Abstract
A series of novel functionalized mono-, bis- and tris-(S)-{[(2S,4R,8R)-8-ethyl-quinuclidin-2-yl](6-methoxyquinolin-4-yl)}methanamines including ferrocene-containing derivatives was obtained by the reaction of the precursor amine with a variety of acylation agents. Their in vitro antitumor activity was investigated against human leukemia (HL-60), human neuroblastoma (SH-SY5Y), human hepatoma (HepG2) and human breast cancer (MCF-7) cells by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-assay and the 50% inhibitory concentration (IC50) values were determined. Our data indicate that the precursor amine has no antitumor activity in vitro, but the bis-methanamines with ureido-, thioureido and amide-type linkers display attractive in vitro cytotoxicity and cytostatic effects on HL-60, HepG2, MCF-7 and SH-SY5Y cells. Besides 1H- and 13C-NMR methods the structures of the new model compounds were also studied by DFT calculations.
Keywords: quinine, ferrocene, axial, symmetry, anticancer, activity, in vitro assay
1. Introduction
Chemotherapy is one of the most important methods in fighting cancer and several members of modified natural alkaloids serve as deserving drugs against tumors. Well-known representatives of vinca alkaloids such as vinblastine, camptothecine, staurosporine and ellipticin [1,2,3,4] are typical examples. Cinchona alkaloids have been proved to be efficient antimalarial [5] and antibacterial drug candidates [6]. It is well-documented that the application of quinine derivatives in the field of cancer detection [7,8] and in chemotherapy [9,10,11,12,13,14] goes far back to the past. Since ferrocene-based molecules as anti-tumor agents are also promising matherials [15,16,17,18,19,20,21] with a wide range of biological activities [22] first we envisaged the synthesis of novel ferrocene-based mono- and bis-quinines containing amide, urea, thiourea and acylthiourea linkers providing hydrophilic character along with different hydrogen bond profile for the models subjected to in vitro assays. This choice of functional groups can also be reasoned by the following facts: (i) a number of aromatic urea derivatives play important role as anticancer agents [23]; (ii) similarly, urea-based prodrugs have been reported as candidates for melanocyte-directed enzyme therapy [24]; (iii) thiourea based molecules have been proved to be effective agents in the treatment of human promyelocytic leukemia [25]; (iv) the antiproliferative activity [26] and citotoxicity [27] of some acyl-thiourea derivatives are worth to be noted and a few patents have also been published in this field [28,29].
The pronounced efficiency of several drugs with C2-symmetry [30,31,32] and bis-quinolines [33,34,35] encouraged us to construct three ferrocene derivatives containing two quinine units with C2-symmetry and a reference benzene 1,3,5-tricarboxamide incorporating three quinine units with C3-symmetry. Two further purely organic models with one- and two quinine moieties, respectively, were also prepared as additional references.
2. Results and Discussion
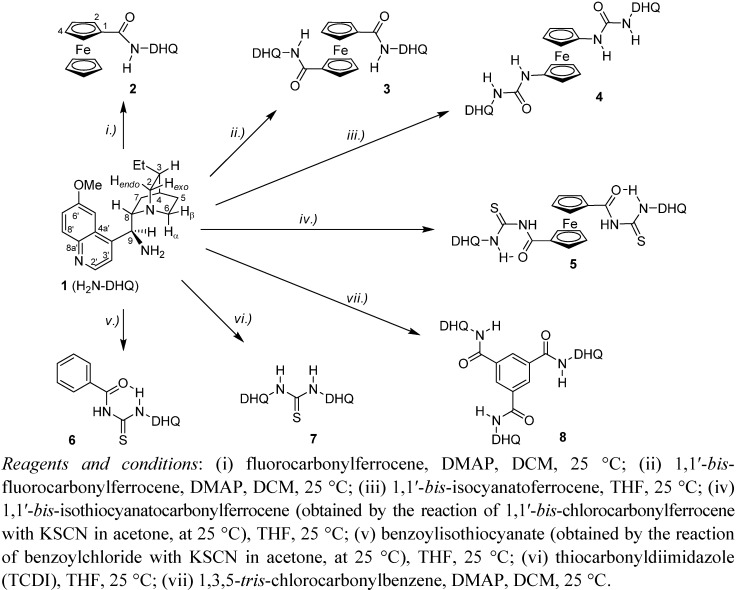
2.1. Synthesis of the Model Compounds
For each synthesis reported in this contribution (S)-{[(2S,4R,8R)-8-ethylquinuclidin-2-yl](6-methoxyquinolin-4-yl)}methanamine (1) [36,37] was used as common precursor serving as source of quinine moiety. In the presence of dimethylaminopyridine (DMAP) the treatment of 1 with the corresponding acylating agent (fluorocarbonylferrocene, 1,1'-bis-fluorocarbonylferrocene, 1,3,5- tris-chlorocarbonylbenzene) in DCM afforded amides 2, 3 and 8. In dry THF the additions of 1 on heterocumulene-type reactants (1,1'-bis-isocyanatoferrocene, benzoylisothiocyanate and ferrocene-1,1'-bis-carbonylisothiocyanate) resulted in the formation of urea- and N-acylisothiocyanate derivatives 4 and 5, 6. The purely organic thiourea model 7 of C2-symmetry was obtained by using thiocarbonyl-diimidazole (TCDI) as reagent. Unstable heterocumulenes 1,1'-bis-isothiocyanato-carbonylferrocene and benzoylisothiocyanate obtained by the reactions of potassium isothiocyanate in acetone at 25 °C with 1,1'-bis-chlorocarbonylferrocene and benzoylchloride, respectively, were used without purification. The low yields of 4–6 (2%–10%) may be ascribed to a variety of competitive transformations including acylation- and bridging reactions along with uncontrolled polymerization processes. Their purification required repeated column chromatography and recrystallization until the 1H-NMR spectra displayed no major impurities.
Scheme 1.
Synthesis of ferrocene-based- and purely organic quinine derivatives.
2.2. Theoretical Calculations
Since the attempts to grow crystals suitable for X-ray analysis have failed so far, the preferred conformations of the new compounds with potential relevance for receptor binding properties were examined by routine DFT calculations [38]. The geometry optimization of the bis- and tris-cinchona derivatives (3–5, 7 and 8) was carried out using the appropriate symmetry constraint (Cn n = 2,3). It was found that in each model compound the N-1 atom is situated in the proximity of the hydrogen atom of the NH group directly attached to the cinchona skeleton (calculated distances: 2.1–2.3 Å) to form a five membered chelate ring representing a hydrophilic segment of the molecule. As evidenced by its downfield-shifted 1H-NMR signal, this NH group is also incorporated in an additional six-membered chelate ring in the acylthiourea derivatives 5 and 6 considerably decreasing the flexibility of these molecules. In the optimized structures of 2–5 the Cp-rings are in the eclipsed position relative to each other and the attached functional groups (amide, urea and acylthiourea) are practically coplanar with them (see Supporting Data for the atomic coordinates).
