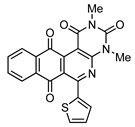 |
8d | 81 |
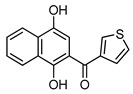 4e |
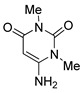 6 |
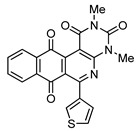 |
8e | 25 |
 4f |
 6 |
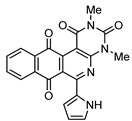 |
8f | 39 |
2.2. Cytotoxic Activities
Compounds 7a, 7c–f, 8a–f were evaluated for in vitro anticancer activity against normal human lung fibroblasts MRC-5 and three human tumor cell lines: AGS gastric adenocarcinoma, SK-MES-1 lung, and J82 bladder carcinoma, in 72-h drug exposure assays. The cytotoxicity of the new compounds was measured using a conventional microculture tetrazolium reduction assay [17,18,19]. The broad variety of the synthesized compounds was designed in order to gain insight upon the influence on the biological activity of phenyl, 3,4,5-trimethoxyphenyl, furan-2-yl, thiophen-2-yl, thiophen-3-yl, and pyrrol-2-yl groups at the 6-position of the benzo[j]phenanthridine- and benzo[g]pyrimido[4,5-c]isoquinolinequinone pharmacophores. The cytotoxic activities of compounds 7 and 8 are collected in Table 2. The cytotoxic activity of analogues 1 and 2 [14] is also included in Table 2 in order to compare the effect of the insertion of aromatic groups at 6-position of the corresponding chromophores. As indicated in Table 2, the tested compounds showed moderate to good activity in the in vitro antitumor screening expressed by the IC50 values. The novel angular quinones are less cytotoxic than the anti-cancer agent etoposide used as reference.
Table 2.
Cytotoxic activity of benzo[j]phenanthridine- and benzo[g]pyrimido[4,5-c]isoquinolinequinone derivatives.
| IC50 ± SEM a (µM) | |||||
|---|---|---|---|---|---|
| Compound | N° | MRC-5 b | AGS c | SK-MES-1 d | J82 e |
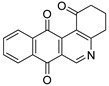 |
1 | 41.3 ± 2.2 | 19.0 ± 0.9 | 51.6 ± 2.7 | 62.6 ± 3.4 |
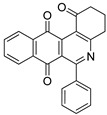 |
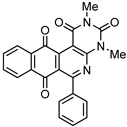
7a | 35.4 ± 2.8 | 21.4 ± 1.3 | >100 | 99.4 ± 6.6 |
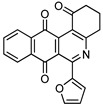 |
7c | >100 | 42.4 ± 2.3 | >100 | >100 |
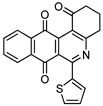 |
7d | 14.3 ± 1.2 | 40.5 ± 2.6 | 64.4 ± 3.2 | 59.7 ± 2.5 |
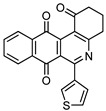 |
7e | 32.3 ± 2.6 | 47.8 ± 2.5 | >100 | >100 |
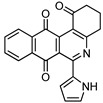 |
7f | 21.3 ± 1.1 | 10.1 ± 0.8 | >100 | >100 |
 |
2 | 72.8 ± 3.4 | 82.1± 4.4 | 67.5 ± 3.6 | 78.6 ± 3.9 |
 |
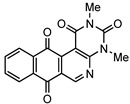
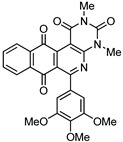
8a | 30.3 ± 3.1 | 4.6 ± 0.2 | 12.4 ± 1.2 | 5.0 ± 0.3 |
 |
8b | 61.1 ± 5.4 | 36.1 ± 2.0 | 85.8 ± 5.1 | >100 |
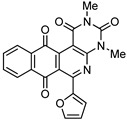 |
8c | 31.8 ± 2.5 | 3.3 ± 0.2 | >100 | >100 |
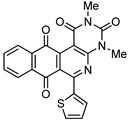 |
8d | >100 | 19.6 ± 1.4 | >100 | 40.3 ± 3.3 |
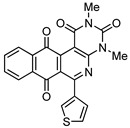 |
8e | 59.1 ± 4.6 | 18.5 ± 1.3 | 59.8 ± 4.5 | 23.4 ± 1.1 |
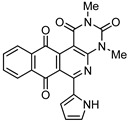 |
8f | 77.8 ± 5.3 | >100 | 74.1 ± 5.8 | >100 |
| etoposide | - | 3.9 ± 0.21 | 0.36 ± 0.15 | 2.8 ± 0.18 | 0.80 ± 0.04 |
a Data represent mean average values for six independent determinations; b Normal human lung fibroblasts cells; c Human gastric adenocarcinoma cell line; d Human lung cancer cell line; e Human bladder carcinoma cell line.
