Abstract
A method for identification of highly fluorescent compounds in vine leaves infected by Plasmopara viticola was developed using reversed phase liquid chromatography with simultaneous diode array and fluorometric detection. Fluorescent compounds were extracted from leaves with a methanol-water mixture (70:30). Separation by HPLC was performed using a C18 column and gradient elution with water-acetonitrile mixtures (20–80% of acetonitrile). The main unknown fluorescent compound was identified by line spectral comparison with a standard obtained by UV photoisomerization of trans-resveratrol glucoside, and its structure was confirmed by liquid chromatography-mass spectrometry. Identification and structural elucidation of the fluorescent compound in the leaves of Vitis vinifera allows early detection of Plasmopara viticola invasion.
Keywords: trans-resveratrol; transformation; 2,4,6-trihydroxyphenanthrene-2-O-glucoside; Plasmopara viticola
1. Introduction
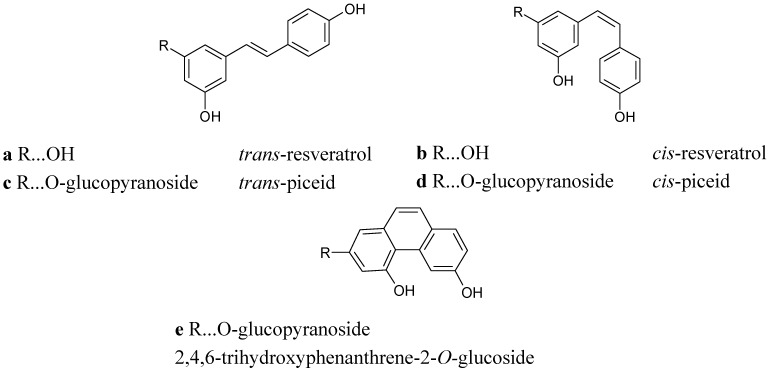
Hydroxylated stilbenes are phytoalexins synthesized by plants, especially in the fruit skins, leaves, and roots, in response to fungal infections and UV light [1,2,3]. Resveratrol (stilbene-3,5,4'-triol) is a constituent of vine plants and wine that has attracted enormous research interest in recent years due to its broad range of biological activities, especially its antioxidant activity. Resveratrol is known to occur in wine in free and glycosidically bound forms. Free trans- and cis-resveratrol (Figure 1a,b) are present in concentration ranges of 0.2–13 mg/L in red wines and 0.1–0.8 mg/L in white wines. For the bound forms of resveratrol, concentrations of the so-called piceids (trans- and cis-resveratrol-3-O-β-glucosides) (Figure 1c,d), are reported to be in a ranges of 0.3–9 mg/L in red and 0.1–2.2 mg/L in white wines [4,5,6]. Ribeiro de Lima et al. [7] determined piceids in Portuguese red wines even in concentrations up to 68 mg/L. Large amounts of cis-and trans-piceid accumulate in healthy berries of different grape varieties [8], as well as following UV light treatment [9] or powdery mildew (Uncinula necator) infection [10]. Interaction between Plasmopara viticola infection and stilbene synthesis in leaves and berries of ten Cabernet Sauvignon clones were recently studied by van Zeller et al. [11]. The isolation and structural characterization of 10 major viniferins in the leaves infected by Plasmopara viticola was very recently described by Mattivi et al. [12]. The literature includes numerous references to content of phytoalexins, and especially trans-resveratrol, in grapevines and wines, but only a few references mention the content of that compound in the leaves, e.g., the first original paper from Langcake and Pryce [1] and more recently that of Babíková et al. [13]. The cis- form of resveratrol is normally present to a lesser extent in grape leaves. The presence of cis-resveratrol in the leaves, berries and wines is, according to the literature, a consequence of biological procedures (so-called “bioproduction”) for treating V. vinifera plants in the vineyards [14]. This means that cis-resveratrol could be a “biomarker” for wines made from grapes not treated with any chemicals and which were under increased impact of “biochemicals” produced most probably by Botrytis cinerea or P. viticola.