2.3. Structure Determination
The supposed structures of the new compounds investigated 2–8 are consistent with their spectral parameters, so only the following remarks are necessary: the C2- and C3-symmetric structures of 3–5, 7 and 8, respectively, are confirmed by the identical spectral data of the two or three chincona residues incorporated in these compounds. In acylthioureas 5 and 6 the presence of the chelate rings gains support from the significantly downfield-shifted 1H-NMR signal of the NH group directly bonded to C-9 atom. The relative configuration of the cinchona unit in each compound investigated was evidenced by DNOE measurements revealing endo position of H-9 in the proximity of H-5α- and H-7α, respectively. Accordingly, NOE’s were detected between H-6α and the proton of the NH group attached to C-9 atom. Their proximity is also reflected from the significant downfield shift of the H-6α signal relative to that of its germinal partner, H-6β (Δδ = 0.6–0.7 ppm). The relative configuration of C-8 centre gains support from the NOE interactions measured between H-2endo- and H-8 atoms. On irradiation of the protons on the ethyl group significant enhancements of the intensity of H-7β and H-8 signals were observed indicating the relative configuration of the C-3 atom.
2.4. In Vitro Activity of the Compounds on Human Tumor Cell Cultures
We have determined the cytotoxic and cytostatic activity of the compounds in vitro on four human tumor cell lines: HL-60 leukemia, HepG2 hepatoma, MCF-7 breast adenocarcinoma and SH-SY5Y neuroblastoma cell cultures and expressed them as IC50 values. Therefore cells were treated with the compounds at 10−4 to 102μM concentration range and the viability of the cells was determined by MTT-assay.
The data summarized in Table 1 show that the precursor amine 1 has no antitumor activity in vitro on the tested human cancer cell cultures. Among the investigated ferrocene derivatives 2–5 the diamide 3 proved to be the most active on each type of tumor cells (the IC50 values of its cytotoxic- and cytostatic effects fall into the ranges between 0.72–1.70 µM and 0.40–1.00 µM, respectively). It is worth to emphasize that the presence of an additional quinine amide moiety in 3 induces a dramatic enhancement in the in vitro antitumor activity compared to that of the analogue mono-amide 2. The outstanding efficiency of 3 can probably be attributed to a cooperation of the two functionalities adopting optimal conformation by practically unrestricted rotation of the two Cp rings.
Table 1.
In vitro cytotoxicity and cytostatic activity of the cinchona derivatives on human tumor cell cultures.
| Cell line | ||||
| HepG2 | SH-SY5Y | HL-60 | MCF-7 | |
| Compd. | Cytotoxicity (IC50a in µM) | |||
| 1 | >100 | >100 | >100 | > 100 |
| 2 | 33.10 ± 3.04 | 29.80 ± 4.24 | 37.70 ± 3.67 | 25.32 ± 4.60 |
| 3 | 0.72 ± 0.01 | 0.78 ± 0.02 | 1.70 ± 0.05 | 0.75 ± 0.02 |
| 4 | 4.24 ± 1.12 | 0.82 ± 0.54 | 0.86 ± 0.02 | 21.70 ± 3.23 |
| 5 | >100 | >100 | 6.70 ± 0.02 | >100 |
| 6 | 17.60 ± 0.25 | 21.20 ± 3.24 | 32.20 ± 4.67 | >100 |
| 7 | 3.34 ± 1.02 | 0.84 ± 0.02 | 1.80 ± 0.56 | 5.34 ± 1.78 |
| 8 | 8.90 ± 0.23 | 1.50 ± 0.02 | 2.30 ± 0.05 | >100 |
| Cytostatic effect (IC50 in µM) | ||||
| 1 | >100 | >100 | >100 | > 100 |
| 2 | 65.00 ± 6.70 | 80.70 ± 5.78 | 41.90 ± 1.45 | 56.00 ± 4.56 |
| 3 | 0.40 ± 0.17 | 0.99 ± 0.10 | 0.76 ± 0.01 | 1.00 ± 0.34 |
| 4 | 3.40 ± 0.12 | 1.30 ± 0.54 | 0.94 ± 0.02 | 5.10 ± 0.67 |
| 5 | >100 | >100 | 6.50 ± 3.56 | 21.80 ± 3.18 |
| 6 | 65.60 ± 3.40 | 82.90 ± 6.78 | >100 | 82.90 ± 7.98 |
| 7 | 4.60 ± 0.02 | 4.20 ± 2.30 | 10.20 ± 1.65 | 3.89 ± 1.18 |
| 8 | 19.60 ± 2.12 | 17.20 ± 3.45 | 4.50 ± 0.01 | 2.36 ± 0.01 |
a The 50% inhibitory concentration (IC50) values were determined from the dose-response curves. The curves were defined using MicrocalTM Origin1 (version 7.5) software.
Significant differences are discernible between the activities of ferrocene-based bis-urea 4 and bis-acylthiourea 5. While 4 shows considerable activities on each investigated cell line, compound 5 has only selective cytotoxic and cytostatic effect on the HL-60 cells. The spectacularly decreased activity of 5 may be associated with the intramolecular hydrogen bonds and the increased rigidity of the two acylthiourea units stabilized by their chelate structure.
Among the purely organic models thiourea 7 of C2-symmetry exhibited significant cytotoxic and cytostatic effects against each tested cancer cell line, especially on HL-60 and SH-SY5Y cultures (cytotoxic effect: IC50 = 1.80 and 0.84 µM, cytostatic activity: 10.20 and 4.20 µM, respectively). Acylthiourea 6 and tris-amide 8 with C3-symmetry also displayed remarkable activities (with higher IC50 values: Between 17.60 and 32.20 µM; 2.30 and 8.90 µM, respectively) without in vitro cytotoxic effect on MCF-7 cells. On the other hand, these molecules were slightly cytostatic on the same cell lines after overnight incubation (Table 1).
3. Experimental
3.1. General
All chemicals were obtained from commercially available sources (Sigma-Aldrich) and–except for THF–used without further purification. THF was purified by distillation from LiAlH4 under inert atmosphere. For the in vitro assays 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide [MTT], RPMI-1640 medium, DMEM medium, fetal calf serum [FCS] and nonessential amino acids were also obtained from Sigma-Aldrich. Melting points (uncorrected) were determined with a Boethius microstage. Merck Kieselgel (230–400 mesh, 60 Ǻ) and alumina (Brockmann I grade, approx. 150 mesh, 58 Ǻ, activated neutral). The reactions were monitored using standard TLC technique and were stopped when no more starting compound was detected.
HL-60 human leukemia cells (ATCC: CCL-240) and the adherent HepG2 human hepatoma cells (ATCC: HB-8065) were cultured in RPMI-1640 medium supplemented with 10% FCS (fetal calf serum, Sigma Ltd.), 2 mM l-glutamine, and 160 μg/mL gentamycin. The adherent MCF-7 human breast adenocarcinoma cells (ATCC: HTB-22) and the adherent SH-SY5Y human neuroblastoma cells were maintained in DMEM medium containing 10% FCS, L-glutamine (2 mM), gentamycin (160 μg/mL), 1 mM pyruvate and 1% nonessential amino acids. Cell cultures were maintained at 37 °C in a humidified atmosphere with 5% CO2.