Comparison of the cytotoxic potency of the previously reported benzo[j]phenanthridine- and benzo[g]pyrimido[4,5-c]isoquinolinequinones 1 and 2 with the 6-aryl-substituted analogs 7a–f/8a–f, evaluated in this study, clearly indicates that the biological activity on normal and human cancer cells depends on the nature of the substituent located at the 6-position.
The initial structure-activity relationship (SAR) was focused on the effects of insertion of aryl-substituents at the 6-position of the benzo[j]phenanthridinequinone chromophore (compound 1). According to the IC50 values it can be deduced that the insertion of a phenyl group in the 6-position of 1, as in quinone 7a, does not induce a significant change on the cytotoxic activity and selectivity index (SI = IC50 fibroblasts/IC50 cancer cells) on human gastric adenocarcinoma cell line. However, the substitution strongly decreases the biological effect, respect to 1, on human lung cancer and bladder carcinoma cell lines.
Comparison of the cytotoxic potency between the furan-2-yl-substituted quinone 7b and 1, reveals that insertion of the furyl group at the 6-position results in a decreasing effect on the potency on human gastric adenocarcinoma cells and the suppression of the cytotoxic activity on normal lung fibroblasts, lung cancer and bladder carcinoma cell lines. The insertion of a thiophen-2-yl or thiophen-3-yl substituent, bonded at the 6-position of the pharmacophore, as in compounds 7c and 7d, promotes an increasing effect on normal lung fibroblasts and no significant changes on the cytotoxicy on the tested cancer cell lines.
The data of Table 2 reveal that on AGS cells analogue 7e shows higher antitumor potency but a similar selectivity index to compound 1. Also observed is that the insertion of the pyrrol-2-yl substituent suppresses the cytotoxic activity on lung cancer and bladder carcinoma cell lines.
We can conclude that among the evaluated 6-arylbenzo[j]phenanthridinequinones, the pyrrolyl-derivative 7e could be of interest for the design of new analogues with antitumor activity on human gastric adenocarcinoma cells.
Next, the SAR analysis was focused on the effects of the insertion of aryl groups at the 6-position of the benzo[g]pyrimido[4,5-c]isoquinolinequinone scaffold (compound 2). The data in Table 2 reveal that the insertion of a phenyl group in the 6-position of 2, as in quinone 8a, has a remarkable effect on the antitumor potency and selectivity index of all the tested cancer cell lines. It can be seen that the effect of such insertion on the cytotoxic activity on gastric and bladder cancer cells, reaches values nearly 18- and 16-times higher than the reference compound 2. Compound 8b was included in the cytotoxic evaluation on the basis of the biological activity of 8a and precedent structure-activity relationships of benzo[c]phenanthridines, which reveal that methoxyphenyl substituents lead to derivatives with enhanced antiproliferative activity on cancer cells [20] respect to the unsubstituted phenyl-derivative. According to the biological evaluation of 8b, the expected enhanced effect respect to compound 8a was not observed. On the contrary, compound 8b displayed significant less cytotoxic activity on the tested cell lines, compared to 8a.
Next we examined the influence on the insertion of heterocyclic groups in the 6-position of the benzo[g]pyrimido[4,5-c]isoquinolinequinone chromophore. The substitution of a furan-2-yl group, as in 8c, resulted in the suppression of the antitumor activity on lung cancer and bladder carcinoma cell lines but in a 25-fold increase in potency on gastric cell line compare to compound 2, with a high selectivity index (9.6). The insertion a thiophenyl substituent, bonded through the 2- or 3-position to the 6-position of the chromophore, as in compounds 8d and 8e, does not induce significant changes on the cytotoxicy of the tested cancer cell lines. Concerning the effect of the pyrrol-2-yl group, as in 8f, a strong decreasing effect on the cytotoxic activity of all the tested cell lines was observed.
3. Experimental
3.1. General
All reagents were commercially available reagent grade and were used without further purification. Melting points were determined on a Stuart Scientific SMP3 apparatus and are uncorrected. The IR spectra were recorded on an FT Bruker spectrophotometer using KBr disks, and the wave numbers are given in cm−1. 1H-NMR spectra were run on Bruker AM-200 and AM-400 instruments in deuterochloroform (CDCl3). Chemical shifts are expressed in ppm downfield relative to tetramethylsilane (TMS, δ scale), and the coupling constants (J) are reported in Hertz. 13C-NMR spectra were obtained in CDCl3 at 50 and 100 MHz. Chemical shifts are reported in δ ppm downfield from TMS, and J-values are given in Hertz. HRMS were obtained on a Thermo Finnigan spectrometer, model MAT 95XP. Silica gel Merck 60 (70–230 mesh) was used for preparative column chromatography and TLC aluminum foil 60F254 for analytical TLC. Acylnaphthohydroquinones 4a–f were prepared according to a recently reported solar photoacylation procedure [16]. The structure of 4a, 4c–f were confirmed by comparison of their spectral properties with those of authentic samples.
