Abstract
Tertiary lymphoid structures (TLS) are transient ectopic lymphoid aggregates that often share structural similarities to conventional secondary lymphoid organs. In a variety of solid cancers, the presence of these structures commonly correlates with high densities of tumor-infiltrating T lymphocytes and prolonged patient survival. These observations suggest that TLS act as sites for the development of beneficial antitumor immune responses. However, few murine tumor models have been described that could enable a more comprehensive understanding of the functionality of TLS in solid cancers. We previously reported that murine B16-F1 melanoma or Lewis Lung carcinoma cells transfected to express the model antigen ovalbumin form intratumoral TLS after implantation into the peritoneal cavity of C57BL/6 mice. In this chapter, we describe immunofluorescent microscopy and flow cytometry approaches for identifying and characterizing intratumoral TLS. Additionally, we describe an adoptive transfer method for demonstrating the infiltration of naïve T cells into B16-OVA melanoma tumors via the lymph node-like vasculature that is an essential functional feature of tumor-associated TLS.
Keywords: Tertiary lymphoid structure, melanoma, CD8+ T lymphocyte, peripheral node addressin, cancer-associated fibroblast, immunofluorescence, flow cytometry, adoptive cell transfer
1. Introduction
It is well-established that the immune system plays an important role in cancer immunosurveillance and tumor eradication. A high-density of CD8+ T lymphocytes infiltrating solid tumors is commonly associated with favorable clinical outcomes and patient survival [Reviewed in (1–3)]. Cytokine infusion, checkpoint blockade inhibition, vaccination and adoptive transfer strategies have all been used to increase the representation of intratumoral CD8+ T lymphocytes and bolster antitumor immunity (4–7). However, only a small fraction of patients responds favorable to these immunotherapies. Gene expression profiling of patients who respond favorably to these immunotherapies have been shown to have a pre-existing immune infiltrate prior to treatment (8–10). This suggests that the limited representation of intratumoral CD8+ T lymphocytes is a fundamental barrier to the success of current cancer immunotherapies. One potential strategy to increase the representation of CD8+ T lymphocytes in solid tumors is by increasing their exogenous infiltration through vaccination or alterations in tumor-associated vasculature. An alternative potential strategy is to enhance the intratumoral immune response by promoting the development of tertiary lymphoid structures (TLS).
TLS were initially identified as ectopic accumulations of immune cells that develop in and/or near chronically inflamed non-lymphoid tissues in association with microbial infections, graft rejection, and autoimmune disorders [Reviewed in (11)]. Some of these TLS have considerable morphological similarities to conventional secondary lymphoid organs. For example, TLS often exhibit organized T- and B-cell compartments, a stromal infrastructure that produces homing chemokines and survival factors, lymphatic vessels, and high endothelial-like vessels that express peripheral node addressin (PNAd) (12–14). Alternatively, TLS-like structures found in adipose tissue display all of the above characteristics expect for discernable T- and B-cell compartmentalization (15, 16). TLS have been documented in association with a wide variety of primary and metastatic solid tumors in humans. Their presence is almost always a favorable prognostic indicator for patient survival (17, 18). Higher densities of TLS correlate with higher representation of tumor-infiltrating CD8+ T lymphocytes (19, 20). Additionally, the presence of TLS associates with infiltrates that display an activated and cytotoxic immune signature (21). These observations suggest that tumor-associated TLS serve as productive sites for in situ activation of CD8+ T lymphocytes, which in turn control tumor growth.
The availability of animal models that could enable a more comprehensive understanding of the functionality of TLS in solid cancers has been limited. Recently, we demonstrated that naïve CD8+ T cells can directly infiltrate tumors formed from two transplantable murine cell lines: B16-F1 melanoma and Lewis Lung carcinoma. Once in the tumor, these naïve cells become activated, proliferate and differentiate into immune effectors (22). This infiltration is dependent on the development of tumor-associated blood vessels that express PNAd and CCL21, the ligands that engage CD62L and CCR7 on naïve and central memory T cells, respectively, and enable them to enter lymph nodes (LN) (23). Interestingly, tumors growing in the peritoneal cavity, but not subcutaneously, develop intratumoral TLS in association with this PNAd+/CCL21+ LN-like tumor vasculature. In this chapter, we describe methods for the development, identification and characterization of intratumoral TLS in intraperitoneal B16 melanoma tumors. Additionally, we describe an adoptive transfer method to demonstrate the function of the PNAd+/CCL21+ LN-like tumor vasculature in promoting naïve T cells infiltration.
2. Materials
2.1. Implantation of murine melanoma tumors
B16-OVA melanoma cell line (see Note 1).
Complete medium: RPMI-1640 supplemented to a final concentration of 5% (v/v) fetal bovine serum (FBS), 2 mM L-glutamine and 15 mM HEPES.
Trypsin-EDTA solution: 0.5% Trypsin-EDTA diluted 1:10 in Ca2+/Mg2+-free Hank’s balanced salt solution with sodium bicarbonate.
PBS: Ca2+/Mg2+-free phosphate-buffer saline, pH 7.4.
15 mL capped conical polystyrene tubes.
T75 cm2 culture flask.
MACS 70 μm Smart Strainers (Miltenyi).
6-8-week-old C57BL/6 mice.
Tribromoethanol working solution: Dissolve 1.25 g of tribromoethanol in 25 mL of tert-amyl alcohol. Dilute 1.25 mL of this solution into 50 mL of water.
1 mL tuberculin syringes pre-attached with a 25G × 5/8-in. needle.
2.2. Preparation of murine melanoma tumors
Tribromoethanol working solution (as described in Section 2.1.9).