The antioxidant activity of resveratrol has been linked to reduced mortality from coronary heart disease (CHD) [15]. Resveratrol also inhibits human platelet aggregation in vitro [16] and modulates eicosanoid synthesis toward a pattern likely to be protective against CHD [17,18]. More recently, Gehm et al. [19] have reported estrogenic properties of resveratrol that may also contribute to the reported cardioprotective effects of wine consumption. The literature includes an increasing number of publications addressing various aspects of biological and health effects of resveratrol, e.g., recent review of Guilford and Pezzuto [20]. The urgent need to exchange experience in this area led to organization of the first International Conference of Resveratrol and Health, held in Copenhagen in 2010. All articles dealing with health aspects of resveratrol were published in a special issue of the Annals of the New York Academy of Sciences in 2011 [21].
Vitis vinifera varieties are highly susceptible to downy mildew (P. viticola) infection, and some fungicides are needed to sustain economic grape production. Fungicides, especially copper derivatives, are directly linked to ecological harm, however, and therefore reasonable alternative mechanisms for grapevine protection are urgently needed. trans-Resveratrol and its derivatives are main phytoalexins of Vitis vinifera plants. trans-Resveratrol is synthesized in the free form by the action of stilbene synthase and then is rapidly glycosylated by a glycosyltransferase to trans-resveratrol glucoside. Both free and glycosylated forms of trans-resveratrol could isomerize to cis- forms either by UV irradiation or in healthy plants most probably by a cis-isomerase, which has not been characterized to date [22]. Further isomerization to a trihydroxy phenanthrene derivative would be catalyzed by cis-isomerase, most probably present together with other enzymes in P. viticola tissues, and/or by the sun’s UV irradiation. The first step in the verification of this hypothesis is to find phenanthrene derivatives both in the healthy and infected grapevine leaves.
Figure 1.
Chemical structures of the stilbene and phenanthrene derivatives.
2. Results and Discussion
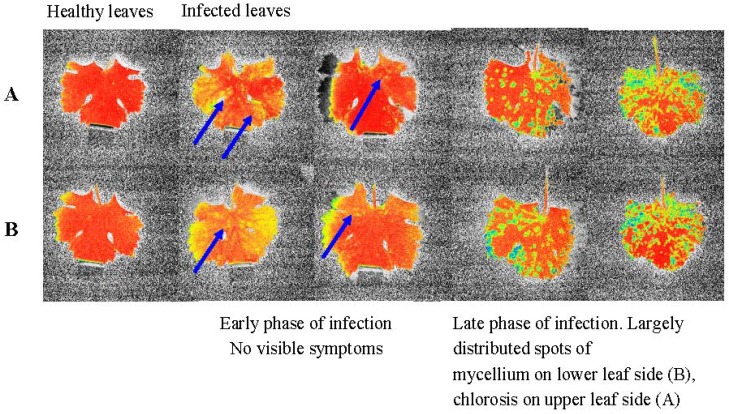
The distribution of fluorescence emission was measured in healthy and infected grape leaves during July 2009 and 2010 at vineyards in Kostice and Lednice, Czech Republic. These vineyards are located at an altitude of 176 m a.s.l and have absolutely no fungicidal protection (biodynamic). Controlled varieties were grafted on Teleki 5C rootstock. The training is central (trunk 0.7 m) with one cane (8 to 10 buds). The varieties are planted 1 m apart. The inter-row width is 2.2 m. Natural occurrence of fungi is an early and significant occurrence, but was an artificial infection of shrubs (mixture of water and spray-pathogen spores, loose-zoospore). From each variety (susceptible varieties: Chardonnay, Sauvignon, Muscatel, Müller Thurgau and Alibernet, resistant varieties: Cerason, Malverina, Hibernal and Laurot), 10 healthy leaves and 10 infected leaves in total were measured. In the case of sensitive varieties in the early stage of infection when no noticeable visible symptoms were yet visible, ΦII showed heterogeneous distribution of small patches on the leaves having locally reduced photosynthetic activity due to infection. As the infection progressed, the number of places with reduced photosynthetic activity increased continuously and their total area was also increasing. In the case of resistant varieties, the places with reduced ΦII at an early stage of infection were far more extensive. While in such cases there was no expansion of the affected areas, the plants localized the infection into a large spot on the leaf, which was followed by necrosis. Necrosis of infected tissue is clearly visible in Figure 2 for the Sauvignon variety.
