Abstract
Purpose
Stress contributes to many psychiatric disorders; however, responsivity to stressors can vary depending on previous or current stress exposure. Relatively innocuous heterotypic (differing in type) stressors can summate to result in exaggerated neuronal and behavioral responses. Here we investigated the ability of prior high dietary sodium chloride (salt) intake, a dehydrating osmotic stressor, to enhance neuronal and behavioral responses of mice to an acute psychogenic swim stress (SS). Further, we evaluated the contribution of the osmo-regulatory stress-related neuropeptide arginine vasopressin (VP) in the hypothalamic paraventricular nucleus (PVN), one of only a few brain regions that synthesize VP. The purpose of this study was to determine the impact of high dietary salt intake on responsivity to heterotypic stress and the potential contribution of VPergic-mediated neuronal activity on high salt-induced stress modulation, thereby providing insight into how dietary (homeostatic) and environmental (psychogenic) stressors might interact to facilitate psychiatric disorder vulnerability.
Approach
Salt loading (SL) with 4% saline for 7 days was used to dehydrate and osmotically stress mice prior to exposure to an acute SS. Fluid intake and hematological measurements were taken to quantify osmotic dehydration, and serum corticosterone levels were measured to index stress axis activation. Immunohistochemistry (IHC) was used to stain for the immediate early gene product c-Fos to quantify effects of SL on SS-induced activation of neurons in the PVN and extended amygdala – brain regions that are synaptically connected and implicated in responding to osmotic stress and in modulation of SS behavior, respectively. Lastly, the role of VPergic PVN neurons and VP type 1 receptor (V1R) activity in the amygdala in mediating effects of SL on SS behavior was evaluated by quantifying c-Fos activation of VPergic PVN neurons and, in functional experiments, by nano-injecting the V1R selective antagonist dGly[Phaa1,d-tyr(et), Lys, Arg]-VP bilaterally into the amygdala prior to the SS.
Findings
SL increased serum osmolality (P < 0.01), which positively correlated with time spent mobile during, and time spent grooming after a SS (P < 0.01, P < 0.01), and SL increased serum corticosterone levels (P < 0.01). SL alone increased c-Fos immunoreactivity among PVN neurons (P = 0.02), including VP positive neurons (P < 0.01). SL increased SS-induced c-Fos activation of PVN neurons as well (P <0.01). In addition, SL and SS each increased the total number of PVN neurons that were immunoreactive for VP (P < 0.01). An enhancing effect of SL and SS was observed on c-Fos positive cell counts in the central (P = 0.02) and basolateral (P < 0.01) nuclei of the amygdala and bilateral nano-injections of V1R antagonist into the amygdala reduced time spent mobile both in salt loaded and control mice during SS (P < 0.05, P < 0.05).
Summary
Taken together, these data indicate that neuronal and behavioral responsivity to an acute psychogenic stressor is potentiated by prior exposure to high salt intake. This synergistic effect was associated with activation of PVN VP neurons and depended, in part, on activity of V1 receptors in the amygdala. Findings provide novel insight into neural mechanisms whereby prior exposure to a homeostatic stressor such as osmotic dehydration by excessive salt intake increases responsivity to a perceived stress. These experiments show that high dietary salt can influence stress responsivity and raise the possibility that excessive salt intake could be a contributing factor in the development of stress-related psychiatric disorders.
Keywords: Salt diet, stress coping, arginine vasopressin, psychiatric, paraventricular nucleus, hyperosmolality, dehydration
1. INTRODUCTION
Although stress is an established risk factor for development of psychiatric disorders, many individuals experiencing one or more major life stressors do not develop a mental health condition. A number of factors have begun to emerge as determinants of how responsive individuals are to stress. These include unique genetic profiles, early traumatic life experiences and recent psychological or physiological stress events (Cohen et al., 1995; Heim and Nemeroff, 2001). High intake of dietary sodium chloride (salt) is a common physiological osmotic stressor that is known to dramatically increase the risk and severity of multiple disease modalities, most notably cardiovascular diseases such as high blood pressure (Toney et. al., 2003; Osborn et. al., 2007; Toney and Stocker, 2010; He et al., 2013). How excessive dietary salt intake may enhance one’s responsivity to psychological stress is, however, not well understood. In the United States, people consume ~3,400 mg of sodium a day - more than twice the recommended daily allowance according to the American Heart Association (American Heart Association, 2017) - a clear need exists to explore if high salt intake negatively affects psychological health as dramatically as it does cardiovascular health.
Animal models of psychiatric disorders often deploys osmotic stressors, such as water deprivation, as well as psychological stressors, such as swim stress (SS), to investigate neural mechanisms involved in maladaptive stress coping (Monteiro et al., 2015). These studies reveal that a relatively innocuous stimulus experienced on a daily basis can become psychologically damaging if paired with a heterotypic stressor, i.e. a different type of stress. High salt intake is an osmotic stress that activates arginine vasopressin (VP)-synthesizing neurons of the hypothalamic paraventricular nucleus (PVN), one of a few brain regions that produces this osmo-regulatory stress-related neuropeptide (Brown et al., 2013). VP release may then further increase sympathetic neural outflow and induce activation of the endocrine hypothalamic-pituitary-adrenal (HPA) stress axis, both hallmarks of the integrated stress response (Smith and Vale, 2006).
SS is a psychological stressor that activates PVN neurons and stimulates release of VP within not only the PVN, but also limbic forebrain regions such as the amygdala (Wotjak et al., 2001; Ebner et al., 2002; Hernández et al., 2016). Increased extracellular VP within the amygdala binds to its type 1 receptor (V1R) – a receptor implicated in development of stress-related psychiatric disorders (Stoop et al., 2015). Given that amygdalar V1R activation modulates behavioral coping responsivity to stress stimuli, it is possible that activation of VP neurons by multiple stress stimuli could yield exaggerated neuronal and behavioral responses (Ebner et al., 2002). By acting upon stress-sensitive neurons within, and circuits emanating from the PVN, osmotic stressors may have the capacity to promote exaggerated responses to heterotypic stressors, especially if the latter also are capable of exciting those specific PVN neurons responsive to osmotic stress.
The purpose of the current study was to expand upon research investigating heterotypic stress synergism by determining the impact of excessive intake of dietary salt (a physiological osmotic stress) on behavioral and neuronal responsivity to an acute psychological stressor. To test the hypothesis that activation of stress sensitive neural circuits by high dietary salt enhances responsivity to an acute psychogenic stressor, we utilized chronic salt loading (SL) as an osmotic stressor and SS as an acute psychogenic stressor. Literature evidence not only indicates that SL and SS activate the PVN and stimulate VP-synthesizing neurons, but also directly implicates VP as a significant modulator of swimming behaviors (Ebner et. al., 2002; Choe et. al., 2015). Time spent mobile during SS was evaluated as a primary measure of active behavioral stress coping (de Kloet and Molendjik, 2016; Commons et. al., 2017). Others have shown that increased mobility time during a SS is indicative of increased stress responsivity (Lu et. al., 2008), and active stress coping is associated with aggression, irritability, impaired impulse control, and hypertensive cardiovascular responses to stress (Keay and Bandler, 2001). It should be noted that other inescapable psychogenic stressors that allow quantification of behavior response, such as tail suspension, cause profound physiological stress by spiking cerebral blood pressure and altering baroreceptor sensitivity (Brizzee and Walker, 1985; Wilkerson et. al., 2002). Therefore, SS was determined to be a psychogenic stressor well suited to testing our working hypothesis. We additionally sought to determine osmotic and psychological stress sensitive brain regions involved in potentiating behavioral responses to stress, and we investigated VP as a mediator of neural responses to osmotic stress, psychological stress, and their combination. Ultimately, a greater understanding of neural mechanisms that cross-sensitize stress responsivity could aid development of new treatments for stress related psychiatric disorders that consider high dietary salt as a major environmental potentiating stressor.
2. MATERIALS AND METHODS
2.1 Animals
Studies used adult C57BL/6J male mice (3 – 5 months post-natal) acquired from Jackson Laboratories that were housed with same sex peers in a temperature-controlled (24°C) vivarium maintained on a 14-hour/10-hour light/dark cycle in plastic cages (29 cm × 18 cm × 13 cm) containing rodent bedding (Sani-chips; Harlan Teklad, Madison, WI). Animals had ad libitum access to food (irradiated rodent sterilizable diet; Harlan Teklad) and to tap water except where noted.
Experimental procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, 8th Ed., and were approved by the Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
2.2 Salt Loading
SL of mice was accomplished by providing only 2% or 4% saline (NaCl) drinking solution ad libitum for 7 consecutive days. Control mice drank tap water ad libitum.
