Abstract
Objective:
Determine the effects of arthritis on the trans-synovial clearance of small and large model compounds following local delivery to the knee joint in a rat model.
Design
Intra-articular delivery was studied in rat knee joints in an osteoarthritis model of knee joint instability (medial collateral ligament and meniscus transection model or MMT). Fluorescently-labeled 10 kDa or 500 kDa dextran was injected in the arthritic or unoperated control (naive) joints 3 weeks after surgical destabilization, and the temporal clearance pattern was evaluated via in vivo regional fluorescence imaging, dextran concentrations in plasma and draining lymph nodes, and by quantification of fluorescence in histological synovium sections. Together these data were used to evaluate the effect of osteoarthritis and solute size on the rate of drug clearance from the joint.
Results:
Clearance of 10 kDa dextran from the joint space quantified using in vivo fluorescence imaging of the knee joint region was not significantly different between naive and MMT joints. In contrast, clearance of 500 kDa dextran was significantly reduced for MMT joints when compared to naive joints by fluorescence in vivo imaging. Drug accumulation in lymph nodes and plasma were lower for the 500 kDa dextran as compared to 10 kDa dextran, and lymph node levels were further reduced with the presence of osteoarthritis. Furthermore, synovium was significantly thicker in MMT joints than in naive joints and image analysis of joint tissue sections revealed different trans-synovial distributions of 10 and 500 kDa dextran.
Conclusion:
Large macromolecules were retained in the arthritic joint longer than in the healthy joint, while smaller molecules are cleared similarly in healthy and arthritic joints. In vivo fluorescence imaging, plasma and lymph node concentrations, and spatial distributions of drug fluorescence identified differences in higher molecular weight clearance between naive and arthritic disease states. Findings may relate to a thickening of synovium for joints with induced arthritis, and support the concept that intra-articular drug delivery effectiveness may vary with the state of joint pathology.
Keywords: dextran, MMT, in vivo imaging, lymph node, osteoarthritis, rodent model, surgical model, knee joint
Graphical Abstract
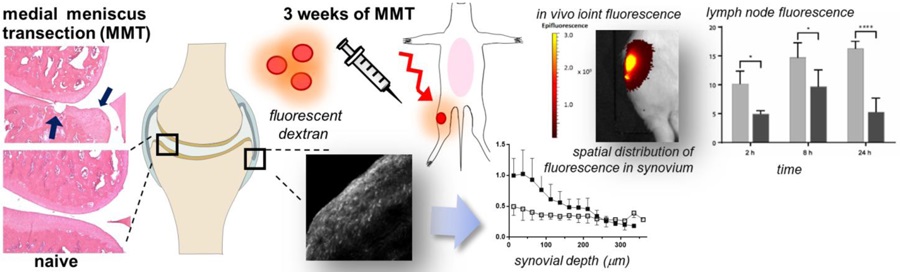
INTRODUCTION
Osteoarthritis (OA) is a degenerative disease of diarthrodial joints projected to impact nearly 67 million Americans or 25% of the US population [1]. The disease is characterized by progressive deterioration of the cartilage lining, subchondral bone destruction and thickening of the joint capsule [2, 3]. These tissue changes lead to symptomatic joint pain and dysfunction, loss of range of motion, and restrictions on daily activities [4]. The most widely-prescribed pharmacological treatment for OA is the oral administration of non-steroidal anti-inflammatory drugs (NSAIDs); intra-articular (IA) injections of corticosteroids or hyaluronic acid are commonly used when NSAIDs are no longer effective [5]. While corticosteroids and many disease-modifying drugs have benefits when administered directly to the affected joint, these benefits are limited by a rapid clearance of lower molecular weight (MW) drugs through the vasculature and lymphatics of the joint synovium [2, 6, 7].
In order to increase the residence time of small-molecule drugs that may have efficacy in treating arthritis, a broad set of drug delivery strategies have been developed [8]. These strategies include encapsulation of the drug in natural materials, such as chitosan, alginate, albumin protein, and elastin-like polypeptides, as well as synthetic materials such as polymers and lipids [9–17]. The drug carrier’s capacity to increase drug residence in the joint is dependent on its ability to reduce the rate of drug release and transport across the synovium. Additionally, micron- and nano-sized carriers may be distributed throughout the joint by different mechanisms, and subsequently cleared through different strategies [18]. Some studies have shown that arthritis pathology can affect the transport of solutes from the joint, by modifying the intra-articular pressure, synovial permeability and thickness, and lymphatic vessel function and distribution [19–21].
Historically, the trans-synovial clearance of solutes from the joint was first studied using radiolabeled drugs and sacrificial tracking. These radioisotopes provided a high signal-to-noise ratio such that clearance kinetics, derived from the diminishing isotope detected in the joint cavity, could be readily determined. Comparisons between naive and diseased joints were made by associating functional measures, like clearance kinetics, to markers of disease state (biomarkers, degree of joint effusion, pain, etc.) [6, 22–24]. More recently, far-red fluorescent dyes in conjunction with in vivo imaging systems have been used to study joint clearance of labeled compounds in living organisms [25, 26, 27]. With such an approach, our laboratory previously utilized Cy7 fluorophore-labeled silk particles and in vivo imaging to demonstrate increased residence time for micron-sized particles in the knee joints of naive rats [26]. With many particle-forming depots as in this prior study, polydispersity in the particle formation and lack of spatial resolution afforded with in vivo fluorescence imaging limits an ability to reveal mechanisms that govern transport of molecules and particles of varying size and physicochemical characteristics. Alternatively, precisely controlled and labeled dextrans offer various advantages over microparticles and have been used previously to study lymphatic transport mechanisms in non-musculoskeletal systems [28].
In this study, we employed fluorescently-labeled dextrans of two molecular weights to understand how arthritis affects trans-synovial solute transport following IA delivery. Osteoarthritis (OA) was induced in a rat model of surgically-induced knee joint instability (transection of the medial meniscus and medial collateral ligament, or MMT [29,30]). After development of OA, knee joints received an injection of fluorescently labeled dextran and longitudinal fluorescence imaging of the knee joint was performed in combination with measures of plasma and lymph node concentrations of injected dextrans. Lastly, a novel method to quantify trans-synovial flow with greater spatial resolution was developed utilizing image analysis of joint synovium in order to track solute transport through the joint tissues. Results illustrate that larger molecules are retained in the arthritic joint longer than in the healthy joint, with a pattern for clearance to lymphatics and plasma that differ from that of lower molecular weight solutions. Measures of trans-synovial transport of macromolecules by fluorescence microscopy revealed differences in the spatial distribution of small and large molecular weight solutes, and differences with disease, that are consistent with physiological restrictions to transport in the diseased synovium. The mechanism for longer retention times in the arthritic joint were found to arise, in part, from increased synovial thickness and a clear localization of the fluorescence at the intimal surface.