The IR spectra were run in KBr disks on a Bruker IFS-55 FT-spectrometer controlled by Opus 3.0 software. Optical rotations were measured with a Zeiss Polamat A polarimeter. The 1H- and 13C-NMR spectra were recorded in CDCl3 or DMSO-d6 solution in 5 mm tubes at RT, on a Bruker DRX-500 spectrometer at 500.13 (1H) and 125.76 (13C) MHz, with the deuterium signal of the solvent as the lock and TMS as internal standard. DEPT spectra were run in a standard manner, using only a Θ = 135° pulse to separate the CH/CH3 and CH2 lines phased “up” and “down”, respectively. The 2D-COSY, HMQC and HMBC spectra were obtained by using the standard Bruker pulse programs. The exact mass measurements were performed using a Q-TOF Premier mass spectrometer (Waters Corporation, 34 Maple St, Milford, MA, USA) in positive electrospray mode.
The precursor [(S)-((2S,4R,8R)-8-ethylquinuclidin-2-yl](6-methoxyquinolin-4-yl)methanamine (1) was prepared according to the method described by Brunner et al. [36] and is simply referred to as “amine” in each procedure described below.
3.2.Synthesis of the Novel Quinine Derivatives
3.2.1. N-{(S)-[(2S,4R,8R)-8-Ethylquinuclidin-2-yl](6-methoxyquinolin-4-yl)methyl)}ferrocene-carboxamide (2)
The amine (0.70 g, 2.2 mmol), fluorocarbonylferrocene (0.50 g, 2.2 mmol; prepared from ferrocene carboxylic acid according to the method reported by Galow et al. [39]) and dimethylaminopyridine (DMAP; 0.26 g, 2.2 mmol) were dissolved in DCM (10 mL). the solution was stirred at RT under argon for 45 min and evaporated to dryness. The residue was subjected to column chromatography on silica [eluent: DCM/MeOH (30:1)] followed by recrystallization from dry ether (using 25 mL for 100 mg substance) to obtain the product as light yellow powder (592 mg, 51%). mp. 184–186 °C; [α]D26: −46.4° (EtOH c = 0.23 g/100 mL); IR (cm−1): 3313, 1635, 1530, 1512, 1242, 1174, 1029, 581, 489; 1H-NMR (DMSO-d6): 8.74 (d, 1H, J = 4.5 Hz, H-2'); 7.93 (d, 1H, J = 9.2 Hz, H-8'); 7.90 (br s, 1H, NH); 7.88 (d, J = 7.25 Hz, 1H, H-5'); 7.60 (d, J = 4.5 Hz, H-3'); 7.40 (d, 1H, J = 4.5 Hz, H-3'); 7.38 (dd, 1H, J = 9.2 Hz and 2.5 Hz, H-7'); 5.77 (br ~d, J~9 Hz, 1H, H-9); 4.77 and 4.76 (2 × br s, 2 × 1H, H-2,5, η5-C5H4); 4.27 (br s, 2H, H-3,4, η5-C5H4); 4.03 (s, 5H, η5-C5H5); 3.95 (s, 3H, OCH3); 3.45 (br qa, J = 8.6 Hz, 1H, H-8); 3.23 (br ~t, J ~ 12 Hz, 1H, partly overlapped by the HDO signal of the solvent, H-6α); 3.12 (dd, J = 13.2 Hz and 9.5 Hz, 1H, H-2exo); 2.75 (ddd, J = 12.5 Hz, 11.4 Hz and 4.5 Hz, 1H, H-6β); 2.59 (ddd, J = 12.5 Hz, 11.2 Hz, 4.5 Hz, 1H, H-5β); 2.44 (br d, J = 13.2 Hz, 1H, H-2endo); 1.55 (br ~s, 1H, H-4); 1.53–1.48 (overlapping m’s, 2H, H-5β, H-7β); 1.43 (m, 1H, H-5α); 1.35 (m, 1H, H-3); 1.31 (m, 1H, CH3-CHAHB); 1.23 (m, 1H, CH3-CHAHB); 0.82 (t, 3H, J = 7.2 Hz, CH3); 0.68 (dd, J = 13.5 Hz and 8.1 Hz, 1H, H-7α); 13C-NMR (DMSO-d6): 169.2 (C=O); 158.1 (C-6'); 148.5 (C-2'); 146.2 (C-4'); 145.0 (C-8a'); 132.1 (C-8'); 129.3 (C-4a'); 122.0 (C-7'); 120.9 (C-3'); 103.9 (C-5'); 77.0 (C-1, η5-C5H4); 70.8 (two coalesced lines, C-3,4, η5-C5H4); 70.1 (η5-C5H5); 69.2 and 69.0 (C-2,5, η5-C5H4); 58.5 (C-8); 58.1 (C-2); 56.4 (OCH3); 50.0 (C-9); 41.9 (C-6); 37.8 (C-3); 29.1 (C-5); 27.9 (CH3-CH2); 27.0 (C-7); 25.5 (C-4); 12.9 (CH3-CH2); HRMS exact mass calculated for C31H36N3O256Fe: 538.2157 [MH]+; found: 538.2162.
3.2.2. N-{(S)-[(2S,4R,8R)-8-Ethylquinuclidin-2-yl](6-methoxyquinolin-4-yl)methyl)}ferrocene-1,1'-bis-carboxamide (3)
The amine (467 mg, 1.4 mmol), 1,1'-bis-fluorocarbonylferrocene (200 mg, 2.9 mmol; prepared from ferrocene dicarboxylic acid [39]) and DMAP (176 mg, 2.9 mmol) were dissolved in DCM (6 mL) and the solution was stirred under argon for 45 min. The residue obtained by the evaporation of the reaction mixture was purified by flash column chromatography on silica using DCM/MeOH (5:1) as eluent followed by recrystallization from Et2O to yield the product as brownish yellow powder (134 mg 21%). mp. 137.5–139.5 °C; [α]D26: −62.1° (EtOH c = 0.22 g/100 mL); IR (cm−1): 3248, 1645, 1623, 1608, 1533, 1509, 1229, 1175, 1030, 485; 1H-NMR (DMSO-d6): 8.71 (d, 1H, J = 4.5 Hz, H-2'); 8.02 (br s, 1H, NH); 7.93 (d, 1H, J = 9.2 Hz, H-8'); 7.85 (br s, 1H, H-5'); 7.63 (d, 1H, J = 4.5 Hz, H-3'); 7.40 (dd, 1H, J = 9.2 Hz and 2.5 Hz, H-7'); 5.72 (br ~d, J~8 Hz, 1H, H-9); 4.64 (br s, 2H, H-2,5, η5-C5H4); 4.53 and 4.50 (2 × br s, 2 × 1H, H-3,4, η5-C5H4); 3.94 (s, 3H, OCH3); 3.57 (br~t, J ~ 8 Hz,, 1H, H-8); 3.38 (m, 1H, H-6α); 3.16 (dd, J = 12.7 Hz and 7.3 Hz, 1H, H-2exo); 2.68 (ddd, J = 12.5 Hz, 11.2 Hz and 4.7 Hz, 1H, H-6β); 2.54 (br d, J = 12.7 Hz, 1H, H-2endo); 1.63–1.59 (overlapping m’s, 3H, H-4, H-5β, H-7β); 1.50-1.46 (overlapping m’s, 2H, H-3, H-5α); 1.42 (m, 1H, CH3-CHAHB); 1.35 (m, 1H, CH3-CHAHB); 0.83 (t, 3H, J = 7.2 Hz, CH3); 0.74 (dd, J = 13.2 Hz and 7.7 Hz, 1H, H-7α); 13C-NMR (DMSO-d6): 169.1 (C=O); 158.2 (C-6'); 148.6 (C-2'); 145.9 (C-4'); 145.1 (C-8a'); 132.2 (C-8'); 128.3 (C-4a'); 122.2 (C-7'); 121.0 (C-3'); 103.8 (C-5'); 78.0 (C-1, η5-C5H4); 72.4 (two coalesced lines, C-3,4, η5-C5H4); 70.7 (two coalesced lines, C-2,5, η5-C5H4); 58.7 (C-8); 58.0 (C-2); 56.5 (OCH3); 49.7 (C-9); 41.9 (C-6); 37.6 (C-3); 28.8 (C-5); 27.8 (CH3-CH2); 27.4 (C-7); 25.9 (C-4); 12.7 (CH3-CH2); HRMS exact mass calculated for C52H61N6O456Fe: 889.4104 [MH]+; found: 889.4130.