Figure 2.
Infection by Plasmopara viticola progress in the leaves of susceptible variety Sauvignon. Upper row A illustrates increasing chlorosis on the upper side of the leaf, lower row B illustrates the growth of mycelium on the lower leaf side. The arrows highlight the spots where the infection (necrosis) started.
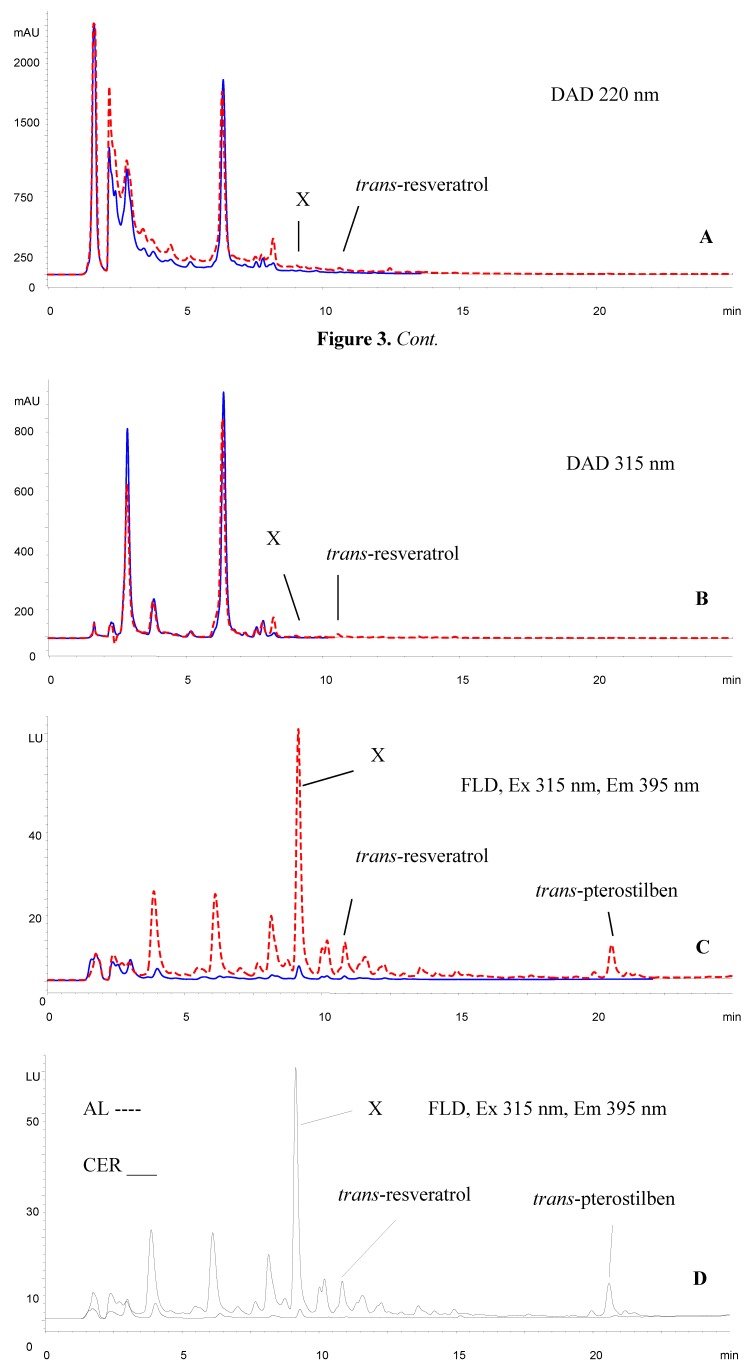
For all leaves, significant ΦII changes in leaf area were correlated with the infected sites. The samples for determining phenolic compounds and photosynthetic pigments were taken in the areas with reduced photosystem II activity (low ΦII). Methanolic extracts of the samples were measured by UV (DAD) and fluorescence detection, whereby four peaks were clearly visible in the retention times window starting at 4 min. The largest, most distinct peak had a retention time of 9.15 min (see Figure 3).
Figure 3.
