Abstract
Originating in the grapes, anthocyanins and their derivatives are the crucial pigments responsible for the red wine color. During wine maturation and aging, the concentration of monomeric anthocyanins declines constantly, while numerous more complex and stable anthocyanin derived pigments are formed, mainly including pyranoanthocyanins, polymeric anthocyanins produced from condensation between anthocyanin and/or flavan-3-ols directly or mediated by aldehydes. Correspondingly, their structural modifications result in a characteristic variation of color, from purple-red color in young red wines to brick-red hue of the aged. Because of the extreme complexity of chemical compounds involved, many investigations have been made using model solutions of know composition rather than wine. Thus, there is a large amount of research still required to obtain an overall perspective of the anthocyanin composition and its change with time in red wines. Future findings may well greatly revise our current interpretation of the color in red wines. This paper summarizes the most recent advances in the studies of the anthocyanins derived pigments in red wines, as well as their color evolution.
Keywords: anthocyanin, red wine, pyranoanthocyanin, polymeric anthocyanins, wine maturation and aging
1. Introduction
The color of red wines is one of the first features perceived by consumers that can greatly influence their commercial acceptance [1,2]. Extracted from red grape berries, monomeric anthocyanins contribute crucially to the young red wine color [3,4,5,6]. Meanwhile, the intramolecular or intermolecular interaction of anthocyanins themselves or with other organic chemicals, especially the phenolic compounds, such as sell-association and copigmentation, can further enhance their color expression [7,8,9,10,11,12,13].
During wine maturation and aging, the concentration of monomeric anthocyanins in red wines declines constantly, especially the acylated anthocyanins [2,14,15,16,17]. A series of mechanisms might be related to such changes, such as their adsorption by yeast, their degradation and oxidation, their precipitation with proteins, polysaccharides or condensed tannins, and the progressive and irreversible formation of more complex and stable anthocyanin derived pigments, such as various pyranoanthocyanins, polymeric anthocyanins produced from condensation between anthocyanin and/or flavan-3-ols directly or mediated by aldehydes, as well as their further derivatives [2,14,15,18,19,20,21,22,23,24,25]. Such variations can result in the significant changes of the color, the mouth feel and the flavor properties of red wines [2,4,6,14,15,26,27,28,29].
Until now, numerous mechanisms were proposed to support the formation of such anthocyanin derived pigments, most of which were conducted from studies carried out in model solutions [14,24,25]. However, because the anthocyanin make-up of red wine is extremely complex, as well as other phenolic or non-phenolic compounds, most of the previous studies were conducted in model solutions, which had relatively simple and defined chemical compositions. Although these surveys were under conditions that cannot fully represent the real complexion in red wines, it was still necessary to discover these reactions one by one. As a lot of new research is undertaken, the model wine solutions became to be more complex, which made the studies more difficult [30,31,32,33]. However, they could show us some bigger pieces in the jigsaw puzzle of anthocyanins and their variation in red wines. Furthermore, when future findings in this field give us the intact picture, they might change our current understanding dramatically.
The application of modern analysis methods, especially the solid-phase extraction (SPE) and/or high-performance or high-pressure liquid chromatography (HPLC), greatly facilitate the separation of anthocyanin derivatives in red wines [34,35,36,37]. With the help of various mass spectrometry (MS), such as electrospray ionization mass spectrometry (ESI-MS), matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), atmospheric pressure photo ionization quadrupole time-of-flight mass spectrometry (APPI-QqTOF MS), as well as the advances in nuclear magnetic resonance (NMR), the anthocyanins derivatives in red wines can be identified in structure quickly and correctly [38,39,40,41,42,43,44]. With the help of such techniques, many of the previous proposed reaction mechanisms for the formation of polymeric anthocyanins and other new pigments have been verified [14,24,25,45,46].
The aim of this paper is to summarize both of the basic knowledge and the newest achievements in the field of the formation of anthocyanins derived pigments, their color evolution in aged red wines, and the effects of different aging practices on them.
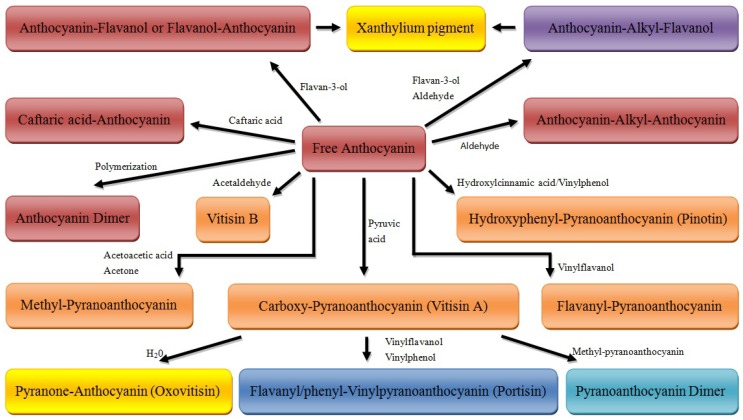
2. Pyranoanthocyanins and Related Pigments in Wines
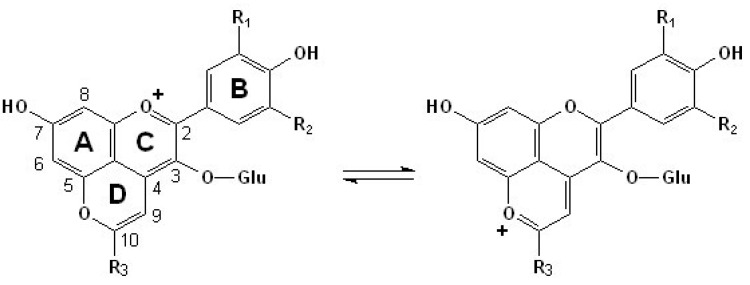
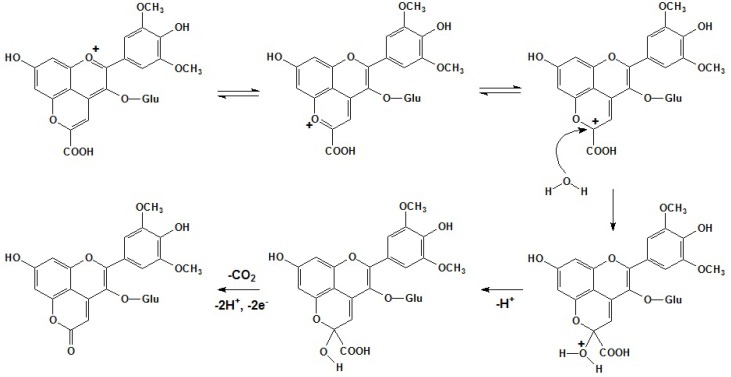
The direct reaction between free anthocyanins and certain yeast by-products, such as acetaldehyde, pyruvic acid and vinylphenols can lead to the formation of another group of stabilized pigments, the pyranoanthocyanins [47,48,49,50,51,52]. Generally, pyranoanthocyanins constitute one of the most important classes of anthocyanin-derived pigments occurring naturally in red wine [53,54,55,56]. Normally, they are cycloaddition products, which have an additional pyran ring between the C4 position in the C ring and the hydroxyl group on the C5 position in the A ring of the anthocyanin molecule [47,52]. More generally speaking, the pyranoanthocyanin structure can be formed through the reaction of an anthocyanin molecule with a compound containing a polarizable double-bound [57]. Thus, compared to the free anthocyanins, pyranoanthocyanins have two heteroaromatic rings, and they have a dynamic equilibrium among different flavylium cation forms, as shown in Figure 1. These new pigments are mainly formed from grape anthocyanins during the fermentation of must and later during the maturation and aging of red wines [47,52].
Figure 1.
General structures of pyranoanthocyanins derived from anthocyanidin-3-O-glucoside in red wines and the dynamic equilibrium between their different flavylium cation forms. The R1 and R2 groups could be H, OH, OCH3. The R3 groups could be H, COOH, CH3, (vinyl)phenols, (vinyl)flavanols [47,48].
To date a great number of pyranoanthocyanins have been identified from red wines, especially in the aged red wines, including carboxy-pyranoanthocyanins (A type vitisins), B type vitisins, methyl-pyranoanthocyanins, hydroxyphenyl-pyranoanthocyanins (pinotins), flavanyl-pyranoanthocyanin, and their second generated pigments, such as flavanyl/phenyl-vinylpyranoanthocyanins (portisins), pyranone-anthocyanins (oxovitisins), pyranoanthocyanin dimers and others [25,47]. Very interestingly, some studies even reported that pyranoanthocyanins represented a very big part of total pigment content in rosé wines (partially fermented with skins of red grapes, V. vinifera cv. Garnacha) and in blanc de noir (fermented without skins of red grapes, V. vinifera cv. Monastrell) base and sparkling wines [58].
Such pyranoanthocyanins are highly stable and resistant to sulfur dioxide bleaching and oxidative degradation, therefore they can significantly contribute to the color stability of red wines [47,52,59]. However, most pyranoanthocyanins possess yellow to orange color and contribute to the tawny color shift associated with red wine aging, except for the newly found A type portisins, which are blue [47,48,50,52,60]. Detailed information of some pyranoanthocyanins that can be frequently detected in aged red wines is summarized in Table 1, as shown below.
Table 1.
The mass spectral and UV-vis data of some major pyranoanthocyanins that can be detected in various aged red wines [61,62,63,64,65,66,67].
| Compounds | Molecular ion M+ (m/z) | Fragment ion M+ ( m/z) | λmax (nm) |
|---|---|---|---|
| Vitisin A type | |||
| Cyanidin-3-O-glucoside-pyruvic acid | 517 | 359 | 503 |
| Cyanidin-3-O-acetylglucoside-pyruvic acid | 559 | 359 | 505 |
| Cyanidin-coumaroylglucoside-pyruvic acid | 661 | 359 | 507 |
| Delphinidin-3-O-glucoside-pyruvic acid | 533 | 371 | 507 |
| Delphinidin-3-O-acetylglucoside-pyruvic acid | 575 | 371 | 509 |
| Delphinidin-3-O-coumaroylglucoside-pyruvic acid | 679 | 371 | 511 |
| Peonidin-3-O-glucoside-pyruvic acid | 531 | 369 | 509 |
| Peonidin-3-O-acetylglucoside-pyruvic acid | 573 | 369 | 510 |
| Peonidin-3-O-coumaroylglucoside-pyruvic acid | 677 | 369 | 511 |
| Petunidin-3-O-glucoside-pyruvic acid | 547 | 385 | 508 |
| Petunidin-3-O-acetylglucoside-pyruvic acid | 589 | 385 | 509 |
| Petunidin-3-O-coumaroylglucoside-pyruvic acid | 693 | 385 | 510 |
| Malvidin-3-O-glucoside-pyruvic acid | 561 | 399 | 513 |
| Malvidin-3-O-acetylglucoside-pyruvic acid | 603 | 399 | 516 |
| Malvidin-3-O-coumaroylglucoside-pyruvic acid | 707 | 399 | 513 |
| Vitisin B type | |||
| Malvidin-3-O-glucoside-acetaldehyde | 517 | 355 | 490 |
| Malvidin-3-O-acetylglucoside-acetaldehyde | 559 | 355 | 494 |
| Malvidin-3-O-coumaroylglucoside-acetaldehyde | 663 | 355 | 497 |
| Pinotin type | |||
| Delphinidin-3-O-glucoside-4-vinylcatechol | 597 | 435 | 510 |
| Delphinidin-3-O-acetylglucoside-4-vinylcatechol | 639 | 435 | 512 |
| Delphinidin-3-O-coumaroylglucoside-4-vinylcatechol | 743 | 435 | 514 |
| Peonidin-3-O-glucoside-4-vinylcatechol | 595 | 433 | 504 |
| Peonidin-3-O-acetylglucoside-4-vinylcatechol | 637 | 433 | 506 |
| Peonidin-3-O-coumaroylglucoside-4-vinylcatechol | 741 | 433 | 508 |
| Petunidin-3-O-glucoside-4-vinylcatechol | 611 | 449 | 510 |
| Petunidin-3-O-acetylglucoside-4-vinylcatechol | 653 | 449 | 512 |
| Petunidin-3-O-coumaroylglucoside-4-vinylcatechol | 757 | 449 | 516 |
| Malvidin-3-O-glucoside-4-vinylcatechol | 625 | 463 | 512 |
| Malvidin-3-O-acetylglucoside-4-vinylcatechol | 667 | 463 | 514 |
| Malvidin-3-O-coumaroylglucoside-4-vinylcatechol | 771 | 463 | 514 |
| Delphinidin-3-O-glucoside-4-vinylphenol | 581 | 419 | 504 |
| Delphinidin-3-O-acetylglucoside-4-vinylphenol | 623 | 419 | 506 |
| Delphinidin-3-O-coumaroylglucoside-4-vinylphenol | 727 | 419 | 506 |
| Peonidin-3-O-glucoside-4-vinylphenol | 579 | 417 | 499 |
| Peonidin-3-O-acetylglucoside-4-vinylphenol | 621 | 417 | 504 |
| Peonidin-3-O-coumaroylglucoside-4-vinylphenol | 725 | 417 | 505 |
| Petunidin-3-O-glucoside-4-vinylphenol | 595 | 433 | 504 |
| Petunidin-3-O-acetylglucoside-4-vinylphenol | 636 | 433 | 506 |
| Petunidin-3-O-coumaroylglucoside-4-vinylphenol | 741 | 433 | 507 |
| Malvidin-3-O-glucoside-4-vinylphenol | 609 | 447 | 504 |
| Malvidin-3-O-acetylglucoside-4-vinylphenol | 651 | 447 | 507 |
| Malvidin-3-O-coumaroylglucoside-4-vinylphenol | 755 | 447 | 509 |
| Malvidin-3-O-caffeoylglucoside-4-vinylphenol | 771 | 447 | 532 |
| Delphinidin-3-O-glucoside-4-vinylguaiacol | 611 | 451 | 502 |
| Peonidin-3-O-glucoside-4-vinylguaiacol | 609 | 447 | 499 |
| Petunidin-3-O-glucoside-4-vinylguaiacol | 625 | 463 | 502 |
| Malvidin-3-O-glucoside-4-vinylguaiacol | 639 | 477 | 504 |
| Malvidin-3-O-acetylglucoside-4-vinylguaiacol | 681 | 477 | 506 |
| Malvidin-3-O-coumaroylglucoside-vinylguaiacol | 755 | 477 | 508 |
| Flavanyl-pyranoanthocyanin type | |||
| Delphinidin-3-O-glucoside-4-vinyl(epi)catechin | 777 | 615 | 501 |
| Delphinidin-3-O-acetylglucoside-4-vinyl(epi)catechin | 819 | 615 | 503 |
| Peonidin-3-O-glucoside-4-vinyl(epi)catechin | 775 | 613 | 199 |
| Peonidin-3-O-acetylglucoside-4-vinyl(epi)catechin | 817 | 613 | 501 |
| Petunidin-3-O-glucoside-4-vinyl(epi)catechin | 791 | 629 | 502 |
| Petunidin-3-O-acetylglucoside-4-vinyl(epi)catechin | 833 | 629 | 504 |
| Malvidin-3-O-glucoside-4-vinyl(epi)catechin | 805 | 643 | 503 |
| Malvidin-3-O-acetylglucoside-4-vinyl(epi)catechin | 847 | 643 | 508 |
| Malvidin-3-O-coumaroylglucoside-4-vinyl(epi)catechin | 951 | 643 | 503 |
2.1. Structures and Formation of Vitisins
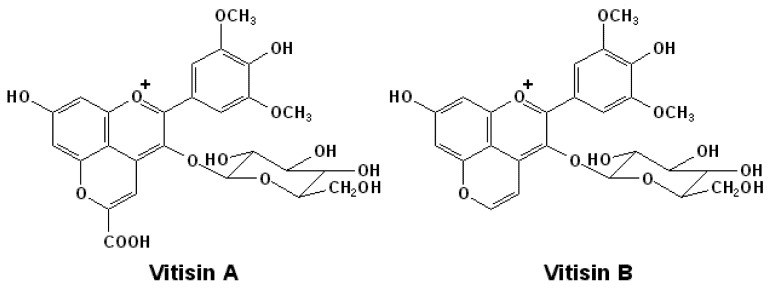
Vitisins, the first group of pyranoanthocyanins to be identified in red wines are usually the most abundant pyranoanthocyanins [50,68,69]. The precursors for these pigments are usually secondary metabolites derived from the glycolysis cycle of yeast metabolism during alcoholic fermentation. For example, the most well known, vitisin A, is formed from malvidin-3-O-glucoside and pyruvic acid, and vitisin B is synthesized as the product of malvidin-3-O-glucoside and acetaldehyde, as shown in Figure 2 [48]. Vitisin A and other similar pyranoanthocyanins arising from reactions between α-keto-acids and anthocyanins can also be grouped as carboxy-pyranoanthocyanins, whereas vitisin B or its homologues differ from carboxy-pyranoanthocyanins by lacking the carboxyl group on the D ring of the pyranoanthocyanin molecule [24,70,71]. Other secondary metabolites from yeast, such as acetone, acetoin, oxalacetic acid, acetoacetic acid and diacetyl, are also likely to react with free anthocyanins to form similar pyranoanthocyanins. For example, acetone or acetoacetic acid can react with an anthocyanin by a similar mechanism to form a similar product with a methyl moiety, which can be termed a methyl-pyranoanthocyanin [24,47,60,72].
