Abstract
Purpose
Cytoreductive therapy is beneficial in patients with essential thrombocythemia (ET) at high risk of thrombosis. However, its value in those lacking high-risk features remains unknown. This open-label, randomized trial compared hydroxycarbamide plus aspirin with aspirin alone in patients with ET age 40 to 59 years and without high-risk factors or extreme thrombocytosis.
Patients and Methods
Patients were age 40 to 59 years and lacked a history of ischemia, thrombosis, embolism, hemorrhage, extreme thrombocytosis (platelet count ≥ 1,500 × 109/L), hypertension, or diabetes requiring therapy. In all, 382 patients were randomly assigned 1:1 to hydroxycarbamide plus aspirin or aspirin alone. The composite primary end point was time to arterial or venous thrombosis, serious hemorrhage, or death from vascular causes. Secondary end points were time to first arterial or venous thrombosis, first serious hemorrhage, death, incidence of transformation, and patient-reported quality of life.
Results
After a median follow-up of 73 months and a total follow-up of 2,373 patient-years, there was no significant difference between the arms in the likelihood of patients reaching the primary end point (hazard ratio, 0.98; 95% CI, 0.42 to 2.25; P = 1.0). The incidence of significant vascular events was low, at 0.93 per 100 patient-years (95% CI, 0.61 to 1.41). There were also no differences in overall survival; in the composite end point of transformation to myelofibrosis, acute myeloid leukemia, or myelodysplasia; in adverse events; or in patient-reported quality of life.
Conclusion
In patients with ET age 40 to 59 years and lacking high-risk factors for thrombosis or extreme thrombocytosis, preemptive addition of hydroxycarbamide to aspirin did not reduce vascular events, myelofibrotic transformation, or leukemic transformation. Patients age 40 to 59 years without other clinical indications for treatment (such as previous thrombosis or hemorrhage) who have a platelet count < 1,500 × 109/L should not receive cytoreductive therapy.
INTRODUCTION
Essential thrombocythemia (ET) is a chronic myeloid malignancy characterized by thrombocytosis and, in most patients, a somatic mutation affecting JAK2 (50% to 60%), CALR (25% to 35%), or MPL (5% to 10%). A major complication is thrombosis, most frequently arterial. High risk of thrombosis is associated with age older than 60 years or history of prior thrombosis1-3; other factors may include the JAK2 V617F mutation, cardiovascular risk factors, or increased white cell count.4-6 Patients have increased risk of hemorrhage, especially with marked thrombocytosis, whereas platelet count is not associated with thrombotic risk.5,7 A minority of patients transform to myelofibrosis (MF), acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), or polycythemia vera (PV).8
Patients with ET at high risk of thrombosis developed fewer thrombotic events when treated with the cytoreductive agent hydroxycarbamide.9 In the Primary Thrombocythemia-1 (PT-1) high-risk study, the composite vascular end point of arterial thrombosis, venous thrombosis, serious hemorrhage, or death from vascular events was reduced in patients randomly assigned to hydroxycarbamide compared with those who received anagrelide.8 Hydroxycarbamide therapy was associated with lower risks of arterial thrombosis, serious hemorrhage, MF, and treatment intolerance, which established hydroxycarbamide as first-line treatment for patients requiring cytoreduction. Aspirin reduced the risk of thrombotic events in PV10 and, although prospective data in ET are lacking, aspirin has been recommended to reduce vascular risk.11,12
For patients with ET who lack features associated with high vascular risk, the rate of thrombosis is low (< 2% per year),13,14 but may be higher in the subgroup age 40 to 59 years.2 On the basis of these data, patients age 40 to 59 years were designated intermediate risk in the PT-1 study. There are no randomized data indicating whether cytoreductive therapy is of value in this group. Although some authors suggest that hydroxycarbamide might increase the risk of AML or nonhematologic malignancies,15,16 this has not been confirmed.3,17,18 Moreover, the chronic disease course of these patients, with complications that occur at a low rate but have major clinical consequences, means that clinical studies should follow a substantial cohort of patients over a prolonged time period. We report the results of the PT-1 intermediate-risk prospective, randomized trial comparing hydroxycarbamide plus aspirin with aspirin alone in patients with ET age 40 to 59 years and lacking high-risk factors.