HPLC chromatograms of the extracts of the Alibernet variety (susceptible) leaves. Comparison of healthy leaves (blue line) and leaves infected by Plasmopara viticola (red dashed line). (A) Chromatogram of the extract at 220 nm. (B) The same chromatogram at 315 nm. (C) Chromatogram of the extract using FLD detector. Compound X is 2,4,6-trihydroxyphenanthrene-2-O-glucoside. (D) Comparison of the susceptible infected variety Alibernet (dashed line) and resistant infected variety Cerason (solid line).
Formation of phenanthrene derivatives as final products of the UV photo isomerization reaction of trans-resveratrol to cis-resveratrol is described in the literature, whereby the latter undergoes a photo cyclization reaction to phenanthrene [23,24]. Without elucidating any structure for a new compound, Merás et al. have postulated that possible phenanthrene derivatives could occur as a result of UV irradiation of the piceid solution [25].
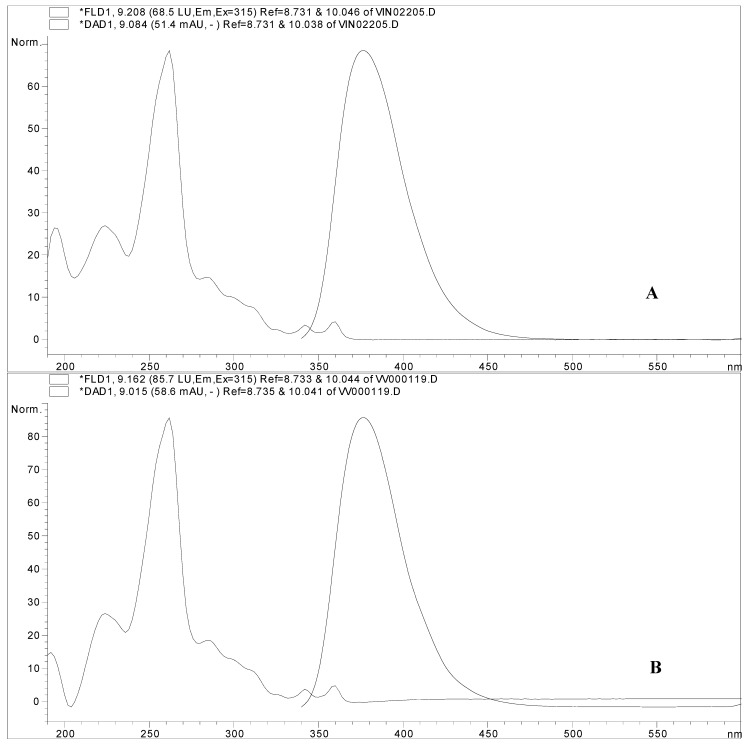
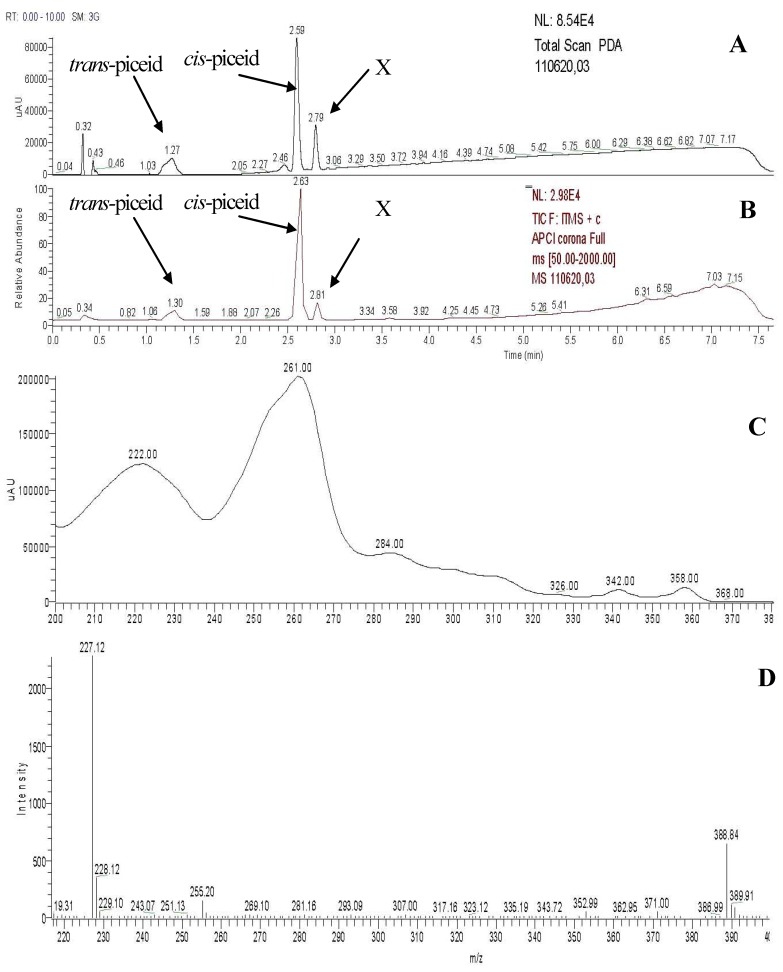
To test the possibility of trans-piceid phototransformation into our highly fluorescent compound, an experiment was performed whereby trans-piceid was irradiated in an open vial at 254 nm for 4 h. After irradiation, there appeared in addition to the two already existing components (trans- and cis-piceid) a new component (x), which eluted also at the retention time of 9.15 min overlapping with the peak and UV spectra of the unknown substance (Figure 4).
Figure 4.
(A) UV spectrum of irradiated trans-piceid standard (dashed line) and its emission spectrum (solid line). (B) UV spectrum of the Alibernet variety leaves extract (dashed line) and its emission spectrum (solid line), which is peak X in the chromatogram shown in Figure 3.
Figure 5.
LC-MS analysis of the irradiated trans-piceid standard. (A) Total scan from the photo diode array detector. (B) Full spectrum from LC-MS APCI measurement in the positive mode. (C) UV spectrum of the peak with the retention time of 2.80 min. (D) Mass spectrum from LC-MS APCI measurement in the positive mode of the peak with the retention time of 2.80 min.