2.3 Swim Stress and Grooming
Behavioral experiments were conducted on the seventh day of the SL protocol. Mice were transferred from the animal colony to the procedure room at least one hour prior to testing. Mice were then subjected to a five minute SS. Cylindrical acrylic tanks, each with a 20 cm inner diameter and a height of 25 cm, were used for the SS. Tanks were filled to a height of 18 cm with tap water (~25°C) and placed in a white container with three walls (L: 27 cm, W: 27 cm, H: 61 cm) and a transparent acrylic top to shield mice from possible ambient disturbances during the swim. A digital camera was positioned directly above the acrylic top and used to record swimming behavior for offline scoring. Mobility was defined as active leg movement beyond that required to prevent submersion below the surface, and was assessed by an observer blinded to the treatment condition. After five minutes, mice were thoroughly dried using an absorbent laboratory pad.
Once dried, grooming behavior was assessed. Mice were placed in a clean transparent cage (29 cm × 18 cm × 13 cm) containing fresh rodent bedding. A plastic filtered top was placed on the cage without a chow rack, water, or chow. Behavior was recorded for five minutes using a digital camera positioned along the 29 cm-long side of the cage. Grooming behavior was defined as stroking or licking of the nose, paws, face, or body and was scored by an observer blinded to the treatment condition.
2.4 Brain Histology and Immunohistochemistry
Approximately 2 h after exposure to the SS, mice were anesthetized with ~3% isoflurane and perfused transcardially with 20 mL of 0.1 M phosphate-buffered saline (PBS) followed by 50 mL of 4% paraformaldehyde (PFA) in 0.1 M PBS. Brains were removed and post fixed in 4% PFA for 4 h at room temperature (~22.5 °C), then transferred to 30% sucrose in 0.1 M PBS at 4 °C for ≥ 2 days. Brains were sectioned at 30 μM in the coronal plane using a sliding microtome (Leica) and saved as three sets of alternate sections. Prior to staining, sections were stored at − 20°C in polyvinyl-pyrrolidone (PVP-40) cryoprotectant.
Immunohistochemical staining for the immediate early gene product c-Fos was performed as previously described (Bardgett, et al., 2014b) and was used to detect activated neurons in the PVN and amygdala. Sections were removed from cryoprotectant, rinsed (3×, 5 min in 0.1 M PBS) and incubated for 30 min in 0.5% sodium borohydride in 0.1 M PBS to remove auto fluorescent aldehydes generated during fixation. After additional rinsing (3×, 5 min in 0.1 M PBS), sections were incubated in 0.1 M PBS containing 3% goat serum and 0.3% Triton-X 100 (blocking solution) for 2 h. Sections were then incubated in blocking solution containing polyclonal rabbit anti-c-Fos antibody (1:10,000; Millipore) and polyclonal guinea pig anti-VP (1:1,000: Peninsula Labs) for 72 h at 4 °C on an oscillating platform. After rinsing (3×, 5 min in 0.1 M PBS), sections were transferred to blocking solution containing biotinylated goat anti-rabbit IgG secondary antibody (1:250; Thermofisher) and goat anti-guinea pig IgG secondary antibody (1:250; Thermofisher) conjugated to AlexaFluor-488 and incubated (~22.5 °C) for 2 h. Sections were then rinsed (3×, 5 min in 0.1 M PBS) and c-Fos immunoreactivity revealed by incubation in streptavidin-AlexaFluor-594 conjugate (1:250; Thermofisher) for 10 min at room temperature. Sections underwent three final rinses (5 min in 0.1 M PBS) and were mounted on slides using ProLong Diamond to preserve fluorescence.
Tissue sections stained for c-Fos and VP were examined with a scanning confocal microscopy system (Prairie Technologies) using a 43 series ion laser (Melles Griot) and Sapphire 561 laser (Coherent) to emit 488 and 561 nm wavelengths. Images were captured using a 16-bit Cascade II digital camera (Photometrics, Inc.) and analyzed using NIS-Elements Advanced Research 3.2 software. Nuclei expressing c-Fos were declared “c-Fos positive” if peak fluorescence intensity was ≥ 10,000 (A.U.). c-Fos positive cells were counted and compared between control and SL mice with and without exposure to SS. Among PVN neurons, co-localized c-Fos and VP immunoreactivity was also quantified for statistical analysis.
2.5 Hematology
As previously described (Stocker et al., 2005; Bardgett el. al., 2014a), blood for hematology analysis was collected via a cardiac puncture while mice were anesthetized with isoflurane immediately prior to perfusion fixation. Blood samples were taken from behaviorally naïve mice, and from SS exposed mice two hours after behavioral testing. In all cases, blood was drawn immediately prior to transcardiac perfusion. Hematocrit was measured by loading microhematocrit capillary tubes with fresh blood and spinning on a micro centrifuge (Damon IEC Division) for 3 min. The remaining blood samples were spun for 8 minutes at 5000 RPM and the serum was collected to measure serum osmolality using a Model 3320 Osmometer (Advanced Instruments Inc.) and protein levels using a Master Refractometer (Atago). Bonferroni’s multiple comparisons tests after a two-factor ANOVA (salt loading, swim stress) relieved SS did not alter serum osmolality between SL or not SL mice (P > 0.05), so these data were combined (Table 1).
Table 1.
Graded dietary salt intake induces osmotic dehydration and increases the stress hormone corticosterone.
| Salt Loading Treatment | Fluid Intake (mL/day) | Serum Osmolality (mOsm/kg) | Hematocrit (%) | Serum Protein (g/mL) | Serum Corticosterone (ng/mL) |
|---|---|---|---|---|---|
| Tap Water | 7.0 ± 0.5 (n = 6) |
330 ± 2.6 (n = 13) |
45 ± 0.6 (n = 13) |
5.5 ± 0.2 (n = 16) |
97 ± 16 (n = 5) |
| 2% Saline | 14 ± 1.9## (n = 10) |
333 ± 3.5## (n = 16) |
48 ± 1.1* (n = 11) |
6.2 ± 0.2 (n = 16) |
76 ± 12## (n = 5) |
| 4% Saline | 3.6 ± 0.6** (n = 11) |
388 ± 9.0** (n = 13) |
51 ± 2.0** (n = 4) |
6.6 ± 0.3** (n = 16) |
335 ± 78* (n = 6) |
Effects of graded dietary salt on fluid intake, hematologic measures and the stress hormone corticosterone. Values were obtained after the 7-day SL protocol. Water drank is the estimated daily volume consumed per mouse (mL/day). Data are mean ± SEM.
P < 0.01 significant difference from tap water controls;
P < 0.01 significant difference from tap water controls;
P < 0.01 significant difference from 4% saline treatment with Dunnett’s post hoc multiple comparisons test after a one-factor ANOVA. Values in parentheses are sample sizes.
2.6 Corticosterone ELISA
Behaviorally naïve mice were SL with either 2% or 4% saline for one week as described above. Under isoflurane anesthesia mice were decapitated and trunk blood was collected. After clotting for 1 h at room temperature, samples were centrifuged at 3500 rpm for 1 h at 4 °C. Serum was stored at −80 °C until analysis. All serum collection occurred between 1100 and 1500 h.
Serum corticosterone was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Sciences, Farmingdale, NY). Spectroscopic measurements of absorbance at 405 nm with correction at 595 nm was quantified using a Varioskan Flash plate reader (Thermo Scientific).
2.7 Indwelling Amygdala Cannulae
Mice were anesthetized with 2 – 3% isoflurane in oxygen and mounted in a stereotaxic head frame (Kopf). Stainless steel guide cannulae (25G) were lowered into the amygdaloid complex bilaterally at the following stereotaxic coordinates with respect to bregma: AP; −1.6 mm, ML; ± 3.1, DV; −4 mm. Cannula were anchored in place with two small screws and cranio-plastic cement as previously described by our laboratory (Toney and Porter, 1993). A 32G stainless steel obturator was placed in the lumen of each guide cannula and secured in place. Each mouse received post-operative injections of Combi-Pen-84® antibiotic (30,000 U, subcutaneous inj.) and buprenorphine (0.1 mg/kg, intraperitoneal inj.), and Neo-Predef® topical antiseptic powder at skin tissue surrounding the guide cannula. Prior to experiments, mice were singly housed in the animal facility for 2-5 days of post-operative recovery.