PATIENTS AND METHODS
Patients
Patients were recruited from 140 hospitals in the United Kingdom, Ireland, Australia, France, and New Zealand between July 21, 1997, and July 31, 2012. Research ethics committees in each country approved the study protocol, and all participants gave written informed consent.
Eligible patients met Polycythemia Vera Study Group diagnostic criteria for ET (listed in the Data Supplement) and were either newly diagnosed or previously treated. Patients were classified as intermediate risk if they were age 40 to 59 years and did not meet any high-risk criteria: a history of ischemia, thrombosis, or embolism; hemorrhage caused by ET; hypertension or diabetes requiring pharmacologic therapy; current or previous platelet counts ≥ 1,000 × 109/L. The exclusion for extreme thrombocytosis reflects the increased risk of hemorrhage associated with acquired von Willebrand syndrome, such that cytoreduction is warranted in these patients.5,12,19 During the study (on May 6, 2004), the upper limit of the platelet count for eligibility was increased to 1,500 × 109/L. Exclusion criteria are listed in the Data Supplement.
Randomization
Eligible patients were randomly assigned in a 1:1 ratio to receive aspirin alone or hydroxycarbamide plus aspirin. Patients were entered into the trial by fax or by telephoning the Clinical Trial Service Unit (CTSU), Oxford, United Kingdom, until June 23, 2010, and subsequently at the Cambridge Clinical Trials Unit (CCTU), Cambridge, United Kingdom. Random assignment was accomplished by the central computer at CTSU and CCTU, using a minimization algorithm20 to ensure that equal numbers of patients were allocated to each arm, overall and within subgroups of previous treatment (none, aspirin only, cytoreductive therapy only, or both). A random number generator was used to assign the initial patients, then by minimization with a random component. At CTSU, treatment allocations were assigned by the randomization program and communicated by staff to the enrolling doctor or nurse by fax or telephone, followed by a confirmation letter to the responsible clinician. At CCTU random assignments and treatment allocations were handled by fax to the treating clinician.
Procedures
The study was open label. Patients assigned to hydroxycarbamide plus aspirin were treated with 0.5 to 2 g oral hydroxycarbamide once per day, adjusted to maintain platelet counts within the range of 200 to 400 × 109/L. All patients were advised to take aspirin 75 mg once per day (100 mg in Australia) or an alternative antiplatelet agent if aspirin was contraindicated. Visit frequency, which was at the physician’s discretion, was recommended to be at a minimum of every 3 months. Details of principal end point diagnoses and adverse events were recorded annually in both arms, and quality-of-life data were recorded annually for the first 5 years (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 [EORTC QLQ-C30], version 2).
Peripheral blood samples for central molecular analysis were collected at trial entry from 259 patients for DNA extraction (Strangeways Research Laboratory, Cambridge, United Kingdom) and screened for JAK2 V617F, CALR, and MPL mutations at the Department of Haematology, University of Cambridge (described in the Data Supplement). For 177 patients, a bone marrow trephine biopsy performed at trial entry was reviewed centrally by two hematopathologists for diagnostic accuracy (123 patients had a peripheral blood smear provided).
Outcomes
The composite primary end point was time from random assignment until the patient died as a result of thrombosis or hemorrhage or had a serious hemorrhage or thrombotic event (see the Data Supplement). Secondary end points were time to the first arterial or venous thrombotic event or to the first serious hemorrhage; time to death; incidence of transformation to MF, AML, MDS, or PV; and patient-reported quality of life. Full end point definitions are listed in the Data Supplement. End points that occurred before May 31, 2013, and were reported before October 31, 2013, were validated after independent evaluation by two clinicians who were blinded to the patients’ treatment assignments; this included review of peripheral blood smears and/or bone marrow samples for transformations to MF, AML, and MDS. The study chairman resolved any disagreements.