To confirm that this new peak is the assumed phenanthrene derivative, LC-MS measurements were carried out using the Accela Fleet ion trap LC-MS instrument and APCI ionization technique in the positive mode. By LC-MS measurement of the peak with the retention time 2.80 min, we observed two distinct ions: a molecular ion at m/z 389 [M+H]+ and an ion at [M–162]+ (Figure 5, Table 1). The fragment with m/z 162 is anhydroglucose and it is typical for the fragmentation of glucosides. There is ion 135+ missing in the fragmentation spectrum, which is typical for trans- or cis-resveratrol. In UV spectrum there is a maximum at 261 nm, which is typical for trihydroxy phenanthrene derivative—see Montsko et al. [24].
Table 1.
MS/MS measurements of 2,4,6-trihydroxyphenanthrene-2-O-glucoside.
| MS technique | Parent ion | Fragments ions |
|---|---|---|
| + APCI | 389+ | 371+ (M-H2O) |
| 389+ | 353+ (M-2H2O) | |
| 389+ | 335+ (M-3H2O) | |
| 389+ | 227+ (M-162) | |
| 227+ | 209+ (M-H2O) | |
| 227+ | 199+, 181+, 157+ |
This molecular ion and typical fragmentation provide convincing results, as the observed data and fragmentation pattern are consistent with the proposed structure. Therefore, according to all experimental data, our main unknown fluorescent compound must be 2,4,6-trihydroxyphenanthrene-2-O-glucoside (Figure 1e). This trihydroxyphenanthrene O-glucoside was recently obtained from a multilayer countercurrent chromatography fraction after careful purification using a series of separation steps [26]. As to the hypothetical formation of 2,4,6-trihydroxyphenanthrene-2-O-glucoside from cis-piceid, photochemical reactions as well as enzymatic activities can be considered likely. To the best of our knowledge, 2,4,6-trihydroxyphenanthrene-2-O-glucoside is reported here for the first time as a natural product whose amount is significantly increased in relation to the impact of P. viticola. The concentration of 2,4,6-trihydroxyphenanthrene-2-O-glucoside in the non-infected leaves is in the range of 2.36–14.24 μg.g−1 f.w. for susceptible varieties and in the range of 2.76–8.31 μg.g−1 f.w. for resistant varieties.