3.2.3. 1,1'-(Ferrocene-1,1'-diyl)-bis-{3-[(S)-((2S,4R,8R)-8-ethylquinuclidin-2-yl](6-methoxyquinolin-4-yl)methyl)}urea (4)
The amine (7.58 g, 23 mmol) and 1,1'-diisocyanatoferrocene [40] (2.6 g, 9.7 mmol) were stirred in dry THF at RT under argon overnight. After the evaporation of the reaction mixture the crude product was dissolved in DCM/MeOH (10:1) and the solution was passed through Celite and concentrated in vacuo. The residue was column chromatographed on silica with DCM/MeOH (10:1) and crystallized by EtOH (15 mL) to obtain the pure product as yellow powder (358 mg, 2%). mp. 230 °C (dec.); [α]D26: −107.0° (EtOH c = 0.02 g/100 mL); IR (cm−1): 3277, 3244, ~3100–2100 (diffuse), 1678, 1622, 1584, 1510, 1247, 1083, 1029, 599, 486; 1H-NMR (CDCl3): 11.20 (br s, 1H, NH, bonded to η5-C5H4); 8.81 (s, 1H, NH, bonded to C-9); 8.76 (d, 1H, J = 4.5 Hz, H-2'); 8.03 (d, 1H, J = 9.2 Hz, H-8'); 7.98 (br s, 1H, H-5'); 7.46 (d, 1H, J = 4.5 Hz, H-3'); 7.40 (dd, 1H, J = 9.2 Hz and 2.5 Hz, H-7'); 5.93 (br ~d, J ~ 9 Hz, 1H, H-9); 5.02 and 4.13 (2 × br s, 2 × 1H, H-2,5, η5-C5H4); 4.51 (br qa, J = 8.8 Hz, 1H, H-8); 4.10 (s, 3H, OCH3); 4.04 (br ~t, J~13 Hz, 1H, H-6α); 3.83 and 3.56 (2 × br s, 2 × 1H, H-3,4, η5-C5H4); 3.74 (dd, J = 13.2 Hz and 9.5 Hz, 1H, H-2exo); 3.24–3.16 (overlapping m’s, 2H, H-2endo, H-6α); 2.18 (ddd, J = 12.5 Hz, 11.2 H and 4.5 Hz, 1H, H-6β); 2.03 (br s, H-4); 1.93–1.87 (m, 4H, H-3, H-5α, H-5β, H-7β); 1.49–1.45 (m, 2H, CH3-CH2); 0.94 (t, 3H, J = 7.2 Hz, CH3); 0.87 (dd, J = 13.2 and 8.8 Hz, 1H, H-7α); 13C-NMR (CDCl3): 159.0 (C-6'); 155.2 (C=O); 148.0 (C-2'); 145.4 (C-8a'); 142.6 (C-4'); 132.2 (C-8'); 128.5 (C-4a'); 122.8 (C-7'); 119.7 (C-3'); 102.5 (C-5'); 99.6 (C-1, η5-C5H4); 64.2 and 62.4 (C-3,4, η5-C5H4); 59.0 (two coalesced lines, C-2,5, η5-C5H4); 64.9 (C-8); 56.7 (C-2); 56.4 (OCH3); 50.0 (C-9); 41.2 (C-6); 35.3 (C-3); 26.9 (CH3-CH2); 25.34 (C-7); 25.30 (C-4); 25.2 (C-5); 11.9 (CH3-CH2); HRMS exact mass calculated for C52H63N8O456Fe: 919.4322 [MH]+; found: 919.4352.
3.2.4. 1,1'-(Ferrocene-1,1'-dicarbonyl-diyl)-bis-{3-[(S)-((2S,4R,8R)-8-ethylquinuclidin-2-yl](6-methoxyquinolin-4-yl)methyl)}thiourea (5)
1,1'-bis-Isothiocyanatocarbonylferrocene was prepared from 1,1'-bis-chlorocarbonylferrocene (2.18 g, 7 mmol) according to the procedure employing potassium isothiocyanate as reagent and acetone as solvent [41]. This reactive intermediate was used without purification after acetone was removed by distillation and the residue was dissolved in THF. The amine (5.39 g, 17 mmol) and 1,1'-bis-(isothiocyanatocarbonyl)ferrocene dissolved in 100 mL of dry THF were stirred overnight at RT under argon. The reaction mixture was concentrated in vacuo. The residue was subjected to column chromatography on silica using DCM/MeOH (15:1) as eluent. The partially purified product was dissolved in EtOH and slowly precipitated by water. The precipitate was filtered off then the chromatography and the recrystallization were repeated in order to get rid of the traces of isothiocyanate reagent to afford the pure product as brick-red powder (120 mg, 2%). mp. 167–169 °C; [α]D26: −128.6° (EtOH c = 0.22 g/100 mL); IR (cm−1): 3157, 1669, 1621, 1540, 1508, 1226, 1160, 1027, 592, 490; 1H-NMR (DMSO-d6): 11.62 (br s, 1H, NH inside the chelate); 10.70 (br s, 1H, NH outside the chelate); 8.74 (d, 1H, J = 4.5 Hz, H-2'); 7.98 (d, 1H, J = 9.2 Hz, H-8'); 7.83 (br s, 1H, H-5'); 7.58 (d, 1H, J = 4.5 Hz, H-3'); 7.44 (dd, 1H, J = 9.2 Hz and 2.5 Hz, H-7'); 5.97 (br ~d, J~10 Hz, 1H, H-9); 5.16 and 5.08 (2 × br s, 2H, H-2,5, η5-C5H4); 4.53 and 4.50 (2 × br s, 2 × 1H, H-3,4, η5-C5H4); 3.97 (s, 3H, OCH3); 3.50 (br t, J = 10.0 Hz, 1H, H-8); 3.23–3.16 (overlapping m’s, 2H, H-2exo and H-6α); 2.76 (ddd, J = 12.5 Hz, 11.2 H and 4.5 Hz, 1H, H-6β); 2.53 (dd, J = 13.8 Hz and 4.9 Hz, 1H, H-2endo); 1.68 (m, 1H, H-5β); 1.60 (br s, 1H, H-4); 1.50 (m, 1H, H-5α); 1.44 (m, 1H, H-3); 1.28 (br dd, J = 13.3 Hz and 10.0 Hz, 1H, H-7β); 1.27–1.22 (overlapping m’s, 2H, CH3-CH2); 0.95 (dd, J = 13.5 Hz and 8.2Hz, 1H, H-7α); 0.76 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (DMSO-d6): 180.4 (C=S); 171.6 (C=O); 158.2 (C-6'); 148.5 (C-2'); 144.5 (C-4'); 145.3 (C-8a'); 132.3 (C-8'); 128.7 (C-4a'); 122.0 (C-7'); 121.9 (C-3'); 104.0 (C-5'); 75.6 (C-1, η5-C5H4); 74.89 and 74.85 (C-3,4, η5-C5H4); 72.4 and 72.0 (C-2.5, η5-C5H4); 60.4 (C-8); 57.8 (C-2); 56.9 (OCH3); 56.5 (C-9); 42.2 (C-6); 37.6 (C-3); 28.7 (C-5); 27.5 (C-4); 26.1 (CH3-CH2); 25.7 (C-7); 12.5 (CH3-CH2); HRMS exact mass calculated for C54H63N8O4S256Fe: 1007.3763 [MH]+; found: 1007.3776.