Figure 2.
The structures of vitisin A and vitisin B generated from malvidin-3-O-glucoside [47,70,71].
Besides malvidin-3-O-glucoside, other anthocyanin flavylium ions in red wines can also form the vitisin-like pyranoanthocyanins, even the acylated ones. Until now, almost the whole series of such pyranoanthocyanins from anthocyanidin-3-O-glucoside have been reported in wine [61,62,63,64]. Structurally, vitisins apparently have a greater conjugation system than their precursors as so should have a longer maximum absorption wavelength than free anthocyanins. However, in reality result is reversed and surprisingly vitisins have a lower maximum absorption wavelength and appear orange under the same pH conditions. The possible reason is that the new formed pyran ring may balance with the B ring contribution resulting in a small decrease in the overall maximum absorption wavelength [73].
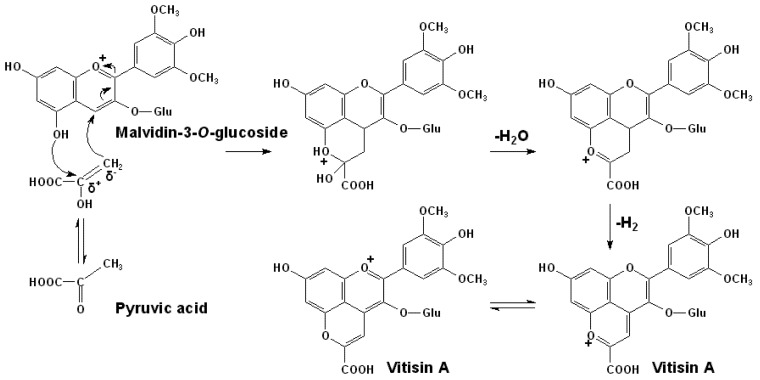
For the formation of vitisin A, under acidic conditions such as exist in red wines, the pyruvic acid in its enolic form can react with malvidin-3-O-glucoside at the nucleophilic C5 hydroxyl group and the electrophilic C4 position with the enolic form’s double bond to form the additional pyran ring. This cycloaddition step is followed by further dehydration and re-aromatization to yield the malvidin-3-O-glucoside pyruvic adduct, vitisin A, as shown in Scheme 1 [47,48,74].
Scheme 1.
Normally, vitisins are formed early to middle of the fermentation when the pyruvic acid concentration was more elevated [24,75]. Several factors affect their formation of vitisins. For example, the maximum vitisin A formation was found to occur under conditions of low pH, higher pyruvic acid concentration and low temperature [76,77]. Furthermore, some other factors that can influent the above factors may also affect the production of vitisin A. For example, the yeast strain used in the alcohol fermentation, the lactic acid bacteria used in malolactic fermentation and the content of sulfite dioxide [77,78]. However, other studies also reported that trace amounts of vitisins could be detected and identified in fresh red grape skins or juice, showing the evidence of their existence in grape berries [79,80]. However, the presence of vitisins in grapes may also be artifacts, which should not originate in biosynthesis from grape skins.
Furthermore, vitisins may also participate in the formation of polymers with wine tannins to form more complicated pigments. The existence of (+)-catechin-(C4-C6/C8)-vitisins in red wine has been reported, and it was proposed that they resulted from cycloaddition of pyruvic acid (vitisin A) or acetaldehyde (vitisin B) to the anthocyanin moiety of the (+)-catechin-(C4-C6/C8)-anthocyanins adducts, rather than direct reactions between the nucleophilic C8 or C6 positions of vitisins and the electrophilic position of flavan-3-ols [81].
2.2. Structures and Formation of Pinotins
In red wines, both hydroxycinnamic acids and 4-vinylphenols can react with free anthocyanins to form the hydroxyphenyl-pyranoanthocyanins, some of which are also named as pinotins, since they were firstly isolated from Pinotage wines [24,47,82,83,84]. The basic structures of well known hydroxyphenyl-pyranoanthocyanins are illustrated in Figure 3.
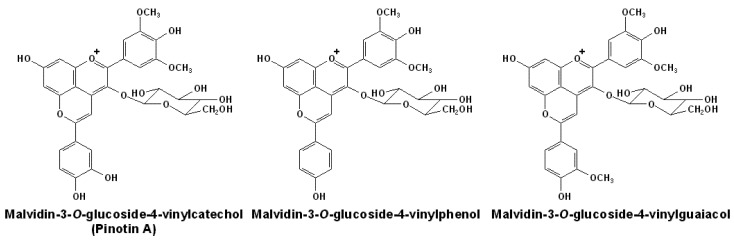
Figure 3.
The structures of hydroxyphenyl-pyranoanthocyanins generated from malvidin-3-O-glucoside [24,61,82].
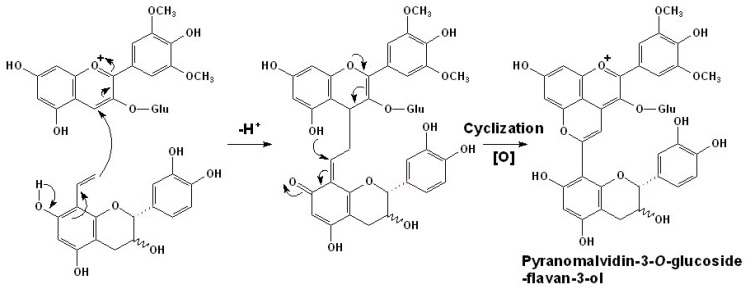
In red wines, the main hydroxycinnamic acids present are p-coumaric, caffeic, ferulic and sinapic acids, whereas 4-vinylphenol and 4-vinylguaiacol may normally arise from the decarboxylation of p-coumaric and ferulic acid, respectively [85]. In a previously proposed mechanism, hydroxyphenyl-pyranoanthocyanins result from the cycloaddition of the ethylenic bond of the 4-vinylphenol molecule at C4 and C5 positions of the anthocyanin molecule followed by an oxidation process, resulting in a pyran ring, and hydroxycinnamic acids cannot be involved in the reactions without the enzymatic decarboxylation via the Saccharomyces cerevisiae cinnamate decarboxylase (CD) during alcoholic fermentation [85,86].
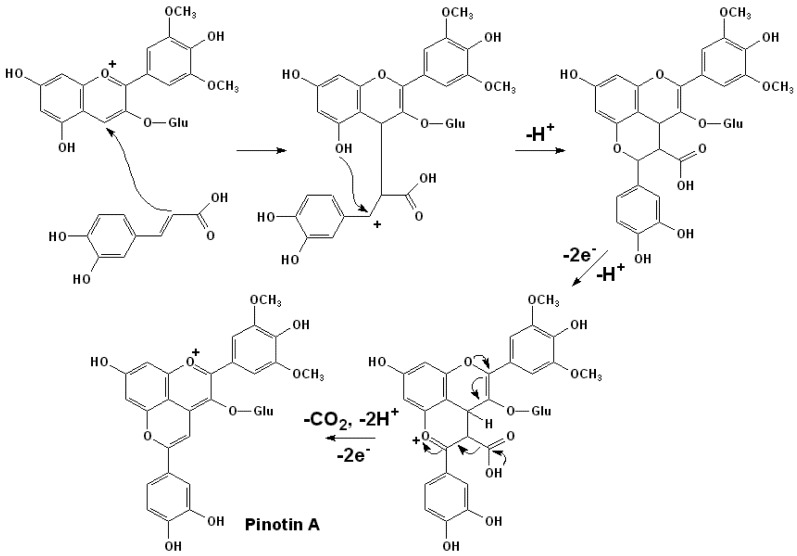
However, a relatively new mechanism was reported to result in the formation of such group pyranoanthocyanins. In the formation of pinotin A (pyranomalvidin-3-O-glucoside-catechol), the best known pigment in this category, the nucleophilic C2 position of the caffeic acid initially attacks the electrophilic C4 position of a malvidin-3-O-glucoside to form an electro deficient intermediate. Subsequently, the hydroxyl group at the C5 position of the anthocyanin moiety will intramolecularly react with this intermediate carbonium ion to form a pyran ring. After the further oxidation and decarboxylation, the final products are formed, as shown in Scheme 2. Thus, without the enzymatic decarboxylation by yeast CD into their respective 4-vinylphenols, but with the additional non-enzymatic decarboxylation, hydroxycinnamic acids with electron-donor substituent, such as p-coumaric, ferulic, caffeic and sinapic acids can also participate in the formation of pinotin-like pigments with free anthocyanins [47,51,87].
Scheme 2.
Until now, almost the whole series of the phenyl-pyranoanthocyanin derivatives of anthocyanidin-3-O-glucoside and their acylated derivates are detected and identified in red wines [87,88]. At the acidic pH of red wines, these pigments absorb in the visible spectrum at λmax 505–508 nm, showing it a red-orange color [47,61,62,63,64]. The concentration of hydroxycinnamic acids or 4-vinylphenols is more important than that of the anthocyanidin-3-O-glucosides in the formation of such kinds of pyranoanthocyanins [89]. Unlike vitisins, they tend to accumulate post alcohol fermentation. For example, the most rapid synthesis of pinotin A was observed in 2.5 to 4 year old wines where the malvidin-3-O-glucoside had decreased to an extremely low level while the concentration of caffeic acid remained very stable. A minimum concentration of 5–10 mg/L of malvidin-3-O-glucoside is necessary for such reactions [90].
2.3. Structures and Formation of Flavanyl-Pyranoanthocyanins
Flavanyl-pyranoanthocyanins, also known as vinylflavanol-pyranoanthocyanins or pyrano-anthocyanin-flavanols, bearing a pyranoanthocyanin moiety directly linked to flavanols, are another group of pyranoanthocyanins naturally occurring in red wines or synthesized in wine model solutions by reactions of anthocyanins with flavanols mediated by acetaldehyde, as shown in Figure 4 [24,47]. Similar to vitisins or pinotins, these pigments possess a hypsochromically shifted maximum of absorption in the visible region to values of 490–511 nm. Compared to the red-purple hue of the genuine anthocyanins, this hypsochromic shift of λmax results in a more orange color, which is also more stable at varying pH values [91].
Figure 4.
The structures of flavanyl-pyranoanthocyanins generated from malvidin-3-O-glucoside and 8-vinylcatechin or 8-vinylprocyanidin [47,92].
Pigments of such class, such as adducts of malvidin-3-O-glucoside and vinylcatechin, vinylepicatechin, and vinylprocyanidin B2, were first detected and identified in model solutions [91]. Later, flavanyl-pyranoanthocyanins derived from malvidin-3-O-glucoside (as well as its acylated products) and the vinyl derivatives of (+)-catechin, (−)-epicatechin, and oligomeric procyanidins up to tetramers were detected in red wines by HPLC-MS/MS analysis [93]. It is proposed that they are formed by the cycloaddition reaction between vinylflavanols and anthocyanins, by a mechanism similar to that of hydroxyphenyl-pyranoanthocyanins with vinylphenols, as shown in Scheme 3 [92]. However, as important precursors, vinylflavanol adducts do not occur naturally in grapes. It is believed that vinylflavanol adducts arise from the dehydration of flavanol-ethanol adducts and the decomposition of the methylmethine-linked flavanol adducts, both of which can result from the reaction between flavanols and acetaldehyde [92,93,94,95].
Scheme 3.
Besides pyranoanthocyanin-flavanol monomers, pyranoanthocyanin-procyanidin dimers and pyranoanthocyanin-flavanols with more polymerized structures have also been identified from some Port wines and red wines [63,66,67,93]. Sometimes, malvidin-3-O-glucoside can react with 8-vinylcatechin to produce various more complicate pigments, including the tree-like pyranoanthocyanin. However, such products usually cannot be detected in wine samples because of their trace levels [92]. Further, such pyranoanthocyanin-flavanols were much more stable against degradation and bleaching by sulfur dioxide than the free anthocyanins [66].
2.4. Structures and Formation of Portisins
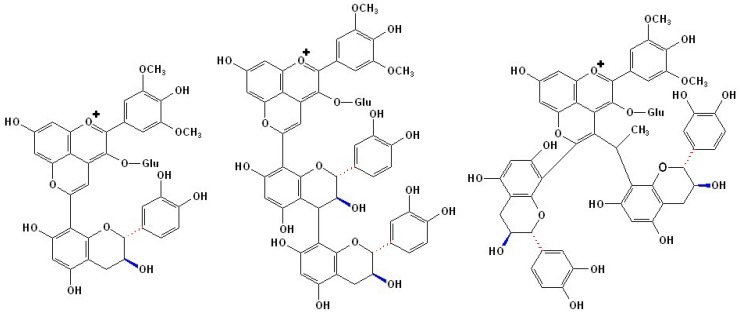
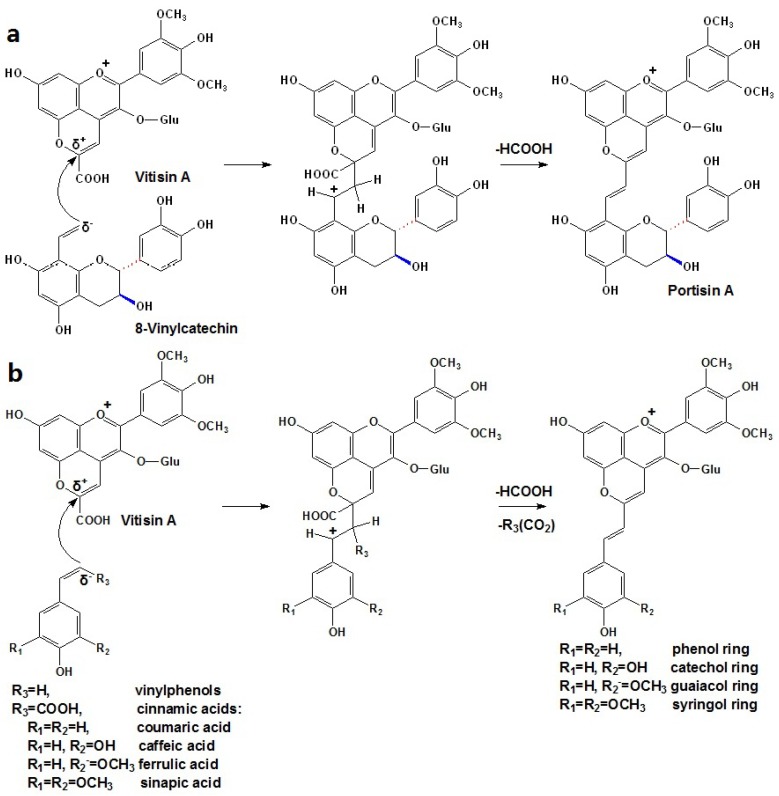
Also known as flavanyl/phenyl-vinylpyranoanthocyanins, the portisins were firstly isolated and identified in Port red wines [24,47,96,97]. As a group of significant anthocyanin derivatives, pyranoanthocyanins, especially the carboxy-pyranoanthocyanins, can also react with other compounds during wine aging to generate some other pyranoanthocyanins with more complicated structures. Structurally, they bear a pyranoanthocyanin moiety linked through a vinyl bridge to a flavanol or phenol unit [98]. As may be expected portisins, compared with vitisins, have maximum light absorption at a longer wavelength, close to 570 nm, and therefore present a blue-violet color [96,97,98,99]. Furthermore, portisins seems to have higher resistance to the attack of water or sulfite dioxides than their monomeric anthocyanin precursors, which could be explained by a higher protection of their chromophore groups against water or bisulfite nucleophilic attack [99,100].