Cannulated mice were provided either 4% saline or tap water as described in the SL protocol. All injections of the VP V1 receptor antagonist dGly[Phaa1,d-tyr(et), Lys, Arg]-VP (Bachem, Inc.) were bilaterally delivered into the amygdala by inserting a stainless-steel injection cannula (32G) of a predetermined length through indwelling guide cannulae. Antagonist was dissolved to a concentration of 100 nM in sterilized saline. Antagonist concentration was determined as a dose (0.02 pmol or 22 pg) that alone did not alter animal behavior. All injections into the amygdaloid complex were in a volume of 200 nL delivered over a period of 1 min using a 1 μL Hamilton glass syringe connected to the injector by polyethylene (PE-10) tubing.
Immediately after bilateral amygdala injections, each mouse was placed in a transparent observation chamber (L: 18 cm, W: 13 cm, H: 11 cm) with a transparent top. Each chamber was a three-walled white acrylic shield (L: 27 cm, W: 27 cm, H: 61 cm) prevent ambient activity from influencing behavior during the observation time. Activity was recorded with a digital camera for 15 min while viewing through the 18 cm side of the observation chamber. To evaluate general locomotor activity, the number of times mice crossed from one side of the cage to the other (cage crosses) was quantified. Rearing behavior, and time spent grooming were also assessed at a later time by an observer blinded to treatment conditions.
After 15 minutes, mice were removed from their observation chambers and immediately subjected to an acute 5 min SS as described above. Upon completion, mice were thoroughly dried and briefly anesthetized 30 min later to collect trunk blood samples as described above. For histological confirmation of amygdala injection sites, 75 nL of 0.2% rhodamine fluorescent latex nanospheres was delivered with the same injector used to deliver VR1 antagonist or vehicle.
2.8 Data Analysis
Statistical analysis was performed using Prism 7.0 (GraphPad, San Diego, CA). Drinking solution consumed by each animal was estimated as the volume difference before and after the 7-day SL protocol divided by the number of mice per cage (Table 1). Hematology data were compared using a one-factor analysis of variance (ANOVA), followed by Dunnet’s multiple comparisons tests (Table 1). Time spent mobile during (Figure 1.A) and time spent grooming after (Figure 1.B) SS were analyzed using an unpaired Student’s t-test. Pearson’s Correlation was used to evaluate the relationship between serum osmolality and time spent mobile during the SS (data not shown).
Figure 1.
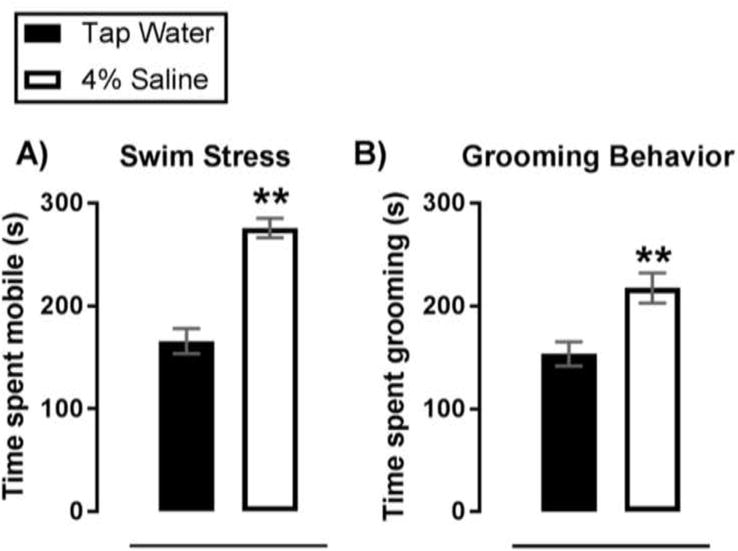
Chronic SL increases active coping in response to a acute swim stress. Summary data of time spent mobile during (A) and time spent grooming after (B) an acute SS. Data are mean ± SEM. **P < 0.01 significant difference from tap water controls with Dunnett’s post hoc multiple comparisons test after a one-factor ANOVA. Sample size per data point are as follows: (A) n = 9 – 11; (B) n = 8 – 11.
Neuronal nuclei stained for c-Fos and somata stained for VP or both (c-Fos/VP double labeling) in the PVN (Figure 2) and supraoptic nucleius (SON; Supplemental table 1) were counted from a representative section through each nucleus. c-Fos positive neuronal nuclei in the basolateral and central sub-regions of the amygdala were similarly counted (Figure 3). Distribution of immunoreactive staining within the PVN and SON (plate 38) and basolateral and central amygdala (plate 43) was determined according to histological plates in the mouse brain atlas of Paxinos and Franklin (1997). For each brain region, nuclei from each hemisphere were counted and averaged. Data were analyzed using a two-factor ANOVA (SL, swim stress) followed by a Dunnett’s or Bonferroni’s multiple comparison test.
Figure 2.
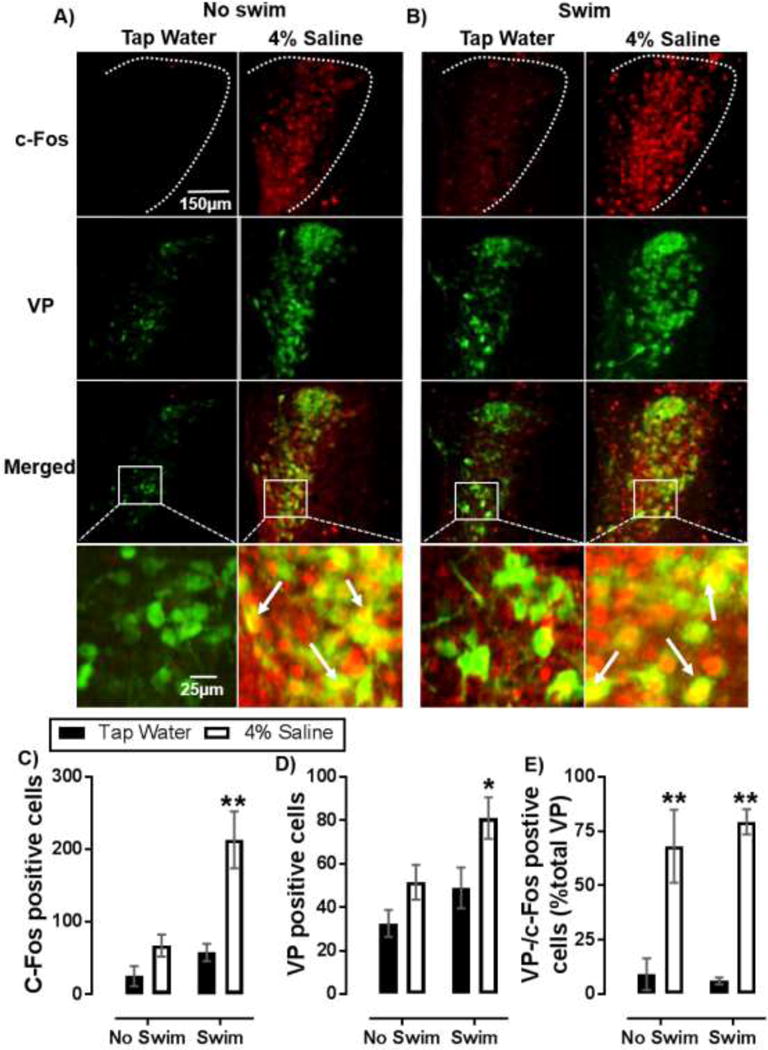
Salt loading increases PVN VP neuron recruitment in response to acute swim stress. Representative images of c-Fos (red), VP (green), or c-Fos/VP co-immunoreactivity (double labelled-yellow) in the PVN of mice that underwent SL without (A) or with (B) subsequent exposure to acute SS. (C) Summary data of c-Fos positive cell counts. (D) Summary data of VP positive cell counts. (E) Percent of activated (c-Fos positive) VP cells (double labelled- yellow). White arrows indicate cells co-stained for c-Fos and VP. Data are mean ± SEM. *P < 0.05 significant difference from within swim treatment tap water controls; **P < 0.01 significant difference from within swim treatment tap water controls using Bonferroni’s post hoc multiple comparisons test after a two-factor (SL, swim stress) ANOVA. Sample size per data point are n = 4 – 5.
Figure 3.