MATERIALS AND METHODS
Reagents and materials
Two tracer molecules of widely separated molecular weights (10 kDa, 500 kDa) were used here to test if differences in spatial localization and systemic clearance of these sized molecules could be quantified with fluorescence detection as described here. Dextran-Texas Red (10,000 Da), dextran-fluorescein (500,000 Da), and Slide-A-Lyzer™ dialysis cassettes (3500 MWCO) were purchased from Life Technologies (Watham, MA). Both dextran-dye conjugates are expected to be of higher MW than reported due to the conjugated dye, by an amount that does not exceed 12% of the reported MW. CF™633 succinimidyl ester (CF633), sodium bicarbonate, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). Plasma collection tubes (EDTA-coated Vacutainer™, 4 mL) were ordered from Becton Dickinson (Franklin Lakes, NJ). Cryovials (2 mL) were purchased from Corning (Corning, NY) for storage of physiological fluids.
Conjugation of CF633 fluorophore to 500 kDa dextran
Dextran-fluorescein (500 kDa) was labeled with CF633 to allow for imaging in the near infrared range. Prior to conjugation a 10 mM dextran-fluorescein stock solution was prepared in a 0.1M sodium bicarbonate solution and a 10 mM dye stock solution of CF633 succinimidyl ester was prepared in DMSO. CF633 was then added to the dextran solution at a 50:1 molar ratio, and the resulting mixture was stirred (1h at room temperature) and dialyzed (4 L of distilled water for 2h) to remove unreacted CF633 (MWCO 3500 Da). The dialysate was then dried (24 h, Freezone 2.5L, Labconco; Kansas City, MO), resuspended in 0.5 mL sterile PBS, and a calibration curve of fluorescence at 630/650 nm (ex/em) against dextran concentration (w/v) was determined (Enspire® Multimode Plate Reader, Perkin Elmer; Waltham, MA). In our prior work, we similarly coupled Cy7 to primary amines of silk proteins and observed the conjugation to be very stable with less than 10% of coupled Cy7 released over 7 days and only in a protease buffer [26].
Surgical model of osteoarthritis (OA)
Sprague-Dawley male rats (n=48, 200–225g; Charles River Laboratories, Wilmington, MA) were acquired at Duke University following approval by the institutional IACUC committee. Rats were split into groups of (1) unoperated controls (naive) and (2) those receiving surgery to model OA. All animals in the OA group underwent surgery for medial meniscus transection (MMT) of the right knee as described previously [30]. With this procedure, surgical instability of the knee is induced by transection of the medial collateral ligament (MCL) and radial transection of the middle third of the meniscus. Rats in the OA group were allowed free cage activity for a period of 3 weeks following surgery. Naive and MMT animals then received an IA injection of labeled dextran for longitudinal in vivo fluorescence imaging, and sacrificed at 2, 8, or 24 h after IA injection as illustrated in Figure 1 (n=4 per group at each time point for both 10 kDa and 500 kDa).
Figure 1:
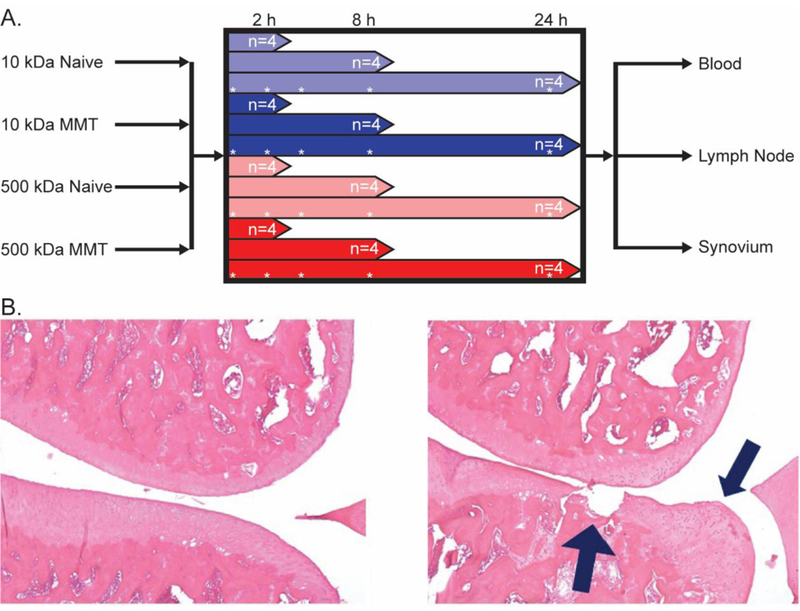
A. Study design, animals were grouped by sacrifice point post-injection. At time of sacrifice, plasma as well as synovial and lymph tissue was collected. Asterisks represent in vivo imaging timepoints B. Histology of sham (Left) and MMT-operated (Right) rats 21 days post-surgery. Frontal sections of the medial aspect of rat knee joints were stained with Toluidine Blue. Sham exhibits normal cartilage, subchondral bone and synovium. MMT surgery. Prominent chondrophyte/osteophyte present adjacent to severe cartilage erosion. Chondrocyte loss is complete to the deep zone and significant proteoglycan loss can be detected all the way to the tidemark.
In vivo imaging of intra-articularly injected macromolecule
Naive and MMT animals were grouped into cohorts based on sacrifice point: 2, 8, or 24 h after IA injection. Under 1–3% isoflurane anesthesia, animals received a 30 µL IA injection of a sterile solution containing 10 kDa dextran-Texas Red (1 mM, or 30 nmoles) or 500 kDa dextran-CF633 (25 μM or 0.75 nmoles). Immediately following injection, animals were imaged longitudinally at 0+ (~ 10 min), 1, 2, 4, 8, and 24 h (or until sacrifice) using the IVIS Kinetic (Perkin Elmer; Waltham, MA); animals receiving 10 kDa dextran-Texas Red were imaged at 605/700 nm (ex/em) and those injected with 500 kDa dextran-CF633 were imaged at 640/700 nm (ex/em). Region-of-interest (ROI) analysis was utilized to measure temporal fluorescence emission from the rat knee joints as described previously [26]. In brief, total joint fluorescence at t = 1h (TF0) was obtained for each animal to allow for distribution of the injected dextran in the space. Total fluorescence (TF) in the joint ROI at each timepoint was then normalized by this baseline value to obtain a normalized fluorescence in the ROI (NFK = TF/ TF0). Values for NFK over time were numerically fit to a mono-exponential decay equation (NFK (t) = N0*e−kt where N0 is the estimated value at time zero; k = decay rate constant; t = time in hours). The decay rate constant from each fit was used to determine a decay half-life (t1/2) for each MW dextran. After imaging at terminal time points, animals were sacrificed; blood and tissue from the lymph nodes and synovium were collected as described below.