3.2.5. 1-Benzoyl-3-[(S)-((2S,4R,8R)-8-ethylquinuclidin-2-yl](6-methoxyquinolin-4-yl)methyl)thiourea (6)
The amine (3.00 g, 9.2 mmol) and benzoylisothiocyanate (1.51 g, 9.2 mmol) were dissolved in dry THF (100 mL). The reaction mixture was stirred overnight at RT and evaporated to dryness. The residue was purified by flash column chromatography on silica using DCM/MeOH (80:1) as eluent to obtain the product as glassy transparent substance (484 mg, 10%). mp. 92–94 °C; [α]D26: −217.3° (EtOH c = 0.31 g/100 mL); IR (cm−1): 3162, 1667, 1621, 1542, 1507, 1258, 1147, 1027; 1H-NMR (CDCl3): 11.50 (br s, 1H, NH inside the chelate); 11.20 (br s, 1H, NH outside the chelate); 8.64 (d, 1H, J = 4.5 Hz, H-2'); 7.88 (d, 1H, J = 9.2 Hz, H-8'); 7.84 (d, 2H, J = 7.3 Hz, H-2,6, Ph); 7.70 (br s, 1H, H-5'); 7.54 (t, 1H, J = 7.3 Hz, H-4, Ph); 7.51 (d, 1H, J = 4.5 Hz, H-3'); 7.35 (dd, 1H, J = 9.2 Hz and 2.5 Hz, H-7'); 5.78 (br ~d, J~8 Hz 1H, H-9); 3.86 (s, 3H, OCH3); 3.28 (br ~t, J ~ 8 Hz, 1H, H-8); 3.10–3.05 (overlapping m’s, 2H, H-2exo and H-6α); 2.58 (ddd, J = 12.5 Hz,11.2 Hz and 4.5 Hz, 1H, H-6β); 2.36 (dd, J = 13.8 Hz and 4.9 Hz, 1H, H-2endo); 1.54 (m, 1H, H-5β); 1.47 (m, 1H, H-4); 1.37 (m, 1H, H-5α); 1.28 (m, 1H, H-3); 1.15–1.05 (m, 3H, H-7β, CH3-CH2); 0.79 (dd, J = 13.5 Hz and 8.2Hz, 1H, H-7α); 0.67 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): 180.4 (C=S); 169.0 (C=O); 158. 0 (C-6'); 148.6 (C-2'); 145.5 (C-4'); 145.0 (C-8a'); 133.9 (C-4, Ph); 133.0 (C-1, Ph); 132.3 (C-8'); 129.5 (C-2,6, Ph); 129.3 (C-3,5, Ph); 128.7 (C-4a'); 122.0 (two coalesced lines, C-3', C-7'); 103.5 (C-5'); 60.0 (C-8); 57.7 (C-2); 56.4 (OCH3); 55.7 (C-9); 41.9 (C-6); 37.6 (C-3); 29.0 (C-5); 27.7 (C-4); 26.1 (CH3-CH2); 25.7 (C-7); 12.8 (CH3-CH2); HRMS exact mass calculated for C28H33N4O2S: 489.2324 [MH]+; found: 489.2323.
3.2.6. 1,3-Bis-{(S)-[(2S,4R 8R)-8-ethylquinuclidin-2-yl](6-methoxyquinolin-4-yl)methyl)}thiourea (7)
The amine (7.58 g, 23 mmol) and thiocarbonyldiimidazole (TCDI; 2.08 g, 12 mmol) were stirred in dry THF (150 mL) under argon. After TCDI was slowly dissolved the solution was evaporated and the residue was subjected to flash column chromatography on silica using DCM/MeOH (15:1) as eluent. The resulted oily substance was crystallized by water-ethanol and thoroughly washed with boiling water to obtain the product as white powder (171 mg, 2%). mp. 137–138 °C; [α]D26: −141.8° (EtOH c = 0.21 g/100 mL); IR (cm−1): 3265, 1622, 1541, 1509, 1257, 1082, 1031; 1H-NMR (DMSO-d6): 8.58 (d, 1H, J = 4.5 Hz, H-2'); 7.94 (br s, 1H, NH); 7.82 (d, 1H, J = 9.2 Hz, H-8'); 7.70 (br s, 1H, H-5'); 7.29 (dd, 1H, J = 9.2 Hz and 2.5 Hz, H-7'); 7.25 (d, 1H, J = 4.5 Hz, H-3'); 5.12 (br d, J = 10.0 Hz,1H, H-9); 3.81 (s, 3H, OCH3); 3.00 (dd, J = 12.9 Hz and 10.2 Hz, 1H, H-2exo); 2.95 (br t, J = 10.0 Hz, 1H, H-8); 2.85 (br ~t, J ~ 12 Hz, 1H, H-6α); 2.35 (br ~t, J~12 Hz 1H, H-6β); 2.23 (br d, J = 12.9 Hz, 1H, H-2endo); 1.38 (m, 2H, H-4, H-5β); 1.28 (m, 1H, H-3); 1.24 (m, 1H, H-5α); 1.10–1.05 (m, 2H, CH3-CH2); 0.99 (br dd, J = 13.3 Hz and 10.3Hz, 1H, H-7β); 0.69 (br d, J = 13.5 Hz, 1H, H-7α); 0.65 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (DMSO-d6): 183.0 (C=S); 157.9 (C-6'); 148.3 (C-2'); 147.0 (C-4'); 145.0 (C-8a'); 132.0 (C-8'); 128.9 (C-4a'); 121.9 (C-7'); 121.4 (C-3'); 104.1 (C-5'); 60.5 (C-8); 57.8 (C-2); 57.0 (C-9); 56.5 (OCH3); 41.6 (C-6); 37.7 (C-3); 29.0 (C-5); 27.6 (C-4); 26.2 (CH3-CH2); 25.9 (C-7); 12.6 (CH3-CH2); HRMS exact mass calculated for C41H53N6O2S: 693.3951 [MH]+; found: 693.3959.