Portisins are considered to be derived through condensation of anthocyanin-pyruvic acid adducts (vitisin A type pyranoanthocyanins) and vinylphenolic compounds. In the formation of portisin A, a vitisin A molecule reacts through its carbon at the C10 position with the vinyl group of a 8-vinyl-flavanol adduct, which may be derived either from the cleavage of ethyl-linked flavanol oligomers, or from the cleavage of anthocyanin-ethyl-flavanol pigments. After the loss of a formic acid group and further oxidation, a vinyl bridge is formed and a new pigment is the result, as shown in Scheme 4a [96,100,101]. The extended conjugation of the π electrons in the newly formed structure may confer a higher stability of the molecule and may be the origin of its blue color. Although these compounds are in very low concentration in red wines, their possible chromatic contribution should not be overlooked [96,97,98,99]. Until now, many A type portisins has been identified in some Port wine fractions, including the catechin-vinylpyrano derivatives of petunidin, peonidin and malvidin-3-O-glucosides, malvidin-3-O-acetylglucoside and peonidin, as well as malvidin-3-O-coumaroylglucoside [102].
Scheme 4.
Formation mechanism of flavanyl/phenyl-vinylpyranoanthocyanin derived from carboxy-pyranoanthocyanins: (a) A type portisins (flavanyl-vinylpyranoanthocyanin); (b) B type portisins (phenyl-vinylpyranoanthocyanin) [96,103].
Recently, a new group of portisins, B type, were detected in aged Port wines. In these new pigments, the flavanol moiety is replaced by a phenolic moiety with different hydroxylation and methoxylation patterns [98,103]. Correspondingly, they are proposed to be formed by the reaction of carboxy-pyranoanthocyanins with cinnamic acids or vinylphenols. This mechanism is similar to that for portisin A, but it involves a further decarboxylation, as shown in Figure 8b [103]. However, the colors of B type portisins are different from those of A type portisins, by showing a hypsochromic shift of the maximum absorption wavelength in the visible spectrum [73,103].
2.5. Structures and Formation of Oxovitisins
Besides portisins, carboxy-pyranoanthocyanin, because of their electrophilic character, can also be involved in the formation of other pyranoanthocyanin pigments. More recently, a new class pyranoanthocyanins, named as oxovitisins (pyranone-anthocyanin) was detected in an aged Port wine. These new pigments display only a pronounced broad band around 370 nm in the UV-Vis spectrum and therefore contribute yellow color to a wine. Generally, they are direct derivatives of carboxy-pyranoanthocyanins, especially vitisin A. In their formation, a water molecule firstly attacks the positively charged C10 position of the pyranoanthocyanin precursor, leading to their hemiacetal formation. Then the resulting intermediates undergo a series of decarboxylation, oxidation, and dehydration reactions to produce the final new and neutral pyran-2-one structures, as shown in Scheme 5 [104]. However, it was recently reported that vitisin B types are not in equilibrium with the hemiacetal forms resulting from the nucleophilic attack by water, thus these vitisins may not readily take part in the formation of such a class of pigments [47,104,105].
Scheme 5.
Formation mechanism of oxovitisins (pyranone-anthocyanin) derived from carboxy-pyranoanthocyanins [104].
2.6. Pyranoanthocyanin Dimers
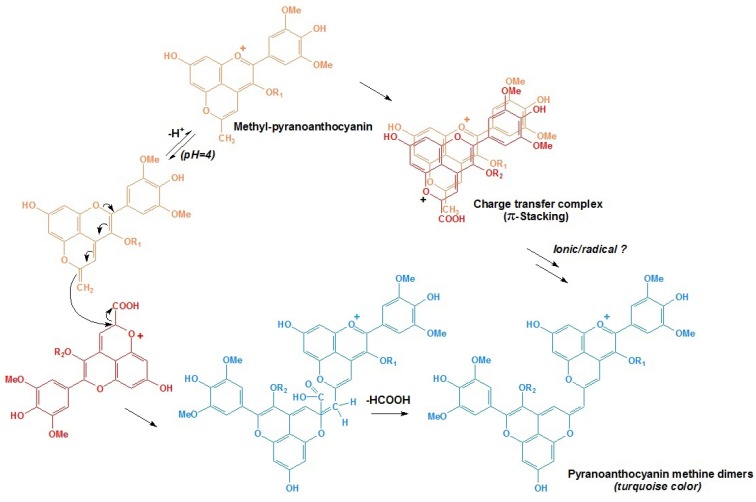
More recently, a new class of pyranoanthocyanin dimers that present an outstanding, rare turquoise color (maximum visible absorption wavelength at ~730 and ~680 nm) was identified in an aged Port wine [106]. They were shown to be formed from reactions between carboxy-pyranoanthocyanins and methyl-pyranoanthocyanins. Two mechanisms for their synthesis have been proposed, as shown in Scheme 6. In the first pathway, after the deprotonation of the methyl group of the methyl-pyranoanthocyanin, a methylene group is formed at C10 position of the E ring. Then, the nucleophilic double bond of this methylene group attacks the electrophilic carbon at C10 position of the carboxy-pyranoanthocyanin molecule. After losing formic acid, the pyranoanthocyanin methane dimer with a structure of two pyranoanthocyanin moieties linked through a methine group can be formed. The second pathway involves the formation of a charge-transfer complex between the two precursors which are stabilized by the π-stacking of the aromatic rings. Through further ionic or radical reactions, condensation occurs between both the two precursors. Although there are still some unclear assumptions in the second mechanism, it was proposed that the charge-transfer complex pathway seemed to be more likely [47,106,107].
Scheme 6.
Proposed two pathways for the formation of pyranoanthocyanin dimers [47,106].
3. Polymeric Anthocyanins
Besides their important role as cofactors in copigmentation, phenolic or polyphenolic compounds extracted from the red grape berry skins and seeds, or even from the oak wood, can also be directly involved in the wine coloration by forming polymeric pigments with anthocyanins [14,24,45,108,109]. The formation of such pigments occurs by direct polymerization of anthocyanins and flavan-3-ols or proanthocyanidins, as well as by the formation of the ‘bridge’ mediated polymeric pigments from anthocyanins themselves or with flavan-3-ols or proanthocyanidins [14,24,45].
These polymeric pigments are more stable than the monomeric anthocyanins and help stabilize wine color. Polymerization results in the chromophore of the anthocyanin being protected from water and nucleophilic attack, oxidation or other chemical modifications, such as the bleaching of sulfur dioxide [14,110,111]. In addition, as a red wine ages the proportion of monomeric anthocyanins soon decreases as the colored polymeric anthocyanins form. Consequently this process affects the anthocyanin equilibrium while retaining the red color of anthocyanins in the polymer [112]. Besides, such polymeric pigments are also important to the mouth feel of red wines, since they are more soluble than the pure polymeric proanthocyanidins and limit the precipitation of these condensed tannins. It is estimated that about 25% anthocyanins may have polymerized with flavonoid compounds by the end of alcohol fermentation, and this level will rise to more 40% after one year’s aging. From then on, the polymerization will continue at a decreasing rate until there has been total polymerization after several years [14,113]. It is obvious that after long time, though there are almost no monomeric anthocyanins in old red wines, the wines are still red or red-brown in hue [114]. So with time polymerization shifts the absorption prosperities of anthocyanin chromophore from red to brown, or even orange and yellow [115]. The color density also diminishes with time, as a result of the loss of monomeric anthocyanins and the slow precipitation of large pigment polymers.
Scheme 7.
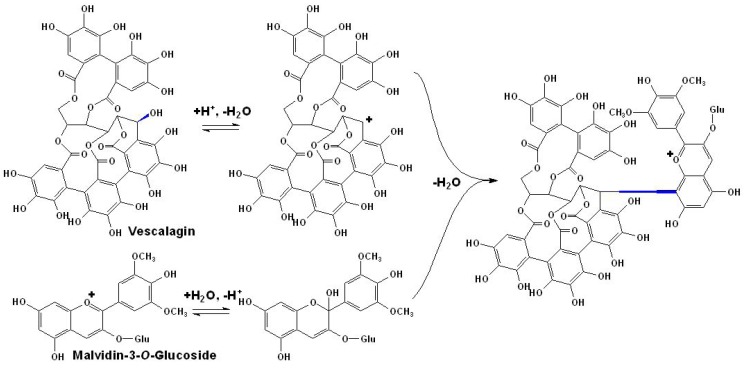
Formation mechanism of ‘anthocyanoellagitannin’ pigment derived from malvidin-3-O-glucoside and (−)-vescalagin [109].
It is worth to point that besides flavonoids, anthocyanins may react with some non-flavonoid phenolic compounds to form complicated pigments. In a research conducted in an acidic organic solution, malvidin-3-O-glucoside can react with (−)-vescalagin to form desired condensation product. The visible absorption band of such so-called ‘anthocyanoellagitannin’ pigment is bathochromically shifted by more than 20 nm, presenting a deeper red-purple color [109]. Although such anthocyanin derivatives have not been observed in red wines, considering (−)-vescalagin or its epimer (-)-castalagin are found in relatively high amounts in fagaceous hardwoods, such as in Quercus (oak) and Castanea (chestnut) species (up to 6% by weight of dry heartwood), it is not hard to imagine the detection and identification of pigments of this class in the red wines after wood aging in the near future [109]. The proposed formation mechanism of the ‘anthocyanoellagitannin’ pigment was illustrated in Scheme 7, as shown below [109].
3.1. Directly Condensed Products of Anthocyanins and Flavanols
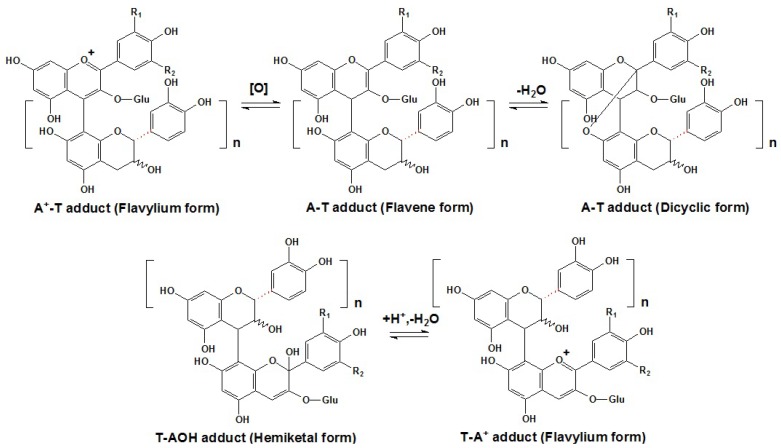
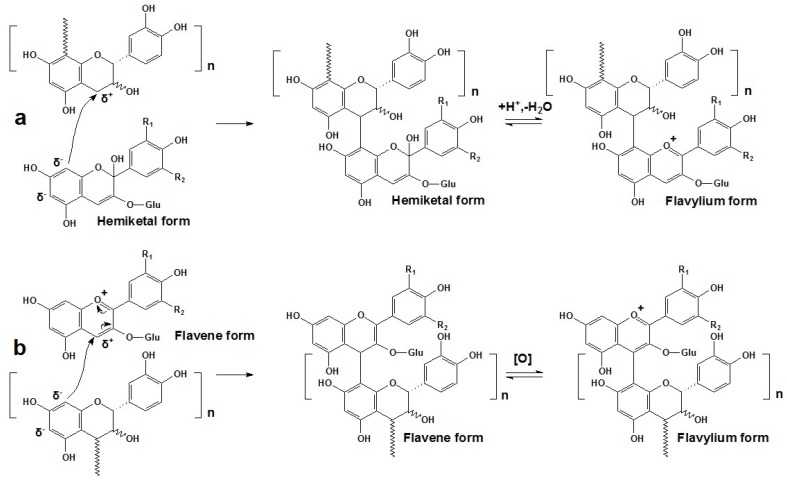
At red wine pH, free anthocyanins in the flavylium form can act as electrophiles through their C2 or C4 positions in the C ring, or act as nucleophiles in the hemiketal form through their C6 or C8 positions in the A ring [14,46,116,117,118,119]. Thus, free anthocyanins can condense with tannins (primarily flavan-3-ols and oligomeric proanthocyanidins) directly to generate either T-A or A-T type anthocyanin/tannin adducts [14,46,112,116,118,119,120]. As with monomeric anthocyanins, these polymeric pigments can also occur in a dynamic equilibrium among some molecular forms, mainly the quinoidal base, the flavylium cation and the hemiketal or carbinol pseudobase, as shown in Scheme 8 [116]. However, the presence of oxygen or oxidants is necessary for the color recovery [121,122]. Additionally, acidic pH conditions and high temperatures can also promote such reactions [117,122].
Scheme 8.
The equilibria among the different structural forms of T-A or A-T type anthocyanin/tannin adducts in aged red wines [113].
The abundant and colorless anthocyanins in hemiketal forms can generate the T-A adducts with tannins. They are formed by the attack of the nucleophilic C8 or C6 position in the A ring of the anthocyanin molecule to the electrophilic C4 position in the C ring of a flavan-3-ol or a terminal unit of oligomeric proanthocyanidin. After dehydration, the T-A adducts can generate colored flavylium chromophores and enhance the color expression, since the colorless hemiketal forms of free anthocyanins are modified to their corresponding colored flavylium forms of polymeric anthocyanins, as shown in Scheme 9a [14,46,112,118,119,120,123]. The presence of such adducts has been extensively reported in red wines, but their exact structures were only elucidated recently by MS and NMR [112,118,119,124,125]. For example, (+)-catechin-(C4α→C8)-malvidin-3-O-glucoside is the most well known polymerized pigment in this category [118,119]. It is reported that the oligomeric proanthocyanidins can possess up to eight flavan-3-ol units, but it is possible that new pigments containing proanthocyanidins with higher polymerization may be discovered [123].
Scheme 9.
Proposed mechanism of T-A and A-T adducts formation [112,118,119,120,123].
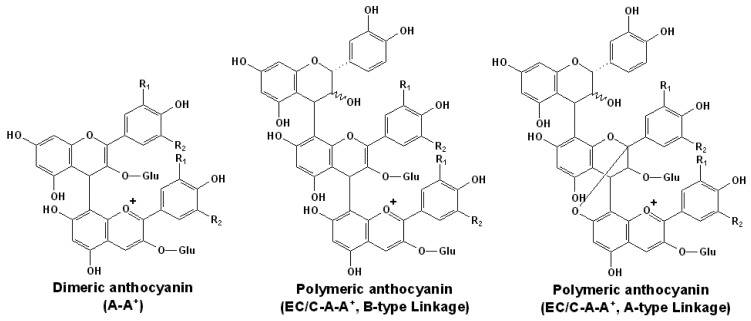
In contrast, when the electrophilic C4 position in the C ring of an anthocyanin in the flavylium form is attacked by the nucleophilic C8 or C6 position in the A ring of a flavan-3-ol or a terminal unit of an additional proanthocyanidin, an A-T adduct can be produced. Initially, this adduct is generated as a colorless flavene complex [126]. It is thought that the subsequent oxidation can rearrange the molecule to the colored flavylium state, as shown in Figure 13b [127,128]. Some of them can even form the anthocyanin-flavan-3-ol dimer, which contains both carbon-carbon and ether interflavanoid linkages [129]. Besides, the diglucosidic anthocyanins can also participate in such polymerization [130]. Because the C4 position of the flavylium anthocyanin is utilized in the A-T type adduct, anthocyanins in the bisulfite addition compound form, induced by the addition of sulfur dioxide to the C4 carbon, may prevent such A-T polymerization [131,132]. Additionally, anthocyanin-anthocyanin dimers can also be formed in red wines. Interestingly, some of them have the structures that are similar to B-type proanthocyanidins, but others can form an additional intramolecular bond by an additional dehydration reaction to produce an A-type proanthocyanidin like structure, as shown in Figure 5 [133,134,135,136]. For example, besides the normal malvidin-3-O-glucoside-(C4-C8)-malvidin-3-O-glucoside, the bicyclic malvidin-3-O-glucoside-(C2-O-C7, C4-C8)-malvidin-3-O-glucoside was also detected in red wine fractions [133,134,135]. Detailed mass spectrometry information of some major products from direct condensation reactions of anthocyanins and/or flavan-3-ols that can be detected in aged red wines or be synthesized in model wine solutions is summarized in Table 2, as shown below [128,134,135].