Ex vivo analysis of plasma and lymph tissue
At each terminal time point (2, 8, or 24 h), 4 mL of blood was collected in EDTA-coated Vacutainer™ tubes, allowed to settle for 15 min, and then centrifuged (3500 RCF, 4°C, 10 min, Model GS15R, Beckman) to collect plasma. Plasma was transferred to 2 mL cryovials for storage at −80°C. To analyze dextran concentrations, 100 µL of plasma was transferred from each cryovial to 500 µL microcentrifuge tubes and multiple tubes were placed within the test chamber of the IVIS Kinetic for fluorescence readings at 605/700 nm (ex/em, Texas Red) or 640/700 nm (ex/em, CF633). Standard curves were generated from cryovials containing 0–0.5 µM dextran in 100 µL plasma collected from separate animals to allow for the calculation of dextran concentrations in plasma (CP).
At the time of sacrifice, the ipsilateral lumbar lymph node was harvested via anterior dissection, from just beneath fatty tissue posterior to the peritoneal cavity. Collected lymph node and tissue was placed in a separate microcentrifuge tube and filled with 250 µL of PBS. Tissue suspensions were homogenized (Tissue-Tearor 398, Biospec; Bartlesville, OK) and centrifuged (13,000 RCF, 4°C, 10 min, Model GS15R, Beckman). Fluorescence intensity in the resulting supernatant (triplicates of 60 µL) was quantified using an Enspire Multimode plate reader. Standard curves were generated from lumbar lymph nodes harvested from separate animals that were suspended in 200 µL of PBS with 0–100 nM CF633 or 0–10 µM dextran-Texas Red, homogenized, and centrifuged. These standard curves were then used to calculate dextran concentration in the harvested lymph nodes (CL).
Processing and imaging joint synovium cross sections
Post-sacrifice, ipsilateral knee joints were harvested to procure synovium and confluent tissues for image analysis of fluorescence distribution in tissue sections. The quadriceps and patellar tendon were retracted and a scalpel used to cut closely along the medial and lateral surfaces of the femoral head; synovial tissue was identified as the highly vascularized, fibrous tissue flanking either side of the patella, patella tendon, and fat pad. Tissue was embedded in OCT medium and snap frozen in isopentane suspended in liquid nitrogen. Cryosections were taken (14 µm, Leica CM 1850 Cryostat, Leica Microsystems Inc., Buffalo Grove, IL) transverse to the patellar ligament to obtain cross-sections of synovium from both medial and lateral regions of the joint. Unstained tissue sections were imaged (LSM 510, Carl Zeiss AG, Oberkochen, Germany) using a 543 or 633 nm excitation for detection of 10 kDa Texas Red-dextran or 500 kDa CF633-dextran, respectively. Given variability amongst tissue sections and between CF633 and Texas-Red fluorophores, different detector gain settings were used to visualize fluorescence across the full spatial field. Images were processed with custom-written algorithms in ImageJ (NIH, Bethesda, MD) and MATLAB (MathWorks Inc., Natick, MA) as described below to locate the synovial boundary and calculate spatial distribution of fluorescence intensity throughout the tissue.
Image analysis of dextran penetration in synovium
Digital fluorescence images of tissue sections (12-bit, 2048 × 2048) were converted to 16-bit, greyscale images, and pixel intensities were normalized to detector gain levels using a linear polynomial fit for each fluorophore. The free surface of the synovium was identified via manual trace (segmented line tool, ImageJ), the manually-identified trace was fit to a cubic spline to obtain x-y coordinate data for each surface point, and the average distance to the onset of muscle autofluorescence (488/520 nm (ex/em), Figure 2) was used to define a synovium thickness. Projections were generated in the x-direction from each surface point to the synovium-muscle interface, and a normalized intensity was calculated at each pixel within a single projection was recorded. Data for fluorescence intensity within each projection were binned into 25 µm intervals, corresponding to intervals of successively increasing depths of 25 µm from the synovial surface. Bins were numbered in ascending order away from the synovial surface, and bins of the same number were grouped for all projections. In this manner, an averaged profile for normalized fluorescence intensity (NFs) was obtained as a function of distance from the synovial surface for each section studied (Figure 2B). Profiles were used to test for NFs decay from the synovial surface as a function of dextran MW, to compare naive versus MMT, and to compare across different times (see Statistical Analysis).
Figure 2:
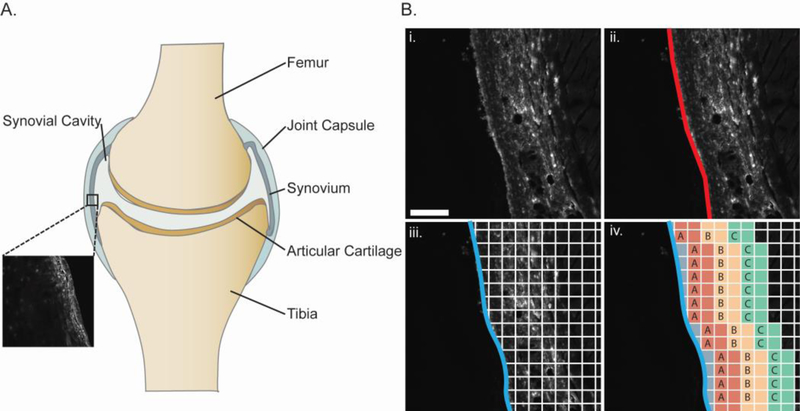
A. Representative schematic describing basic anatomy of a diarthrodial joint; the inset image depicts the synovial tissue that was analyzed in the adjacent panel B. (i) Representative image of synovial tissue section after scaling to 16-bit greyscale (scale bar = 100 μm) showing method used to calculate trans-synovial transport of fluorescently-labeled dextrans. (ii) Synovial boundary manually marked (red) was fit to a cubic spline (iii) and tissue depths were gridded and pixel intensity values obtained at grid points along each horizontal profile. (iv) Profiles were split into discrete bins by depth and assigned to grouped bins.