Surgical scissors, forceps and tweezers.
50 mL capped conical polystyrene tubes.
Platform rocker.
PBS (see 2.1).
Fixative solution: 4% (w/v) paraformaldehyde (PFA) in PBS.
Cryopreservation solution: 30% (w/v) sucrose in PBS.
25mm × 20mm × 5mm Cryomolds (Tissue-Tek).
O.C.T. compound (Tissue-Tek).
Superfrost Plus Microscope Slides (Fisher Scientific).
Cryostat pre-set to −20°C.
Dry ice.
2.3. Immunofluorescent staining
Glass coplin jar.
Slide humidity chamber.
−20°C freezer.
KimWipes.
Rat anti-mouse CD16/CD32 monoclonal blocking antibody (BioXcell).
Methanol, histological grade, 98%.
Staining buffer: 5% (w/v) bovine serum albumin (BSA) in PBS.
Avidin/Biotin blocking kit (Vector Laboratories).
Quenching solution: 3% hydrogen peroxide, 0.1% (w/v) sodium azide in PBS.
Tris-NaCl blocking buffer (TNB): 0.1 M Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% (w/v) tyramide signal amplification blocking reagent (PerkinElmer).
Tyramide signal amplification (TSA) plus biotin kit (Perkin Elmer).
Monoclonal antibodies for immunofluorescent staining (Table 1).
DyLight-550 conjugated Streptavidin (ThermoFisher Scientific).
ProLong Gold Antifade Mountant with DAPI (ThermoFisher Scientific).
25 × 50 Premium Cover glasses.
Horseradish-peroxidase conjugated streptavidin.
Table 1:
Antibodies for detecting TLS in tumors by immunofluorescence
| Specificity | Clone | Conjugate | Supplier | Working Dilution |
|---|---|---|---|---|
| PNAd | MECA-79 | Biotin | BioLegend | 1:50 |
| CD31 | 390 | Alexa Fluor 647 | BioLegend | 1:100 |
| Podoplanin | 8.1.1 | eFluor 660 | ThermoScientific/eBioscience | 1:100 |
| B220 | RA3–6B2 | Alexa Fluor 488 | BioLegend | 1:100 |
| CD11c | N418 | Biotin | BioLegend | 1:50 |
| CD3 | 17A2 | Alexa Fluor 647 | BioLegend | 1:50 |
2.4. Preparation of tumor tissue
10× MACS buffer: 0.5% (w/v) BSA, 2 mM EDTA.
10× PBS.
Awesome MACS buffer: 100 mL of 10× MACS buffer, 100 mL of 10× PBS; 10 mL of 200 mM L-glutamine solution, 10 mL of 100× sodium pyruvate solution, 10 mL of 100× non-essential amino acid solution, 20 mL of 50× essential amino acid solution and 4.5 grams of glucose in 1 L final volume. Adjust pH to 7.4 and filter solution with a 0.22 μm steritop filter.
Digestion buffer: 10 mL of FBS, 5 mL of 200 mM L-glutamine, 5 mL of 100× sodium pyruvate, 5 mL of 100× non-essential amino acids, 10 mL of 50× essential amino acids, 500 μL of 10 mg/mL gentamicin and 7.5 mL of 1M HEPES in 500 mL final volume of L-glutamine-free and sodium pyruvate-free Dulbecco’s Modified Eagles Medium (DMEM).
Working buffer: 0.42 U/mL of Liberase™ (Sigma) and 60 U/mL of DNase I (Sigma) in Digestion buffer.
Harvest buffer: 50 mL of FBS, 5 mL of 200 mM L-glutamine, 5 mL of 100× sodium pyruvate, 5 mL of 100× non-essential amino acids, 10 mL of 50× essential amino acids, 500 μL of 10 mg/mL gentamicin and 7.5 mL of 1M HEPES in 500 mL final volume of DMEM.
MACS 70 μm Smart Strainers (Miltenyi).
Red Blood Cell Lysing Buffer Hybri-Max (Sigma).
Water or bead bath set at 37°C.
Mouse CD45 MicroBeads (Miltenyi Biotec).
Anti-biotin MicroBeads (Miltenyi Biotec).
LS columns (Miltenyi Biotec).
MACS manual magnetic separator (Miltenyi Biotec).
Monoclonal antibodies for enrichment (Table 2).
Monoclonal antibodies for cell surface staining (Table 3).
Anti-mouse CD16/CD32 monoclonal blocking antibody (BioXcell).
Polystyrene 96-well, No Lid, V-bottom plate.
5 mg/mL DAPI viability dye.
Table 2:
Antibodies for magbead enrichment of stromal populations for flow cytometry
| Specificity | Clone | Conjugate | Supplier | Working Dilution |
|---|---|---|---|---|
| Podoplanin | 8.1.1 | Biotin | BioLegend | 1:1000 |
| CD31 | 390 | Biotin | BioLegend | 1:1000 |
Table 3:
Antibodies for flow cytometry staining of cell surface markers on stromal and lymphocyte populations
| Specificity | Clone | Conjugate | Supplier | Working Dilution |
|---|---|---|---|---|
| CD45 | 30-F11 | Alexa Fluor 488 | BioLegend | 1:2000 |
| CD3 | 17A2 | Alexa Fluor 647 | BioLegend | 1:500 |
| CD19 | 6D5 | PE-Cy7 | BioLegend | 1:1000 |
| CD8α | 53–6.7 | APC-eFluor780 | ThermoScientific/ eBioscience | 1:1000 |
| Podoplanin | 8.1.1 | APC | BioLegend | 1:1000 |
| CD31 | 390 | PerCP-Cy5.5 | BioLegend | 1:1000 |
| Ter119 | Ter119 | PE-Cy7 | BioLegend | 1:500 |
| Thy1.1 | HIS51 | PE | ThermoScientific/ eBioscience | 1:1000 |
2.5. Adoptive transfer of CD8+ T lymphocytes
6-8-week-old C57BL/6 mice bearing 10-14 days-old B16-OVA tumors (Section 3.1).