3.2.7. N-{(S)-[(2S,4R,8R)-8-Ethylquinuclidin-2-yl](6-methoxyquinolin-4-yl)methyl)}benzene-1,3,5-tris-carboxamide (8)
The amine (3.60 g 11 mmol), pyridine (0.89 mL 11 mmol) and DMAP (224 mg 1.8 mmol) were dissolved in dry DCM (100 mL). During vigorous stirring 1,3,5-tris-chlorocarbonylbenzene (0.66 mL, 3.7 mmol) was added to the solution in one portion. After stirring for 24 h the solution was poured onto ice. DCM was distilled off at atmospheric pressure. The resulted precipitate was filtered off and dried to yield the product as white powder (3.60 g 87%). mp. 245 °C (dec.); [α]D26: −122.9° (EtOH c = 0.26 g/100 mL); IR (cm−1): 3305, 1658, 1621, 1508, 1229, 1029, 1029; 1H-NMR (CDCl3): 8.65 (d, 1H, J = 4.5 Hz, H-2'); 8.48 (s, 1H, H-2,4,6, Ph); 8.01 (d, 1H, J = 9.2 Hz, H-8'); 7.95 (br s, 1H, NH); 7.67 (br s, 1H, H-5'); 7.37 (dd, 1H, J = 9.2 Hz and 2.5 Hz, H-7'); 7.33 (d, 1H, J = 4.5 Hz, H-3'); 5.45 (br ~d, J ~ 10 Hz, 1H, H-9); 3.94 (s, 3H, OCH3); 3.19 (dd, J = 13.9 Hz and 9.6 Hz, 1H, H-2exo); 3.09–3.04 (overlapping m’s, 2H, H-6α and H-8); 2.67 (m, 1H, H-6β); 2.38 (dd, J = 13.9 Hz and 5.0 Hz, 1H, H-2endo); 1.64 (br s,1H, H-4); 1.62 (m, 1H, H-5β); 1.53 (m, 1H, H-5α); 1.43 (m, 1H, H-3); 1.35 (ddd, J = 13.3 Hz, 10.3 Hz and 5.0 Hz, 1H, H-7β); 1.25 and 1.19 (2 × m, 2 × 1H, CH3-CH2); 0.96 (dd, J = 13.3 Hz and 6.4 Hz, 1H, H-7α); 0.78 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): 165.8 (C=O); 158.3 (C-6'); 148.0 (C-2'); 145.8 (C-8'); 145.6 (C-4a); 134.7 (C-1,3,5 Ph); 132.3 (C-8'); 129.4 (C-2,4,6, Ph); 128.6 (C-4a'); 122.0 (C-7'); 119.1 (C-3'); 102.1 (C-5'); 60.7 (C-8); 56.7 (C-2); 56.0 (OCH3); 51.7 (C-9); 41.4 (C-6); 37.5 (C-3); 29.0 (C-5); 27.7 (CH3-CH2); 26.1 (C-4); 25.5 (C-7); 12.3 (CH3-CH2); HRMS exact mass calculated for C69H82N9O6: 1132.6421 [MH]+; found: 1132.6388.
3.3. In Vitro Cytostatic and Cytotoxic Activity of the Compounds
The cells were grown to confluency and were plated into 96-well plate with initial cell number of 5.0–7.5 × 103 per well. After 24 h incubation at 37 °C, cells were treated with the compounds in 200 μL final volume containing 1.0 v/v% DMSO. Cells were incubated with the compounds at 10−4–102 μM concentration range for overnight. Control cells were treated with serum free medium (RPMI-1640 or DMEM) only or with DMSO (c = 1.0 v/v%) at 37 °C for overnight. After incubation the cells were washed twice with serum free (RPMI-1640 or DMEM) medium. To determine the in vitro cytostatic effect, cells were cultured for a further 72 h in serum containing medium. To measure the in vitro cytotoxicity of the compounds, MTT-assay was carried out immediately after the overnight treatment: The cell viability was determined by the following method using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide [42,43]. The solution of MTT (45 μL, 2 mg/mL) was added to each well which was reduced by the respiratory chain [42,43] and other electron transport systems [44] to form precipitated violet formazan crystals within the cell [45]. The amount of these crystals can be determined by spectrophotometry serving as an estimate for the number of mitochondria and hence the number of living cells in the well [46]. After 4 h of incubation the cells were centrifuged for 5 min (900 g) and supernatant was removed. The obtained formazan crystals were dissolved in 50 μL of DMSO and the optical density (OD) of the samples was measured at λ = 540 and 620 nm, respectively, employing ELISA Reader instrument (iEMS Reader, Labsystems, Finland). OD620 values were subtracted from OD540 values and the percent of cytostasis or cytotoxicity was calculated using equation “Cytostatic effect/Cytotoxicity (%) = [1 − (ODtreated/ODcontrol)] × 100” (where ODtreated and ODcontrol correspond to the optical densities of the treated and the control cells, respectively). In each case two independent experiments were carried out with 4–8 parallel measurements. The 50% inhibitory concentration (IC50) values were determined from the dose-response curves. The curves were defined using MicrocalTM Origin1 (version 7.5) software: cytostasis (%) or cytotoxicty (%) was plotted as a function of concentration, fitted to a sigmoidal curve and, based on this curve, the half maximal inhibitory concentration (IC50) value was determined representing the concentration of a compound required for 50% inhibition in vitro and expressed in micromoles.
4. Conclusions
Among the novel compounds reported in this contribution the bis- and tris-quinine derivatives exerted a dose-dependent in vitro antitumor activity at micromolar concentrations on the investigated tumor cell cultures. The ferrocene-based bis-amide 3 of pronounced activity can be considered as a promising lead structure for the development of a novel class of therapeutical agents. The highly promising results also obtained for a metallocene model containing two ureido functional groups (compound 4) suggest that 1,1'-disubstituted ferrocene unit with easily rotating Cp-rings seems to be highly beneficial to the desired activity allowing the molecule to adopt a conformation in which the two cooperating groups are situated in optimal distance from each other. On the other hand, the presence of rigid acylthiourea moiety stabilized by chelate structure dramatically decreases the activity on the investigated cell lines.