Statistical analysis.
IVIS imaging.
An unpaired t-test was used to detect evidence of a difference in the half-life of fluorescence decay (t1/2) between naive and MMT conditions within each dextran molecular weight. NFK values were evaluated with a two-way analysis of variance (ANOVA) and Bonferroni’s post-hoc test to detect differences in NFK between naive and MMT groups, and separately for each dextran MW (GraphPad Prism, La Jolla CA). Plasma and lymph tissue fluorescence detection. Comparisons of CP were performed by two-way ANOVA with Bonferroni’s post-hoc test to test for differences between naive and MMT groups, and separately for each molecular weight (GraphPad Prism). Comparisons of CL were performed by two-way ANOVA to detect differences between naive and MMT conditions for each injected dextran (GraphPad Prism). Spatial measures of trans-synovial fluorescence detection in synovium. Values for NFS projections were numerically fit to a linear or first-order exponential model (Matlab, Mathworks, Inc., Natick, MA) and parameters describing the fit were reported for naive and MMT synovium at both 2h and 24h. Comparisons of the numerical fit between 2h and 24h were performed by ANCOVA (JMP 10 software, JMP, Cary, NC) for each injected dextran and within MMT and naïve joints. An unpaired t-test was used to detect differences in thickness between naive and MMT synovium pooled across timepoints and MW treatment (GraphPad Prism).
RESULTS
Intra-articular clearance of labelled-dextrans in naive and OA joints.
In vivo imaging of knee joints following IA injection of fluorescently-labeled dextrans confirmed an ability to detect fluorescence in the joint ROI that decreased with time (Figure 3A). The ROI normalized fluorescence, NFK, was higher in MMT joints when compared to naive joints at 2 h after delivery of the 10 kDa dextran (p<0.05, ANOVA). Similarly, NFK was elevated in MMT joints when compared to naive joints after receiving an IA injection of the 500 kDa dextran at both 8 h (p<0.05) and 24 h (p<0.01) post injection. The t1/2 of NFK for the 10 kDa dextran of 3.26 h (R2 = 0.92) in naive joints was not found to differ statistically from values of 4.62 h (R2 = 0.86) in MMT joints; there was evidence of a significant difference in t1/2 between naive and MMT joints, however, for joints receiving the 500 kDa dextran with values for t1/2 of NFK of 17.3 h (R2 = 0.67) and 40.1 h (R2 = 0.6) respectively (p<0.05, t-test).
Figure 3:
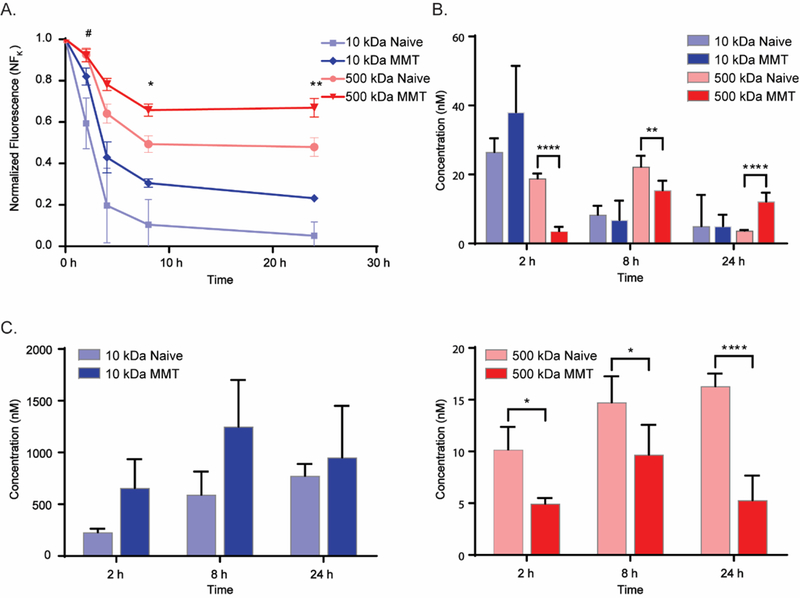
A. Knee total normalized fluorescence (NFK) following intra-articular injection of 10 kDa (1 mM) or 500 kDa (25 μM) labeled dextrans in naive and MMT joints. There was evidence of elevated NFK in MMT joints receiving 10 kDa dextran, when compared to naive joints at 2 h after IA injection (# = p<0.05, 2-way ANOVA, 2 h timepoint). There was also evidence of elevated NFK in MMT joints receiving an IA injection of 500 kDa dextran, compared to naive joints, at both 8 and 24 h post injection (* = p<0.05, ** = p<0.01, 2-factor ANOVA). B. Plasma dextran concentrations (CP ) at terminal time points. There was evidence of differences in CP between naive and MMT animals receiving the 500 kDa dextran at 2, 8, and 24 h. C. Lymph dextran concentrations (CL) of labeled 10 kDa (Left) and 500 kDa (Right) dextrans at terminal time points. There was evidence of lowered CL in 500 kDa dextran injected MMT animals as compared to naive at 2, 8, and 24 h post injection (*=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001, ANOVA; Bonferonni’s post-hoc test). Data are plotted as mean ± SD. In fluids from plasma and lymph nodes, results were not normalized for the injected dose so differences between the two solute sizes were not tested.
Ex vivo analysis of fluorescence in plasma
Plasma fluorescence values (CP ) values for 10 kDa-injected animals appear to peak at 2 h and decline at 8 and 24 h after injection, with no evidence of significant differences between naive and MMT groups. In contrast, values for Cp in animals receiving the 500 kDa dextran injection were significantly different between MMT and naive animals at 2 h (p<0.0001), 8 h (p<0.01), and 24 h (p<0.0001) after injection (Figure 3B). Of note, Cp was lower in MMT animals as compared to naive at both 2 and 8 h post-injection, and higher at the 24 h timepoint in the MMT animals.