6-8-week-old congenic Thy1.1 mice (see Note 2).
Tribromoethanol working solution (see Section 2.1.9).
CTL medium: 50 mL of FBS, 5 mL of 200 mM L-glutamine, 5 mL of 100× sodium pyruvate, 5 mL of 100× non-essential amino acids, 10 mL of 50× essential amino acids, 500 μL of 10 mg/mL gentamicin, 7.5 mL of 1 M HEPES and 454 μL of 55 mM 2-meraptoethanol in a final volume of 500 mL of RPMI-1640.
Autoclaved 7 mL Dounce homogenizer.
MACS 70 μm Smart Strainers (Miltenyi).
Red Blood Cell Lysing Buffer Hybri-Max (Sigma).
Awesome MACS buffer (Section 2.4.3).
Naïve CD8+ T cell isolation kit (Miltenyi Biotec).
LS columns (Miltenyi Biotec).
MACS manual magnetic separator (Miltenyi Biotec).
Heating lamp with a 250W (infrared) bulb.
Tailveiner restrainer (BrainTree Scientific, Inc.).
1 mL tuberculin syringes pre-attached with a 25G × 5/8-in. needle.
3. METHODS
3.1. Implantation of subcutaneous and intraperitoneal murine melanoma tumors
Culture B16-OVA melanoma cells in Complete Medium in a T75 flask to 60-70% confluency.
Aspirate supernatant, rinse flask with 5 mL room temperature Trypsin-EDTA solution and aspirate again. Add 5 mL Trypsin-EDTA solution and tilt to cover the bottom of the flask. Incubate 2-3 min at 37°C, or until cells have fully detached.
Add 5 mL cold Complete Medium and transfer cell suspension to a 15 mL conical tube. Rinse flask with 5 mL cold Complete Medium and add to the 15 mL conical tube. Centrifuge 5 min at 600 × g at 4°C.
Aspirate supernatant, resuspend in 5 mL cold Complete Medium, centrifuge 5 min at 600 × g at 4°C.
Aspirate supernatant, resuspend in 5 mL cold PBS, centrifuge for 5 min at 600 × g at 4°C. Repeat once more.
Resuspend in 5 mL cold PBS, pass through MACS Smart Strainer into a new 15 mL conical tube, count live cells and adjust to 2 × 106 cells/mL with cold PBS.
For subcutaneous tumors only, anesthetize mice by intraperitoneal injection of 15 μL tribromoethanol working solution per g of mouse weight (see Note 3).
Subcutaneously or intraperitoneally inject 200 μL (4 × 105 cells) of cell suspension into C57BL/6 mice with a 1 mL syringe with a 25G × 5/8-in. needle. Allow subcutaneous tumors to grow for 14 days and intraperitoneal tumors to grow for 12 days (see Note 4).
3.2. Preparation of murine melanoma tumors for immunofluorescence microscopy
Anesthetize mice by intraperitoneal injection of 25 μL tribromoethanol working solution per g of mouse weight. Euthanize by cervical dislocation and dissect out tumor with surgical scissors and forceps.
Transfer each tumor to a separate 50 mL conical tube containing 10 mL room temperature PBS. Rock on platform rocker for 5 min at room temperature.
Using forceps, transfer tumor to a new 50 mL conical tube containing 30 mL room temperature fixative solution. Cap and gently rock for 1 h at room temperature (see Note 5).
Using forceps, transfer tumor to a new 50 mL conical tube containing 30 mL cold cryopreservation solution and gently rock overnight at 4°C.
Aspirate cryopreservation solution, transfer tumor to Cryomold with forceps, embed it in OCT and place on dry ice for 30 min.
Cut 7-μm sections with a −20°C cryostat and mount on Superfrost Plus microscope slides.
3.3. Immunofluorescent staining of cell surface markers on formalin-fixed tumor sections
Immerse slides in a coplin jar containing cold methanol 10 min at −20°C.
Remove slides, wipe off excess methanol with KimWipes, and air-dry 10 min at room temperature.
Immerse slides in a coplin jar containing PBS 10 min at room temperature.
Remove slides, place in a humidity chamber, and pipette 200 μL 0.5 μg/mL anti-CD16/32 antibody in staining buffer uniformly across sections. Incubate 15 min at room temperature to block Fc receptors.
Immerse slides in a coplin jar containing PBS 5 min at room temperature. Remove slides, blot off liquid with KimWipes and place in humidity chamber.
Pipette 200 μL Avidin D solution from Avidin/Biotin blocking kit uniformly across sections. Incubate 15 min at room temperature to block endogenous biotin. Wash as in Step 5.
Pipette 200 μL biotin solution from Avidin/Biotin blocking kit uniformly across sections. Incubate 15 min at room temperature to block Avidin D. Wash as in Step 5.
Pipette 200 μL quenching solution from Avidin/Biotin blocking kit uniformly across sections. Incubate 30 min at room temperature to block endogenous peroxidases. Wash as in Step 5.
Pipette 200 μL biotin conjugated anti-PNAd antibody in TNB buffer uniformly across sections. Incubate overnight at 4°C. Wash as in Step 5.