Acknowledgments
This work was financially supported by the Hungarian Scientific Research Fund (OTKA K-83874) and the European Union and the European Social Fund (grant agreement no. TÁMOP 4.2.1/B-09/KMR-2010-0003). The nondifferentiated SH-SY5Y cell line was a kind gift from Zsolt Datki [47,48,49,50] (Department of Medical Chemistry, University of Szeged, Szeged, Hungary).
Footnotes
Sample Availability: Samples of the compounds 2–8 are available from the authors.
References and Notes
- 1.Ding Y., Bao Y., An L. Progress in antitumor agents, vinblastine analogues. Zhongguo Yiyao Gongye Zazhi. 2005;36:424–428. [Google Scholar]
- 2.Gao H. Research status of antitumor drug camptothecin and its derivatives. Hebei Yiyao. 2008;30:1786–1788. [Google Scholar]
- 3.Prudhomme M. Staurosporines and structurally related indolocarbazoles as antitumor agents. Anticancer Agents Nat. Prod. 2005:499–517. [Google Scholar]
- 4.Ohashi M., Oki T. Ellipticine and related anticancer agents. Expert Opin. Ther. Pat. 1996;6:1285–1294. doi: 10.1517/13543776.6.12.1285. [DOI] [Google Scholar]
- 5.Kaur K., Jain M., Reddy R.P., Jain R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010;45:3245–3264. doi: 10.1016/j.ejmech.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Wolf R., Baroni A., Greco R., Donnarumma G., Ruocco E., Tufano M.A., Ruocco V. Quinine sulfate and bacterial invasion. Ann. Clin. Microbiol. Antimicrob. 2002:1–5. doi: 10.1186/1476-0711-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelsey F.E., Brunschwig A. Concentration of quinine in gastrointestinal cancers; preliminary report. Cancer Res. 1947;7:355–356. [PubMed] [Google Scholar]
- 8.Kim J., Lee K., Jung W., Lee O., Kim T., Kim H., Lee J., Passaro D.J. Validity of serum pepsinogen levels and quininium resin test combined for gastric cancer screening. Cancer Detect. Prev. 2005;29:570–575. doi: 10.1016/j.cdp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Lehnert M., Dalton W.S., Roe D., Emerson S., Salmon S.E. Synergistic inhibition by verapamil and quinine of P-glycoprotein-mediated multidrug resistance in a human myeloma cell line model. Blood. 1991;77:348–354. [PubMed] [Google Scholar]
- 10.Taylor C.W., Dalton W.S., Mosley K., Dorr R.T., Salmon S.E. Combination chemotherapy with cyclophosphamide, vincristine, adriamycin, and dexamethasone (CVAD) plus oral quinine and verapamil in patients with advanced breast cancer. Breast Cancer Res. Treat. 1997;42:7–14. doi: 10.1023/A:1005716214718. [DOI] [PubMed] [Google Scholar]
- 11.Genne P., Dimanche-Boitrel M.T., Mauvernay R.Y., Gutierrez G., Duchamp O., Petit J.M., Martin F., Chauffert B. Cinchonine, a potent efflux inhibitor to circumvent anthracycline resistance in vivo. Cancer Res. 1992;52:2797–2801. [PubMed] [Google Scholar]
- 12.Baraniak D., Kacprzak K., Celewicz L. Synthesis of 3′-azido-3′-deoxythymidine (AZT) cinchona alkaloid conjugates via click chemistry: Toward novel fluorescent markers and cytostatic agents. Bioorg. Med. Chem. Lett. 2011;21:723–726. doi: 10.1016/j.bmcl.2010.11.127. [DOI] [PubMed] [Google Scholar]
- 13.Sohue N. Quinine derivatives and the transplantable tumor. III. The effect of quinine derivatives upon the growth rate of Fujinawa's rat sarcoma in the tissue culture. Folia Pharm. Jpn. 1941;31:1–7. [Google Scholar]
- 14.Sakai S., Minoda K., Saito G., Akagi S., Ueno A., Fukuoka F. The anticancer action of quinoline derivatives. Gann. 1955;46:605–616. [PubMed] [Google Scholar]
- 15.Fiorina V.J., Dubois R.J., Brynes S. Ferrocenyl polyamines as agents for the chemoimmunotherapy of cancer. J. Med. Chem. 1978;21:393–395. doi: 10.1021/jm00202a016. [DOI] [PubMed] [Google Scholar]
- 16.Koepf-Maier P., Koepf H., Neuse E.W. Ferrocenium salts—The first antitumor iron compounds. Angew. Chem. Int. Ed. 1984;96:446–447. doi: 10.1002/ange.19840960626. [DOI] [Google Scholar]
- 17.Neuse E.W., Kanzawa F. Evaluation of the activity of some water-soluble ferrocene and ferricenium compounds against carcinoma of the lung by the human tumor clonogenic assay. Appl. Org.-Met. Chem. 1990;4:19–26. [Google Scholar]
- 18.Snegur L.V., Nekrasov S., Gumenyuk V.V., Zhilina Z.V., Morozova N.B., Skviridova I.K., Rodina I.A., Sergeeva N.S., Shchitkov K.G., et al. Ferrocenylalkylazoles, a new class of low-toxicity compounds with antitumor activity. Rossiiskii Khim. Zhurn. 1998;42:178–183. [Google Scholar]
- 19.Osella D., Ferrali M., Zanello P., Laschi F., Fontani M., Nervi C., Cavigiolio G. On the mechanism of the antitumor activity of ferrocenium derivative. Inorg. Chim. Acta. 2000;306:42–48. doi: 10.1016/S0020-1693(00)00147-X. [DOI] [Google Scholar]
- 20.Gormen M., Pigeon P., Top S., Vessieres A., Plamont M.A., Hillard E.A., Jaouen G. Facile synthesis and strong antiproliferative activity of disubstituted diphenylmethylidenyl-[3]ferrocenophanes on breast and prostate cancer cell lines. Med. Chem. Commun. 2010;1:149–151. doi: 10.1039/c0md00026d. [DOI] [Google Scholar]
- 21.Monserrat J.P., Chabot G.G., Hamon L., Quentin L., Scherman D., Jaouen G., Hillard E.A. Synthesis of cytotoxic ferrocenyl flavones via a ferricenium-mediated 1,6-oxidative cyclization. Chem. Commun. 2010;46:5145–5147. doi: 10.1039/c0cc01290d. [DOI] [PubMed] [Google Scholar]
- 22.Hillard E.A., Vessieres A., Thouin L., Jaouen G., Amatore C. Ferrocene-mediated proton-coupled electron transfer in a series of ferrocifen -type breast-cancer drug candidates. Angew. Chem. Int. Ed. 2006;45:285–290. doi: 10.1002/anie.200502925. [DOI] [PubMed] [Google Scholar]
- 23.Li H., Lv P., Yan T., Zhu H. Urea derivatives as anticancer agents. Anticancer Agents Med. Chem. 2009;9:471–480. doi: 10.2174/1871520610909040471. [DOI] [PubMed] [Google Scholar]
- 24.Jordan A.M., Khan T.H., Malkin H., Osborn H.M.I. Synthesis and analysis of urea and carbamate prodrugs as candidates for melanocyte-directed enzyme prodrug therapy (MDEPT) Bioorg. Med. Chem. 2002;10:2625–2633. doi: 10.1016/S0968-0896(02)00097-4. [DOI] [PubMed] [Google Scholar]