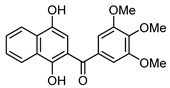
(1,4-Dihydroxynaphthalen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4b). This compound was prepared in 60% yield from 1,4-naphthoquinone (3, 500 mg; 3.16 mmol) and 3,4,5-trimethoxy-benzaldehyde (620 mg, 3.16 mmol); orange solid, m.p.: 189–191 °C. IR (KBr): νmax cm–1: 3463 (OH), 1579 (C=O). 1H-NMR (CDCl3 + DMSO-d6): δ 3.93 (s, 9H, 3 × OMe), 7.03 (s, 2H, 2′ - and 6′ -H), 7.12 (s, 1H, 3-H), 7.56 (t, 1H, J = 7.6 Hz, 6- or 7-H), 7.66 (t, 1H, J = 7.6 Hz, 7- or 6-H), 8.18 (d, 1H, J = 8.0 Hz, 5- or 8-H), 8.43 (d, 1H, J = 8.4 Hz, 8- or 5-H), 9.25 (s, 1H, OH), 13.38 (s, 1H, OH); 13C-NMR (CDCl3 + DMSO-d6): δ 55.8 (2 × C). 60.3, 106.4 (2 × C), 106.6, 111.3, 121.9, 123.7, 125.3, 125.7, 128.9, 129.5, 133.0, 140.4, 144.1, 152.3 (2 × C), 156.6, 199.2. HRMS (M+): m/z calcd for C20H18O6: 354.11034; found: 354.10955.
General procedure for the synthesis of 6-aryl-substituted benzo[j]phenanthridine- and benzo[g]pyrimido[4,5-c]isoquinolinequinones: A suspension of 4, the respective enaminone 5 or 6, silver (I) oxide, anhydrous MgSO4 (0.3 g) and dichloromethane (20 mL) was vigorously stirred at room temperature after completion of the reaction as indicated by thin layer chromatograpy (TLC). The mixture was filtered through celite, thoroughly washed with CH2Cl2 and the filtrate was evaporated under reduced pressure. Column chromatography of the residue (70:30 petroleum ether/ethyl acetate) yielded pure samples of the corresponding N-heterocyclic quinone.
6-Phenyl-3,4-dihydrobenzo[j]phenanthridine-1,7,12(2H)-trione (7a). Prepared from 4a (50 mg; 0.19 mmol), enaminone 5 (42.1 mg; 0.38 mmol), Ag2O (396.9 mg; 1.71 mmol); (4 h, 30.1 mg, 45%), yellow solid m.p.: 250–252 °C. IR (KBr): νmax cm–1: 1676 (C=O), 1665 (C=O). 1H-NMR (400 MHz, CDCl3): δ 2.31 (q, 2H, J = 6.4 Hz, 3-H), 2.97 (t, 2H, J = 6.4 Hz, 2-H), 3.21 (t, 2H, J = 6.4 Hz, 4-H), 7.49 (m, 5H, arom), 7.79 (m, 2H, 8- and 9-H), 8.08 (m, 1H, 7- or 10-H), 8.15 (m, 1H, 10- or 7-H). 13C-NMR (100 MHz, CDCl3): δ 21.59, 33.28, 39.19, 125.64, 126.87, 127.16, 127.96, 128.23 (2C), 128.59 (2C), 129.25, 133.57, 134.12, 134.31, 134.53, 140.26, 144.53, 163.32, 166.74, 182.05, 183.77, 197.95. HRMS (M+): m/z calcd for C23H15NO3: 353.10519; found 353.10471.