Pipette 200 μL horseradish-peroxidase conjugated streptavidin in TNB buffer uniformly across sections. Incubate 45 min at room temperature. Wash as in Step 5 (see Note 6).
Pipette 200 μL 0.5 μg/mL biotin conjugated tyramide from TSA kit uniformly across sections. Incubate 10 min at room temperature. Wash as in Step 5.
Pipette 200 μL 0.5 μg/mL of DyLight550 conjugated streptavidin in staining buffer uniformly across sections. Incubate 1 h at room temperature. Wash as in Step 5.
Pipette cell surface marker antibody cocktail in staining buffer (Table 1) (see Note 7) uniformly across sections. Incubate 1 h at room temperature. Wash as in Step 5.
Apply ProLong Gold DAPI Antifade Mountant and apply coverslip.
3.4. Identification and characterization of TLS in B16 melanoma by Immunofluorescence
Acquisition and analysis of immunofluorescent images can be performed with any fluorescent microscope and image software package, respectively. We use a Zeiss AxioImager with Apotome and an AxioCam MRm camera, and Zeiss Zen image analysis software. We define intratumoral TLS to have the following features:
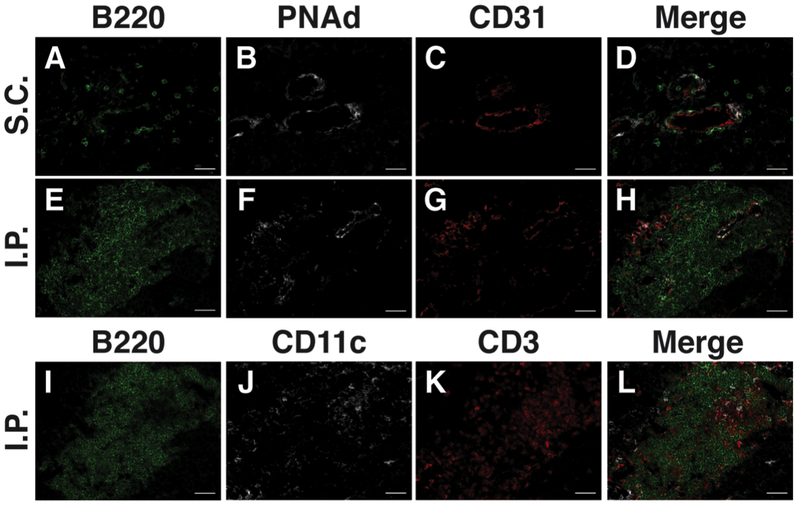
Aggregates of 50 or more B220+ B lymphocytes in juxtaposition to PNAd+ CD31+ vasculature. Note that such aggregates are present in intraperitoneal tumors (Fig. 1E-H), but lacking in subcutaneous tumors (Fig. 1A-D), despite the presence of PNAd+ vasculature and small numbers of B220+ B lymphocytes.
T lymphocytes and CD11c+ antigen-presenting cells intermingled with B lymphocyte aggregates (Fig. 1I-L)
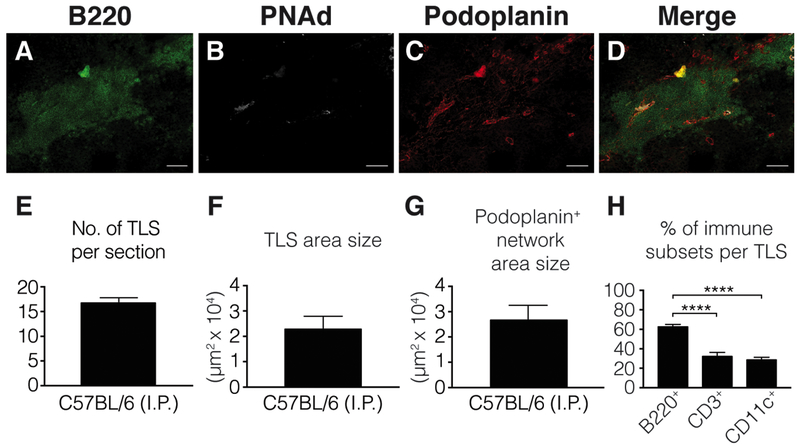
A co-extensive reticular network of podoplanin+ fibroblasts (Fig. 2A-D).
Additional characteristics of TLS in B16-OVA tumors are shown in Fig. 2E-H.
Figure 1:
B16-OVA tumors grown in the subcutaneous (A-D) or intraperitoneal (E-L) cavities of C57BL/6 mice were harvested, histologically prepared and stained for immunofluorescent microscopy as outlined in Sections 3.1–3.3. Tumor sections were stained with antibodies of the indicated specificities (Table 1). (E-H) and (I-L) are serial sections of a single B16-OVA intraperitoneal tumor. Abbreviations: S.C., subcutaneous; I.P., intraperitoneal. Scale bar = 50 μm.
Figure 2:
A B16-OVA tumor growing in the intraperitoneal cavity of a C57BL/6 mouse was harvested, histologically prepared and stained for immunofluorescent microscopy (A-D), as outlined in Sections 3.1–3.3. Tumor sections were stained with antibodies of the indicated specificities (Table 1). Scale bar = 100 μm. (F-G) Area measurements were done by drawing a perimeter around TLS and podoplanin+ structures with the polygon tool in Zen software. Frequency of TLS (E) and individual immune subsets (H) was done by using the manual count tool in Zen software. Abbreviation: I.P., intraperitoneal. Error bars represent mean ± SEM. ns: P>0.05, *P<0.05, **P<0.01, ***P<0.001, ****P,0.0001. P values in (H) were calculated using the Mann-Whitney test.