Table 2.
The mass spectral data of some major products from direct condensation reactions of anthocyanins and/or flavan-3-ols [128,134,135].
| Compounds | Molecular ion M+ (m/z) | Fragment ion M+ (m/z) |
|---|---|---|
| Delphinidin-glucoside-(epi)catechin | 753 | 591,573,465,439,303 |
| Petunidin-glucoside-(epi)catechin | 767 | 605,587,453,359,329 |
| Peonidin-glucoside-(epi)catechin | 751 | 589,571,463,437 |
| Malvidin-glucoside-gallocatechin | 797 | 635,617,509,467,373 |
| Malvidin-glucoside-(epi)catechin | 781 | 619,601,467,373,331 |
| Malvidin-acetylglucoside-(epi)catechin | 823 | 619,601,467,493,331 |
| Malvidin-coumaroylglucoside-(epi)catechin | 927 | 619,493,467,451,331 |
| Malvidin-glucoside-PC dimer | 1069 | 907,781,619 |
| Malvidin-glucoside-malvidin-glucoside | 985 | 823,661,535,331 |
| Malvidin-glucoside-malvidin-acetylglucoside | 1027 | 865,823,661,331 |
| Malvidin-glucoside-petunidin-acetylglucoside | 1013 | 851,809,647,331 |
| Malvidin-glucoside-delphinidin-acetylglucoside | 999 | 837,795,633,331,303 |
| Malvidin-acetylglucoside-malvidin-acetylglucoside | 1069 | 865,661,331 |
| Malvidin-glucoside-peonidin-acetylglucoside | 997 | 835,631,303 |
| Malvidin-acetylglucoside-petunidin-acetylglucoside | 1055 | 851,647,521,317 |
| Malvidin-glucoside-malvidin-coumaroylglucoside | 1131 | 969,823,661,535,331 |
| Malvidin-acetylglucoside-malvidin-coumaroylglucoside | 1173 | 969,865,661,535,331 |
| Malvidin-glucoside-delphinidin-coumaroylglucoside | 1103 | 941,795,633,507,331 |
| Malvidin-glucoside-petunidin-coumaroylglucoside | 1117 | 955,809,647,317 |
| Malvidin-glucoside-peonidin-coumaroylglucoside | 1101 | 939,793,631,505,331 |
| Malvidin-coumaroylglucoside-malvidin-coumaroylglucoside | 1277 | 969,661,639 |
| Malvidin-glucoside-delphinidin-glucoside | 957 | 795,633,507,331,303 |
| Malvidin-glucoside-cyanidin-glucoside | 941 | 779,617,491,449 |
| Malvidin-glucoside-petunidin-glucoside | 971 | 809,647,521,331,317 |
| Malvidin-glucoside-peonidin-glucoside | 955 | 793,631,505,331 |
| Malvidin-glucoside-cyanidin-coumaroylglucoside | 1087 | 925,779,617,493,287 |
| (Epi)catechin-delphinidin-glucoside-malvidin-glucoside | 1245 | 1083,795,921,903,633 |
| (Epi)catechin-cyanidin-glucoside-malvidin-glucoside | 1229 | 1067,917,904 |
| (Epi)catechin-petunidin-glucoside-malvidin-glucoside | 1259 | 1097,971,935,747,671 |
| (Epi)catechin-peonidin-glucoside-malvidin-glucoside | 1243 | 1081,1063,919 |
| (Epi)catechin-malvidin-glucoside-malvidin-glucoside | 1273 | 1111,949,931,823,661 |
| (Epi)gallocatechin-delphinidin-glucoside-malvidin-glucoside | 1261 | 1099,937 |
| (Epi)gallocatechin-petunidin-glucoside-malvidin-glucoside | 1275 | 1113,951,647 |
| (Epi)gallocatechin-malvidin-glucoside-malvidin-glucoside | 1289 | 1127,965,823,535,331 |
As well as the above flavan-3-ol monomers and oligomeric proanthocyanidins can also undergo a similar condensation generating larger condensed tannins, which might be more complex than those extracted from grape berries [137]. However, these large polymers are easier to precipitate and harder to condense with anthocyanins than the smaller proanthocyanidins [138].
Figure 5.
Structures of some directly condensed oligomeric anthocyanins [133,134,135].
3.2. Acetaldehyde or Glyoxylic Acid Mediated Polymeric Products
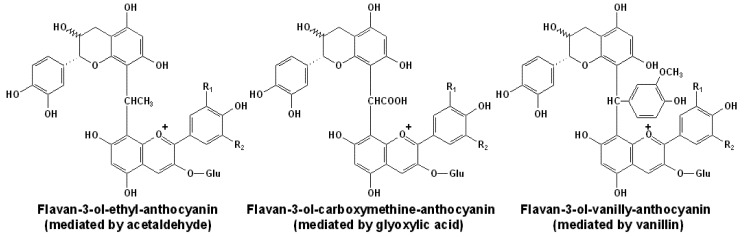
Besides the direct condensation with flavonoids, anthocyanins can also form reddish or violet polymeric pigments via the mediation of aldehydes, showing in Figure 6 [91,139,140,141,142]. Studies indicated that the anthocyanin moiety in such polymeric pigment was more protected against water attack and the color of such pigment showed more stability with regard to bleaching by sulfite dioxide than that of monomeric anthocyanins. However, they are more sensitive to degradation in aqueous solutions [143].
Figure 6.
Structures of some mediated condensed oligomeric anthocyanins [91,139,140,141,142].
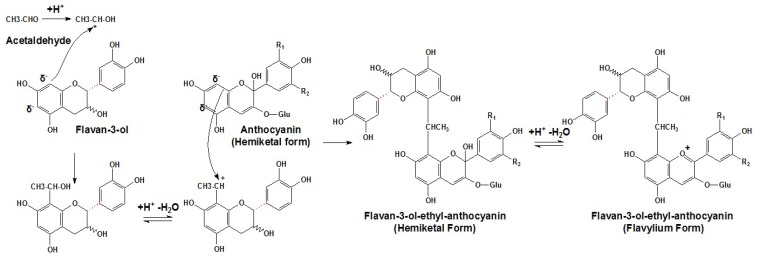
Arising from different sources, such as yeast or the oxidation of ethanol, acetaldehyde is the most abundant aldehyde in red wine [139]. At the low pH value of red wines, a small proportion of acetaldehyde exists as the protonated form, as a reactive carbonium ion state. In such state, acetaldehyde can react with the nucleophilic C8 or C6 position in the A ring of a flavan-3-ol molecule or a terminal unit of proanthocyanidin. After dehydration, the acetaldehyde linked flavanol adduct can give rise to a new carbonium ion that can attack the nucleophilic C8 position in the A ring of an anthocyanin molecule. After deprotonation, the resulting compound can form a violet quinoidal base of the cross-linked flavanol-ethyl-anthocyanin adduct, as shown in Scheme 10 [32,144,145].
Scheme 10.
Proposed mechanism of acetaldehyde mediated tannin–anthocyanin additions [32,144,145].
As the flavanol moiety can be attached at the C8 or C6 position, whereas the anthocyanin moiety in the flavylium state can be only attached at the C8 position, such indirect polymerized pigments have two different structural isomers, involving the C8-(CH-CH3)-CH8 and C6-(CH-CH3)-CH8 isomers [24,140,146]. Because the ethyl (or methylmethine) bridge has an asymmetric carbon, each of the above mentioned regioisomers has two diastereoisomers differing in the stereochemistry (R or S) of the methine carbon [117,140]. This reaction mechanism has been demonstrated in numerous studies using the NMR analysis of indirect condensed products, such as the ethyl-linked malvidin-3-O-glucoside-(epi)catechin adducts [147]. However, recently it has also been demonstrated that the C6 position in the A ring of the hemiketal form of an anthocyanin can also participate in such condensation, albeit to a lesser extent than the C8 position [148]. The CH3CH linked new pigments can cause rapid and spectacular color augmentation with shifts toward violet [93,141,149].
Additionally, ethyl-linked pigments usually undergo further polycondensation, which means the degree of polymerization can be very high. Previous studies have already identified trimeric and tetrameric pigments in model solutions [95]. It is important to note that anthocyanins are only present at the terminal points of the linear oligomers linked by ethyl bridging, thus there are no ethyl-linked pigments containing more than two anthocyanin units [95,150]. Recently, some more complicated ethyl bridged flavanol-anthocyanin oligomeric adducts, including (+)-catechin-ethyl-(+)-catechin-malvidin-3-O-glucoside and ethyl-[(+)-catechin-malvidin-3-O-glucoside]2, were obtained by hemisynthesis and identified in aged table wine and aged Port wine, showing the possible existence of more complicated condensed anthocyanin pigments [148,150].
The polymerization of monomeric flavan-3-ols and oligomeric proanthocyanidins with anthocyanins in the presence of acetaldehyde occurs at different rates. For example, when malvidin-3-O-glucoside participates in the acetaldehyde mediated polymerization, the reaction rates decrease in the following order: procyanidin C1, procyanidin B1, procyanidin B2, procyanidin B2-3′-O-galloyl, procyanidin B3, (−)-epicatechin and (+)-catechin [151]. When procyanidin B2 participates in the acetaldehyde mediated polymerization, the reaction rates decrease as the following order: peonidin-3-O-glucoside, cyanidin-3-O-glucoside and malvidin-3-O-glucoside. The products are transient and they later evolve to form substances with a greater degree of condensation [149]. Therefore, these reactions do not only contribute to the color change observed during wine aging, but do contribute to the decline in astringency. Though cool aging temperatures can retard the aldehyde mediated polymerization between anthocyanins and flavan-3-ols, it can limit the fast formation of excessively large pigments, their subsequent precipitation, and hence color loss [140,152]. Furthermore, the presence of oxygen and lower pH values can promote such reactions, since the formation of acetaldehyde and its protonated form are favored under such conditions, respectively [121,140,153]. However, previous studies have only focused on the reactions involving smaller proanthocyanidins and the monomeric flavan-3-ols in model solutions, so the relative importance of the bigger polymeric proanthocyanidins is still unclear.
Additionally, besides acetaldehyde, some minor aldehydes in red wine, such as propionaldehyde, isovaleraldehyde, isobutyraldehyde, benzaldehyde, formaldehyde, 2-methybutyraldehyde, vanillin, furfural and hydroxymethylfurfural can also mediate the condensation to add further complexity through the similar mechanism described for acetaldehyde [33,60,142,154,155,156]. For example, the glyceraldehyde and dihydroxyacetone generated from the oxidation of glycerol, which is the second most common alcohol in red wines, can promote the formation of the novel cross-linked anthocyanin based pigments [33]. Propionaldehyde can mediate condensation reaction between malvidin-3-O-glucoside and (+)-catechin to lead to the formation of malvidin-3-O-glucoside-(C8→)-propyl-(C8→)-(+)-catechin [60]. In the presence of furfural or its derivative 5-hydroxymethylfurfural, the reactions between (+)-catechin and anthocyanins were also detected to produce various furfuryl or 5-hydroxymethylfurfuryl group linked oligomers [154].
Also glyoxylic acid bearing an aldehyde moiety can mediate indirect condensation of anthocyanins and flavan-3-ols. Similar to the acetaldehyde-mediated polymerization, glyoxylic acid from iron-catalyzed oxidation of tartaric acid attacks the nucleophilic C8 or C6 positions in the A ring of anthocyanins and flavan-3-ols to produce a carboxy methane bridged oligomers [141]. Considering the relative amounts of the tartaric acid and ferrous ions in wine, this reaction may be quite important during wine aging [157]. It probably competes with the acetaldehyde-mediated polymerization and contributes considerably to color alteration in the aging wine [158].
Detailed mass spectrometry information of some major products from aldehyde-mediated condensation reactions of anthocyanins and/or flavan-3-ols that can be detected in aged red wines or be synthesized in model wine solutions is summarized in Table 3, as shown below [63,64,66,128]. Although most of them are synthesized in model solutions, which usually are detected in trace amounts or cannot be detected in most aged red wines, they may also contribute considerably to the wine color evolution and mouth feel of red wines, as well as the diversity of anthocyanin derivative pigments [47,79,138,144,154].
Table 3.
The mass spectral data of some major products from aldehyde-mediated condensation reactions of anthocyanins and/or flavan-3-ols [63,64,66,128].
| Compounds | Molecular ion M+ (m/z) | Fragment ion M+ (m/z) |
|---|---|---|
| Petunidin-glucoside-8-ethyl-(epi)catechin | 795 | 633,505,481,435,328 |
| Malvidin-glucoside-8-ethyl-gallocatechin | 825 | |
| Malvidin-glucoside-8-ethyl-(epi)catechin | 809 | 657,517,357,341,331 |
| Malvidin-acetylglucoside-8-ethyl-(epi)catechin | 851 | |
| Malvidin-coumaroylglucoside-8-ethyl-(epi)catechin | 955 | 803,665,647,357,341 |
| Peonidin-glucoside-8-ethyl-(epi)catechin | 779 | |
| Peonidin-coumaroylglucoside-8-ethyl-(epi)catechin | 925 | 635,617,327 |
| Malvidin-glucoside-4-methyl-(epi)catechin | 795 | 505 |
| Malvidin-glucoside-4-2methylpropyl-(epi)catechin | 837 | 547 |
| Malvidin-glucoside-4-3methylbutyl-(epi)catechin | 851 | 561 |
| Malvidin-glucoside-4-2methylbutyl-(epi)catechin | 851 | 561 |
| Malvidin-glucoside-4-benzyl-(epi)catechin | 871 | 581 |
| Malvidin-glucoside-4-propyl-(epi)catechin | 823 | 533 |
| Malvidin-glucoside-4-ethyl-PC dimer | 1097 | 519 |
| Malvidin-glucoside-4-3methylbutyl-PC dimer | 1139 | |
| Malvidin-glucoside-4-benzyl-PC dimer | 1159 | 707 |
| Malvidin-glucoside-4-propyl-PC dimer | 1111 | 959 |
| Malvidin-glucoside-4-ethyl-epicatechin-3-O-gallate | 961 | 799,519 |
| Malvidin-glucoside-4-ethyl-B2-3'-O-gallate | 1249 | |
| Malvidin-glucoside-4-3methylbutyl-epicatechin-3-O-gallate | 1003 | |
| Malvidin-glucoside-4-3methylbutyl-B2-3'-O-gallate | 1291 | |
| Malvidin-glucoside-4-benzyl-epicatechin-3-O-gallate | 1023 | 719 |
| Malvidin-glucoside-4-benzyl-PB2-3'-O-gallate | 1311 | 419,581 |
| Malvidin-glucoside-4-propyl-epicatechin-3-O-gallate | 975 | 813,671 |
| Malvidin-glucoside-4-propyl-PB2-3'-O-gallate | 1263 | 821 |
| Malvidin-glucoside-4-methyl-epicatechin-3-O-gallate | 947 | 785,343 |
| Malvidin-glucoside-4-methyl-PB2-3'-O-gallate | 1235 | 1073,793 |
| Malvidin-glucoside-4-isobutyl-epicatechin-3-O-gallate | 989 | 385 |
| Malvidin-glucoside-4-isobutyl-PB2-3'-O-gallate | 1277 | 835 |
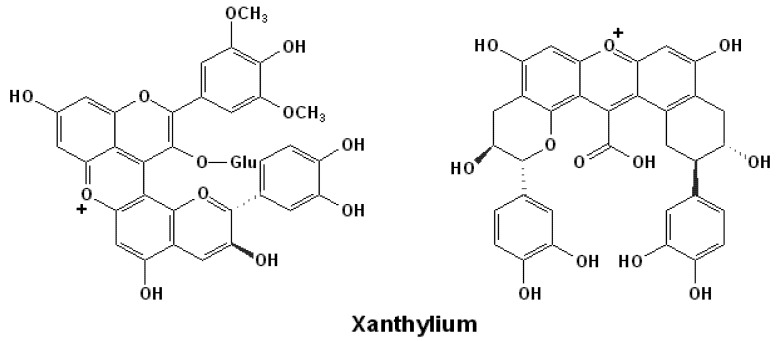
3.3. Xanthylium Pigments
Xanthylium salts are one group of important polyphenolic pigments which are considered to be derived by direct or indirect condensation of flavonoids, especially with anthocyanins and/or flavan-3-ols. Such condensed flavonoid oligomers can be converted to corresponding xanthene derivatives by subsequent cyclization, which can be further oxidized to form the yellow to red xanthylium pigments, as shown in Figure 7 [159,160,161,162]. Xanthyliums have various sources in red wines.