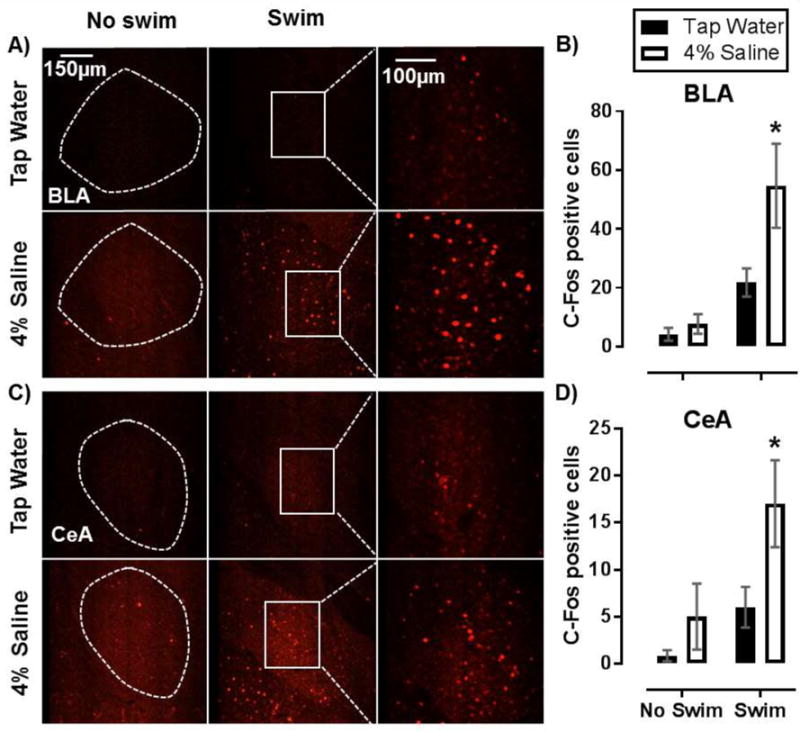
SL increases recruitment of neurons in the basolateral and central nucleus of the amygdala in response to acute swim stress. Representative images of c-Fos positive cells in the basolateral (BLA) (A) and central (CeA) (C) nucleus of the amygdala in mice that either underwent SL alone, acute SS alone, or SL with acute SS. Dotted line represents outline of BLA and CeA. (B, D) Summary data of c-Fos positive cell counts from the BLA (B) and CeA (D). Data are mean ± SEM. *P < 0.05 significant difference from within swim treatment tap water controls determined using Bonferroni’s post hoc multiple comparisons test after a two-factor (SL, swim stress) ANOVA. Sample size per data point are n = 3 – 4.
Data from mice with indwelling amygdala cannulae, including cage crosses, time spent grooming, rearing events, and time spent mobile during SS were analyzed using a two-factor ANOVA (SL, vehicle/V1R antagonist) followed by Bonferroni’s multiple comparison test (Figures 4.A–C, Supplemental Figure 1.A,B). Post SS corticosterone levels from tap water treated mice that received either nanoinjections of vehicle or V1R antagonist were analyzed with student’s T- test (data in text). All data are expressed as mean ± SEM. P ≤ 0.05 was a priori considered statistically significant.
Figure 4.
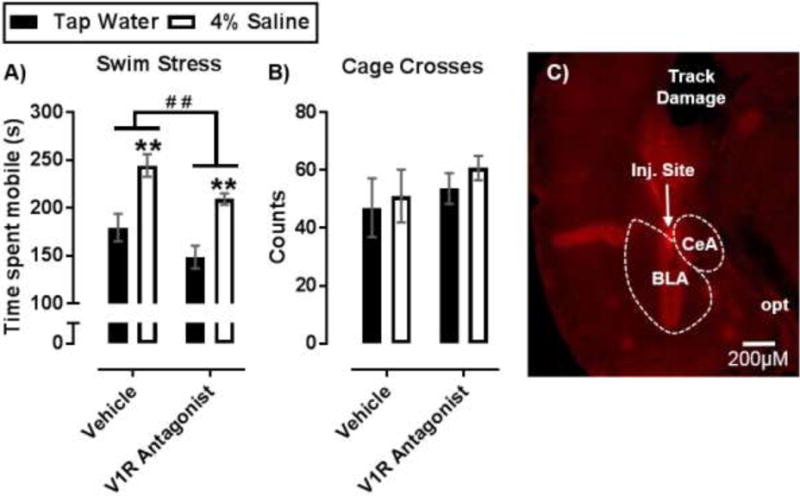
Effect of intra-amygdala nano-injections of V1R antagonist on SL-induced active coping behavior. (A) Time (s) spent mobile during SS after bilateral nano-injections of a VP V1R antagonist into the amygdala. (B) Cage crossings in an observation chamber over 15 min as a measure of generalized locomotor activity. (C) Histological verification of nano-injection sites in the CeA and BLA. Image shows cannula track (track damage), optic nerve (opt) injection site (inj. site), and amygdala sub regions; Data are mean ± SEM. **P < 0.01 significant difference from within drug treatment group compared to tap water controls with Bonferroni’s post hoc multiple comparisons test. # # P < 0.01 indicated significant main effect of V1R antagonist with a two-factor ANOVA. Sample size per data point are n = 5 – 8.
3. RESULTS
3.1 SL induced osmotic dehydration and corticosterone release
Initial experiments confirmed intake of drinking solutions and hematological effects of SL in mice treated with 2% or 4% saline (Table 1). Mice that drank 2% saline ingested a greater volume than control mice that drank tap water (P < 0.01). Mice that drank 4% saline showed a non-significant trend to ingest less than tap water controls (P = 0.10). Hematological measures revealed that SL with 2% and 4% saline significantly increased hematocrit compared to mice consuming tap water (P = 0.05, P < 0.01; respectively), and serum protein concentrations trended upward with 2% saline (P = 0.06), reaching significance in the group treated with 4% saline (P < 0.01). These data suggest SL with 2% and 4% saline induced hypovolemia. Unlike hematocrit, serum osmolality and corticosterone concentration increased only with 4% saline treatment in comparison to tap water treatment (P < 0.01, P < 0.01; respectively). These data suggest that SL with 4% saline induced osmotic dehydration, a substantial physiological stress. Because a major goal of this study was to investigate the role of dietary salt as a stress stimulus, remaining experiments focused on effects of SL with 4% saline as it induces a robust increase in serum corticosterone levels.
3.2 SL increased active stress coping during acute SS
To determine enhancing effects of high dietary salt intake on responsivity of mice to a psychogenic stressor, stress coping behaviors of mice that underwent SL were determined during and after SS. SL increased time spent mobile during (p < 0.01) and time spent grooming after (P < 0.01) an acute SS (Figure 1.A, B). Both behaviors indicate an increase in active stress coping (Commons, et. al., 2017; Kalueff et al., 2016). Further, positive correlations between serum osmolality and time spent mobile during SS (r = 0.92, P < 0.01) or time spent grooming after SS (r = 0.69, P = 0.02) were identified (data not shown). These correlations suggest that SL-induced body fluid hyperosmolality (and/or accompanying hypovolemia) is linked to enhanced active stress coping responses to a psychogenic stress, potentially by activation of brain regions such as the PVN where neurons respond both to hyperosmolality and to SS (Ebner et al., 2002; Bourque, 2008; Toney et. al., 2012). Of note, Bonferroni’s multiple comparisons tests after a two-factor ANOVA (SL, SS) indicated no difference in serum osmolality among salt loaded mice that underwent a SS compared to those that did not (P > 0.05). Therefore, it is highly unlikely that greater swimming and grooming was for the purpose of water consumption, as increased fluid intake during the SS would yield reduced serum osmolality.
3.3 SL enhances PVN neural circuit recruitment in response to acute SS
To investigate possible limbic neural circuits participating in increased active stress coping effects of SL, c-Fos immunoreactive neurons (c-Fos positive) were counted in mice that underwent SL alone (Figure 2.A) and mice that underwent SL followed by exposure to SS (Figure 2.B). Because the PVN serves as a central hub for integration of stress inputs, this region was of particular interest. In the PVN, SL and swim stress each significantly increased the number of c-Fos positive neuronal nuclei [F(1,13) = 20.2, P < 0.01, F(1,13) = 16.5, P < 0.01; respectively]. A significant interaction showed SL potentiated the effect of SS on c-Fos activation [F(1,13) = 6.62, P = 0.02] (Figure 2.C). Specifically, mice that drank 4% saline showed greater swim stress-induced c-Fos labeling compared to those that drank tap water prior to SS (P < 0.01) (Figure 2.C). Therefore, our data implicate neural circuits sensitive to osmotic dehydration in the recruitment of psychogenic SS activated PVN neurons.