3. Experimental
3.1. Standards
Both trans-resveratrol and trans-piceid were purchased from Sigma-Aldrich (Prague, Czech Republic).
3.2. Sample Preparation
Using a Open FluorCam imaging fluorometer (P.S. Instruments, Ltd., Brno, Czech Republic), the distribution parameter ΦII was measured. Red wine varieties Cerason and Laurot originated from interspecific crosses ‘Merlan’ (‘Merlot’ × Seibel 13 666) × ‘Fratava’ (Blaufränkisch × St. Laurent); white wine variety Malverina originated from crosses Rakish (Villard blanc × Frühroter Veltliner) × Merlan (Merlot × Seibel 13 666) and Hibernal originated from crosses Chancellor × Riesling Weiss.
For the analysis of phenolic compounds (especially the two isomers of resveratrol) in leaves, three samples (the tissue in the center of the infection, the tissue 1 cm distant from the infection, and healthy tissue) from each measured leaf were taken as cuts of circular shape using a cork borer. Diameter of the cuts was 14 mm and the area was 154 mm2. Samples were weighed, placed in vials, and 0.5 mL of 70% methanol was added. Extraction was for 24 h at room temperature in darkness, with occasional shaking. The methanolic extract was then collected, passed through a glass filter, and analyzed using HPLC. After extraction, the cut leaf samples were lyophilized and dry cuts were extracted using ethyl acetate. Ethyl acetate was then evaporated with nitrogen flow and the residue dissolved in 0.5 mL of methanol. Extracts were stored at −20 °C until HPLC measurement. Both extracts were analysed separately.
3.3. Photo Isomerization Experiments
The solution of trans-piceid in a 4 mL open vial in 50% methanol was irradiated by UV lamp (Camag, Switzerland, Catalog No. 29010, Serial No. 921022) at 254 nm and laboratory temperature.
3.4. Samples Analysis
The samples were analyzed using an HP 1050 (Ti-series) HPLC instrument (Hewlett Packard, Palo Alto, CA, USA) on a 3 μm, 150 mm × 2 mm, Luna C18(2) column (Phenomenex, Torrance, CA, USA) with water-acetonitrile-o-phosphoric acid mobile phase. Mobile phase A used 5% of acetonitrile + 0.1% of o-phosphoric acid; mobile phase B used 80% of acetonitrile + 0.1% of o-phosphoric acid. The gradient was increased from 20% of B to 80% of B during 20 min and from 80% of B to 100% of B during 5 min. Flow rate was 0.250 mL min−1 and column temperature 25 °C. Injection volume was 5 μL. Also used were an HP G1315B diode array detector (DAD, Hewlett-Packard), with detection wavelengths at 220 and 315 nm, and scanning range 190–600 nm, as well as an HP G1321A fluorescence detector (FLD, Hewlett-Packard), with excitation wavelength 315 nm, emission wavelength 395 nm, and scanning of emission in the range of 300–600 nm. Finally, the method was validated in terms of linearity, limits of detection, and repeatability, and it was applied to the analysis of leaves extracts samples. Detection limits for piceid and 2,4,6-trihydroxyphenanthrene-2-O-glucoside were calculated, and were found to be 0.128 μg/mL for trans-piceid and 19.2 ng/mL for 2,4,6-trihydroxyphenanthrene-2-O-glucoside.
LC-MS was performed using an LCQ Accela Fleet (Thermo Fisher Scientific, San Jose, CA, USA) equipped with electro-spray (ESI), atmospheric pressure chemical (APCI) and atmospheric pressure photo (APPI) ionization sources and a photodiode array detector. A 1.9 μm, 50 mm × 2.1 mm, Hypersil Gold column (Thermo Electron Corporation, Bellefonte, PA, USA) was used with water-acetonitrile-formic acid mobile phase. Mobile phase A used 5% of acetonitrile + 0.1% of formic acid; mobile phase B used 80% of acetonitrile + 0.1% of formic acid. The gradient was increased from 15% of B in 1 min to 100% of B in 6.5 min and decreased to 15% of B in 6.51 min then held up to 10 min. Injection volume was 10 μL and flow rate 0.400 mL min−1. APCI capillary temperature was 275 °C, APCI vaporizer temperature 400 °C, sheath gas flow 58 L min−1, auxiliary gas flow 10 L min−1, source voltage 6 kV, source current 5 μA, and capillary voltage 10 V.