Figure 7.
The A-T adducts can generate yellow-orange xanthylium pigments by further structural rearrangements. After the dehydration reaction between the hydroxyl groups on the C5 position in the A ring of the anthocyanin moiety and the C8 position in the A ring of the flavan-3-ol unit, a new heterocyclic pyran ring is formed between the two parent rings and the xanthylium structure is generated [108,115,152,163,164]. However, they are also proposed to be formed directly from oligomeric flava-3-ols [157,165].
The glyoxylic acid mediated dimer of flavan-3-ols and anthocyanins can also generate xanthylium pigments by a similar mechanism. For example, carboxymethane bridged anthocyanins and/or (+)-catechin dimers can be converted into a xanthene derivative by a dehydration reaction involving the hydroxyl groups on the C7 positions, and the product can be further oxidized to xanthylium pigments [166,167]. Similarly xanthylium can also be synthesized from formaldehyde or 2-methybutyraldehyde mediated flavonoid dimers, such as (+)-catechin-furfuryl/-hydroxymethylfurfuryl-malvidin-3-O-glucoside dimers [154].
4. Influence of Aging Practices on Anthocyanins and Their Derivatives in Red Wines
During wine aging, the concentrations of monomeric and copigmented anthocyanins decrease progressively [14,16,24,25,168]. In particular, acylated anthocyanins disappear a little more quickly than the non-acylated ones. It has been suggested that acylated anthocyanins might undergo hydrolysis into their non-acylated forms during aging. But, different non-acylated free anthocyanins almost share the same depleting kinetics, and pyranoanthocyanins show a similar or lower disappearance rate than their corresponding free precursors [16]. While the monomeric anthocyanins decline more, polymeric anthocyanins are formed helping to maintain the red wine color, albeit with a change of hue.
Oxygen is very important for the evolution of anthocyanins in red wines, particularly during wine aging [121]. Whereas strong oxygenation increases wine oxidation, mild oxygenation during storage can improve wine quality by stabilizing wine color [169]. As already discussed, oxygen is involved in a range of mechanisms, such as the formation of direct or indirect polymeric anthocyanin pigments [119,170]. Previous research has been principally focused on wine aging in wood barrels with permeation of oxygen and more recently has been expanded to include aging in stainless steel tanks with controlled oxygenation [170].
Micro-oxygenation is one of the techniques used to promote the evolution of anthocyanins in red wines [169,171,172,173,174,175]. It involves the formation of micro-bubbles through the injection of gaseous oxygen into the wine by means of a micro-diffuser, or the controlled diffusion of oxygen through a permeable membrane [173]. Research results indicate that the effect of this technique on wine color can be compared to that of the maturation in oak barrels, especially with the addition of oak chips or staves [172,174,175]. Normally, the application of micro-oxygenation can result in a lower concentration of monomeric anthocyanins and a higher concentration of polymeric anthocyanins and pyranoanthocyanins in red wines [169,171,172,173,174,175]. It can be followed by aging in barrels or bottles to further modify the color properties of the wine. Red wines with lower phenolic content have been found to be less influenced by micro-oxygenation and may even suffer over-oxygenation [169]. Thus, such enological techniques need to be use after careful consideration.
When aging in oak, besides oxygen permeation, many compounds, such as gallic, ferulic, vanillic, syringic, ellagic acids, ellagitannins and tannins are extracted into wine, thereby introducing desirable oak aromas and flavors into red wines, as well as involving in a number of reactions with wine phenolics, such as anthocyanins and tannins modifying the palate structure of the wine [14,24]. Though aging in oak barrels is traditional and is beneficial, oak chips can be used in wine aging, frequently in conjunction with micro-oxygenation, to reduce costs [172,174,176,177]. In some research, red wines previously in contact with oak chips or staves lost their monomeric anthocyanins more quickly and showed a more rapid increase in polymerization than those aged in barrels. In some cases this resulted in the premature development of red-brown hues [176,177]. Some researchers have suggested that the barrel wood type had a significant influence on the chromatic characteristics of red wines. For example, maturation and aging in chestnut barrels has produced different results to those from oak aging because of their greater quality of oxygen penetrating through chestnut than oak barrels [178]. Some studies showed that the red wines aged with French oak lost slightly more monomeric anthocyanins than those aged in Hungarian or American oak, whereas others have reported that red wine aged in Hungarian oak barrels suffered a slightly higher loss of monomeric anthocyanins than those treated with French and American oak wood [176,177]. This perhaps is not surprising given the natural variation in wood composition combined with differences in seasoning and coopering by the different barrel makers. So perhaps it is not surprising that some studies have also suggested that the type of container, an oak barrel or a stainless-steel tank, has little influence on the anthocyanin fingerprints in red wines [170].
After fermentation the lees are not always removed immediately and some wines are aged with lees for 3–8 months, the technique of bâtonnage [179]. During this time, though the yeast cell walls could well adsorb some anthocyanins, the dead yeast cells may also release some compounds that could contribute to wine aging by improving wine stabilization in terms of color and proteins [179,180]. Some studies also revealed that aging over lees appears to have a protective effect on the total monomeric anthocyanin content [172,179,180]. Besides, as mentioned above, pyruvic acid and acetaldehyde can be involved in the formation of pyranoanthocyanins, and acetaldehyde also contribute to the synthesis of bridged anthocyanin pigments.
During aged with lees, some polysaccharide can be released from the cell wall after yeast autolysis [181]. As one of the major polysaccharide groups present in wine, mannoproteins are highly glycosylated and play a series of important roles in wines, such as to adsorb ochratoxin A, to enhance malolactic bacteria growth, to inhibit tartaric salts crystallization, to prevent protein haziness, to enhance and interact with some wine aromas, as well as to react with anthocyanins, tannins or other phenolics in wines [181,182,183,184,185,186,187,188]. In recent years some researches aimed to evaluate the influence of mannoproteins in the color stability in red wines were conducted by using commercial mannoproteins or mannoprotein overproducing yeast strain. The results usually suggested that there was no influence on color stability but these mannoproteins can delay the tannin polymerization in red wines [189,190,191,192]. Considering the crucial role of tannins in the formation of polymeric anthocyanin pigments, they may take effect in the long-term stability of the wine color.
5. Conclusions
During winemaking and wine aging, anthocyanins in red wines undergo complicated chemical changes to form diverse anthocyanin derivatives, as summarized in Scheme 11 [14,24,57]. Accurate knowledge of their variation is crucial for better understanding and control of color evolution. Numerous by-products of yeast metabolism not only play a role in copigmentation, which is the first step in the formation of stable pigments in red wines, but also in the further formation of the new pigments, such as the pyranoanthocyanins and the polymeric anthocyanin pigments [193]. Although some of these pigments have been detected in only very small quantities in red wines, they have unique spectroscopic features and together may contribute noticeably to the overall hue and stability of color of aged red wines. Thus, factors such as yeast species and strains, temperature, pH, and the concentration of sulfur dioxide during fermentation, as well as various enological practices will all influence the profiles of anthocyanin and their derivatives, leading to diverse color expression.
Scheme 11.
The areas for future research into anthocyanin derivatives, their chemistry and behavior during red wine maturation and aging is still expanding and there is much to be done. Some of the fields requiring extensive investigation include:
(1) Identification of new pyranoanthocyanins in aged red wines, especially the new pigments from second generation of carboxy- or methyl-pyranoanthocyanins.
(2) Identification of more complicated polymeric anthocyanins in aged red wines, especially the ones with higher polymerization degree and new configurations.
(3) Quantification of the amounts of such pigments and their extinction coefficients to enable the assessment of their contribution to visual wine color.
(4) New enology practices to improve the production and stability of anthocyanin derivatives, as well as total wine color.
Acknowledgements
This study was supported by the Special Funds of Modern Industrial Technology System for Agriculture (nycytx-30).
Footnotes
Sample Availability: Not available.
References and Notes
- 1.Mazza G., Francis F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995;35:341–371. doi: 10.1080/10408399509527704. [DOI] [PubMed] [Google Scholar]
- 2.Wrolstad R.E., Durst R.W., Lee J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Tech. 2005;16:423–428. doi: 10.1016/j.tifs.2005.03.019. [DOI] [Google Scholar]
- 3.Degenhardt A., Hofmann S., Knapp H., Winterhalter P. Preparative isolation of anthocyanins by high-speed countercurrent chromatography and application of the color activity concept to red wine. J. Agric. Food Chem. 2000;48:5812–5818. doi: 10.1021/jf0007481. [DOI] [PubMed] [Google Scholar]
- 4.Robinson W.B., Weirs L.D., Bertino J.J., Mattick L.R. The relation of anthocyanin composition to color stability of New York State wines. Am. J. Enol. Vitic. 1966;17:178–184. [Google Scholar]
- 5.Busse-Valverde N., Gómez-Plaza E., López-Roc J.M., Gil-Muňoz R., Bautista-Ortín A.B. The extraction of anthocyanins and proanthocyanidins from grapes to wine during fermentative maceration is affected by the enological technique. J. Agric. Food Chem. 2011;59:5450–5455. doi: 10.1021/jf2002188. [DOI] [PubMed] [Google Scholar]
- 6.Jensen J.S., Demiray S., Egebo M., Meyer A.S. Prediction of wine color attributes from the phenolic profiles of red grapes (Vitis vinifera) J. Agric. Food Chem. 2008;56:1105–1115. doi: 10.1021/jf072541e. [DOI] [PubMed] [Google Scholar]
- 7.González-Manzano S., Santos-Buelga C., Dueñas M., Rivas-Gonzalo J.C., Escribano-Bailón T. Colour implications of self-association processes of wine anthocyanins. Eur. Food Res. Technol. 2008;226:483–490. doi: 10.1007/s00217-007-0560-9. [DOI] [Google Scholar]
- 8.Miniati E., Damiani P., Mazza G. Copigmentation and self-association of anthocyanins in food model systems. Ital. J. Food Sci. 1992;4:109–116. [Google Scholar]
- 9.Dangles O., Saito N., Brouillard R. Anthocyanin intramolecular copigment effect. Phytochemistry. 1993;34:119–124. [Google Scholar]
- 10.Malien-Aubert C., Dangles O., Amiot M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra- and intermolecular copigmentation. J. Agric. Food Chem. 2001;49:170–176. doi: 10.1021/jf000791o. [DOI] [PubMed] [Google Scholar]
- 11.Eiro M.J., Heinonen M. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. J. Agric. Food Chem. 2002;50:7461–7466. doi: 10.1021/jf0258306. [DOI] [PubMed] [Google Scholar]
- 12.Boulton R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001;52:67–87. [Google Scholar]
- 13.Cavalcanti R.N., Santos D.T., Meireles M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems-An overview. Food Res. Int. 2011;44:499–509. doi: 10.1016/j.foodres.2010.12.007. [DOI] [Google Scholar]
- 14.Jackson R.S. Wine Science: Principle and Applications. 3rd. Elsevier-Academic Press; Oxford, UK: 2008. pp. 287–295. [Google Scholar]
- 15.Brouillard R., Chassaing S., Fougerousse A. Why are grape/fresh wine anthocyanins so simple and why is it that red wine color lasts so long? Phytochemistry. 2003;64:1179–1186. doi: 10.1016/s0031-9422(03)00518-1. [DOI] [PubMed] [Google Scholar]
- 16.Monagas M., Bartolomé B., Gómez-Cordovés C. Evolution of polyphenols in red wines from Vitis vinifera L. during aging in the bottle. Eur. Food Res. Technol. 2005;220:331–340. doi: 10.1007/s00217-004-1109-9. [DOI] [Google Scholar]
- 17.Gómez-Míguez M., González-Miret M.L., Heredia F.J. Evolution of colour and anthocyanin composition of Syrah wines elaborated with pre-fermentative cold maceration. J. Food Eng. 2007;79:271–278. doi: 10.1016/j.jfoodeng.2006.01.054. [DOI] [Google Scholar]
- 18.Vasserot Y., Caillet S., Maujean A. Study of anthocyanin adsorption by yeast lees. Effect of some physicochemical parameters. Am. J. Enol. Vitic. 1997;48:433–437. [Google Scholar]
- 19.Morata A., Gómez-Cordovés M.C., Suberviola J., Bartolomé B., Colomo B., Suárez J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003;51:4084–4088. doi: 10.1021/jf021134u. [DOI] [PubMed] [Google Scholar]
- 20.Tseng K.-C., Chang H.-M., Wu J.S.-B. Degradation kinetics of anthocyanin in ethanolic solutions. J. Food Process. Preserv. 2006;30:503–514. doi: 10.1111/j.1745-4549.2006.00083.x. [DOI] [Google Scholar]
- 21.Kader F., Irmouli M., Zitouni N., Nicolas J.-P., Metche M. Degradation of cyanidin 3-glucoside by caffeic acid o-quinone. Determination of the stoichiometry and characterization of the degradation products. J. Agric. Food Chem. 1999;47:4625–4630. doi: 10.1021/jf981400x. [DOI] [PubMed] [Google Scholar]
- 22.Cheynier V., Fulcrand H., Guyot S., Oszmianski J., Moutounet M. Reactions of enzymically generated quinones in relation to browning in grape musts and wines. In: Lee C.Y., Whitaker J.R., editors. Enzymatic Browning and Its Prevention. 1st. ACS Publications; Washington, DC, USA: 1995. pp. 130–143. [Google Scholar]