We next sought to determine the neuropeptide phenotype of c-Fos positive PVN neurons by co-staining for the stress neuroactive peptide VP, the release of which is stimulated both by body fluid hyperosmolality and by SS (Ebner et al., 2002; Bourque, 2008). We found that VP positive PVN cell counts increased both with SL and acute SS [F(1, 13)= 8.74, P = 0.01, F(1, 13) = 6.99, P = 0.02; respectively] (Figure 2.D), suggesting that SL and acute SS each can induce non-VP neurons to express VP to immune-detection threshold, likely by stress induction of VP gene expression. Analysis further revealed that SL also increased the percent of VP positive neurons co-labeled with c-Fos [F(1, 19) = 53.18, P < 0.01] (Figure 2.E), suggesting that neurons induced to express VP were among the population of neurons activated by SS. Within swim group comparisons showed that a greater percentage of VP neurons were co-labeled with c-Fos in mice that drank 4% saline compared to tap water controls (P < 0.01, P < 0.01; respectively). These data suggest that SL likely drives VP production to enable sustained VP release and therefore persistent receptor tonus at downstream synaptic targets of SS-sensitive neurons in the PVN.
To determine if the effect of SL and SS-induced c-Fos activation was specific to the PVN or simply reflected a general increase in activation of body fluid regulating brain regions that synthesize VP, c-Fos activated cells were evaluated in the supraoptic nucleus (SON) (Supplemental Table 1). In the SON, SL alone increased c-Fos positive cell counts [F(1, 10) = 106.2, P < 0.01], while swim stress showed no effect [F(1, 10) = 0.11, P = 0.75] (Supplemental Table 1). SL increased VP positive cell counts and VP positive neurons co-labeled with c-Fos [F(1, 10) = 25.9, P < 0.01; F(1, 10) = 107, P < 0.01], while swim stress had no effect on either measure [F(1, 10) = 1.25, P = 0.29; F(1, 10) = 0.04, P = 0.84]. Using the SON as a hypothalamus specific control indicates that the PVN is likely an especially important brain region for integration of heterotypic stress sensitive inputs.
3.4 SL enhances amygdala recruitment by acute SS
Because PVN neurons project monosynaptically to the amygdala to influence behavioral responses to stress, we evaluated c-Fos activation in the basolateral amygdala (BLA) (Figure 3.A) and central nucleus of the amygdala (CeA) (Figure 3.C) in mice after SL and/or swim stress exposure. In the BLA, SL and swim stress each significantly increased c-Fos positive cell counts [F(1, 9) = 5.97, P = 0.04, F(1, 9) = 18.79, P < 0.01; respectively]. A trend toward an interaction suggests that SL may have potentiated swim stress induced c-Fos induction in the BLA [F(1, 9) = 3.90, P = 0.08] (Figure 3.B), as c-Fos cell counts following SS were greater in mice that drank 4% saline compared to tap water drinking controls (P < 0.01). Evaluation of the CeA showed swim stress and SL increased c-Fos-activation [F(1, 9) = 5.36, P = 0.05; F(1, 9) = 5.11, P = 0.05] (Figure 3.D). In the 4% saline group, SS again resulted in a greater increase in c-Fos counts compared to tap water controls (P = 0.05). These data suggest that the combination of SL and SS lead to exaggerated amygdala activation, which may contribute to enhanced active stress coping behavior.
3.5 V1R antagonist injections into the amygdala attenuate active coping behavior during SS
Given that VP positive projections from the PVN have been identified in the amygdala (Brown et al., 2013), we sought to determine if SL induced increases in VP signaling within the amygdala contributed to observed increases in active coping behavior during acute SS. Indwelling cannulae were surgically implanted for bilateral nano-injections of vehicle or VP receptor 1 (V1R) antagonist into the amygdaloid complex of awake mice. The CeA and BLA were simultaneously targeted as both regions showed greater c-Fos expression when SL and SS were combined (Figure 4.C). Data from vehicle injection studies revealed that SL with 4% saline increased time spent mobile during SS [F(1, 21) = 29.54, P < 0.01], replicating our earlier finding (Figure 1.A). A main effect of V1R antagonist showed a reduction in time spent mobile during SS [F(1, 21) = 8.12, P = 0.01]. Within drug group comparisons showed more mobility in 4% saline treated mice compared to tap water controls (P < 0.01) (Figure 4.A). Interestingly, no interaction between SL and V1R antagonist treatment was observed [F(1, 21) = 0.03, P = 0.86]. This suggests that VP receptor activation within the amygdala broadly mediates swimming behavior, which is consistent with literature evidence (Ebner et. al., 2002). However, data also indicate that V1R activation is not solely responsible for SL-induced enhancement of active coping during SS.
To determine if effects of SL on SS behavior were due to a general increase in locomotor activity or if effects of amygdala V1R blockade were due to a generalized suppression of activity, mouse behavior in an observation chamber was analyzed immediately after nano- injections of vehicle or V1R antagonist. No effect of SL [F(1, 22) = 0.45, P = 0.51] or V1R antagonist was observed [(1, 22) = 0.96, P = 0.34] on locomotor activity as assessed by evaluating cage crossing events within the observation chamber (Figure 4.B). In addition, neither grooming time nor rearing events were altered by SL [F(1,22) = 0.14, P = 0.71; F(1,22) = 1.58, P = 0.22; respectively] or intra-amygdala injections of V1R antagonist [F(1,22) = 2.01, P = 0.16; F(1,22) = 1.00, P = 0.33] (Supplemental figures 1.A, B). These data suggest that effects of SL and V1R antagonist were specific to active coping behaviors during acute SS. To certify that V1R antagonism in amygdala was not reducing time spent mobile during a SS in a SL- independent manner by altering serum corticosterone, we measured serum levels after a SS in water treated mice alone. V1R antagonism did not alter corticosterone levels in mice treated with vehicle (Mean ± SEM: 210 ± 32, n = 5) compared to mice treated with V1R after SS (Mean ± SEM: 186 ± 11, n = 4) (P = 0.53, Student’s T-test). Lastly, injection site placement in the amygdala was verified by histology (Figure 4.C).
4. DISCUSSION
Psychological stress is a contributing factor to the development of many psychiatric disorders, notably anxiety, post-traumatic stress disorder, and depression (Heim and Nemeroff, 2001; Shin and Liberzon et. al., 2010; Pittenger and Duman, 2007). A greater understanding of neural mechanisms that mediate stress responsivity and coping, and the environmental factors that enhance these mechanisms, could aid prevention or treatment of stress-related mental health disorders. Toward that end, we have shown that l osmotic dehydration stress, induced by chronic SL through ingestion of hypertonic saline, can increase serum osmolality and corticosterone levels in mice (Table 1), and increase time spent mobile during and time spent grooming after a psychogenic SS (Figure 1.A, B). Increased active stress coping responses to SS positively related with recruitment of PVN neurons in mice that underwent SL prior to exposure to SS (Figure 2.C). The total number of VP positive PVN neurons increased both with SL and SS (Figure 2.D), and PVN-VP neuron activation was pronounced in all animals that underwent SL (Figure 2.E). The latter is consistent with a high level of VP release and receptor tonus downstream of the PVN. Activation of the basolateral and central amygdaloid nuclei was found in mice that underwent SL and SS (Figure 3.B, D), potentially due to an increase in amygdala VP activation of V1R. Blockade of V1R in the amygdala reduced time spent mobile during the SS; however, reduced swimming behavior was observed in mice regardless of whether they did or did not undergo SL (Figure 4.A). This supports the conclusion that VP receptor activation within the amygdala mediates swimming behavior in manner not specific to SL. Together these data indicate that excessive dietary salt intake increases stress responsivity by increasing active coping to an acute psychogenic stress. The underlying mechanism potentially involves increased VPergic neurotransmission, which likely participates in conjunction with yet to be identified mechanisms including intrinsic neuroadaptions and potentiation of other neurotransmitters systems.
Consumption of hypertonic saline is a potent physiological stressor that can induce osmotic dehydration (Choe et al., 2015), which is consistent with our results showing that chronic SL with 4% saline significantly increased serum osmolality, plasma protein concentration, and hematocrit (Table 1). Unlike in rats, SL with just 2% saline is insufficient to induce osmotic dehydration in mice (Choe et al., 2015; Krause et al., 2017). This species difference is likely due to superior renal sodium excretory capacity of mice, making them more resistant to dehydration compared to rats (Bankir and de Rouffignac, 1985). Of note is that 4% saline treated mice showed the highest osmolality yet consumed less fluid than 2% saline treated mice. Consumption of 4% saline is likely aversive and mice in this treatment condition were likely hypovolemic and hyperosmotic due to a combination of reduced fluid intake and consumption of hypertonic solutions.