4. Conclusions
This work demonstrates identification of highly fluorescent compounds in vine leaves infected by Plasmopara viticola using reversed phase liquid chromatography with simultaneous diode array and fluorometric detection. 2,4,6-Trihydroxyphenanthrene-2-O-glucoside was identified for the first time as a main fluorescent natural product arising in relation to Plasmopara viticola impact on the vine leaves. The identification was performed by line spectral comparison with a standard obtained by UV photo isomerization of trans-resveratrol glucoside and its structure was confirmed by liquid chromatography-mass spectrometry.
Acknowledgements
This study was funded by the Grant Agency of the Czech Republic, grant number 525/09/0365, and by CzechGlobe—Centre for Global Climate Change Impacts Studies, Reg. No. CZ.1.05/1.1.00/02.0073.
Footnotes
Sample Availability: Samples of the extracts and irradiated trans-piceid are available from the authors.
References and Notes
- 1.Langcake P., Pryce R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant. Pathol. 1976;9:77–86. doi: 10.1016/0048-4059(76)90077-1. [DOI] [Google Scholar]
- 2.Langcake P., Pryce R.J. The production of resveratrol and the viniferins by grapevines in response to ultraviolet irradiation. Phytochemistry. 1977;16:1193–1196. [Google Scholar]
- 3.Jeandet P., Bessis R., Maume B.F., Meunier P., Peyron D., Trollat P. Effect of enological practices on the resveratrol isomer content of wine. J. Agric. Food Chem. 1995;43:316–319. doi: 10.1021/jf00050a010. [DOI] [Google Scholar]
- 4.Lamuela-Raventós R.M., Romero-Pérez A.I., Waterhouse A.L., de la Torre-Boronat M.C. Direct HPLC analysis of cis- and trans-resveratrol and piceid isomers in Spanish red Vitis vinifera wines. J. Agric. Food Chem. 1995;43:281–283. doi: 10.1021/jf00050a003. [DOI] [Google Scholar]
- 5.Goldberg D.M., Ng E., Karumanchiri A., Diamandis E.P., Soleas G.J. Resveratrol glucosides are important components of commercial wines. Am. J. Enol. Viticult. 1996;47:415–420. [Google Scholar]
- 6.Sato M., Suzuki Y., Okuda T., Yokotsuka K. Content of resveratrol, piceid, and their isomers in commercially available wines made from grapes cultivated in Japan. Biosci.Biotechnol. Biochem. 1997;61:1800–1805. doi: 10.1271/bbb.61.1800. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro de Lima M.T., Waffo-Téguo P., Teissedre P.L., Pujolas A., Vercauteren J., Cabanis J.C., Mérillon J.M. Determination of stilbenes (trans-astringin, cis-and trans-piceid, and cis- and trans-resveratrol) in Portuguese wines. J. Agric. Food Chem. 1999;47:2666–2670. doi: 10.1021/jf9900884. [DOI] [PubMed] [Google Scholar]
- 8.Gatto P., Vrhovsek U., Muth J., Segala C., Romualdi C., Fontana P., Pruefer D., Stefanini M., Moser C., Mattivi F., Velasco R. Ripening and genotype control stilbene accumulation in healthy grapes. J. Agric. Food Chem. 2008;56:11773–11785. doi: 10.1021/jf8017707. [DOI] [PubMed] [Google Scholar]
- 9.Adrian M., Jeandet P., Douillet-Breuil A.C., Tesson L., Bessis R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000;48:6103–6105. doi: 10.1021/jf0009910. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Perez A.I., Lamuela-Raventos R.M., Andres-Lacueva C., de La Torre-Boronat M.C. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J. Agric. Food Chem. 2001;49:210–215. doi: 10.1021/jf000745o. [DOI] [PubMed] [Google Scholar]
- 11.van Zeller de Macedo Basto Goncalves M.I., Bavaresco L., Civardi S., Ferrari F. Interactions between Plasmopara viticola infection and stilbene synthesis in leaves and berries of ten ‘Cabernet Sauvignon’ clones. Vitis. 2011;50:119–122. [Google Scholar]