- 23.Sarni-Manchado P., Cheynierm V., Moutounet M. Reaction of polyphenoloxidase generated caftaric acid o-quinone with malvidin 3-O-glucoside. Phytochemistry. 1997;45:1365–1369. doi: 10.1016/S0031-9422(97)00190-8. [DOI] [Google Scholar]
- 24.Monagas M., Bartolomé B. Anthocyanins and anthocyanin-derived compounds. In: Victoria Moreno-Arribas M., Carmen Polo M., editors. Wine Chemistry and Biochemistry. 1st. Springer Science+Business Media, LLC; 233 Spring Street, New York, NY, USA: 2009. pp. 439–462. [Google Scholar]
- 25.Ribéreau-Gayon P., Glories Y., Maujean A., Dubourdieu D. Handbook of Enology Volume 2 The Chemistry of Wine Stabilization and Treatments. 2nd. John Wiley & Sons Ltd; Chichester, UK: 2005. pp. 141–204. [Google Scholar]
- 26.Guadalupe Z., Ayestarán B. Changes in the color components and phenolic content of red wines from Vitis vinifera L. Cv. “Tempranillo” during vinification and aging. Eur. Food Res. Technol. 2008;228:29–38. doi: 10.1007/s00217-008-0902-2. [DOI] [Google Scholar]
- 27.Vidal S., Francis L., Noble A., Kwiatkowski M., Cheynier V., Waters E. Taste and mouth-feel properties of different types of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta. 2004;513:57–65. doi: 10.1016/j.aca.2003.10.017. [DOI] [Google Scholar]
- 28.Vidal S., Francis L., Williams P., Kwiatkowski M., Gawel R., Cheynier V., Waters E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004;85:519–525. doi: 10.1016/S0308-8146(03)00084-0. [DOI] [Google Scholar]
- 29.Dufour C., Sauvaitre I. Interactions between anthocyanins and aroma substances in a model system. Effect on the flavor of grape-derived beverages. J. Agric. Food Chem. 2000;48:1784–1788. doi: 10.1021/jf990877l. [DOI] [PubMed] [Google Scholar]
- 30.Picinelli A., Bakker J., Bridle P. Model wine solutions: Effect of sulphur dioxide on colour and composition during ageing. Vitis. 1994;33:31–35. [Google Scholar]
- 31.Saucier C., Bourgeois G., Vitry C., Roux D., Glories Y. Characterization of (+)-catechin-acetaldehyde polymers: A model for colloidal state of wine polyphenols. J. Agric. Food Chem. 1997;45:1045–1049. doi: 10.1021/jf960597v. [DOI] [Google Scholar]
- 32.Pissarra J., Lourenço S., González-Paramás A.M., Mateus N., Santos-Buelga C., De Freitas V. Formation of new anthocyanin-alkyl/aryl-flavanol pigments in model solutions. Anal. Chim. Acta. 2004;513:215–221. doi: 10.1016/j.aca.2003.09.039. [DOI] [Google Scholar]
- 33.Laurie V.F., Waterhouse A.L. Glyceraldehyde bridging between flavanols and malvidin-3-glucoside in model solutions. J. Agric. Food Chem. 2006;54:9105–9111. doi: 10.1021/jf0617770. [DOI] [PubMed] [Google Scholar]
- 34.Sun B., Leandro M.C., De Freitas V., Spranger M.I. Fractionation of red wine polyphenols by solid-phase extraction and liquid chromatography. J. Chromatogr. A. 2006;1128:27–38. doi: 10.1016/j.chroma.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Jeffery D.W., Mercurio M.D., Herderich M.J., Hayasaka Y., Smith P.A. Rapid isolation of red wine polymeric polyphenols by solid-phase extraction. J. Agric. Food Chem. 2008;56:2571–2580. doi: 10.1021/jf073478w. [DOI] [PubMed] [Google Scholar]
- 36.Dugo P., Favoino O., Lo Presti M., Luppino R., Dugo G., Mondello L. Determination of anthocyanins and related components in red wines by micro- and capillary HPLC. J. Sep. Sci. 2004;27:1458–1466. doi: 10.1002/jssc.200401822. [DOI] [PubMed] [Google Scholar]
- 37.Vergara C., Mardones C., Hermosín-Gutiérrez I., Von Baer D. Comparison of high-performance liquid chromatography separation of red wine anthocyanins on a mixed-mode ion-exchange reversed-phase and on a reversed-phase column. J. Chromatogr. A. 2010;1217:5710–5717. doi: 10.1016/j.chroma.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Mazzuca P., Ferranti P., Picariello G., Chianese L., Addeo F. Mass spectrometry in the study of anthocyanins and their derivatives: Differentiation of Vitis vinifera and hybrid grapes by liquid chromatography/electrospray ionization mass spectrometry and tandem mass spectrometry. J. Mass Spectrom. 2005;40:83–90. doi: 10.1002/jms.778. [DOI] [PubMed] [Google Scholar]
- 39.Monagas M., Núñez V., Bartolomé B., Gómez-Cordovés C. Anthocyanin-derived pigments in Graciano, Tempranillo, and Cabernet Sauvignon wines produced in Spain. Am. J. Enol. Vitic. 2003;54:163–169. [Google Scholar]
- 40.Wang J., Sporns P. Analysis of anthocyanins in red wine and fruit juice using MALDI-MS. J. Agric. Food Chem. 1999;47:2009–2015. doi: 10.1021/jf981008j. [DOI] [PubMed] [Google Scholar]
- 41.Ivanova V., Dörnyei Á., Stefova M., Stafilov T., Vojnoski B., Kilár F., Márk L. Rapid MALDI-TOF-MS detection of anthocyanins in wine and grape using different matrices. Food Anal. Methods. 2011;4:108–115. doi: 10.1007/s12161-010-9143-7. [DOI] [Google Scholar]
- 42.Gómez-Ariza J.L., García-Barrera T., Lorenzo F. Anthocyanins profile as fingerprint of wines using atmospheric pressure photoionisation coupled to quadrupole time-of-flight mass spectrometry. Anal. Chim. Acta. 2006;570:101–108. doi: 10.1016/j.aca.2006.04.004. [DOI] [Google Scholar]
- 43.Ferrari E., Foca G., Vignali M., Tassi L., Ulrici A. Adulteration of the anthocyanin content of red wines: Perspectives for authentication by Fourier Transform-Near InfraRed and 1H NMR spectroscopies. Anal. Chim. Acta. 2011;701:139–151. doi: 10.1016/j.aca.2011.05.053. [DOI] [PubMed] [Google Scholar]
- 44.Košir I.J., Lapornik B., Andrenšek S., Wondra A.G., Vrhovšek U., Kidrič J. Identification of anthocyanins in wines by liquid chromatography, liquid chromatography-mass spectrometry and nuclear magnetic resonance. Anal. Chim. Acta. 2004;513:277–282. doi: 10.1016/j.aca.2003.12.013. [DOI] [Google Scholar]
- 45.Castañeda-Ovando A., Pacheco-Hernández Ma.d.L., Páez-Hernández Ma.E., Rodríguez J.A., Galán-Vidal C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- 46.Nave F., Teixeira N., Mateus N., De Freitas V. The fate of flavanol-anthocyanin adducts in wines: Study of their putative reaction patterns in the presence of acetaldehyde. Food Chem. 2010;121:1129–1138. doi: 10.1016/j.foodchem.2010.01.060. [DOI] [Google Scholar]
- 47.De Freitas V., Mateus N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011;401:1463–1473. doi: 10.1007/s00216-011-5175-0. [DOI] [PubMed] [Google Scholar]
- 48.Fulcrand H., Benabdeljalil C., Rigaud J., Chenyier V., Moutounet M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry. 1998;47:1401–1407. doi: 10.1016/s0031-9422(97)00772-3. [DOI] [PubMed] [Google Scholar]
- 49.Canals R., Llaudy M.C., Valls J., Canals J.M., Zamora F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2005;53:4019–4025. doi: 10.1021/jf047872v. [DOI] [PubMed] [Google Scholar]
- 50.Bakker J., Timberlake C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997;45:35–43. doi: 10.1021/jf960252c. [DOI] [Google Scholar]
- 51.Schwarz M., Wabnitz T.C., Winterhalter P. Pathway leading to the formation of anthocyanin-vinylphenol adducts and related pigments in red wines. J. Agric. Food Chem. 2003;51:3682–3687. doi: 10.1021/jf0340963. [DOI] [PubMed] [Google Scholar]
- 52.Rentzsch M., Schwarz M., Winterhalter P. Pyranoanthocyanins-An overview on structures, occurrence, and pathways of formation. Trends Food Sci. Technol. 2007;18:526–534. doi: 10.1016/j.tifs.2007.04.014. [DOI] [Google Scholar]
- 53.Vivar-Quintana A.M., Santos-Buelga C., Francia-Aricha E., Rivas-Gonzalo J.C. Formation of anthocyanin-derived pigments in experimental red wines. Food Sci. Tech. Int. 1999;5:347–352. doi: 10.1177/108201329900500407. [DOI] [Google Scholar]
- 54.Mateus N., Silva A.M.S., Vercauteren J., De Freitas V. Occurrence of anthocyanin-derived pigments in red wines. J. Agric. Food Chem. 2001;49:4836–4840. doi: 10.1021/jf001505b. [DOI] [PubMed] [Google Scholar]
- 55.Hayasaka Y., Asenstorfer R.E. Screening for potential pigments derived from anthocyanins in red wine using nanoelectrospray tandem mass spectrometry. J.Agric. Food Chem. 2002;50:756–761. doi: 10.1021/jf010943v. [DOI] [PubMed] [Google Scholar]
- 56.Alcalde-Eon C., Escribano-Bailón M.T., Santos-Buelga C., Rivas-Gonzalo J.C. Separation of pyranoanthocyanins from red wine by column chromatography. Anal. Chim. Acta. 2004;513:305–318. doi: 10.1016/j.aca.2003.10.076. [DOI] [Google Scholar]
- 57.Von Baer D., Rentzsch M., Hitschfeld M.A., Mardones C., Vergara C., Winterhalter P. Relevance of chromatographic efficiency in varietal authenticity verification of red wines based on their anthocyanin profiles: Interference of pyranoanthocyanins formed during wine ageing. Anal. Chim. Acta. 2008;621:52–56. doi: 10.1016/j.aca.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 58.He J., Carvalho A.R.F., Mateus N., De Freitas V. Spectral features and stability of oligomeric pyranoanthocyanin-flavanol pigments isolated from red wines. J. Agric. Food Chem. 2010;58:9249–9258. doi: 10.1021/jf102085e. [DOI] [PubMed] [Google Scholar]
- 59.Mateus N., Carvalho E., Carvalho A.R.F., Melo A., González-Paramás A.M., Santos-Buelga C., Silva A.M.S., De Freitas V. Isolation and structural characterization of new acylated anthocyanin-vinyl-flavanol pigments occurring in aging red wines. J. Agric. Food Chem. 2003;51:277–282. doi: 10.1021/jf020695i. [DOI] [PubMed] [Google Scholar]
- 60.Pozo-Baón M.Á., Monagas M., Polo M.C., Gómez-Cordovés C. Occurrence of pyranoanthocyanins in sparkling wines manufactured with red grape varieties. J. Agric. Food Chem. 2004;52:1300–1306. doi: 10.1021/jf030639x. [DOI] [PubMed] [Google Scholar]
- 61.Mateus N., De Pascual-Teresa S., Rivas-Gonzalo J.C., Santos-Buelga C., De Freitas V. Structural diversity of anthocyanin-derived pigments in port wines. Food Chem. 2002;76:335–342. doi: 10.1016/S0308-8146(01)00281-3. [DOI] [Google Scholar]
- 62.Monagas M., Núñez V., Bartolomé B., Gómez-Cordovés C. Anthocyanin-derived pigments in Graciano, Tempranillo, and Cabernet Sauvignon wines produced in Spain. Am. J. Enol. Vitic. 2003;54:163–169. [Google Scholar]
- 63.De Villiers A., Vanhoenacker G., Majek P., Sandra P. Determination of anthocyanins in wine by direct injection liquid chromatography-diode array detection-mass spectrometry and classification of wines using discriminant analysis. J. Chromatogr. A. 2004;1054:195–204. [PubMed] [Google Scholar]
- 64.Boido E., Alcalde-Eon A., Carrau F., Dellacassa E., Rivas-Gonzalo J.C. Aging effect on the pigment composition and color of Vitis vinifera L. cv. Tannat wines. Contribution of the main pigment families to wine color. J. Agric. Food Chem. 2006;54:6692–6704. doi: 10.1021/jf061240m. [DOI] [PubMed] [Google Scholar]
- 65.He J., Santos-Buelga C., Mateus N., De Freitas V. Isolation and quantification of oligomeric pyranoanthocyanin-flavanol pigments from red wines by combination of column chromatographic techniques. J. Chromatogr. A. 2006;1134:215–225. doi: 10.1016/j.chroma.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 66.Chinnici F., Sonni F., Natali N., Galassi S., Riponi C. Colour features and pigment composition of Italian carbonic macerated red wines. Food Chem. 2009;113:651–657. doi: 10.1016/j.foodchem.2008.07.055. [DOI] [Google Scholar]
- 67.Aguirre M.J., Isaacs M., Matsuhiro B., Mendoza L., Santos L.S., Torres S. Anthocyanin composition in aged Chilean Cabernet Sauvignon red wines. Food Chem. 2011;129:514–519. doi: 10.1016/j.foodchem.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Romero C., Bakker J. Interactions between grape anthocyanins and pyruvic acid, with effect of pH and acid concentration on anthocyanin composition and color in model solutions. J. Agric. Food Chem. 1999;47:3130–3139. doi: 10.1021/jf981286i. [DOI] [PubMed] [Google Scholar]
- 69.Bakker J., Bridle P., Honda T., Kuwano H., Saito N., Terahara N., Timberlake C.F. Identification of an anthocyanin occurring in some red wines. Phytochemistry. 1997;44:1375–1382. [Google Scholar]
- 70.Jordheim M., Fossen T., Andersen Ø.M. Preparative isolation and NMR characterization of carboxypyranoanthocyanins. J. Agric. Food Chem. 2006;54:3572–3577. doi: 10.1021/jf053240c. [DOI] [PubMed] [Google Scholar]
- 71.Oliveira J., Mateus N., Silva A.M.S., De Freitas V. Equilibrium forms of Vitisin B pigments in an aqueous system studied by NMR and visible spectroscopy. J. Phys. Chem. B. 2009;113:11352–11358. doi: 10.1021/jp904776k. [DOI] [PubMed] [Google Scholar]
- 72.Lu Y., Foo L.Y. Unusual anthocyanin reaction with acetone leading to pyranoanthocyanin formation. Tetrahedron Lett. 2001;42:1371–1373. doi: 10.1016/S0040-4039(00)02246-2. [DOI] [Google Scholar]
- 73.Carvalho A.R.F., Oliveira J., De Freitas V., Mateus N., Melo A. A theoretical interpretation of the color of two classes of pyranoanthocyanins. J. Mol. Struct. Theochem. 2010;948:61–64. doi: 10.1016/j.theochem.2010.02.020. [DOI] [Google Scholar]
- 74.Morata A., Calderón F., González M.C., Gómez-Cordovés M.C., Suárez J.A. Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. Food Chem. 2007;100:1144–1152. doi: 10.1016/j.foodchem.2005.11.024. [DOI] [Google Scholar]
- 75.Romero C., Bakker J. Effect of acetaldehyde and several acids on the formation of vitisin A in model wine anthocyanin and colour evolution. Int. J. Food Sci. Technol. 2000;35:129–140. doi: 10.1046/j.1365-2621.2000.00372.x. [DOI] [Google Scholar]
- 76.Romero C., Bakker J. Effect of storage temperature and pyruvate on kinetics of anthocyanin degradation, vitisin A derivative formation, and color characteristics of model solutions. J. Agric. Food Chem. 2000;48:2135–2141. doi: 10.1021/jf990998l. [DOI] [PubMed] [Google Scholar]
- 77.Morata A., Gómez-Cordovés M.C., Calderón F., Suárez J.A. Effects of pH, temperature and SO2 on the formation of pyranoanthocyanins during red wine fermentation with two species of Saccharomyces. Int. J. Food Microbiol. 2006;106:123–129. doi: 10.1016/j.ijfoodmicro.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 78.Monagas M., Gómez-Cordovés C., Bartolomé B. Evaluation of different Saccharomyces cerevisiae strains for red winemaking. Influence on the anthocyanin, pyranoanthocyanin and non-anthocyanin phenolic content and colour characteristics of wines. Food Chem. 2007;104:814–823. doi: 10.1016/j.foodchem.2006.12.043. [DOI] [Google Scholar]