To further index the effect of SL as a stressor, circulating levels of corticosterone were measured to assess HPA axis activation (Table 1). Mice that drank 4% saline had increased corticosterone, suggesting that SL itself constituted an osmotic stressor (Smith and Vale, 2006). This is consistent with reports that hyperosmotic stimuli can induce robust increases in plasma corticosterone levels (Lauand et. al., 2007). Of note is that mice consuming 4% saline presented with hypovolemia, as well as hyperosmolality. Therefore, increased concertation of serum corticosterone observed in 4% salt loaded mice could involve concentration of the hormone due to reduced blood volume. However, mice treated with 2% saline also presented with hypovolemia without hyperosmolality or high corticosterone. This suggests that hypovolemia is not sufficient to elevate serum corticosterone. It is granted that there could be threshold effects such that the greater degree of hypovolemia and hyperosmolality in 4% salt loaded mice might have been necessary to support a significant increase of serum corticosterone. In rats, chronic SL with 2% saline has been shown to increase, decrease, or not alter corticosterone levels (Dohanics et al., 1990; Amaya et al., 2001; Elias et al., 2002). A possible explanation for these discrepancies is that effects of dietary salt on HPA axis activation might be transient, phasic, or function in a “dose”-dependent manner. It is important, however, to recognize that corticosterone levels can rise in response to numerous stimuli including innocuous stimulants such as feeding, novelty, and social support (Shin and Liberzon, 2010). Therefore, increased serum corticosterone in the current study is interpreted only as a positive indicator of HPA axis activation by our SL protocol.
After first establishing that SL with 4% saline was an effective stressor, we next sought to identify the enhancing effect of SL on behavioral responses to a psychogenic stress, specifically using SS. While originally developed as a predictive screen for antidepressants, SS serves as an effective psychogenic stressor that allows behavioral responsiveness to stress and coping styles to be quantified (de Kloet and Molendjik, 2016; Commons et. al., 2017). Increased time spent mobile during a swim stress is indicative of enhanced active stress coping. We found that 4% SL increased time spent mobile during and time spent grooming after SS (Figure 1.A, B), both signs of enhanced active coping responses to a stressful experience (Commons et al. 2017; Kalueff et al., 2016; Lu et al., 2008). Physiological stressors that drive endocrine and sympathetic nervous system limbs of the stress response tend to increase active coping behaviors. Water deprivation and exposure to cold stress are two examples of physiological stressors that increase stress axis and sympathetic activity as well as time spent mobile during SS (Hata et al., 1999; Zhang et al., 2016). From an evolutionary perspective, SL, water deprivation, and cold stress all require animals to actively engage with their environment to survive. By activating neurons in brain regions such as the PVN, these physiological stressors induce autonomic nervous system and endocrine changes to heighten arousal and improve coping with environmental stressors (Fiedler et al., 2006; Prabha et al., 2011; Bardgett et al., 2014a; Wilson, 2017). It is tempting to speculate that an exaggerated response to SS by prior SL may manifest as panic- or anxiety-like behavior. This possibility is strengthened by the fact that time spent mobile during SS is increased by anxiogenic drugs and decreased by anxiolytic drugs (Hata et al., 1999; Skrebuhhova et al., 1999). However, it should be noted that acute osmotic dehydration via bolus administration of hypertonic saline has been reported to increase social behaviors, which may be viewed as an anxiolytic effect (Krause et al., 2011). Clearly, additional studies are warranted to further elucidate mechanisms underlying modulatory effects of acute vs chronic osmotic stress on endocrine and behavioral responses to psychogenic stress. This is underscored by recent evidence that SL of mice for 5 days with 2% saline accelerates recovery of the HPA axis corticosterone response to acute restraint (Krause et al., 2017).
Of special note is that SS employed in the present study is both a psychogenic stressor and a requirement to measure changes in active stress coping associated with an osmotic SL stress. Therefore, a limitation of our behavioral results is that we are only able to speak to the effect of SL on behavior in the context of the behavioral model explored (i.e. SS). It is, however, possible to evaluate the independent and combined effect of SS and SL on neuronal activation. Therefore, a key factor of our experimental design was to evaluate neuron activation (i.e., c-Fos expression) in all stress specific contexts studied. Immunostaining for c-Fos was used to index the extent to which SL increased SS-induced PVN neuronal recruitment. The PVN is a hub for integrating physiological and psychological stress inputs (Smith and Vale, 2006; Toney and Stocker, 2010). Unexpectedly, we found that our SS protocol alone did not significantly increase c-Fos positive neurons in the PVN. While somewhat surprising, this is consistent with literature evidence (Yanagida et. al., 2016). Experiments using rats have shown that SS increases c-Fos positive cell counts in the PVN; however, these experiments generally conditioned the rodent towards increased immobility by conducting an initial 15 minute swim 24 hr prior to a 5 minute SS (Duncan et. al., 1996). A prior SS was excluded from the current study as our primary interest was investigating the effects of a standing osmotic stress on responses to a single psychogenic swim stress. Strikingly, SL substantially enhanced the number of c-Fos positive PVN neurons after a single SS (Figure 2.C). One possible explanation for this potentiating effect is that SL stimulated dendritic release of VP within the PVN, a well- documented neuronal excitatory phenomenon that could have lowered the firing threshold for swim stress sensitive PVN neurons (Wotjak et al., 2001; Ribeiro et al., 2015).
Immunostaining for VP revealed that SL increased the total number of VP positive neurons in the PVN (Figure 2.D), which suggests an osmotic stressor can elicit a “state-dependent” change by inducing non-VP positive cells to express VP. Of special interest is the fact that exposure to an acute SS also increased the total number of VP neurons in the PVN (Figure 2.D), and the combination of SL combined with swim stress induced the greatest increase in VP positive cells. Both stressors combined appear to induce a populational recruitment of VP cells, which is consistent with previous reports (Burbach et al., 1984; Wotjak et al., 2001); however, a novel aspect of our findings is that SL and swim stress induce additive neurochemical plasticity in PVN neurons. The translational impact of this finding is strengthened by literature evidence that state-specific changes in PVN neurochemistry and neuronal function can be long lasting (Burbach et al., 1984). Hypothetically, previous stress exposure might increase the total number of VP cells in the PVN such that future stress exposure, capable of maximally activating the available pool of PVN-VP cells, would elicit greater VP-driven responses at downstream targets. While intriguing, future studies are needed to determine if a greater level of VP-tonus could increase the likelihood of developing a psychiatric disorder.
Dual c-Fos/VP staining showed that SL activated the majority of available VP PVN neurons (Figure 2.E). As an acute psychogenic SS triggers an expansion of the available VP-containing neuronal reservoir, activation of this reservoir by SL would ostensibly more effectively signal to downstream neurons. Additionally, we found that potentiating effects of SL on SS induced c- Fos expression were specific to the PVN, as the SON did not show an expansion of an active VP positive neuron pool following a swim stress (Supplemental Table 1). This fact is consistent with previous studies suggesting that SS does not activate VP cells in the SON (Wotjak et al., 2001).
Along with the PVN, SL enhanced c-Fos cell counts in the basolateral and central nucleus of the amygdala in response to an acute SS (Figure 3.B, D). This is in accord with reports that the amygdala modulates stress coping behaviors, such as mobility during SS (Ebner et al., 2002). A role for VP as a mediator of stress coping in the amygdala is strengthened by accounts of intraventricular VP administration dose dependently increasing time spent mobile during SS (Yang et al., 2012). Because the amygdala receives PVN inputs sensitive to hyperosmotic stimulation and mediates drinking behavior in salt depleted rats (Lawrence and Pittman, 1984; Hu et al., 2015), and because there is evidence of a VPergic PVN-to-amygdala projection (Hernández et al., 2016), it is possible that a PVN-amygdala vasopressinergic monosynaptic pathway is driven by SL and experience potentiated activity during subsequent SS to augment release of VP in the amygdala. Alternatively, extrahypothalamic VP-synthesizing neurons, potentially within the amygdala itself, have been identified and may be an additional or alternative source of VP in the amygdala (Caffé et. al., 1987). Identification of input source(s) of amygdala VP is of fundamental importance. Future studies utilizing retrograde tracers paired with dual immunohistochemical staining are needed to determine brain regions upstream of the amygdala that both synthesize and which could either persistently tonically release VP during chronic stress and/or acutely increase VP release during acute stress.