Ex vivo analysis of fluorescence in lymph nodes
Lymph node fluorescence values were used as an indicator of dextran transport from IA injection through lymphatic vessels in both naive and OA joints. For joints injected with 10 kDa dextran, there was a trend towards higher concentrations of dextrans (CL) in ipsilateral lumbar lymph nodes of MMT joints than naive joints at multiple timepoints (Figure 3C); nevertheless, these trends were not found to contribute to significant differences between MMT and naive joints at p<0.05. Conversely, CL for ipsilateral lymph nodes of animals injected with 500 kDa dextran were reduced in MMT animals as compared to naive animals at 2 h (p<0.05), 8 h (p<0.05) and 24 h (p<0.0001).
Fluorescence imaging analysis of trans-synovial diffusion
Fluorescence microscopy of thin sections of the infrapatellar synovium revealed that MMT animals had significantly thicker synovium compared to naive (350 ± 120 µm vs. 182 ± 51 µm respectively, p<0.0001). The normalized fluorescence intensity (NFs) was most often maximum at the intimal surface of the synovial lining (Figure 4A). The fluorescence profile for 10 kDa dextran was found to distribute spatially through the synovial thickness with a nearly linear and monotonic decay (Figure 4B; R2 = 0.97 and 0.96, NFs fit to linear model for healthy and MMT synovium, respectively). For the 10 kDa injectate, there was no evidence of a statistical difference in the fluorescence profile across the synovium between 2h or 24 h post-injection for both MMT and healthy joints. In contrast, the fluorescence profile for 500 kDa dextran resembles an exponentially decaying function with increasing synovial depth, in both naive and MMT joints. It was thus necessary to use a first-order exponential function to numerically match the NFs profiles for the 500 kDa dextran (R2 = 0.85 and 0.97 for fit to exponential curve for healthy and MMT synovium, respectively). For MMT joints, the exponential dependence of 500 kDa dextran on depth is even more pronounced, with values for NFs at 2 h that are approximately half that in healthy synovium (Figure 4C). For the 500 kDa injectate, there was no evidence of a statistical difference in the fluorescence profile across the synovium in MMT joints between 2h and 24 post-injection; however, the fluorescence profile changed significantly from 2h to 24h for the same molecule in the naive joint (p<0.0001, ANCOVA), indicating that the MMT synovium did contribute to delayed transport of the higher molecular weight dextran.
Figure 4:
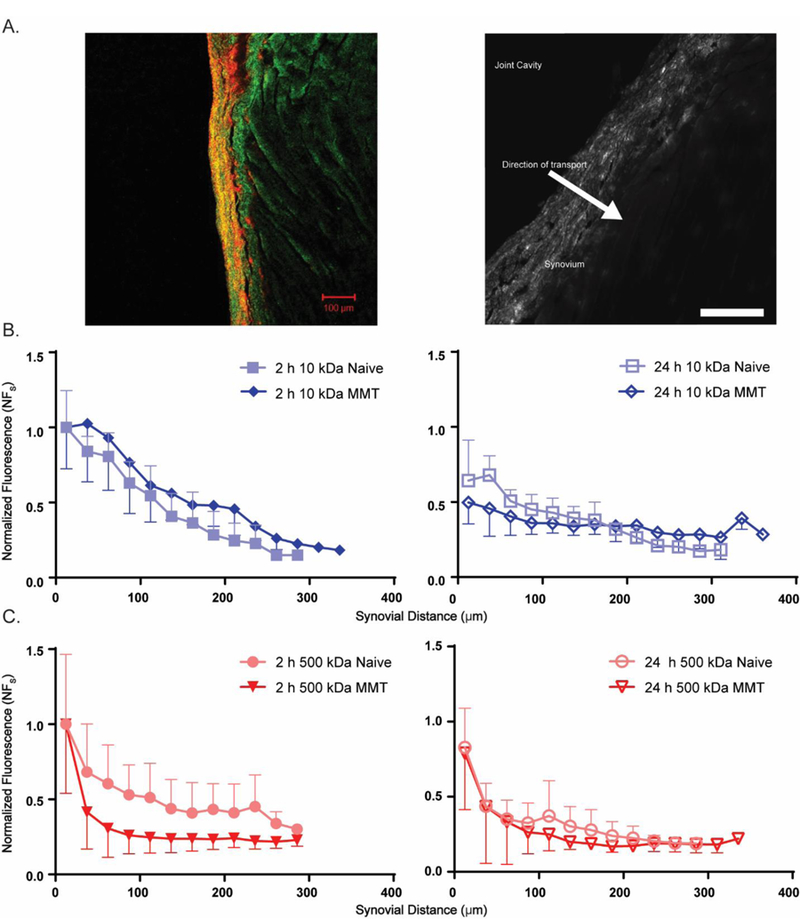
A. Fluorescence micrograph (Left) of rat synovium with 543 nm excitation (red, Texas red) and 488 nm excitation of muscle autofluorescence (green). Greyscale image (Right) showing trans-synovial transport of fluorescently-labeled dextrans. Bright fluorescence was typically observed at the synovial intima (most superficial part of the synovium) with decreasing fluorescence in the direction of transport towards the sub-synovium (scale bar = 100 μm). B. NFs values for 10 kDa dextran transport across naive and MMT joint synovium following intra-articular injection. Decreasing NFs is observed with distance from the synovial intima. C. NFs values for 500 kDa dextran transport across joint synovium as described above. An exponential profile is observed with decreasing NFs with distance from the synovium surface. The fluorescence profile for the 500 kDa dextran measured at 2h post-injection differed from that at 24h post-injection, for the naïve joints only (ANCOVA, p< 0.0001, not shown). The fluorescence profile did not differ with time for 10 kDa dextrans in any joint, nor for 500 kDa dextrans in the MMT joints. Data represent mean ± SD.
DISCUSSION
In vivo imaging of labeled dextrans after intra-articular injection
In vivo imaging of labeled dextrans following IA injection demonstrated a more rapid fluorescence decay in the joint space for 10 kDa molecules, as compared to the 500 kDa molecules. This finding, in naive joints, was associated with t1/2 of 3.26 h and 17.3 h for 10 kDa and 500 kDa dextrans respectively, corroborating many prior studies showing short joint residence times for small molecular weight compounds measured using alternate methods [31, 32, 33]. Solute transport through synovium is a complex process that will depend on molecular weight, steric hindrance, charge, carrier molecules (e.g., albumin) and more. Dextrans are strongly hydrophilic molecules which ensure that their clearance from the intra-articular space is primarily dependent on diffusion and convection in the mobile phase of the synovial fluid, rather than restricted by solubility due to hydrophobic interactions. Furthermore, in vivo measures of fluorescence within a region-of-interest (ROI), as performed here, cannot distinguish amongst tissues of the synovium, adjacent muscle and fat, and intra-articular structures. For these reasons, direct measures of t1/2 cannot be made for comparison against prior studies for molecular transport; nevertheless, the approximately 5-fold differences in t1/2 between 10 kDa and 500 kDa molecules provides some support for the utility of dextran transport and fluorescence in vivo imaging for studying intra-articular clearance.