3.5. Preparation, enrichment and cell surface marker staining of tumor-associated hematopoietic and stromal cells for flow cytometry
Anesthetize mice by intraperitoneal injection of 25 μL tribromoethanol working solution per g of mouse weight. Euthanize by cervical dislocation and dissect out tumor with surgical scissors and forceps. Transfer to a weigh boat filled with cold Digestion Buffer (see Note 8).
Aspirate Digestion Buffer from weigh boat and mince tumor with scissors into ∼1 mm3 pieces. Flush minced tumor into a 15 mL conical tube with 5 mL of Working Buffer (see Note 9). Incubate 30 min in a 37°C water bath. Pipette suspension gently at 5 min intervals to break up aggregates (see Note 10).
Pass suspension through MACS Smart Strainer into a new 15 mL conical tube. Wash digestion tube with 5 mL cold Harvest Buffer and pass through filter. Centrifuge 5 min at 600 × g at 4°C.
Aspirate supernatant, resuspend in 5 mL cold Harvest Buffer, centrifuge 5 min at 600 × g at 4°C and aspirate supernatant.
Resuspend in 2 mL Red Blood Cell Lysing Buffer Hybri-Max. Incubate 1 min at 37°C.
Add 8 mL cold Harvest Buffer, centrifuge 5 min at 600 × g at 4°C and aspirate supernatant. Repeat this step twice.
Resuspend in 5 mL cold Awesome MACS buffer (see Note 8) and count viable cells. A typical cell yield for a B16-OVA tumor is 5 × 107. Centrifuge 5 min at 600 × g at 4°C and aspirate supernatant.
Resuspend in 80 μL cold Awesome MACS buffer and 20 μL CD45 MicroBeads per 107 cells. Mix by inverting the 15 mL conical tube and incubate on ice for 15 min. Centrifuge 5 min at 600 × g at 4°C and aspirate supernatant. Resuspend in cold Awesome MACS buffer to 2 × 108 cells/mL. Proceed immediately to magnetic separation.
Rinse LS column in a MACS manual magnetic separator with 3 mL of cold Awesome MACS buffer.
Pipette cell suspension onto column and allow to flow by gravity. Collect CD45Neg cells that flow through into a new 15 mL conical tube. Rinse column with 3 mL of cold Awesome MACS buffer three times, collecting CD45Neg cells that flow though into same collection tube. Count viable cells, cap tube and keep on ice. A typical cell yield of CD45Neg cells from a B16-OVA tumor is 4 × 107.
Remove column from magnetic separator and insert into a new 15 mL conical tube. Apply 3 mL of cold Awesome MACS buffer and firmly flush out CD45Pos cells with supplied plunger. Count viable cells, cap tube and keep on ice. A typical cell yield of CD45Pos cells from a B16-OVA tumor is 1 × 107.
Centrifuge CD45Neg cells 5 min at 600 × g at 4°C. Aspirate supernatant, and resuspend in cold Awesome MACS buffer at 107 cells/mL. Add biotinylated anti-podoplanin and/or anti-CD31 (Table 2) to a final concentration of 0.5 μg/mL. Incubate on ice for 30 min. Centrifuge 5 min at 600 × g at 4°C and aspirate supernatant.
Resuspend CD45Neg cells in 80 μL cold Awesome MACS buffer and 20 μL of anti-biotin MicroBeads per 107 cells. Mix by inverting the tube and incubate 15 min on ice. Centrifuge 5 min at 600 × g at 4°C and aspirate supernatant. Resuspend in cold Awesome MACS buffer to 2 × 108 cells/mL.
Repeat Steps 9-11 with a new LS column using these CD45neg cells as input. Discard cells that flow through while the column is in the magnetic separator, and collect the podoplanin+ and/or CD31+ cells that are eluted with the plunger after the column is removed from the separator.
Centrifuge the CD45Pos cells collected in Step 11 and enriched stromal fraction collected in Step 14 5 min at 600 × g at 4°C. Aspirate supernatant and resuspend in 5 mL of cold Awesome MACS buffer. Centrifuge 5 min at 600 × g at 4°C and aspirate supernatant.
Resuspend CD45Neg cells in 100 μL cold Awesome MACS buffer and pipet entire suspension to a 96-well plate. Resuspend CD45Pos cells in 1 mL cold Awesome MACS buffer and pipet 100 μL (1 × 106 cells) to same 96-well plate. Centrifuge 5 min at 600 × g at 4°C and aspirate supernatant.
Resuspend both fractions in 100 μL cold Awesome MACS buffer containing 0.5 μg/mL of anti-CD16/32. Incubate 15 min at 4°C, centrifuge 5 min at 600 × g at 4°C and aspirate supernatant.
Resuspend CD45Pos cells in 100 μL cold Awesome MACS buffer with CD45, CD3, CD8α and CD19 antibodies, and the CD45Neg cells in 100 μL of cold awesome MACS buffer with CD45, Ter119, CD31 and gp38 antibodies (Table 3). Incubate 30 min at 4°C, centrifuge 5 min at 600 × g at 4°C and aspirate supernatant.
Resuspend both fractions in 5 mL cold Awesome MACS buffer. Centrifuge 5 min at 600 × g at 4°C and aspirate supernatant. Repeat this step twice.
Resuspend both fractions in 1 mL cold Awesome MACS buffer containing 0.2 μg/mL DAPI. Incubate 30 min at 4°C, and analyze by flow cytometry.