- 79.Revilla I., Pérez-Magariño S., González-SanJosé M.L., Beltrán S. Identification of anthocyanin derivatives in grape skin extracts and red wines by liquid chromatography with diode array and mass spectrometric detection. J. Chromatogr. A. 1999;847:83–90. doi: 10.1016/S0021-9673(99)00256-3. [DOI] [PubMed] [Google Scholar]
- 80.Wang H., Race E.J., Shrikhande A. Characterization of anthocyanins in grape juices by ion trap liquid chromatography−mass spectrometry. J. Agric. Food Chem. 2003;51:1839–1844. doi: 10.1021/jf0260747. [DOI] [PubMed] [Google Scholar]
- 81.Nave F., Teixeira N., Mateus N., De Freitas V. Hemisynthesis and structural characterization of flavanol-(4, 8)-vitisins by mass spectrometry. Rapid Commun. Mass Spectrom. 2010;24:1964–1970. doi: 10.1002/rcm.4599. [DOI] [PubMed] [Google Scholar]
- 82.Schwarz M., Jerz G., Winterhalter P. Isolation and structure of pinotin A, a new anthocyanin derivative from pinotage wine. Vitis. 2003;42:105–106. [Google Scholar]
- 83.Hakånsson A.E., Pardon K., Hayasaka Y., De Sa M., Herderich M. Structures and colour properties of new red wine pigments. Tetrahedron Lett. 2003;44:4887–4891. doi: 10.1016/S0040-4039(03)01090-6. [DOI] [Google Scholar]
- 84.Fulcrand H., Dos Santos P.-J.C., Sarni-Manchado P., Cheynier V., Favre-Bonvin J. Structure of new anthocyanin-derived wine pigments. J. Chem. Soc. Perkin Trans. 1. 1996;7:735–739. [Google Scholar]
- 85.Chatonnet P., Dubourdieu D., Boidron J.-N., Lavigne V. Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J. Sci. Food Agric. 1993;62:191–202. doi: 10.1002/jsfa.2740620213. [DOI] [Google Scholar]
- 86.Morata A., González C., Suárez-Lepe J.A. Formation of vinylphenolic pyranoanthocyanins by selected yeasts fermenting red grape musts supplemented with hydroxycinnamic acids. Int. J. Food Microbiol. 2007;116:144–152. doi: 10.1016/j.ijfoodmicro.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 87.Schwarz M., Winterhalter P. A novel synthetic route to substituted pyranoanthocyanins with unique colour properties. Tetrahedron Lett. 2003;44:7583–7587. doi: 10.1016/j.tetlet.2003.08.065. [DOI] [Google Scholar]
- 88.Cameira-dos-Santos P.J., Brillouet J.-M., Cheynier V., Moutounet M. Detection and partial characterisation of new anthocyanins derived pigments in wine. J. Sci. Food Agric. 1996;70:204–208. doi: 10.1002/(SICI)1097-0010(199602)70:2<204::AID-JSFA484>3.0.CO;2-F. [DOI] [Google Scholar]
- 89.Rentzsch M., Schwarz M., Winterhalter P., Hermosín-Guitiérrez I. Formation of hydroxyphenyl-pyranoanthocyanins in Grenache wines: Precursor levels and evolution during aging. J. Agric. Food Chem. 2007;55:4883–4888. doi: 10.1021/jf0702491. [DOI] [PubMed] [Google Scholar]
- 90.Schwarz M., Hofmann G., Winterhalter P. Investigations on anthocyanins in wines from Vitis vinifera cv. pinotage: Factors influencing the formation of pinotin A and its correlation with wine age. J. Agric. Food Chem. 2004;52:498–504. doi: 10.1021/jf035034f. [DOI] [PubMed] [Google Scholar]
- 91.Francia-Aricha E.M., Guerra M.T., Rivas-Gonzalo J.C., Santos-Buelga C. New anthocyanin pigments formed after condensation with flavanols. J. Agric. Food Chem. 1997;45:2262–2266. [Google Scholar]
- 92.Cruz L., Teixeira N., Silva A.M.S., Mateus N., Borges J., De Freitas V. Role of vinylcatechin in the formation of pyranomalvidin-3-glucoside-(+)-catechin. J. Agric. Food Chem. 2008;56:10980–10987. doi: 10.1021/jf8021496. [DOI] [PubMed] [Google Scholar]
- 93.Asenstorfer R.E., Hayasaka Y., Jones G.P. Isolation and structures of oligomeric wine pigments by bisulfite-mediated ion-exchange chromatography. J Agric. Food Chem. 2001;49:5957–5963. doi: 10.1021/jf011261a. [DOI] [PubMed] [Google Scholar]
- 94.Cruz L., Borges E., Silva A.M.S., Mateus N., De Freitas V. Synthesis of a new (+)-catechin-derived compound: 8-Vinylcatechin. Lett. Org. Chem. 2008;5:530–536. doi: 10.2174/157017808785982211. [DOI] [Google Scholar]
- 95.Es-Safi N.E., Fulcrand H., Cheynier V., Moutounet M. Studies on the acetaldehyde-induced condensation of (-)-epicatechin and malvidin 3-O-glucoside in a model solution system. J. Agric. Food Chem. 1999;47:2096–2102. doi: 10.1021/jf9806309. [DOI] [PubMed] [Google Scholar]
- 96.Mateus N., Silva A.M.S., Rivas-Gonzalo J.C., Santos-Buelga C., De Freitas V. A new class of blue anthocyanin-derived pigments isolated from red wines. J. Agric. Food Chem. 2003;51:1919–1923. doi: 10.1021/jf020943a. [DOI] [PubMed] [Google Scholar]
- 97.Mateus N., Oliveira J., Santos-Buelga C., Silva A.M.S., De Freitas V. NMR structure characterization of a new vinylpyranoanthocyanin-catechin pigment (a portisin) Tetrahedron Lett. 2004;45:3455–3457. doi: 10.1016/j.tetlet.2004.03.007. [DOI] [Google Scholar]
- 98.Mateus N., Oliveira J., Pissarra J., González-Paramás A.M., Rivas-Gonzalo J.C., Santos-Buelga C., Silva A.M.S., De Freitas V. A new vinylpyranoanthocyanin pigment occurring in aged red wine. Food Chem. 2006;97:689–695. doi: 10.1016/j.foodchem.2005.05.051. [DOI] [Google Scholar]
- 99.Oliveira J., Santos-Buelga C., Silva A.M.S., De Freitas V., Mateus N. Chromatic and structural features of blue anthocyanin-derived pigments present in Port wine. Anal. Chim. Acta. 2006;563:2–9. doi: 10.1016/j.aca.2005.11.027. [DOI] [Google Scholar]
- 100.Mateus N., De Freitas V. Evolution and stability of anthocyanin-derived pigments during port wine aging. J. Agric. Food Chem. 2001;49:5217–5222. doi: 10.1021/jf0106547. [DOI] [PubMed] [Google Scholar]
- 101.Oliveira J., De Freitas V., Silva A.M.S., Mateus N. Reaction between hydroxycinnamic acids and anthocyanin-pyruvic acid adducts yielding new portisins. J. Agric. Food Chem. 2007;55:6349–6356. doi: 10.1021/jf070968f. [DOI] [PubMed] [Google Scholar]
- 102.Mateus N., Oliveira J., González-Paramás A.M., Santos-Buelga C., De Freitas V. Screening of portisins (vinylpyranoanthocyanin pigments) in port wine by LC/DAD-MS. Food Sci. Technol. Int. 2005;5:353–358. [Google Scholar]
- 103.Carvalho A.R.F., Oliveira J., De Freitas V., Mateus N., Melo A. Unusual color change of vinylpyranoanthocyanin-phenolic pigments. J. Agric. Food. Chem. 2010;58:4292–4297. doi: 10.1021/jf904246g. [DOI] [PubMed] [Google Scholar]
- 104.He J., Oliveira J., Silva A.M.S., Mateus N., De Freitas V. Oxovitisins: A new class of neutral pyranone-anthocyanin derivatives in red wines. J. Agric. Food. Chem. 2010;58:8814–8819. doi: 10.1021/jf101408q. [DOI] [PubMed] [Google Scholar]
- 105.Asenstorfer R.E., Lee D.F., Jones G.P. Influence of structure on the ionisation constants of anthocyanin and anthocyanin-like wine pigments. Anal. Chim. Acta. 2006;563:10–14. doi: 10.1016/j.aca.2005.09.040. [DOI] [Google Scholar]
- 106.Oliveira J., Azevedo J., Silva A.M.S., Teixeira N., Cruz L., Mateus N., de Freitas V. Pyranoanthocyanin dimers: A new family of turquoise blue anthocyanin-derived pigments found in port wine. J. Agric. Food Chem. 2010;58:5154–5159. doi: 10.1021/jf9044414. [DOI] [PubMed] [Google Scholar]
- 107.Chassaing S., Isorez G., Kueny-Stotz M., Brouillard R. En route to color-stable pyranoflavylium pigments-a systematic study of the reaction between 5-hydroxy-4-methylflavylium salts and aldehydes. Tetrahedron Lett. 2008;49:6999–7004. doi: 10.1016/j.tetlet.2008.09.139. [DOI] [Google Scholar]
- 108.Liao H., Cai Y., Haslam E. Polyphenol interactions. Anthocyanins: Co-pigmentation and colour changes in red wines. J. Sci. Food Agric. 1992;59:299–305. doi: 10.1002/jsfa.2740590305. [DOI] [Google Scholar]
- 109.Quideau S., Jourdes M., Lefeuvre D., Montaudon D., Saucier C., Glories Y., Pardon P., Pourquier P. The chemistry of wine polyphenolic C-glycosidic ellagitannins targeting human topoisomerase II. Chem. Eur. J. 2005;11:6503–6513. doi: 10.1002/chem.200500428. [DOI] [PubMed] [Google Scholar]
- 110.Jurd L. Review of polyphenol condensation reactions and their possible occurrence in the aging of wines. Am. J. Enol. Vitic. 1969;20:191–195. [Google Scholar]
- 111.Somers T.C. The polymeric nature of wine pigments. Phytochemistry. 1971;10:2175–2186. doi: 10.1016/S0031-9422(00)97215-7. [DOI] [Google Scholar]
- 112.Salas E., Le Guernevé C., Fulcrand H., Poncet-Legrand C., Cheynier V. Structure determination and colour properties of a new directly linked flavanol-anthocyanin dimer. Tetrahedron Lett. 2004;45:8725–8729. doi: 10.1016/j.tetlet.2004.09.127. [DOI] [Google Scholar]
- 113.Somers T.C. Pigment phenomena-From grapes to wine. In: Webb A.D., editor. Grape and Wine Centennial Symposium Proceedings; Davis, CA, USA: University of California; 1982. pp. 254–257. [Google Scholar]
- 114.Puértolas E., Saldaña G., Alvarez I., Raso J. Effect of pulsed electric field processing of red grapes on wine chromatic and phenolic characteristics during aging in oak barrel. J. Agric. Food Chem. 2010;58:2351–2357. doi: 10.1021/jf904035v. [DOI] [PubMed] [Google Scholar]
- 115.Santos-Buelga C., Francia-Aricha E.M., De Pascual-Teresa S., Rivas-Gonzalo J.C. Contribution to the identification of the pigments responsible for the browning of anthocyanin-flavanol solutions. Eur. Food Res. Technol. 1999;209:411–415. [Google Scholar]
- 116.Remy S., Fulcrand H., Labarbe B., Cheynier V., Moutounet M. First confirmation in red wine of products resulting from direct anthocyanin-tannin reactions. J. Sci. Food Agric. 2000;80:745–751. doi: 10.1002/(SICI)1097-0010(20000501)80:6<745::AID-JSFA611>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 117.Escribano-Bailón T., Dangles O., Brouillard R. Coupling reactions between flavylium ions and catechin. Phytochemistry. 1996;41:1583–1592. [Google Scholar]
- 118.Salas E., Fulcrand H., Meudec E., Cheynier V. Reactions of anthocyanins and tannins in model solutions. J. Agric. Food Chem. 2003;51:7951–7961. doi: 10.1021/jf0345402. [DOI] [PubMed] [Google Scholar]
- 119.Salas E., Atanasova V., Poncet-Legrand C., Meudec E., Mazauric J.P., Cheynier V. Demonstration of the occurrence of flavanol-anthocyanin adducts in wine and in model solutions. Anal. Chim. Acta. 2004;513:325–332. doi: 10.1016/j.aca.2003.11.084. [DOI] [Google Scholar]
- 120.He F., Pan Q.-P., Shi Y., Duan C.-Q. Chemical synthesis of proanthocyanidins in vitro and their reactions in aging wines. Molecules. 2008;13:3007–3032. doi: 10.3390/molecules13123007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Atanasova V., Fulcrand H., Cheynier V., Moutounet M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta. 2002;458:15–27. doi: 10.1016/S0003-2670(01)01617-8. [DOI] [Google Scholar]
- 122.Sáenz-López R., Fernández-Zurbano P., Tena M.T. Analysis of aged red wine pigments by capillary zone electrophoresis. J. Chromatogr. A. 2004;1052:191–197. doi: 10.1016/j.chroma.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 123.Hayasaka Y., Kennedy J.A. Mass spectrometric evidence for the formation of pigmented polymers in red wine. Aust. J. Grape Wine Res. 2003;9:210–220. doi: 10.1111/j.1755-0238.2003.tb00272.x. [DOI] [Google Scholar]
- 124.Vivar-Quintana A.M., Santos-Buelga C., Rivas-Gonzalo J.C. Anthocyanin-derived pigments and colour of red wines. Anal. Chim. Acta. 2002;458:147–155. doi: 10.1016/S0003-2670(01)01619-1. [DOI] [Google Scholar]
- 125.Monagas M., Núñez V., Bartolomé B., Gómez-Cordovés C. Anthocyanin-derived pigments in Graciano, Tempranillo and Cabernet Sauvignon wines produced in Spain. Am. J. Enol. Vitic. 2003;54:163–169. [Google Scholar]
- 126.Santos-Buelga C., Francia-Aricha E.M., De Pascual-Teresa S., Rivas-Gonzalo J.C. Contribution to the identification of the pigments responsible for the browning of anthocyaninflavanol solutions. Eur. Food Res. Technol. 1999;209:411–415. doi: 10.1007/s002170050518. [DOI] [Google Scholar]
- 127.Mateus N., Silva A.M.S., Santos-Buelga C., Rivas-Gonzalo J.C., De Freitas V. Identification of anthocyanin-flavanol pigments in red wines by NMR and mass spectrometry. J. Agric. Food Chem. 2002;50:2110–2116. doi: 10.1021/jf0111561. [DOI] [PubMed] [Google Scholar]
- 128.Pati S., Losito I., Gambacorta G., La Notte E., Palmisano F., Zambonin P.G. Simultaneous separation and identification of oligomeric procyanidins and anthocyanin-derived pigments in raw red wine by HPLC-UV-ESI-MSn. J. Mass Spectrom. 2006;41:861–871. doi: 10.1002/jms.1044. [DOI] [PubMed] [Google Scholar]
- 129.Remy-Tanneau S., Le Guernevé C., Meudec E., Cheynier V. Characterization of a colorless anthocyanin-flavan-3-ol dimer containing both carbon-carbon and ether interflavanoid linkages by NMR and mass spectrometry. J. Agric. Food Chem. 2003;51:3592–3597. doi: 10.1021/jf021227b. [DOI] [PubMed] [Google Scholar]
- 130.Bishop P.D., Nagel C.W. Characterization of the condensation product of malvidin 3,5-diglucoside and catechin. J. Agric. Food Chem. 1984;32:1022–1026. doi: 10.1021/jf00125a019. [DOI] [Google Scholar]
- 131.Berke B., Chèze C., Vercauteren J., Deffieux G. Bisulfite addition to anthocyanins: Revisited structures of colorless adducts. Tetrahedron Lett. 1998;39:5771–5774. doi: 10.1016/S0040-4039(98)01205-2. [DOI] [Google Scholar]
- 132.Kalbasi A., Cisneros-Zevallos L. Fractionation of monomeric and polymeric anthocyanins from concord grape (Vitis labrusca L.) juice by membrane ultrafiltration. J. Agric. Food Chem. 2007;55:7036–7042. doi: 10.1021/jf0706068. [DOI] [PubMed] [Google Scholar]
- 133.Salas E., Dueñas M., Schwarz M., Winterhalter P., Cheynier V., Fulcrand H. Characterization of pigmets from different high speed countercurrent chromatography wine fractions. J. Agric. Food Chem. 2005;53:4536–4546. doi: 10.1021/jf0478096. [DOI] [PubMed] [Google Scholar]
- 134.Alcalde-Eon C., Escribano-Bailón M.T., Santos-Buelga C., Rivas-Gonzalo J.C. Identification of dimeric anthocyanins and new oligomeric pigments in red wine by means of HPLC-DAD-ESI/MSn. J. Mass Spectrom. 2007;42:735–748. doi: 10.1002/jms.1208. [DOI] [PubMed] [Google Scholar]
- 135.Pati S., Liberatore M.T., Gambacorta G., Antonacci D., La Notte E. Rapid screening for anthocyanins and anthocyanin dimers in crude grape extracts by high performance liquid chromatography coupled with diode array detection and tandem mass spectrometry. J. Chromatogr. A. 2009;1216:3864–3868. doi: 10.1016/j.chroma.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 136.Vidal S., Meudec E., Cheynier V., Skouroumounis G., Hayasaka Y. Mass spectrometric evidence for the existence of oligomeric anthocyanins in grape skins. J. Agric. Food Chem. 2004;52:7144–7151. doi: 10.1021/jf048939h. [DOI] [PubMed] [Google Scholar]