6-(3,4,5-Trimethoxyphenyl)-3,4-dihydrobenzo[j]phenanthridine-1,7,12(2H)-trione (7b). Prepared from 4b (50 mg; 0.14 mmol), enaminone 5 (78.40 mg; 0.71 mmol), Ag2O (163.48 mg; 0.71 mmol); (1 h, 58.1 mg, 93%), yellow solid m.p.: 243–244 °C. IR (KBr): νmax cm–1: 1708 (C=O), 1675 (C=O). 1H-NMR (400 MHz, CDCl3): δ 2.31 (q, 2H, J = 6.4 Hz, 3-H), 2.96 (t, 2H,, J = 6.4 Hz, 2-H), 3.22 (t, 2H, J = 6.4 Hz, 4-H), 3.88 (bs, 6H, 3′ - and 5′ -OMe), 3.94 (bs, 3H, 4′ -OMe), 6.72 (bs, 2H, 2′ - and 6′ -H), 7.81 (m, 2H, 8- and 9-H), 8.09 (m, 1H, 7- or 10-H), 8.16 (m, 1H, 10- or 7-H); 13C-NMR (100 MHz, CDCl3): δ 21.58, 33.28, 39.17, 56.26 (2C), 60.93, 106.23 (2C), 126.91, 127.02, 128.61, 133.61, 134.10, 134.33, 134.60, 135.47, 139.14, 140.19, 144.62, 153.16, 162.81, 166.57, 178.67, 182.01, 183.84, 197.78. HRMS (M+): m/z calcd for C26H21NO6: 443.13689; found 443.13670.
6-(Furan-2-yl)-3,4-dihydrobenzo[j]phenanthridine-1,7,12(2H)-trione (7c). Prepared from 4c (50 mg; 0.20 mmol), enaminone 5 (87.44 mg; 0.79 mmol), Ag2O (182.32mg; 0.79 mmol); (1 h, 17.49 mg, 26%), yellow solid m.p.: 190–191 °C. 1H-NMR (400 MHz, CDCl3): δ 2.30 (q, 2H, J = 6.7 Hz, 3-H), 2.95 (t, 2H, J = 6.7 Hz, 2-H), 3.20 (m, 2H, J = 6.7 Hz, 4-H), 6.64 (m, 1H, 4′ -H), 7.26 (m, 1H, 3′ - or 5′ -H), 7.66 (m, 1H, 5′ - or 3′ -H), 7.81 (m, 2H, 8- and 9-H), 8.13 (m, 2H, 7- and 10-H); 13C-NMR (100 MHz, CDCl3): δ 21.50, 33.28, 39.19, 106.23, 112.17, 115.03, 126.89, 127.01, 127.37, 127.89 133.91, 134.14, 134.33, 134.51, 134.60, 145.25, 153.15, 166.62, 166.84, 182.05, 197.54. HRMS (M+): m/z calcd for C21H13NO4: 343.08446; found 343.08449.
6-(Thiophen-2-yl)-3,4-dihydrobenzo[j]phenanthridine-1,7,12(2H)-trione (7d). Prepared from 4d (50 mg; 0.19 mmol), enaminone 5 (123.38 mg; 1.11 mmol), Ag2O (257.49 mg; 1.11 mmol); (5 h, 63.15 mg, 95%), yellow solid m.p.: 224–226 °C. 1H-NMR (400 MHz, CDCl3): δ 2.28 (q, 2H, J = 6.7 Hz, 3-H), 2.92 (t, 2H, J = 6.7 Hz, 2-H), 3.16 (t, 2H, J = 6.7 Hz, 4-H), 7.15 (m, 1H, 4′ -H), 7.58 (m, 1H, 3′ - or 5′ -H), 7.74 (m, 1H, 5′ - or 3′ -H), 7.81 (m, 2H, 8- and 9-H), 8.13 (m, 1H, 7- or 10-H), 8.16 (m, 1H, 10- or 7-H); 13C-NMR (100 MHz, CDCl3): δ 21.49, 33.10, 39.20, 124.85, 126.71, 127.13, 127.79, 130.86, 131.67, 133.98, 134.22, 134.49, 142.07, 145.40, 154.16, 155.12, 160.08, 166.52, 182.54, 183.86, 197.58. HRMS (M+): m/z calcd for C21H13NO3S: 359.06161; found 359.06138.
6-(Thiophen-3-yl)-3,4-dihydrobenzo[j]phenanthridine-1,7,12(2H)-trione (7e). Prepared from 4e (50 mg; 0.19 mmol), enaminone 5 (82.19 mg; 0.74 mmol), Ag2O (171.39 mg; 0.74 mmol), (7 h, 10.5 mg, 16%), yellow solid m.p.: 208–210 °C. 1H-NMR (400 MHz, CDCl3): δ 2.29 (q, 2H, J = 6.6 Hz, 3-H), 2.94 (t, 2H, J = 6.6 Hz, 2-H), 3.19 (t, 2H, J = 6.6 Hz, 4-H), 7.26 (m, 1H, 4′ - or 5′ -H), 7.39 (m, 1H, 5′ - or 4′ -H), 7.80 (m, 3H, 2′ -, 8- and 9-H), 8.14 (m, 2H, 7- and 10-H). 13C-NMR (100 MHz, CDCl3): δ 21.59, 33.23, 39.18, 124.87, 124.97, 126.84, 127.11, 127.62 (2C), 128.68, 133.66, 134.06, 134.24, 134.51, 140.76, 144.77, 157.61, 166.93, 182.24, 183.83, 197.81. HRMS (M+): m/z calcd for C21H13NO3S: 359.06161; found 359.06138.