3.6. Cellular characteristics of TLS containing B16 melanoma tumors by flow cytometry
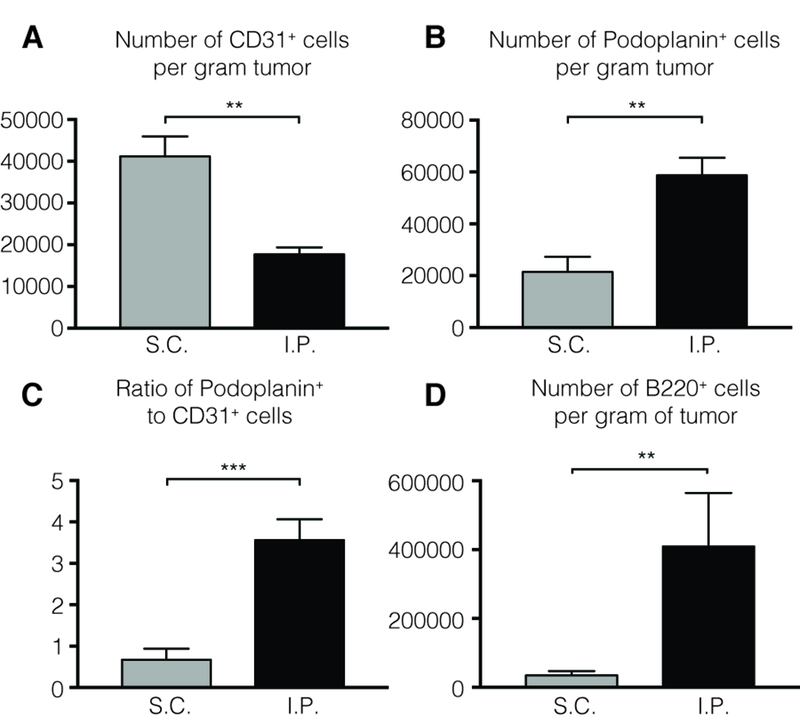
We utilize Beckman Coulter CytoFlex S units for acquisition, and imported FCS files into FlowJo software (TreeStar) for analysis. While flow cytometry does not enable TLS-associated cells to be directly distinguished from non-TLS associated cells in tumors, we have found significant quantitative differences between IP and SC tumors in certain populations that are TLS associated. For comparably sized tumors, the number of CD31+ endothelial cells is actually lower in IP tumors (Fig. 3A). However, the number of podoplanin+ fibroblasts is substantially higher (Fig. 3B). While the podoplanin/CD31 ratio in SC tumors is slightly less than 1, the ratio in IP tumors is about 3.5, consistent with the presence of the reticular network that evident by immunofluorescence (Fig. 3C). This provides a method to further delineate the characteristics of both populations in these different tumor microenvironments. Similarly, the average number of B lymphocytes in IP tumors is about 10-fold greater than in SC tumors (Fig. 3D), and these are largely concentrated in TLS. The number of T cells in both tumors is comparable, about 106 cells.
Figure 3:
Intraperitoneal and subcutaneous B16-OVA tumors grown in C57BL/6 mice were prepared for flow cytometry, as outlined in Sections 3.1 and 3.4. Endothelial cells were gated as live singlet CD45neg Ter119neg CD31pos. Fibroblasts are gated as live singlet CD45neg Ter119neg Podoplaninpos. B cells are gated as live singlet CD45pos CD3neg CD19pos. Abbreviations: S.C., subcutaneous; I.P., intraperitoneal. Error bars represent mean ± SEM. ns: P>0.05, *P<0.05, **P<0.01, ***P<0.001, ****P,0.0001. P values were calculated using the Mann-Whitney test.
3.7. Adoptive transfer of naïve CD8+ T lymphocytes into mice with established tumors
Follow steps in Section 3.1 to grow intraperitoneal or subcutaneous B16-OVA melanoma cells in C57BL/6 mice.
Eighteen hours prior to tumor harvest, anesthetize one or more (see Note 11) congenic Thy1.1 mice by intraperitoneal injection of 25 μL tribromoethanol working solution per g of mouse weight. Euthanize by cervical dislocation and dissect lymph nodes (axillary, brachial, inguinal, cervical and mesenteric) and spleen with surgical scissors and forceps. Pool tissues into an autoclaved Dounce homogenizer containing 5 mL of cold CTL medium and homogenize to create a single-cell suspension.
Pipet suspension through MACS Smart Strainer into a 15 mL conical tube. Wash homogenizer with 5 mL cold CTL medium and pipet through filter. Centrifuge 5 min at 600 × g at 4°C and aspirate supernatant.
Resuspend in 2 mL Red Blood Cell Lysing Buffer Hybri-Max and incubate 1 min in a 37°C water bath. Add 8 mL cold CTL medium, centrifuge 5 min at 600 × g at 4°C and aspirate supernatant.
Resuspend in 5 mL cold Awesome MACS buffer, count viable cells and adjust to 108/400 μL. Add 100 μL of naïve CD8α T-cell biotin-antibody cocktail (supplied with kit) per 108 cells. Mix by inverting tube and incubate 5 min on ice.
Add 200 μL cold Awesome MACS buffer, 200 μL anti-biotin Microbeads and 100 μL of CD44 MicroBeads per 108 cells (both supplied with kit). Mix by inverting tube and incubate 10 min on ice.
Add 10 mL cold Awesome MACS buffer, centrifuge 5 min at 600 × g at 4°C and aspirate supernatant. Resuspend in 500 μL cold Awesome MACS buffer per 108 cells.