Trial Oversight
Annual interim analyses, including details of primary and secondary end points and unexpected or serious toxicities, were assessed by an independent data monitoring committee. Stopping guidelines stated that a difference of at least three standard deviations in an interim analysis of a major end point might be needed to justify halting or modifying the trial prematurely (the Haybittle-Peto rule). The study was funded by the Medical Research Council, United Kingdom, and Cancer Research UK. The funders had no role in study design, data collection, analysis, or interpretation, or writing the report. The corresponding author had full access to all study data and had final responsibility for the decision to submit the report for publication.
Statistical Analysis
Sample size was originally calculated based on the following: if the primary end point were in the order of 4% per annum,2,6 detection of a 50% reduction in complication rate (at 2P = .05, with 80% power) in the hydroxycarbamide-plus-aspirin arm compared with the aspirin-alone arm, the trial was estimated to require a sample size of 280 in each arm observed for a median of 4 years. Based on the possibility of a low event rate, a decision was made in November 2006 that a number-needed-to-treat (NNT) calculation was more appropriate and to continue recruitment until 2012. This duration of follow-up would be the maximum period of time required to generate either a significantly different treatment effect or a point estimate for the NNT to prevent one primary end point per year of > 100, with 95% confidence that NNT is greater than 50.
Rates of the primary and secondary end points and treatment changes were compared between arms on an intention-to-treat basis using Kaplan-Meier curves and log-rank tests, with censoring at the time of death, withdrawal from the study, or last visit for patients lost to follow-up. For the quality-of-life analysis, EORTC QLQ-C30 summary scores21 were calculated for each patient questionnaire and compared between the arms using linear mixed effects models. Hemoglobin, white cell, and platelet counts were analyzed for patients with at least four blood count recordings available in the first 120 months on the study by using linear mixed effects models. Additional details are provided in the Data Supplement.
RESULTS
Patients and Treatment
The PT-1 intermediate-risk trial randomly assigned 382 patients to receive aspirin alone (190 patients) or hydroxycarbamide plus aspirin (192 patients) between July 21, 1997, and July 31, 2012 (Fig 1). Twenty-four patients who were identified after random assignment to be ineligible for trial entry (incorrect diagnosis or risk group) were excluded from the analysis (Fig 1). Median duration of follow-up was 73 months (range, 0 to 187 months): 73 months in the aspirin-alone arm (range, 3 to 183 months) and 73 months in the hydroxycarbamide-plus-aspirin arm (range, 1 to 187 months). Total follow-up was 2,373 patient-years.
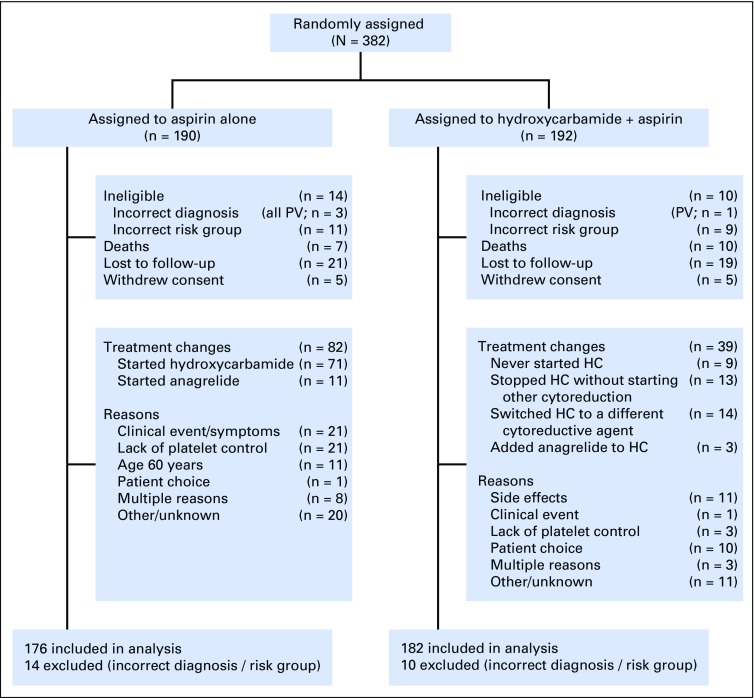
Fig 1.