- 12.Mattivi F., Vrhovsek U., Malacarne G., Masuero D., Zulini L., Stefanini M., Moser C., Velasco R., Guella G. Profiling of resveratrol oligomers, important stress metabolites, accumulating in the leaves of hybrid Vitis vinifera (Merzling × Teroldego) genotypes infected with Plasmopara viticola. J. Agric. Food Chem. 2011;59:5364–5375. doi: 10.1021/jf200771y. [DOI] [PubMed] [Google Scholar]
- 13.Bábíková P., Vrchotová N., Tříska J., Kyseláková M. Content of trans-resveratrol in leaves and berries of interspecific grapevine (Vitis sp.) varieties. Czech J. Food Sci. 2008;26:13–17. [Google Scholar]
- 14.Otreba J.B., Berghofer E., Wendelin S., Eder R. Polyphenole und antioxidative kapazität in österreichischen weinen aus konventioneller und biologischer traubenproduktion. Mitteilungen Klosterneuburg, Rebe und Wein. Obstbau und Früchteverwertung. 2006;56:22–32. [Google Scholar]
- 15.Frankel E.N., Waterhouse A.L., Kinsella J.E. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 16.Orsini F., Pelizzoni F., Verotta L., Aburjai T. Isolation, synthesis, and antiplatelet aggregation activity of resveratrol-3-O-β-D-glucopyranoside and related compounds. J. Nat. Prod. 1997;60:1082–1087. doi: 10.1021/np970069t. [DOI] [PubMed] [Google Scholar]
- 17.Kimura Y., Okuda H., Arichi S. Effects of stilbenes on arachidonate metabolism in leucocytes. Biochim. Biophys.Acta. 1985;834:275–278. doi: 10.1016/0005-2760(85)90167-5. [DOI] [PubMed] [Google Scholar]
- 18.Pace-Asciak C.R., Hahn S., Diamandis E.P., Soleas G., Goldberg D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implication for protection against coronary heart disease. Clin. Chim. Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 19.Gehm B.D., McAndrews J.M., Chien P.-Y., Jameson J.L. Resveratrol, a polyphenolic compound found in grapes and wine is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilford J., Pezzuto J.M. Wine and health: A review. Am. J. Enol. Viticult. 2011;62:471–486. doi: 10.5344/ajev.2011.11013. [DOI] [Google Scholar]
- 21.Vang O., Das D.K., editors. Resveratrol and health. Annals of the New York Academy of Sciences. Wiley-Blackwell; New York, NY, USA: 2011. pp. 1–170. [Google Scholar]
- 22.Chong J., Poutaraud A., Hugueney P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009;177:143–155. doi: 10.1016/j.plantsci.2009.05.012. [DOI] [Google Scholar]
- 23.Vicinelli V., Ceroni P., Maestri M., Lazzari M., Balzani V., Lee S.K., van Heyst J., Vogtle F. Photochemical and photophysical properties of a poly(propylene amine) dendrimer functionalised with E-stilbene units. Org. Biomol. Chem. 2004;2:2207–2213. doi: 10.1039/b404463k. [DOI] [PubMed] [Google Scholar]
- 24.Montsko G., Pour Nikfardjam M.S., Szabo Z., Boddi K., Lorand T., Ohmacht R., Mark L. Determination of products derived from trans-resveratrol UV photoisomerisation by means of HPLC-APCI-MS. J. Photochem. Photobio. A. 2008;196:44–50. doi: 10.1016/j.jphotochem.2007.11.011. [DOI] [Google Scholar]
- 25.Merás I.D., Díaz T.G., Rodríguez D.A. Determination of piceid by photochemically induced fluorescence and second-derivative: Response surface methodology for the optimization of a liquid-liquid extraction procedure for its analysis in wine samples. Talanta. 2008;74:675–682. doi: 10.1016/j.talanta.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 26.Baderschneider B., Winterhalter P. Isolation and characterization of novel stilbene derivatives from Riesling wine. J. Agric. Food Chem. 2000;48:2681–2686. doi: 10.1021/jf991348k. [DOI] [PubMed] [Google Scholar]