Apply suspension to a pre-rinsed LS column (Section 3.5) attached to a magnetic separator. Collect flow-through into a new 15 mL conical tube. Rinse column with 3 mL cold Awesome MACS buffer and collect flow-through into same tube. Centrifuge collection tube 5 min at 600 × g at 4°C and aspirate supernatant.
Resuspend in 5 mL cold PBS, centrifuge for 5 min at 600 × g at 4°C and aspirate supernatant. Resuspend in 5 mL of cold PBS, determine the cell count and adjust to a final concentration of 2 × 107 cells/mL.
Place a single tumor-bearing C57BL/6 mice under an infrared heat lamp until lateral tail veins are apparent (typically 3-5 min).
Restrain mouse in a Tailveiner and inject 200 μL (4 × 106 cells) into the lateral tail vein using a 1 mL syringe with a 25G × 5/8-in. needle. Repeat Steps 10-11 for additional tumor-bearing animals.
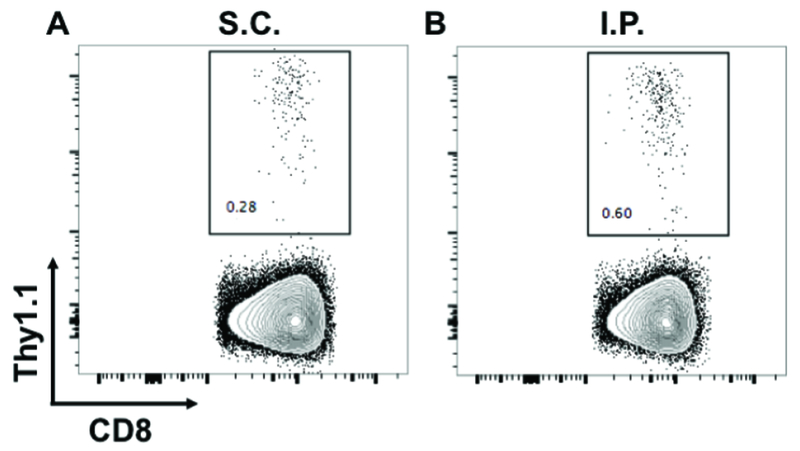
Eighteen h after adoptive transfer (see Note 12), tumor-infiltrating CD8+ T lymphocytes can be isolated and quantitated as described in Section 3.5 using the antibody cocktail described in Table 3, with the addition of anti-Thy1.1. Typical results are shown in Fig 4.
Figure 4:
Naïve CD8+ T cells from congenic Thy1.1 mice were intravenously injected into C57BL/6 mice with established subcutaneous (A) and intraperitoneal (B) B16-OVA tumors. Tumors were harvested 18 h later and infiltrating Thy1.1pos T cells were enumerated by flow cytometry. Numbers on plots indicate the percentage of Thy1.1pos cells out of the live singlet CD45pos CD3pos CD8αpos parent gate. Abbreviations: S.C., subcutaneous; I.P., intraperitoneal.
Acknowledgments
Funding: This work was supported by United States Public Health Service (USPHS) grants R01 CA78400 and R01 CA181794 (V.H.E.). Additional support was provided by USPHS grant P30 CA0044579 to the University of Virginia Cancer Center. A.B.R. was supported by USPHS training grant AI007496 and the Wagner Fellowship. J. D. P. was supported by USPHS training grant CA009109 and the Farrow Fellowship.
Footnotes
We use a B16-F1 melanoma cell line (ATCC) transfected to stably express chicken ovalbumin (B16-OVA). These cells present the OVA257 epitope on H2-Kb (24). This was determined by flow cytometry after incubating cultured B16-OVA cells overnight at 37°C with 100U/mL of murine recombinant IFNγ, staining with anti-mouse SIINFEKL-H-2Kb (BioLegend) and evaluation by flow cytometry.
We utilize congenic Thy1.1 mice to distinguish exogenous versus endogenous CD8+ T lymphocytes. An alternative congenic marker, such CD45.1, can be substituted.
We use tribromoethanol to minimize movement during subcutaneous tumor implantation. This maneuver diminishes spreading of cells after injection, and results in more regularly formed tumors.
These conditions lead to subcutaneous tumors that are 2 mm in each of 2 measurable dimensions. Intraperitoneal tumors grow somewhat faster and may be harvested earlier for that reason. Since intraperitoneal tumors cannot be measured directly, ensure that mice are not allowed to gain more than 5-10% in bodyweight and show signs of discomfort.
Overnight fixation provides excellent preservation of tumor morphology. However, some cell surface marker become undetectable (i.e. CD31). We’ve determined that 1 h fixation still provides excellent tumor morphology and detection of all markers described.
PNAd is expressed at relatively low levels on tumor vasculature when compared to LN-HEV. Tyramide signal amplification enables successful visualization.
We use B220, CD31 and PNAd to identify TLS, CD3 and CD11c to identify T cells and antigen-presenting cells, respectively, and podoplanin to identify fibroblasts. Specific fluorochromes for each are shown in Table 1, and representative staining combinations are shown in Fig. 1 and Fig. 2.
The steps in Sections 3.5–3.6 should be carried as quickly as possible. Isolated endothelial cells and fibroblasts from lymphoid organs and tumors are fragile and significantly lose viability over a few hours. Awesome MACS buffer provides with nutrients to maintain their survival for the duration of the experiment. Enrichment steps and staining of isolated stromal cells should always be carried out in this buffer.
Liberase TM and DNAse I at the concentrations specified in Working Buffer enable high yield isolation of tumor-associated stromal cells from B16 without substantial loss of CD3, CD8, CD4, and B220 on tumor-infiltrating lymphocytes. If you are assessing alternative markers or tumors, optimizing enzyme concentrations and incubation times is recommended.