When comparing fluorescence recordings between naive and MMT joints, there was evidence that dextran clearance from the joint was reduced in pathological joints. This observation was supported by higher values for the half-life of fluorescence decay for 500 kDa dextran in MMT as compared to naive joints, and by higher values of regional fluorescence in the MMT joints at most timepoints for both 10 and 500 kDa solutes. These observations suggest that pathological changes to synovium in the MMT model may impact transport in a size-dependent manner where larger molecular weight solutes encounter greater resistance to joint clearance.
Comparisons between naive and MMT groups in measures of plasma and lumbar lymph node solute concentrations
Plasma dextran concentrations correlated well with findings from in vivo fluorescence imaging, as differences between naive and MMT were shown to be size-dependent. No differences were detected in plasma concentrations (CP) between healthy and MMT animals injected with 10 kDa dextran. In contrast, CP of 500 kDa dextran was reduced in MMT animals at 2 h and 8 h, consistent with the observation of longer durations of regional fluorescence in the knee joints for MMT animals. Similarly, there were no significant differences in lumbar lymph node concentrations of 10 kDa dextran between naive and MMT animals yet there were significantly decreased concentrations of 500 kDa dextran in the lumbar lymph nodes of MMT animals at 2 h, 8 h, and 24 h compared to naive. Combined, these data suggest that the MMT joint pathology caused delayed systemic uptake of labeled 500 kDa dextran. Pathologic changes such as the thicker synovium for MMT joints, as observed here, could contribute to the reduced transport of 500 kDa dextran to the lymphatics and size-selective capillaries. Additionally, changes reported for arthritic pathology in the MMT model such as increased intra-articular pressure (IAP) could contribute to the observed differences in plasma dextran levels; however, work by Levick and co-workers showed that increased IAP (~15–25 cm H2O) will have a limited effect on trans-synovial flow of macromolecules as large as hyaluronan (~ 900 kDa) [34]. However, this same group showed that charge and hydrated diameter of the macromolecule injected could affect trans-synovial exchange, so it may be premature to conclude that a pathological IAP does not have an effect on the results reported here for transport of dextran [35].
An alternative explanation for the decreased CP and CL of 500 kDa dextran is reduced lymphatic vessel function due to joint inflammation. As a result, lower concentrations of 500 kDa dextran would be observed in the lymph nodes and hence peripheral circulation in MMT animals over time. This theory is supported by the work of Zhou and colleagues who studied lymphatic function of the knee joint in a mouse model of chronic inflammatory arthritis [20]. They determined that mice with inflammatory joint disease, following breeding with the human tumor necrosis factor (TNF) transgene (TNF-Tg), had slower lymphatic clearance of indocyanine green (775 Da) which could be restored by the delivery of vascular endothelial growth factor C (VEGF-C). VEGF-C is a potent lymphangiogenic stimulator that acted to increase joint clearance and lymphatic vessel area fraction [36, 37]. Similarly, Shi and coworkers found reduced lymphatic transport of intra-articularly injected indocyanine green into the downstream iliac lymph node of mice in a surgically-induced OA model [19]. Indocyanine green is much smaller than the 10 kDa dextran studied in our work and we find no evidence of statistically significant differences in CL between MMT joints and naive animals. There is a possibility that varying protein composition between diseased and naïve joints could contribute to the prior observations of decreased lymph transport, as indocyanine green could interact with proteins that would affect its measurement and transport from the joint space. This was not directly studied here, however, so we are unable to say how lymph flow independently contributed to our observations of no difference in 10 kDa transport in the MMT joints. Furthermore, differences in synovitis with an inflammatory arthritis model such as the TNF-Tg model, and our surgically-induced MMT model may serve to explain the different findings reported here. As specific pathology in synovium was not characterized in the MMT model, other than synovial thickness, we are unable to compare effects of IAP, capillary density, synovial permeability or other pathological changes amongst these varying models. Future studies should evaluate each of these parameters to elucidate their impact on transport in the arthritic joint.
Microscopic imaging of synovium sections
Microscopic imaging of synovial tissue at sacrifice confirmed evidence of synovium thickening in the MMT knee joints, consistent with a synovial inflammation reported previously for the MMT rat model of OA at 3 weeks post-surgery [38]. The finding for a linear, spatially-varying concentration for the fluorescently labeled 10 kDa dextran in synovium at 2 and 24 h supports the findings of first order clearance kinetics from the knee joint. While no differences were tested between the profiles for naive and MMT synovium for 10 kDa dextran, there were visually consistent observations in decay profiles for naive and MMT synovium by 24 h after fluorophore delivery. This observation is consistent with all complementary measures for transport that demonstrated no disease-dependence for the clearance of the smaller molecular weight solute.
Conversely, the fluorescence profile in the synovium of 500 kDa dextran-injected joints more closely fit an exponential, suggesting that the available interstices and synovial capillaries are significant barriers to transport of large molecules across the synovium. There was some evidence for differences in the spatial distribution of 500 kDa dextran between 2 and 24 h in the naive joints that was not observed in the MMT synovium. Again, this is one more observation that supports the hypothesis that arthritic synovium is less permeable to larger macromolecules.
The approach taken here to study the transport of macromolecules across the synovium of healthy and arthritic rats was adapted from research methods used in the study of drug penetration for topical delivery [39–42]. Much like the skin, the synovial membrane has regions of morphological diversity which can make quantitative analysis of transport phenomena difficult. While identification of a uniquely consistent region of the distal synovium is useful for quantitative comparisons, it may over- or underestimate the transport phenomena occurring more proximal to the patella, a region richer in capillaries and blood vessels. Additionally, transverse joint sections were only taken through the inferior region of the patella tissue. Future studies should evaluate trans-synovial transport within other regions of the synovium, such as that flanking the fat pad, as well as supra-patellar synovial tissues. Differences in macromolecule penetration in these regions may provide insight into the varying transport patterns of the different surfaces of the joint cavity.