6-(1H-Pyrrol-2-yl)-3,4-dihydrobenzo[j]phenanthridine-1,7,12(2H)-trione (7f). Prepared from 4f (50 mg; 0.20 mmol), enaminone 5 (87.75 mg; 0.79 mmol), Ag2O (182.97 mg; 0.79 mmol) (1 h, 7.43 mg, 11%), yellow solid m.p.: 230–232 °C. 1H-NMR (400 MHz, CDCl3) δ: 2.26 (q, 2H, J = 6.6 Hz, 3-H), 2.88 (t, 2H, J = 6.8 Hz, 2-H), 3.10 (t, 2H, J = 6.3 Hz, 4-H), 6.41 (m, 1H, 4′ -H), 7.10 (m, 1H, 3′ - or 5′ -H), 7.53 (m, 1H, 5′ - or 3′ -H), 7.79 (m, 2H, 9- and 10-H), 8.09 (m, 1H, 8 or 11-H), 8.21 (m, 1H, 11 or 8-H). 13C-NMR (100 MHz, CDCl3) δ: 20.43, 32.49, 38.21, 110.66, 118.09, 121.14, 122.39, 124.56, 125.33, 126.33, 129.66, 132.97, 133.13, 133.34, 133.39, 146.26, 150.47, 165.88, 188.04, 183.97, 196.61. HRMS (M+): m/z calcd for C21H14N2O3: 342.10044; found 342.09967.
2,4-Dimethyl-6-phenylbenzo[g]pyrimido[4,5-c]isoquinoline-1,3,7,12(2H,4H)-tetraone (8a). Prepared from 4a (50 mg; 0.19 mmol), aminouracil 6 (80.11 mg; 0.57 mmol), Ag2O (131.74 mg; 0.57 mmol (5 h, 60.7 mg, 81%), yellow solid, m.p.: 261–263 °C. 1H-NMR (400 MHz, CDCl3): δ 3.53 (s, 3H, 4-NMe), 3.79 (s, 3H, 2-NMe), 7.54 (m, 5H, arom.), 7.79 (m, 2H, 8- and 9-H), 8.07 (m, 1H, 7- or 10-H), 8.15 (m, 1H, 10- or 7-H). 13C-NMR (100 MHz, CDCl3): δ 29.36, 30.60, 106.18, 123.20, 126.78, 127.31, 128.27 (2C), 129.28 (2C), 130.18, 133.61, 134.55, 135.33, 135.44, 139.77, 149.99, 151.31, 152.59, 158.80, 165.98, 180.86, 184.46. HRMS (M+): m/z calcd for C23H15N3O4: 397.10626; found 397.10675.
2,4-Dimethyl-6-(3,4,5-trimethoxyphenyl)benzo[g]pyrimido[4,5-c]isoquinoline-1,3,7,12(2H,4H)-tetraone (8b). Prepared from 4b (50 mg; 0.14 mmol), aminouracil 6 (79.65 mg; 0.56 mmol), (5 h, 47 mg, 68%), Ag2O (196.18 mg; 0.85 mmol), yellow solid, m.p.: 290–291 °C. IR (KBr): νmax cm–1: 1673 (C=O). 1H-NMR (400 MHz, CDCl3): δ 3.53 (s, 3H, 4-NMe), 3.79 (s, 3H, 2-NMe), 3.89 (s, 6H, 3′ - and 5′ -OMe), 3.97 (s, 3H, 4′ -OMe), 6.81 (s, 2H, 2′ - and 6′ -H), 7.81 (m, 2H, 8- and 9-H), 8.08 (m, 1H, 7- or 10-H), 8.16 (m, 1H, 10- or 7-H). 13C-NMR (100 MHz, CDCl3): δ 29.18, 30.40, 56.37 (2C), 61.09, 105.86, 106.99 (2C), 123.03, 126.60, 126.99, 133.56, 134.41 (2C), 134.65, 135.12, 140.07, 150.05, 151.12, 152.27, 152.99 (2C), 158.56, 165.24, 180.70, 184.32. HRMS (M+): m/z calcd for C26H21N3O7: 487.13795; found 487.13926.