Cell suspension should initially be pipetted several times with a cut 1000 μL pipette tip. After 10 min, aggregates should easily be pipetted with an un-cut 1000 μL tip. If not, prepare a single-cell suspension using a Dounce homogenizer.
Pooled lymph nodes and spleen from a single congenic Thy1.1 mouse provides 4.0 × 106 naïve CD8+ T cells, sufficient for a two tumor-bearing animal. It is recommended to use three congenic Thy1.1 mice for a maximum of 7 tumor-bearing animals.
7 References
- 1.Vesely M, Kershaw M, Schreiber R, Smyth M (2011) Natural innate and adaptive immunity to cancer. Annu Rev Immunol 29:235–271. [DOI] [PubMed] [Google Scholar]
- 2.Fridman WH, Pagès F, Sautès-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12:298–306. doi: 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 3.Galon J, Angell HK, Bedognetti D, Marincola FM (2013) The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 39:11–26. doi: 10.1016/j.immuni.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Restifo NP, Yang JC, et al. (2008) Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 8:299–308. doi: 10.1038/nrc2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klebanoff CA, Acquavella N, Yu Z, Restifo NP (2011) Therapeutic cancer vaccines: are we there yet? Immunol Rev 239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S, Margolin K (2011) Cytokines in cancer immunotherapy. Cancers 3:3856–3893. doi: 10.3390/cancers3043856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gajewski TF, Louahed J, Brichard VG (2010) Gene signature in melanoma associated with clinical activity. Cancer J 16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8 [DOI] [PubMed] [Google Scholar]
- 9.Ji R- R, Chasalow SD, Wang L, et al. (2012) An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 61:1019–1031. doi: 10.1007/s00262-011-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajewski TF, Schreiber H, Fu Y- X (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14:1014–1022. doi: 10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitzalis C, Jones GW, Bombardieri M, Jones SA (2014) Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 14:447–462. doi: 10.1038/nri3700 [DOI] [PubMed] [Google Scholar]
- 12.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH (1996) Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med 183:1461–1472. doi: 10.1084/jem.183.4.1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley CD, Barone F, Nayar S, et al. (2015) Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol 33:715–745. doi: 10.1146/annurev-immunol-032713-120252 [DOI] [PubMed] [Google Scholar]
- 14.Ruddle NH (2016) High endothelial venules and lymphatic vessels in tertiary lymphoid organs: characteristics, functions, and regulation. Front Immunol 7:491. doi: 10.3389/fimmu.2016.00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randall TD, Mebius RE (2014) The development and function of mucosal lymphoid tissues: a balancing act with micro-organisms. Mucosal Immunol 7:455–466. doi: 10.1038/mi.2014.11 [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Migoni S, Caamaño J (2016) Fat-associated lymphoid clusters in inflammation and immunity. Front Immunol 7:612. doi: 10.3389/fimmu.2016.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieu-Nosjean M- C, Goc J, Giraldo NA, et al. (2014) Tertiary lymphoid structures in cancer and beyond. Trends Immunol 35:571–580. doi: 10.1016/j.it.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 18.Goc J, Fridman W- H, Sautès-Fridman C, Dieu-Nosjean M- C (2013) Characteristics of tertiary lymphoid structures in primary cancers. OncoImmunology 2:e26836. doi: 10.4161/onci.26836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behr DS, Peitsch WK, Hametner C, et al. (2014) Prognostic value of immune cell infiltration, tertiary lymphoid structures and PD-L1 expression in Merkel cell carcinomas. Int J Clin Exp Pathol 7:7610–7621. [PMC free article] [PubMed] [Google Scholar]
- 20.Caro GD, Bergomas F, Grizzi F, et al. (2014) Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res 20:2147–2158. doi: 10.1158/1078-0432.CCR-13-2590 [DOI] [PubMed] [Google Scholar]
- 21.Goc J, Germain C, Vo-Bourgais TKD, et al. (2014) Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 74:705–715. doi: 10.1158/0008-5472.CAN-13-1342 [DOI] [PubMed] [Google Scholar]
- 22.Thompson ED, Enriquez HL, Fu Y- X, Engelhard VH (2010) Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med 207:1791–1804. doi: 10.1084/jem.20092454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peske JD, Thompson ED, Gemta L, et al. (2015) Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun 6:7114. doi: 10.1038/ncomms8114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hargadon KM, Brinkman CC, Sheasley-O’Neill SL, et al. (2006) Incomplete differentiation of tumor-specific CD8+ T cells in tumor-draining lymph nodes. J Immunol 177:6081–6090. [DOI] [PubMed] [Google Scholar]
- 25.Palazón A, Teijeira A, Martínez-Forero I, et al. (2011) Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res 71:801–811. doi: 10.1158/0008-5472.CAN-10-1733 [DOI] [PubMed] [Google Scholar]
- 26.Sasaki K, Zhu X, Vasquez C, et al. (2007) Preferential expression of very late antigen-4 on type 1 CTL cells plays a critical role in trafficking into central nervous system tumors. Cancer Res 67:6451–6458. doi: 10.1158/0008-5472.CAN-06-3280 [DOI] [PubMed] [Google Scholar]
- 27.Scimone ML, Aifantis I, Apostolou I, et al. (2006) A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci 103:7006–7011. doi: 10.1073/pnas.0602024103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walch JM, Zeng Q, Li Q, et al. (2013) Cognate antigen directs CD8+ T cell migration to vascularized transplants. J Clin Invest 123:2663–2671. doi: 10.1172/JCI66722 [DOI] [PMC free article] [PubMed] [Google Scholar]