- 25.Ma Z., Saluta G., Kucera G.L., Bierbach U. Effect of linkage geometry on biological activity in thiourea and guanidine-substituted acridines and platinum-acridines. Bioorg. Med. Chem. Lett. 2008;18:3799–3801. doi: 10.1016/j.bmcl.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cesarini S., Spallarossa A., Ranise A., Schenone S., Rosano C., La Colla P., Sanna G., Busonera B., Loddo R. N-Acylated and N,N'-diacylated imidazolidine-2-thione derivatives and N,N'-diacylated tetrahydropyrimidine-2(1H)-thione analogues: Synthesis and antiproliferative activity. Eur. J. Med. Chem. 2009;44:1106–1118. doi: 10.1016/j.ejmech.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Rao X., Wu Y., Song Z., Shang S., Wang Z. Synthesis and antitumor activities of unsymmetrically disubstituted acylthioureas fused with hydrophenanthrene structure. Med. Chem. Res. 2011;20:333–338. doi: 10.1007/s00044-010-9303-8. [DOI] [Google Scholar]
- 28.Suda Y., Egami K., Fujita H. Preparation of acylthiourea compounds as c-Met kinase inhibitors. PCT Int. Appl. 2009:79. [Google Scholar]
- 29.Ruat M., Faure H., Traiffort E., Schoenfelder A., Mann A., Taddei M., Solinas A., Manetti F. Preparation of aromatic N- acylthiourea and N-acylurea as inhibitors of the Hedgehog protein signalling pathway for the treatment of cancer, neurodegenerative diseases and diabetes. Fr. Demande. 2009;57 [Google Scholar]
- 30.Garcia-Martin F., Cruz L.J., Rodriguez-Mias R.A., Giralt E., Albericio F. Design and synthesis of FAJANU: A de Novo C2 symmetric cyclopeptide family. J. Med. Chem. 2008;51:3194–3202. doi: 10.1021/jm800047b. [DOI] [PubMed] [Google Scholar]
- 31.Manna C.M., Tshuva E.Y. arkedly different cytotoxicity of the two enantiomers of C2-symmetrical Ti(IV) phenolato complexes; mechanistic implications. Dalton T. 2010;39:1182–1184. doi: 10.1039/b920786b. [DOI] [PubMed] [Google Scholar]
- 32.Rabouin D., Perron V., N'Zemba B.C., Gaudreault R., Berube G. A facile synthesis of C2-symmetric 17b-estradiol dimers. Bioorg. Med. Chem. Lett. 2003;13:557–560. doi: 10.1016/S0960-894X(02)00987-3. [DOI] [PubMed] [Google Scholar]
- 33.Raynes K., Galatis D., Cowman A.F., Tilley L., Deady L.W. Synthesis and activity of some antimalarial bisquinolines. J. Med. Chem. 1995;38:204–206. doi: 10.1021/jm00001a026. [DOI] [PubMed] [Google Scholar]
- 34.Ayad F., Tilley L., Deady L.W. Synthesis, antimalarial activity and inhibition of haem detoxification of novel bisquinolines. Bioorg. Med. Chem. Lett. 2001;11:2075–2077. doi: 10.1016/s0960-894x(01)00383-3. [DOI] [PubMed] [Google Scholar]
- 35.Cowman A.F., Deady L.W., Deharo E., Desneves J., Tilley L. Synthesis and activity of some antimalarial bisquinolinemethanols. Aust. J. Chem. 1997;50:1091–1096. doi: 10.1071/C97086. [DOI] [Google Scholar]
- 36.Brunner H., Buegler J. Enantioselective catalysis. 106. 9-Amino-(9-deoxy)cinchona alkaloids and their derivatives. B. Soc. Chim. Belg. 1997;106:77–84. [Google Scholar]
- 37.Vakulya B., Varga S., Csámpai A., Soós T. Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional cinchona organocatalysts. Org. Lett. 2005;7:1967–1969. doi: 10.1021/ol050431s. [DOI] [PubMed] [Google Scholar]
- 38.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Montgomery J.A., Vreven T., Kudin K.N., Burant J.C., et al. GAUSSIAN 03, Rev. A. 1. Gaussian, Inc.; Pittsburgh, PA, USA: 2003. [Google Scholar]
- 39.Galow T.H., Rodrigo J., Cleary K., Cooke G., Rotello V.M. Fluorocarbonylferrocene. A versatile intermediate for ferrocene esters and amides. J. Org. Chem. 1999;64:3745–3746. doi: 10.1021/jo982219q. [DOI] [PubMed] [Google Scholar]
- 40.Van Leusen D., Hessen B. 1,1'-Diisocyanoferrocene and a convenient synthesis of ferrocenylamine. Organometallics. 2001;20:224–226. [Google Scholar]
- 41.Yuan Y., Ye S., Zhang L., Wang B., Xu Y., Wang J., Wang H. Studies on intramolecular hydrogen bonding of 1,1'-bis[N-formyl-N'-p-chlorophenylthiourea]ferrocene. Inorg. Chim. Acta. 1997;256:313–318. doi: 10.1016/S0020-1693(96)05438-2. [DOI] [Google Scholar]
- 42.Slater T.F., Sawyer B., Sträuli U. Studies on succinate-tetrazolium reductase systems: III. Points of coupling of four different tetrazolium salts III. Points of coupling of four different tetrazolium salts. Biochim. Biophys. Acta. 1963;77:383–393. doi: 10.1016/0006-3002(63)90513-4. [DOI] [PubMed] [Google Scholar]
- 43.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y.B., Peterson D.A., Kimura H., Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 1997;69:581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- 45.Altman F.P. Tetrazolium salts and formazans. Prog. Histochem. Cytochem. 1976;9:1–56. doi: 10.1267/ahc.9.1. [DOI] [PubMed] [Google Scholar]
- 46.Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 47.Datki Z., Juhász A., Gálfi M., Soós K., Papp R., Zádori D., Penke B. Method for measuring neurotoxicity of aggregating polypeptides with the MTT assay on differentiated neuroblastoma cells. Brain Res. Bull. 2003;62:223–229. doi: 10.1016/j.brainresbull.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Datki Z., Papp R., Zádori D., Soós K., Fülöp L., Juhász A., Laskay G., Hetényi C., Mihalik E., Zarándi M., Penke B. In vitro model of neurotoxicity of Aβ 1-42 and neuroprotection by a pentapeptide: irreversible events during the first hour. Neurobiol. Dis. 2004;17:507–515. doi: 10.1016/j.nbd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Biedler J.L., Roffler-Tarlov S., Schachner M., Freedman L.S. Multiple Neurotransmitter Synthesis by Human Neuroblastoma Cell Lines and Clones. Cancer Res. 1978;38:3751–3757. [PubMed] [Google Scholar]
- 50.Biedler J.L., Helson L., Spengler B.A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973;33:2643–2652. [PubMed] [Google Scholar]