Utilizing indwelling cannulas, intra-amygdalar nano-injections of the V1R receptor antagonist dGly[Phaa1,d-tyr(et),Lys, Arg]VP reduced time spent mobile during the SS (Figure 4.A); however, this effect was similar in control mice and mice that underwent SL. This suggests that V1R signaling in the amygdala is involved in active coping behaviors during the SS and may not directly participate in the enhancement of active coping caused by SL. Psychogenic stress increases levels of extracellular VP in the central nucleus of the amygdala; therefore, this stress hormone likely modulates coping behaviors in some capacity (Ebner et al., 2002). Alternative stress sensitive neuropeptides, like corticotropin releasing hormone and oxytocin (Stanić et al., 2017), may also play significant roles in stress responsivity to SS. Indeed, SL increases oxytocin (OT) release (Greenwood et. al., 2015) and has been implicated in stress coping behaviors. Of note in this regard is that oxytocin receptors (OTR) are expressed and functionally active in amygdala (Huber et. al., 2005).
It should be noted that neither SL with 4% saline nor V1R antagonist administration in the amygdala altered general locomotor activity (Figure 4.B). Thus recruitment of amygdala V1R and the behavior enhancing effects of SL are likely specific to acute psychogenic stress and not reflective of a generalized deficit in locomotor activity or to negative feedback effects of corticosterone. Lastly, the molecular homology between the V1R, V2R, and OTR, can increase the likelihood of non-specific activity from receptor antagonists (for review: Manning et. al., 2012). This can limit the interpretation of experimental data, as receptor specificity is crucial to identify cellular mechanism. Therefore, the V1R antagonist, dGly[Phaa1,d-tyr(et), Lys, Arg]-VP, used in the current study was chosen due to the high selectivity of this compound for V1R over V2R, specifically 570 times greater selectivity for V1R than V2R (Manning et. al., 1990). This antagonist has been used to study the effects of salt loading (Choe et. al., 2015), and structural analogues of dGly[Phaa1,d-tyr(et), Lys, Arg]-VP, such as Phaa-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-Tyr-NH2, have shown ~8 fold greater selectivity for V1R over OTR (Schmidt et. al., 1991). It is possible that the effects of SL, in part, could be due to activation of oxytocin synthesizing neurons and OTR activation, but it is unlikely that the observed effects of the V1R antagonist were due to activity at the OTR.
5. CONCLUSIONS
Findings presented raise the possibility that two heterotypic stress stimuli, one physiological (SL) and one psychogenic (SS), potentiate neuronal and behavioral responsivity to stress. Enhanced stress responsivity by SL may be due in part to increased PVN-amygdala signaling by the stress neuropeptide VP. From a translational perspective, it is possible that activation of this stress sensitive neural pathway by a postprandial spike in plasma osmolality may sensitize an individual to exhibit exaggerated behavioral responses to a range of psychogenic (perceived) stressors. Psychogenic stress increases salt appetite (Torres et al., 2010), which in turn could further enhance one’s responsivity or overall vulnerability to stress. It is therefore not unreasonable to suggest that long-term activation of VP-PVN neurons could increase the likelihood of developing stress-related mood/psychiatric disorders. Future studies are necessary to further evaluate this possibility.
Supplementary Material
HIGHLIGHTS.
High salt intake (HSI) is proposed to enhance responsivity to psychogenic stress.
HSI is an osmotic stress that increases corticosterone & stress coping behaviors.
HIS enhanced PVN & amygdala neuronal activation by psychogenic stress.
Amygdalar vasopressin receptor 1 activation likely mediates swimming behavior.
HSI may contribute towards the development of stress-linked psychiatric disorders.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) [grant number: HL088052 and HL102310 (GMT); MH093320 and MH106978 (LCD)] and American Heart Association [grant number: 25710176 (GMT)]. Stipend support for NCM and TLG was provided by NIH T32 HL07446 and T32 DA031115, respectively. The authors gratefully acknowledge technical assistance provided by MaryAnn Andrade and Roman Sanchez Martinez.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authorship contributions:
Participated in research design: Mitchell N.C., Gilman T.L., Daws L.C., and Toney G.M.
Conducted experiments: Mitchell N.C. and Gilman T.L.
Performed data analysis: Mitchell N.C. and Gilman T.L.
Wrote or contributed to the writing of the manuscript: Mitchell N.C., Gilman T.L., Daws L.C., and Toney G.M.
References
- Amaya F, Tanaka M, Hayashi S, Tanaka Y, Ibata Y. Hypothalamo-pituitary-adrenal axis sensitization after chronic salt loading. Neuroendocrinology. 2001;73(3):185–193. doi: 10.1159/000054635. [DOI] [PubMed] [Google Scholar]
- American Heart Association. Life style and risk reduction for high blood pressure. http://www.heart.org/idc/groups/heart-public/@wcm/@hcm/documents/downloadable/ucm_300625.pdf (Accessed 11 November 2017)
- Bankir L, de Rouffignac C. Urinary concentrating ability: insights from comparative anatomy. Am J Physiol. 1985;249(6 Pt 2):R643–666. doi: 10.1152/ajpregu.1985.249.6.R643. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Chen QH, Guo Q, Calderon AS, Andrade MA, Toney GM. Coping with dehydration: sympathetic activation and regulation of glutamatergic transmission in the hypothalamic PVN. Am J Physiol Regul Integr Comp Physiol. 2014a;306(11):R804–813. doi: 10.1152/ajpregu.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Sharpe AL, Toney GM. Activation of corticotropin-releasing factor receptors in the rostral ventrolateral medulla is required for glucose-induced sympathoexciation. Am J Physiol Endocrinol Metab. 2014b;307(10):E944–E953. doi: 10.1152/ajpendo.00291.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9(7):519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- Brizzee BL, Walker BR. Altered baroreflex function after tail suspension in the conscious rat. J Appl Physiol. 1985;69(6):2091–2096. doi: 10.1152/jappl.1990.69.6.2091. [DOI] [PubMed] [Google Scholar]
- Brown CH, Bains JS, Ludwig M, Stern JE. Physiological regulation of magnocellular neurosecretory cell activity: Integration of intrinsic, local and afferent mechanisms. J Neuroendocrinol. 2013;25(8):678–710. doi: 10.1111/jne.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach JP, De Hoop MJ, Schmale H, Richter D, De Kloet ER, Ten Haaf JA, De Wied D. Differential responses to osmotic stress of vasopressin-neurophysin mRNA in hypothalamic nuclei. Neuroendocrinol. 1984;39(6):582–584. doi: 10.1159/000124040. [DOI] [PubMed] [Google Scholar]
- Caffé AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septim and ventral hippocampus. J Comp Neurol. 1987;261(2):237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- Choe KY, Han SY, Gaub P, Shell B, Voisin DL, Knapp BA, Barker PA, Brown CH, Cunningham JT, Bourque C. High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron. 2015;85(3):549–560. doi: 10.1016/j.neuron.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Gordon UL. Strategies for measuring stress in studies of psychiatric and physical disorder. In: Cohen S, Kessler RC, Gordon UL, editors. Measuring Stress: A guide for Health and Social Scientists. Vol. 1995. New York, NY: Oxford University Press; 1995. pp. 3–26. [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Neurosci. 2017;8(5):955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Molendijk ML. Coping with the forced swim stressor: Towards understanding an adaptive mechanism. Neural Plasticity. 2016 doi: 10.1155/2016/6503162. ID: 6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohanics J, Kovacs KJ, Folly G, Makara GB. Long-term salt loading impairs pituitary responsiveness to ACTH secretagogues and stress in rats. Peptides. 1990;11(1):59–63. doi: 10.1016/0196-9781(90)90110-q. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Johnson KB, Breese GR. Topographical patterns of brain activity in response to swim stress: assessment of 2-deoxyglucose uptake and expression of fos-like immunoreactivity. J Neurosci. 1993;13(9):3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. Eur J Neurosci. 2002;15:384–388. doi: 10.1046/j.0953-816x.2001.01869.x. [DOI] [PubMed] [Google Scholar]
- Elias LL, Dorival Campos A, Moreira AC. The opposite effects of short- and long- term salt loading on pituitary adrenal axis activity in rats. Horm Metab Res. 2002;34(4):207–211. doi: 10.1055/s-2002-26711. [DOI] [PubMed] [Google Scholar]