- 137.Vidal S., Cartalade D., Souquet J.M., Fulcrand H., Cheynier V. Changes in proanthocyanidin chain length in winelike model solutions. J. Agric. Food Chem. 2002;50:2261–2266. doi: 10.1021/jf011180e. [DOI] [PubMed] [Google Scholar]
- 138.Pérez-Magariño S., González-San José M.L. Evolution of flavanols, anthocyanins, and their derivatives during the aging of red wines elaborated from grapes harvested at different stages of ripening. J. Agric. Food Chem. 2004;52:1181–1189. doi: 10.1021/jf035099i. [DOI] [PubMed] [Google Scholar]
- 139.Timberlake C.F., Bridle P. Interactions between anthocyanins, phenolic compounds and acetaldehyde and their significance in red wines. Am. J. Enol. Vitic. 1976;27:97–105. [Google Scholar]
- 140.Rivas-Gonzalo J.C., Bravo-Haro S., Santos-Buelga C. Detection of compounds formed through the reaction of malvidin 3-monoglucoside and catechin in the presence of acetaldehyde. J. Agric. Food Chem. 1995;43:1444–1449. doi: 10.1021/jf00054a006. [DOI] [Google Scholar]
- 141.Es-Safi N.-E., Le Guernevé C., Cheynier V., Moutounet M. New phenolic compounds formed by evolution of (+)-catechin and glyoxylic acid in hydroalcoholic solution and their implication in color changes of grape-derived foods. J. Agric. Food Chem. 2000;48:4233–4240. doi: 10.1021/jf000283e. [DOI] [PubMed] [Google Scholar]
- 142.Sousa C., Mateus N., Silva A.M.S., González-Paramás A.M., Santos-Buelga C., De Freitas V. Structural and chromatic characterization of a new Malvidin 3-glucoside-vanillyl-catechin pigment. Food Chem. 2007;102:1344–1351. doi: 10.1016/j.foodchem.2006.04.050. [DOI] [Google Scholar]
- 143.Escribano-Bailón T., Áivarez-García M., Rivas-Gonzalo J.C., Heredia F.J., Santos-Buelga C. Color and stability of pigments derived from the acetaldehyde-mediated condensation between malvidin 3-O-glucoside and (+)-catechin. J. Agric. Food Chem. 2001;49:1213–1217. doi: 10.1021/jf001081l. [DOI] [PubMed] [Google Scholar]
- 144.Es-Safi N.E., Cheynier V., Moutounet M. Role of aldehydic derivatives in the condensation of phenolic compounds with emphasis on the sensorial properties of fruit-derived foods. J. Agric. Food Chem. 2002;50:5571–5585. doi: 10.1021/jf025503y. [DOI] [PubMed] [Google Scholar]
- 145.Sun B., Barradas T., Leandro C., Santos C., Spranger I. Formation of new stable pigments from condensation reaction between malvidin 3-glucoside and (-)-epicatechin mediated by acetaldehyde: Effect of tartaric acid concentration. Food Chem. 2008;110:344–351. doi: 10.1016/j.foodchem.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 146.Bakker J., Picinelli A., Bridle P. Model wine solutions: Colour and composition changes during ageing. Vitis. 1993;32:111–118. [Google Scholar]
- 147.Lee D.F., Swinny E.E., Jones G.P. NMR identification of ethyl-linked anthocyanin-flavanol pigments formed in model wine ferments. Tetrahedron Lett. 2004;45:1671–1674. doi: 10.1016/j.tetlet.2003.12.110. [DOI] [Google Scholar]
- 148.Atanasova V., Fulcrand H., Le Guernevé C., Cheynier V., Moutounet M. Structure of new dimeric acetaldehyde malvidin-3-glucoside condensation product. Tetrahedron Lett. 2002;43:6151–6153. doi: 10.1016/S0040-4039(02)01294-7. [DOI] [Google Scholar]
- 149.Dallas C., Ricardo-da-Silva J.M., Laureano O. Products formed in model wine solutions involving anthocyanins, procyanidin B2, and acetaldehyde. Agric. Food Chem. 1996;44:2402–2407. doi: 10.1021/jf940433j. [DOI] [Google Scholar]
- 150.Dueñas M., Salas E., Cheynier V., Dangles O., Fulcrand H. UV-Visible spectroscopic investigation of the 8-8-methylmethine catechin-malvidin 3-glucoside pigments in aqueous solution: Structural transformations and molecular complexation with chlorogenic acid. J. Agric. Food Chem. 2006;54:189–196. doi: 10.1021/jf0516989. [DOI] [PubMed] [Google Scholar]
- 151.Dallas C., Ricardo da Silva J.M., Laureano O. Interactions of oligomeric procyanidins in model wine solutions containing malvidin-3-glucoside and acetaldehyde. J. Sci. Food Agric. 1996;70:493–500. doi: 10.1002/(SICI)1097-0010(199604)70:4<493::AID-JSFA528>3.0.CO;2-7. [DOI] [Google Scholar]
- 152.Baranowski E.S., Nagel C.W. Kinetics of malvidin-3-glucoside condensation in wine model systems. J. Food Sci. 1983;48:419–429. doi: 10.1111/j.1365-2621.1983.tb10756.x. [DOI] [Google Scholar]
- 153.García-Viguera C., Bridle P., Bakker J. The effect of pH on the formation of coloured compounds in model solutions containing anthocyanins, catechin and acetaldehyde. Vitis. 1994;33:37–40. [Google Scholar]
- 154.Es-Safi N.-E., Cheynier V., Moutounet M. Study of the reactions between (+)-catechin and furfural derivatives in the presence or absence of anthocyanins and their implication in food color change. J. Agric. Food Chem. 2000;48:5946–5954. doi: 10.1021/jf000394d. [DOI] [PubMed] [Google Scholar]
- 155.Pissarra J., Mateus N., Rivas-Gonzalo J., Santos Buelga C., De Freitas V. Reaction between malvidin 3-glucoside and (+)-catechin in model solutions containing different aldehydes. J. Food Sci. 2003;68:476–481. [Google Scholar]
- 156.Drinkine J., Lopes P., Kennedy J.A., Teissedre P.-L., Saucier C. Ethylidene-bridged flavan-3-ols in red wine and correlation with wine age. J. Agric. Food Chem. 2007;55:6292–6299. doi: 10.1021/jf070038w. [DOI] [PubMed] [Google Scholar]
- 157.Fulcrand H., Cheynier V., Oszmiansky J., Moutounet M. An oxidised tartaric acid residue as a new bridge potentially competing with acetaldehyde in flavan-3-ol condensation. Phytochemistry. 1997;46:223–227. [Google Scholar]
- 158.Drinkine J., Glories Y., Saucier C. (+)-Catechin-aldehyde condensations: Competition between acetaldehyde and glyoxylic acid. J. Agric. Food Chem. 2005;53:7552–7558. doi: 10.1021/jf0504723. [DOI] [PubMed] [Google Scholar]
- 159.Es-Safi N.-E., Le Guernevé C., Fulcrand H., Cheynier V., Moutounet M. Xanthylium salts formation involved in wine colour changes. Int. J. Food Sci. Tech. 2000;35:63–74. doi: 10.1046/j.1365-2621.2000.00339.x. [DOI] [Google Scholar]
- 160.Santos-Buelga C., Bravo-Haro S., Rivas-Gonzalo J.C. Interactions between catechin and malvidin-3-monoglucoside in model solutions. Eur. Food Res. Technol. 1995;201:269–274. [Google Scholar]
- 161.Es-Safi N.-E., Le Gueraevé C., Labarbe B., Fulerand H., Cheyuier V., Moutouaet M. Structure of a new xanthyfium salt derivative. Tetrahedron Lett. 1999;40:5869–5872. doi: 10.1016/S0040-4039(99)01156-9. [DOI] [Google Scholar]
- 162.Es-Safi N., Le Guernevé C., Labarbe B., Fulcrand H., Cheynier V., Moutounet M. Structure of a new xanthylium salt derivative. Tetrahedron Lett. 1999;40:5869–5872. [Google Scholar]
- 163.Somers T.C. The phenolic nature of wine pigments. Phytochemistry. 1971;10:2175–2186. doi: 10.1016/S0031-9422(00)97215-7. [DOI] [Google Scholar]
- 164.Dueñas M., Fulcrand H., Cheynier V. Formation of anthocyanin-flavanol adducts in model solutions. Anal. Chim. Acta. 2006;563:15–25. [Google Scholar]
- 165.Jurd L., Somers T.C. The formation of xanthylium salts from proanthocyanidins. Phytochemistry. 1970;9:419–427. [Google Scholar]
- 166.George N., Clark A.C., Prenzler P.D., Scollary G.R. Factors influencing the production and stability of xanthylium cation pigments in a model white wine system. Aust. J. Grape Wine Res. 2006;12:57–68. doi: 10.1111/j.1755-0238.2006.tb00044.x. [DOI] [Google Scholar]
- 167.Es-Safi N.E., Le Guernevé C., Fulcrand H., Cheynier V., Moutounet M. New polyphenolic compounds with xanthylium skeletons formed through reaction between (+)-catechin and glyoxylic acid. J. Agric. Food Chem. 1999;47:5211–5217. doi: 10.1021/jf990424g. [DOI] [PubMed] [Google Scholar]
- 168.Alcalde-Eon C., Escribano-Bailón M.T., Santos-Buelga C., Rivas-Gonzalo J.C. Changes in the detailed pigment composition of red wine during maturity and ageing: A comprehensive study. Anal. Chim. Acta. 2006;563:238–254. doi: 10.1016/j.aca.2005.11.028. [DOI] [Google Scholar]
- 169.Cano-López M., Pardo-Mínguez F., Schmauch G., Saucier C., Teissedre P.-L., López-Roca J.M., Gómez-Plaza E. Effect of micro-oxygenation on color and anthocyanin-related compounds of wines with different phenolic contents. J. Agric. Food Chem. 2008;56:5932–5941. doi: 10.1021/jf8006147. [DOI] [PubMed] [Google Scholar]
- 170.Revilla E., López J.F., Ryan J.-M. Anthocyanin pattern of Tempranillo wines during ageing in oak barrels and storage in stainless-steel tanks. Eur. Food Res. Technol. 2005;220:592–596. doi: 10.1007/s00217-005-1148-x. [DOI] [Google Scholar]
- 171.Cano-López M., Pardo-Minguez F., López-Roca J.M., Gómez-Plaza E. Effect of the microoxygenation on anthocyanin and derived pigment content and chromatic characteristics of red wines. Am. J. Enol. Vitic. 2006;57:325–331. [Google Scholar]
- 172.Sartini E., Arfelli G., Fabiani A., Piva A. Influence of chips, lees and micro-oxygenation during aging on the phenolic composition of a red Sangiovese wine. Food Chem. 2007;104:1599–1604. doi: 10.1016/j.foodchem.2007.03.010. [DOI] [Google Scholar]
- 173.Cano-López M., Pardo-Mínguez F., López-Roca J.M., Gómez-Plaza E. Chromatic characteristics and anthocyanin profile of a micro-oxygenated red wine after oak or bottle maturation. Eur. Food Res. Technol. 2007;225:127–132. doi: 10.1007/s00217-006-0390-1. [DOI] [Google Scholar]
- 174.Pérez-Magariño S., Ortega-Heras M., Cano-Mozo E., González-Sanjosé M.L. The influence of oak wood chips, micro-oxygenation treatment, and grape variety on colour, and anthocyanin and phenolic composition of red wines. J. Food Compos. Anal. 2009;22:204–211. [Google Scholar]
- 175.Cano-López M., López-Roca J.M., Pardo-Mínguez F., Gómez-Plaza E. Oak barrel maturation vs. micro-oxygenation: Effect on the formation of anthocyanin-derived pigments and wine colour. Food Chem. 2010;119:191–195. doi: 10.1016/j.foodchem.2009.06.018. [DOI] [Google Scholar]
- 176.Del Álamo Sanza M., Domínguez I.N., Merino S.G. Influence of different aging systems and oak woods on aged wine color and anthocyanin composition. Eur. Food Res. Technol. 2004;219:124–132. [Google Scholar]
- 177.Del Álamo Sanza M., Nevares Domínguez I. Wine aging in bottle from artificial systems (staves and chips) and oak woods Anthocyanin composition. Anal. Chim. Acta. 2006;563:255–263. doi: 10.1016/j.aca.2005.11.030. [DOI] [Google Scholar]
- 178.Gambuti A., Capuano R., Lisanti M.T., Strollo D., Moio L. Effect of aging in new oak, one-year-used oak, chestnut barrels and bottle on color, phenolics and gustative profile of three monovarietal red wines. Eur. Food Res. Technol. 2010;231:455–465. doi: 10.1007/s00217-010-1292-9. [DOI] [Google Scholar]
- 179.Palomero F., Morata A., Benito S., Gonzalez M.C., Suárez-Lepe J.A. Conventional and enzyme-assisted autolysis during ageing over lees in red wines: Influence on the release of polysaccharides from yeast cell walls and on wine monomeric anthocyanin content. Food Chem. 2007;105:838–846. [Google Scholar]
- 180.Moreno-Arribas M.V., Gómez-Cordovés C., Martín-Álvarez P.J. Evolution of red wine anthocyanins during malolactic fermentation, postfermentative treatments and ageing with lees. Food Chem. 2008;109:149–158. doi: 10.1016/j.foodchem.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 181.Feuillat M. Yeast macromolecules: Origin, composition and enological interest. Am. J. Enol. Vitic. 2003;54:211–213. [Google Scholar]
- 182.Baptista A.S., Horii J., Calori-Domingues M.A., Micotti da Glória E., Salgado J.M., Vizioli M.R. The capacity of manno-oligosaccharides, thermolysed yeast and active yeast to attenuate aflatoxicosis. World J. Microb. Biot. 2004;20:475–481. doi: 10.1023/B:WIBI.0000040397.48873.3b. [DOI] [Google Scholar]
- 183.Guilloux-Benatier M., Guerreau J., Feuillat M. Influence of initial colloid content on yeast macromolecule production and on the metabolism of wine microorganisms. Am. J. Enol. Vitic. 1995;46:486–492. [Google Scholar]
- 184.Moine-Ledoux V., Perrin A., Paladin I., Dubourdieu D. Premiers résultats de stabilisation tartrique des vins par addition de mannoprotéines purifies (Mannostab) J. Int. Sci. Vigne Vin. 1997;31:23–31. [Google Scholar]
- 185.Waters E., Pellerin P., Brillouet J. A Saccharomyces mannoprotein that protects wine from protein haze. Carbohyd. Polym. 1994;23:185–191. doi: 10.1016/0144-8617(94)90101-5. [DOI] [Google Scholar]
- 186.Chalier P., Angot B., Delteil D., Doco T., Gunata Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007;100:22–30. doi: 10.1016/j.foodchem.2005.09.004. [DOI] [Google Scholar]
- 187.Escot S., Feulliat M., Dulau L., Charpentier C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001;7:153–159. doi: 10.1111/j.1755-0238.2001.tb00204.x. [DOI] [Google Scholar]
- 188.Poncet-Legrand C., Doco T., Williams P., Vernhet A. Inhibition of grape seed tannin aggregation by wine mannoproteins: Effect of polysaccharide molecular weight. Am. J. Enol. Vitic. 2007;58:87–91. [Google Scholar]
- 189.Guadalupe Z., Palacios A., Ayestarán B. Maceration enzymes and mannoproteins: A possible strategy to increase colloidal stability and color extraction in red wines. J. Agric. Food Chem. 2007;55:4854–4862. doi: 10.1021/jf063585a. [DOI] [PubMed] [Google Scholar]
- 190.Guadalupe Z., Ayestarán B. Effect of commercial mannoprotein addition on polysaccharide, polyphenolic, and colour composition in red wines. J. Agric. Food Chem. 2008;56:9022–9029. doi: 10.1021/jf801535k. [DOI] [PubMed] [Google Scholar]
- 191.Guadalupe Z., Martínez L., Ayestarán B. Yeast mannoproteins in red winemaking. Effect on polysaccharide, polyphenolic and colour composition. Am. J. Enol. Vitic. 2010;61:191–200. [Google Scholar]
- 192.Rodrigues A., Ricardo-Da-Silva J.M., Lucas C., Laureano O. Effect of commercial mannoproteins on wine colour and tannins stability. Food Chem. 2012;131:907–914. doi: 10.1016/j.foodchem.2011.09.075. [DOI] [Google Scholar]
- 193.Brouillard R., Dangles O. Anthocyanin molecular interactions: The first step in the formation of new pigments during wine aging? Food Chem. 1994;51:265–371. [Google Scholar]