MMT model of osteoarthritis in the rat knee joint
While there are many transgenic, surgical, and chemical models of OA in the rat knee joint, we chose to employ the MMT model because of the relatively quick onset of pathological changes. Previously, MMT surgery has been shown to quickly induce changes in the joint; cartilage fibrillation, loss of proteoglycan content, and synovial inflammation are all evident within 3 weeks [43, 44]. Very modest histological changes have been reported for sham-operated rats in prior studies, in rats where the MCL is resected without damage to the meniscus [30]; for example, cartilage lesions of sham-operated animals presented with grades of 0–1 in comparison to grades of 4–5 for MMT-operated animals. Our study of naïve but not sham-operated rats excludes any possible changes in synovium transport due to surgery alone, but can be included in future work to more fully detect the source of changes that were reported here. Further, our studies and others have shown that more severe joint pathologies emerge in the MMT model by week 5 so that our study of animals at post-surgical recovery times of 3 weeks limits our findings to those for only mild OA.
CONCLUSION
Macromolecular clearance from the knee joint is affected by arthritis pathology in a size-dependent manner. Large macromolecules are retained in the arthritic joint longer than in the healthy joint, while smaller molecules are cleared similarly in healthy and arthritic joints. In vivo fluorescence imaging of regional joint concentrations, together with plasma and downstream lymph node concentration data, were used to support these findings. Lastly, this study presents a novel method of studying trans-synovial transport of macromolecules in histological sections that revealed spatial distributions across the synovium that varied between small and large molecular weight solutes, and that differed between naive and arthritic disease states. These differences are consistent with physiological restrictions to transport for larger molecular weight solutions and for diseased synovium. The mechanism for longer retention times in the arthritic joint as observed here arise, in part, from increased synovial thickness and a clear localization of the fluorescence at the intimal surface.
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of Stephen Johnson to veterinary work on this project, and to Dr. Laura Tanenbaum for proof-reading the manuscript.
FUNDING
This work was supported by the National Institutes of Health [R01-AR070975, R01-AR069588, F31-AR063610] and the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no conflicts of interest.
REFERENCESs
- 1.Helmick CG and Watkins-Castillo S, Arthritis, in The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost 2014, United States Bone and Joint Initiative: Rosemont, IL. [Google Scholar]
- 2.Gerwin N, Hops C, and Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev, 2006. 58(2):226–242. [DOI] [PubMed] [Google Scholar]
- 3.Poole A, Howell DS, Etiopathogenesis of Osteoarthritis, in Osteoarthritis. Diagnosis and Medical/Surgical Management 2001, W.B. Saunders Company: Philadelphia. [Google Scholar]
- 4.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, and March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis, 2014. 73(7):1323–1330. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, and Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage, 2008. 16(2):137–162. [DOI] [PubMed] [Google Scholar]
- 6.Wallis WJ, Simkin PA, Nelp WB, and Foster DM. Intraarticular volume and clearance in human synovial effusions. Arthritis Rheum, 1985. 28(4):441–449. [DOI] [PubMed] [Google Scholar]
- 7.Evans CH, Kraus VB, and Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol, 2014. 10(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang ML and Im GI. Drug delivery systems for intra-articular treatment of osteoarthritis. Expert Opin Drug Deliv, 2014. 11(2):269–282. [DOI] [PubMed] [Google Scholar]
- 9.Thakkar H, Sharma RK, Mishra AK, Chuttani K, and Murthy RS. Celecoxib incorporated chitosan microspheres: in vitro and in vivo evaluation. J Drug Target, 2004. 12(9–10):549–557. [DOI] [PubMed] [Google Scholar]
- 10.Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, and Setton LA. A thermally responsive biopolymer for intra-articular drug delivery. J Control Release, 2006. 115(2):175–182. [DOI] [PubMed] [Google Scholar]
- 11.Burgess DJ, Davis SS, and Tomlinson E. Potential use of albumin microspheres as a drug delivery system. I. Preparation and in vitro release of steroids. Int J Pharmaceutics, 1987. 39(1–2):129–136. [Google Scholar]
- 12.Inoue A, Takahashi KA, Arai Y, Tonomura H, Sakao K, Saito M, Fujioka M, Fujiwara H, Tabata Y, and Kubo T. The therapeutic effects of basic fibroblast growth factor contained in gelatin hydrogel microspheres on experimental osteoarthritis in the rabbit knee. Arthritis Rheum, 2006. 54(1):264–270. [DOI] [PubMed] [Google Scholar]
- 13.Tuncay M, Calis S, Kas HS, Ercan MT, Peksoy I, and Hincal AA. Diclofenac sodium incorporated PLGA (50:50) microspheres: formulation considerations and in vitro/in vivo evaluation. Int J Pharm, 2000. 195(1–2):179–188. [DOI] [PubMed] [Google Scholar]
- 14.Natarajan V, Krithica N, Madhan B, and Sehgal PK. Formulation and evaluation of quercetin polycaprolactone microspheres for the treatment of rheumatoid arthritis. J Pharm Sci, 2011. 100(1):195–205. [DOI] [PubMed] [Google Scholar]
- 15.Larsen SW, Frost AB, Ostergaard J, Thomsen MH, Jacobsen S, Skonberg C, Hansen SH, Jensen HE, and Larsen C. In vitro and in vivo characteristics of celecoxib in situ formed suspensions for intra-articular administration. J Pharm Sci, 2011. 100(10):4330–4337. [DOI] [PubMed] [Google Scholar]
- 16.Panusa A, Selmin F, Rossoni G, Carini M, Cilurzo F, and Aldini G. Methylprednisolone-loaded PLGA microspheres: a new formulation for sustained release via intra-articular administration. A comparison study with methylprednisolone acetate in rats. J Pharm Sci, 2011. 100(11):4580–4586. [DOI] [PubMed] [Google Scholar]
- 17.Bonanomi MH, Velvart M, Stimpel M, Roos KM, Fehr K, and Weder HG. Studies of pharmacokinetics and therapeutic effects of glucocorticoids entrapped in liposomes after intraarticular application in healthy rabbits and in rabbits with antigen-induced arthritis. Rheumatol Int, 1987. 7(5):203–212. [DOI] [PubMed] [Google Scholar]