6-(Furan-2-yl)-2,4-dimethylbenzo[g]pyrimido[4,5-c]isoquinoline-1,3,7,12(2H,4H)-tetraone (8c). Prepared from 4c (50 mg; 0.20 mmol), aminouracil 6 (111.04 mg; 0.79 mmol), Ag2O (182.32 mg; 0.79 mmol), (1.5 h, 55.6 mg, 73%) yellow solid, m.p.: 214–216 °C. 1H-NMR (400 MHz, CDCl3): δ 3.51 (s, 3H, 4-NMe), 3.79 (s, 3H, 2-NMe), 6.67 (m, 1H, 4′ -H), 7.38 (m, 1H, 3′ - or 5′ -H), 7.68 (m, 1H, 5′ - or 3′ -H), 7.81 (m, 2H, 8- and 9-H), 8.15 (m, 2H, 7- and 10-H). 13C-NMR (100 MHz, CDCl3): δ 29.14, 30.33, 105.60, 112.41, 116.72 (2C), 126.65, 126.99, 133.68, 134.22 (2C), 134.35, 134.95, 146.00 (2C), 151.84, 152.42, 158.47, 164.05, 180.37, 183.88. HRMS (M+): m/z calcd for C21H13N3O5: 387.08552; found 387.08519.
2,4-Dimethyl-6-(thiophen-2-yl)benzo[g]pyrimido[4,5-c]isoquinoline-1,3,7,12(2H,4H)-tetraone (8d). Prepared from 4d (50 mg; 0.19 mmol), aminouracil 6 (78.41mg; 0.55 mmol) and Ag2O (128.79 mg; 0.55 mmol), (3 h, 60.5 mg, 81%) yellow solid, m.p.: 263–265 °C. 1H-NMR (400 MHz, CDCl3): δ 3.50 (s, 3H, 4-NMe), 3.79 (s, 3H, 2-NMe), 7.18 (m, 1H, 4′ -H), 7.64 (m, 1H, 3′ - or 5′ -H), 7.81 (m, 2H, 8- and 9-H), 8.01 (m, 1H, 5′ - or 3′ -H), 8.13 (m, 1H, 7- or 10-H), 8.17 (m, 1H, 10- or 7-H). 13C-NMR (100 MHz, CDCl3): δ 29.09, 30.37, 105.32, 121.96, 126.36, 127.18, 128.21, 132.52, 133.66, 133.79, 134.30, 134.37, 134.86, 142.17, 150.98, 151.09, 152.03, 157.04, 158.48, 180.95, 184.47. HRMS (M+): m/z calcd for C21H13N3O4S: 403.06268; found 403.06174.
2,4-Dimethyl-6-(thiophen-3-yl)benzo[g]pyrimido[4,5-c]isoquinoline-1,3,7,12(2H,4H)-tetraone (8e). Prepared from 4e (50 mg; 0.19 mmol), aminouracil 6 (52.21 mg; 0.37 mmol) and Ag2O (257.22 mg; 1.11 mmol), (5 h, 18.5 mg, 25%) yellow solid, m.p.: 280–282 °C. 1H-NMR (400 MHz, CDCl3): δ 3.52 (s, 3H, 4-NMe), 3.79 (s, 3H, 2-NMe), 7.35 (m, 1H, 4′ - or 5′ -H), 7.40 (m, 1H, 5′ - or 4′ -H), 7.81 (m, 2H, 8- and 9-H), 7.94 (m, 1H, 2′ -H), 8.14 (m, 2H, 7- and 10-H). 13C-NMR (100 MHz, CDCl3): δ 29.17, 30.36, 105.78, 124.84, 126.54, 127.10, 129.07, 129.49, 133.52, 134.38 (2C), 134.83, 135.05, 140.28, 151.13, 152.51 (2C), 158.57, 159.61, 180.85, 184.38. HRMS (M+): m/z calcd for C21H13N3O4S: 403.06268; found 403.06184.
2,4-Dimethyl-6-(1H-pyrrol-2-yl)benzo[g]pyrimido[4,5-c]isoquinoline-1,3,7,12(2H,4H)-tetraone (8f). Prepared from 4f (50 mg; 0.20 mmol), aminouracil 6 (55.72 mg; 0.40 mmol) and Ag2O (228.71 mg; 0.99 mmol), (3.5 h, 29.8 mg, 39%) red solid, m.p.: 289–291 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.28 (s, 3H, 4-NMe), 3.67 (s, 3H, 2-NMe), 6.28 (s, 1H, 4′ -H), 7.21 (m, 2H, 3′ - or 5′ -H), 7.92 (m, 2H, 8 or 9H), 7.97 (m, 1H, 7- or 10-H), 8.10 (m, 1H, 10- or 7-H), 11.67 (bs, 1H, NH). 13C-NMR (100 MHz, CDCl3) δ: 29.29, 30.81, 104.32, 111.15, 119.27, 121.09, 125.84, 126.13, 127.73, 130.30, 134.69, 135.00, 135.16, 135.30, 151.12, 151.86, 152.94, 153.96, 159.01, 181.38, 185.39. HRMS (M+): m/z calcd for C21H14N4O4: 386.10150; found 386.10104.