- Fiedler J, Jara P, Luza S, Dorfman M, Grouselle D, Rage F, Lara HE, Arancibia S. Cold stress induces metabolic activation of thyrotrophin-releasing hormone- synthesizing neurons in the magnocellular division of the hypothalamic paraventricular nucleus and concomitantly changes ovarian sympathetic activity parameters. J Neuroendocrinol. 2006;18(5):367–376. doi: 10.1111/j.1365-2826.2006.01427.x. [DOI] [PubMed] [Google Scholar]
- Greenwood MP, Mecawi AS, Hoe SZ, Mustafa MR, Johnson KR, Al-Mahmound GA, Elias LL, Paton JF, Antunes-Rodrigues J, Gainer H, Murphy D, Hindmarch CC. A comparison of physiological and transcriptome responses to water deprivation and salt loading in the rat supraoptic nucleus. Am J Physiol Regul Integr Comp Physiol. 2015;308(7):R559–568. doi: 10.1152/ajpregu.00444.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata T, Nishikawa H, Itoh E, Watanabe A. Depressive state with anxiety in repeated cold-stressed mice in forced swimming tests. Jpn J Pharmacol. 1999;79(2):243–249. doi: 10.1254/jjp.79.243. [DOI] [PubMed] [Google Scholar]
- He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomized trails. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hernández VS, Hernández OR, Perez de la Mora M, Gomora MJ, Fuxe K, Eiden LE, Zhang L. Hypothalamic vasopressinergic projections innervate central amygdala GABAergic neurons: implications for anxiety and stress coping. Front Neural Circuits. 2016;10:92. doi: 10.3389/fncir.2016.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Qiao H, Sun B, Jia R, Fan Y, Wang N, Lu B, Yan JQ. AT1 receptor blockade in the central nucleus of the amygdala attenuates the effects of muscimol on sodium and water intake. Neurosci. 2015;307:302–310. doi: 10.1016/j.neuroscience.2015.08.069. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17(1):45–59. doi: 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosc Biobehav Rev. 2001;25(7–8):669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Krause GE, de Kloet AD, Flak JN, Smeltzer MD, Solomon MB, Evanson NK, Woods SC, Sakai RR, Herman JP. Hydration state controls stress responsiveness and social behavior. J Neurosci. 2011;31(14):5470–5476. doi: 10.1523/JNEUROSCI.6078-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause EG, Pati D, Frazier CJ. Chronic salt-loading reduces basal excitatory input to CRH neurons in the paraventricular nucleus and accelerates recovery from restraint stress in male mice. Physiol Behav. 2017;176:189–194. doi: 10.1016/j.physbeh.2017.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauand F, Ruginsk SG, Rodrigues RLP, Reid WL, De Castro M, Elias LLK, Antunes-Rodrigues J. Glucocorticoid modulation of atrial natriuretic peptide, oxytocin, vasopressin, and fos expression in response to osmotic, angiotensinergic and cholinergic stimulation. Neuorsci. 2007;147:247–257. doi: 10.1016/j.neuroscience.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Lawrence D, Pittman QJ. Response of rat paraventricular neurons with central projections to suckling, hemorrhage or osmotic stimuli. Brain Res. 1984;341:176–183. doi: 10.1016/0006-8993(85)91486-6. [DOI] [PubMed] [Google Scholar]
- Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M, Refojo D, Ekker M, Rubenstein JL, Stalla GK, Singewald N, Holsboer F, Wotjak CT, Wurst W, Deussing JM. Conditional mouse mutants highlight mechanisms of corticotropin- releasing hormone effects on stress-coping behaviors. Mol Psychiatry. 2008;13:1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- Manning M, Stoev S, Kolodziejczyk A, Klis WA, Kruszynski M, Misicka A, Olma A, Wo NC, Sawyer WH. Design of potent and selective linear antagonists of vasopressor (V1-receptor) responses to vasopressin. J Med Chem. 1990;33(11):3079–3086. doi: 10.1021/jm00173a027. [DOI] [PubMed] [Google Scholar]
- Monteiro S, Roque S, de Sá-Calcada D, Sousa N, Correia-Neves M, Cerqueira JJ. An efficient chronic unpredictable stress protocol to induce stress-related response in C57BL/6 mice. Front Psychiatry. 2015;6:6. doi: 10.3389/fpsyt.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9(3):228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacol. 2007;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Prabha K, Balan KV, Martin RJ, Lamanna JC, Haxhui MA, Dick TE. Chronic intermittent hypoxia-induced augmented cardiorespiratory outflow mediated by vasopressin-V1A receptor signaling in the medulla. Adv Exp Med Biol. 2011;701:319–325. doi: 10.1007/978-1-4419-7756-4_43. [DOI] [PubMed] [Google Scholar]
- Ribeiro N, Panizza HDN, Santos KMD, Ferreira-Neto HC, Antunes VR. Salt-induced sympathoexcitation involves vasopressin V1a receptor activation in the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2015;309(11):R1369–1379. doi: 10.1152/ajpregu.00312.2015. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Audigier S, Barberis C, Jard S, Manning M, Kolodziejczyk AS, Sawer WH. A radioiodinated linear vasopressin antagonist: a ligand with high affinity and specificity for V1a receptors. FEBS lett. 282(1):77–81. doi: 10.1016/0014-5793(91)80448-c. [DOI] [PubMed] [Google Scholar]
- Skrebuhhova T, Allikmets L, Matto V. Effects of anxiogenic drugs in rat forced swimming test. Methods Find Exp Clin Pharmacol. 1999;21(3):173–178. doi: 10.1358/mf.1999.21.3.534826. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine response to stress. Dialogues Clin Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanić D, Plećaš-Solarović B, Mirković D, Jovanović P, Dronjak S, Marković B, Đordević T, Ignjatović S, Pešić V. Oxytocin in corticosterone-induced chronic stress model: Focus on adrenal gland function. Psychoneuroendocrinol. 2017;80:137–146. doi: 10.1016/j.psyneuen.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol. 2005;563(Pt 1):249–263. doi: 10.1113/jphysiol.2004.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R, Hegoburu C, van den Burg E. New opportunities in vasopressin and oxytocin research: A perspective from the amygdala. Annu Rev Neurosci. 2015;38:369–388. doi: 10.1146/annurev-neuro-071714-033904. [DOI] [PubMed] [Google Scholar]
- Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177(1):43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- Toney GM, Porter JP. Functional roles of brain AT1 and AT2 receptors in the central angiotensin II pressor response in conscious you spontaneously hypertensive rats. Neuropharmacol. 1993;32(6):581–589. doi: 10.1016/0028-3908(93)90054-7. [DOI] [PubMed] [Google Scholar]
- Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol. 2010;588(Pt 18):3375–3384. doi: 10.1113/jphysiol.2010.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney GM, Vallon V, Stockand JD. Intrinsic control of sodium excretion in the distal nephron by inhibitory purinergic regulation of the epithelial NA(+) channel. Curr Opin Nephrol Hypertens. 2012;21(1):52–60. doi: 10.1097/MNH.0b013e32834db4a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres SJ, Turner AI, Nowson CA. Does stress induced salt intake? Br J Nutr. 2010;B103(11):1562–1568. doi: 10.1017/S000711451000098X. [DOI] [PubMed] [Google Scholar]
- Wilson TE. Renal sympathetic nerve, blood flow, and epithelial transport responses to thermal stress. Auton Neurosci. 2017;204:25–34. doi: 10.1016/j.autneu.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Wilkerson MK, Colleran PN, Delp MD. Acute and chronic head-down tail suspension diminishes cerebral perfusion in rats. Am J Physiol Heart Circ Physiol. 2002;282(1):H328–H334. doi: 10.1152/ajpheart.00727.2001. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Naruo T, Muraoka S, Simchen R, Landgraf R, Engelmann M. Forced swimming stimulate the expression of vasopressin and oxytocin in magnocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2001;13:2273–2281. doi: 10.1046/j.0953-816x.2001.01613.x. [DOI] [PubMed] [Google Scholar]
- Yanagida S, Motomura K, Ohashi A, Miura T, Kanba S. Effect of acute imipramine administration on the pattern of forced swim-induced c-Fos expression in the mouse brain. Neurosci Lett. 2016;629:119–124. doi: 10.1016/j.neulet.2016.06.059. [DOI] [PubMed] [Google Scholar]
- Yang J, Pan Y, Yin Z, Hai G, Lu L, Zhao Y, Wang D, Wang H, Wang G. Effect of arginine vasopressin on the behavioral activity in the behavior despair depression rat model. Neuropeptides. 2012;46:141–149. doi: 10.1016/j.npep.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hernández VS, Vázquez-Juárez E, Chay FK, Barrio RA. Thirst is associated with suppression of habenula output and active stress coping: is there a role for a non-canonical vasopressin-glutamate pathway? Front Neur Cir. 2016;10:13. doi: 10.3389/fncir.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