- 18.Horisawa E, Kubota K, Tuboi I, Sato K, Yamamoto H, Takeuchi H, and Kawashima Y. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm Res, 2002. 19(2):132–139. [DOI] [PubMed] [Google Scholar]
- 19.Shi J, Liang Q, Zuscik M, Shen J, Chen D, Xu H, Wang YJ, Chen Y, Wood RW, Li J, Boyce BF, and Xing L. Distribution and alteration of lymphatic vessels in knee joints of normal and osteoarthritic mice. Arthritis Rheumatol, 2014. 66(3):657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q, Guo R, Wood R, Boyce BF, Liang Q, Wang YJ, Schwarz EM, and Xing L. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis Rheum, 2011. 63(8):2318–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh DA, Verghese P, Cook GJ, McWilliams DF, Mapp PI, Ashraf S, and Wilson D. Lymphatic vessels in osteoarthritic human knees. Osteoarthritis Cartilage, 2012. 20(5):405–412. [DOI] [PubMed] [Google Scholar]
- 22.Jacox RF, Johnson MK, and Koontz R. Transport of radioactive sodium across the synovial membrane of normal human subjects. Proc Soc Exp Bio Med, 1952. 80(4):655–657. [DOI] [PubMed] [Google Scholar]
- 23.Harris R, Millard JB, and Banerjee SK. Radiosodium clearance form the knee joint in rheumatoid arthritis. Ann Rheum Dis, 1958. 17:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonas KN, Fraser JR, and Muirden KD. Distribution of biologically labelled radioactive hyaluronic acid injected into joints. Ann Rheum Dis, 1973. 32(2):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butoescu N, Seemayer CA, Palmer G, Guerne PA, Gabay C, Doelker E, and Jordan O. Magnetically retainable microparticles for drug delivery to the joint: efficacy studies in an antigen-induced arthritis model in mice. Arthritis Res Ther, 2009. 11(3):R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mwangi TK, Bowles RD, Tainter DM, Bell RD, Kaplan DL, and Setton LA. Synthesis and characterization of silk fibroin microparticles for intra-articular drug delivery. Int J Pharm, 2015. 485(1–2):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitmire RE, Wilson DS, Singh A, Levenston ME, Murthy N, and Garcia AJ. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials, 2012. 33(30):7665–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy ST, Berk DA, Jain RK, and Swartz MA. A sensitive in vivo model for quantifying interstitial convective transport of injected macromolecules and nanoparticles. J Appl Physiol (1985), 2006. 101(4):1162–1169. [DOI] [PubMed] [Google Scholar]
- 29.Bove SE, Laemont KD, Brooker RM, Osborn MN, Sanchez BM, Guzman RE, Hook KE, Juneau PL, Connor JR, and Kilgore KS. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthritis Cartilage, 2006. 14(10):1041–1048. [DOI] [PubMed] [Google Scholar]
- 30.Allen KD, Mata BA, Gabr MA, Huebner JL, Adams SB Jr., Kraus VB, Schmitt DO, and Setton LA. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res Ther, 2012. 14(2):R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen C, Ostergaard J, Larsen SW, Jensen H, Jacobsen S, Lindegaard C, and Andersen PH. Intra-articular depot formulation principles: role in the management of postoperative pain and arthritic disorders. J Pharm Sci, 2008. 97(11):4622–4654. [DOI] [PubMed] [Google Scholar]
- 32.Burt HM, Tsallas A, Gilchrist S, and Liang LS. Intra-articular drug delivery systems: Overcoming the shortcomings of joint disease therapy. Expert Opin Drug Deliv, 2009. 6(1):17–26. [DOI] [PubMed] [Google Scholar]
- 33.Butoescu N, Jordan O, and Doelker E. Intra-articular drug delivery systems for the treatment of rheumatic diseases: a review of the factors influencing their performance. Eur J Pharm Biopharm, 2009. 73(2):205–218. [DOI] [PubMed] [Google Scholar]
- 34.Levick JR and McDonald JN. Fluid movement across synovium in healthy joints: role of synovial fluid macromolecules. Ann Rheum Dis, 1995. 54(5):417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott D, Coleman PJ, Mason RM, and Levick JR. Action of polysaccharides of similar average mass but differing molecular volume and charge on fluid drainage through synovial interstitium in rabbit knees. Journal of Physiology, 2000. 528(3):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, and Detmar M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood, 2007. 109(3):1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onimaru M, Yonemitsu Y, Fujii T, Tanii M, Nakano T, Nakagawa K, Kohno R, Hasegawa M, Nishikawa S, and Sueishi K. VEGF-C regulates lymphangiogenesis and capillary stability by regulation of PDGF-B. Am J Physiol Heart Circ Physiol, 2009. 297(5):H1685–1696. [DOI] [PubMed] [Google Scholar]
- 38.Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact, 2001. 1(4):363–376. [PubMed] [Google Scholar]
- 39.Touitou E, Dayan N, Bergelson L, Godin B, and Eliaz M. Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release, 2000. 65(3):403–418. [DOI] [PubMed] [Google Scholar]
- 40.Verma DD, Verma S, Blume G, and Fahr A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: a skin penetration and confocal laser scanning microscopy study. Eur J Pharm Biopharm, 2003. 55(3):271–277. [DOI] [PubMed] [Google Scholar]
- 41.Ryman-Rasmussen JP, Riviere JE, and Monteiro-Riviere NA. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol Sci, 2006. 91(1):159–165. [DOI] [PubMed] [Google Scholar]
- 42.Roberts MS, Dancik Y, Prow TW, Thorling CA, Lin LL, Grice JE, Robertson TA, Konig K, and Becker W. Non-invasive imaging of skin physiology and percutaneous penetration using fluorescence spectral and lifetime imaging with multiphoton and confocal microscopy. Eur J Pharm Biopharm, 2011. 77(3):469–488. [DOI] [PubMed] [Google Scholar]
- 43.Mapp PI, Sagar DR, Ashraf S, Burston JJ, Suri S, Chapman V, and Walsh DA. Differences in structural and pain phenotypes in the sodium monoiodoacetate and meniscal transection models of osteoarthritis. Osteoarthritis Cartilage, 2013. 21(9):1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu DG, Ding HF, Mao YQ, Liu M, Yu B, Zhao X, Wang XQ, Li Y, Liu GW, Nie SB, Liu S, and Zhu ZA. Strontium ranelate reduces cartilage degeneration and subchondral bone remodeling in rat osteoarthritis model. Acta Pharmacol Sin, 2013. 34(3):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]