3.2. Anticancer Assay
The cell lines used in this work were obtained from the American Type Culture Collection (ATCC, Manasas, VA, USA). They included MRC-5 normal human lung fibroblasts (CCL-171), AGS human gastric adenocarcinoma cells (CRL-1739), SK-MES-1 human lung cancer cells (HTB-58) and J82 human bladder carcinoma cells (HTB-1). After the arrival of the cells, they were proliferated in the corresponding culture medium as suggested by the ATCC. The cells were stored in medium containing 10% glycerol in liquid nitrogen. The viability of the cells after thawing was higher than 90%, as assessed by trypan blue exclusion test. Cells were sub-cultured once a week and the medium was changed every two days. Cells were grown in the following media: MRC-5, SKMES-1, and J82 in MEM and AGS cells in Ham F-12. The MEM medium contained 2 mM l-glutamine, 1 mM sodium pyruvate and 1.5 g/L sodium hydrogen carbonate. Ham F-12 was supplemented with 2 mM l-glutamine and 1.5 g/L sodium hydrogen carbonate. All media were supplemented with 10% heat-inactivated FBS, 100 IU/mL penicillin and 100 μg/mL streptomycin in a humidified incubator with 5% CO2 in air at 37 °C. For the experiments, cells were plated at a density of 50.000 cells/mL in 96-well plates. One day after seeding, the cells were treated with the medium containing the compounds at concentrations ranging from 0 up to 100 μM during 3 days and finally the MTT reduction assay was carried out. The final concentration of MTT was 1 mg/mL. The compounds were dissolved in DMSO (1% final concentration) and complete medium. Untreated cells (medium containing 1% DMSO) were used as controls. Each experiment was carried out in sextuplicate.
4. Conclusions
We have developed the synthesis of a variety of 6-aryl-substituted benzo[j]phenanthridine- and benzo[g]pyrimido[4,5-c]isoquinolinequinones from 1,4-naphthoquinone, arylaldehydes, aminocyclo- hexenone and aminouracil via an efficient one-step procedure to assemble the respective ABCD-ring systems. The compounds thus obtained were tested against normal human lung fibroblasts (MRC-5) and on gastric adenocarcinoma (AGS), lung cancer (SK-MES-1), and bladder carcinoma (J82) cell lines. Structure-activity relationships within these series of angular quinones reveal that insertion of a pyrrol-2-yl group at the 6-position of the benzo[j]phenanthridinequinone scaffold (i.e., compound 7f) and insertion of a furan-2-yl group in the 6-position (compound 8c) of the benzo[g]pyrimido[4,5-c]isoquinolinequinone scaffold are more significant to increase the potency of these pharmacophores against human gastric adenocarcinoma cell line. Compound 8c is the most promising member because of its potency and high selectivity (9.6) on the human gastric adenocarcinoma cell line.
Acknowledgments
We thank the Fondo Nacional de Ciencia y Tecnología (Grant No. 1100376) for financial support to this study.
Footnotes
Sample Availability: Samples of compounds 7a,7b,7d, 8a–f are available from the authors.
References
- 1.Morton R.A., editor. Biochemistry of Quinones. Academic Press; New York, NY, USA: 1965. [Google Scholar]
- 2.Wakelin L.P.G., Waring M.J. DNA Intercalating Agents. In: Sammes P.G., editor. Comprehensive Medicinal Chemistry. Volume 2. Pergamon Press; Oxford, UK: 1990. pp. 703–724. [Google Scholar]
- 3.Chabner B.A., Allegra C.J., Curt G.A., Calabresi P. Antineoplastic Agents. In: Hardman J.G., Limbird L.E., Molinoff P.B., Ruddon R.W., Gilman A.G., editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th ed. McGraw-Hill; New York, NY, USA: 1996. pp. 1233–1287. [Google Scholar]
- 4.Lown J.W. Anthracycline and Anthracenedione-Based Anticancer Agents. Elsevier; Amsterdam, The Netherlands: 1988. [Google Scholar]
- 5.Carter S.K., Crooke S.T. Mitomycin C: Current Status and New Developments. Academic Press; New York, NY, USA: 1979. [Google Scholar]
- 6.Rao K.V., Cullen W.P. Streptonigrin, an Antitumor Substance, Isolation and Characterization. Antibiot. Ann. 1959;7:950–953. [PubMed] [Google Scholar]
- 7.Liquori A.M., DeLerma B., Ascoli F., Transciatti M. Interaction between DNA and polycyclic aromatic hydrocarbons. J. Mol. Biol. 1962;5:521–526. doi: 10.1016/S0022-2836(62)80125-9. [DOI] [Google Scholar]
- 8.Miller E.C., Miller J.A. Mechanisms of chemical carcinogenesis. Cancer. 1981;47:1055–1064. doi: 10.1002/1097-0142(19810301)47:5+<1055::AID-CNCR2820471302>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Krapcho A.P., Landi J.J., Jr., Hacker M.P., McCormack J.J. Synthesis and antineoplastic evaluations of 5,8-bis[(aminoalkyl)amino]-1-azaanthracene-9,10-diones. J. Med. Chem. 1985;28:1124–1126. doi: 10.1021/jm00146a029. [DOI] [PubMed] [Google Scholar]
- 10.Krapcho A.P., Petry M.E., Getahun Z., Landi J.J., Jr., Stallman J., Polsenberg J.F., Gallagher C.E., Maresch M.J., Hacker M.P. 6,9-Bis[(aminoalkyl)amino]benzo[g]isoquinoline-5,10-diones. A novel class of chromophore-modified antitumor anthracene-9,10-diones: Synthesis and antitumor evaluations. J. Med. Chem. 1994;37:828–837. doi: 10.1021/jm00032a018. [DOI] [PubMed] [Google Scholar]
- 11.Lee H., Hong S.-S., Kim Y.-H. Synthesis and in vitro Evaluation of 3-Substituted-1-azaanthraquinones. Bioorg. Med. Chem. Lett. 1996;6:933–936. doi: 10.1016/0960-894X(96)00156-4. [DOI] [Google Scholar]
- 12.Lee H., Lee S.-I., Yang S.-I. Synthesis and in vitro cytotoxicity of 3-substituted-1,8-diazaanthraquinones produced by Lewis-acid catalyzed hetero Diels-Alder reaction. Bioorg. Med. Chem. Lett. 1998;8:2991–2994. doi: 10.1016/S0960-894X(98)00543-5. [DOI] [PubMed] [Google Scholar]
- 13.Hazlehurst L.A., Krapcho A.P., Hacker M.P. Comparison of aza-anthracenedione-induced DNA damage and cytotoxicity in experimental tumor cells. Biochem. Pharmacol. 1995;50:1087–1094. doi: 10.1016/0006-2952(95)00246-V. [DOI] [PubMed] [Google Scholar]
- 14.Valderrama J.A., Colonelli P., Vásquez D., González M.F., Rodríguez J., Theoduloz C. Studies on quinones. Part 44: Novel angucyclinone N-heterocyclic analogues endowed with antitumoral activity. Bioorg. Med. Chem. 2008;16:10172–10181. doi: 10.1016/j.bmc.2008.10.064. [DOI] [PubMed] [Google Scholar]
- 15.Monsalve A.M., Valderrama J.A., Vásquez D., Ibacache A., Rodríguez J.A., González D., Leiva L., González E. Inhibition of human topoisomerase I and activation of caspase-3 by aza-angucyclinones and arylaminopyrimido[4,5-c]isoquinoline-7,10-quinones. Int. J. Mol. Med. 2012;30:151–156. doi: 10.3892/ijmm.2012.961. [DOI] [PubMed] [Google Scholar]
- 16.Benites J., Rios D., Díaz P., Valderrama J.A. The solar-chemical photo-Friedel–Crafts heteroacylation of 1,4-quinones. Tetrahedron Lett. 2011;52:609–611. doi: 10.1016/j.tetlet.2010.11.149. [DOI] [Google Scholar]
- 17.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of Drug Screening with Panels of Human Tumor Cell Lines Using a Microculture Tetrazolium Assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 18.Van de Loosdrecht A.A., Beelen R.H.J., Ossenkoppele G.J., Broekhoven M.G., Langenhuijsen A.C.M.M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods. 1994;174:311–320. doi: 10.1016/0022-1759(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 19.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., Currens M.J., Seniff D., Boyd M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 20.Kock I., Heber D., Weide M., Wolschendorf U., Clement B. Synthesis and biological evaluation of 11-substituted 6-aminobenzo[c]phenanthridine derivatives, a new class of antitumor agents. J. Med. Chem. 2005;48:2772–2777. doi: 10.1021/jm0490888. [DOI] [PubMed] [Google Scholar]