CONSORT diagram. Details of patients randomly assigned, treated, and followed up in the study. Data were not collected regarding eligibility screening before randomization. Changes to cytoreductive treatment are shown. In the aspirin-alone arm, 71 patients started hydroxycarbamide (HC) and 11 started anagrelide; 23 patients subsequently received one or more additional agents (hydroxycarbamide [14], anagrelide [11], interferon-alfa [1], thalidomide [1]). In the hydroxycarbamide-plus-aspirin arm, nine patients were randomly assigned to hydroxycarbamide but never started it; 13 patients stopped hydroxycarbamide without simultaneously starting a second-line agent; 14 patients switched to an alternative cytoreductive agent (anagrelide [12], interferon-alfa [1], busulphan [1]), and 3 started anagrelide without stopping hydroxycarbamide. PV, polycythemia vera.
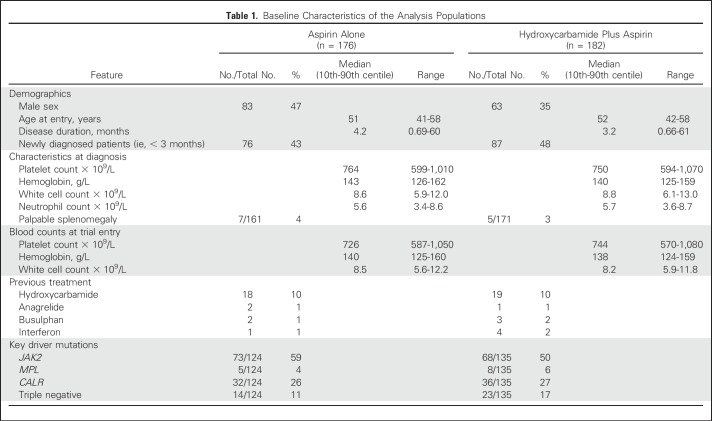
The two arms were well matched with respect to laboratory and clinical features at diagnosis and trial entry (Table 1). Of the 259 patients with molecular information, 141 (54%) had a JAK2 V617F mutation, 68 (26%) a CALR mutation, 13 (5%) an MPL mutation, and 37 (14%) none of these mutations. Of 177 patients for whom a trephine biopsy at trial entry was reviewed centrally, 167 (94%) met British Committee for Standards in Haematology diagnostic criteria for ET,11 other diagnoses being myeloproliferative neoplasm, unclassifiable (5 patients) and primary MF (5 patients). One hundred forty-three (81%) met WHO diagnostic criteria for ET.22
Table 1.
Baseline Characteristics of the Analysis Populations
Changes in cytoreductive treatment were permitted at the discretion of the treating physician and are shown, with reasons, in Figure 1. In the aspirin-alone arm, 82 (47%) of 176 patients in the analysis population started a cytoreductive agent; the median time without cytoreduction from trial entry (ie, time until treatment change, death, consent withdrawal, loss to follow-up, or end of trial) was 36 months. In the hydroxycarbamide-plus-aspirin arm, 39 (21%) of 182 patients in the analysis population had a change in treatment, defined as stopping hydroxycarbamide and/or starting an alternative cytoreductive agent; the median time on trial hydroxycarbamide from trial entry (ie, time until treatment change, death, consent withdrawal, loss to follow-up, or end of trial) was 55 months.
Vascular End Points and Death
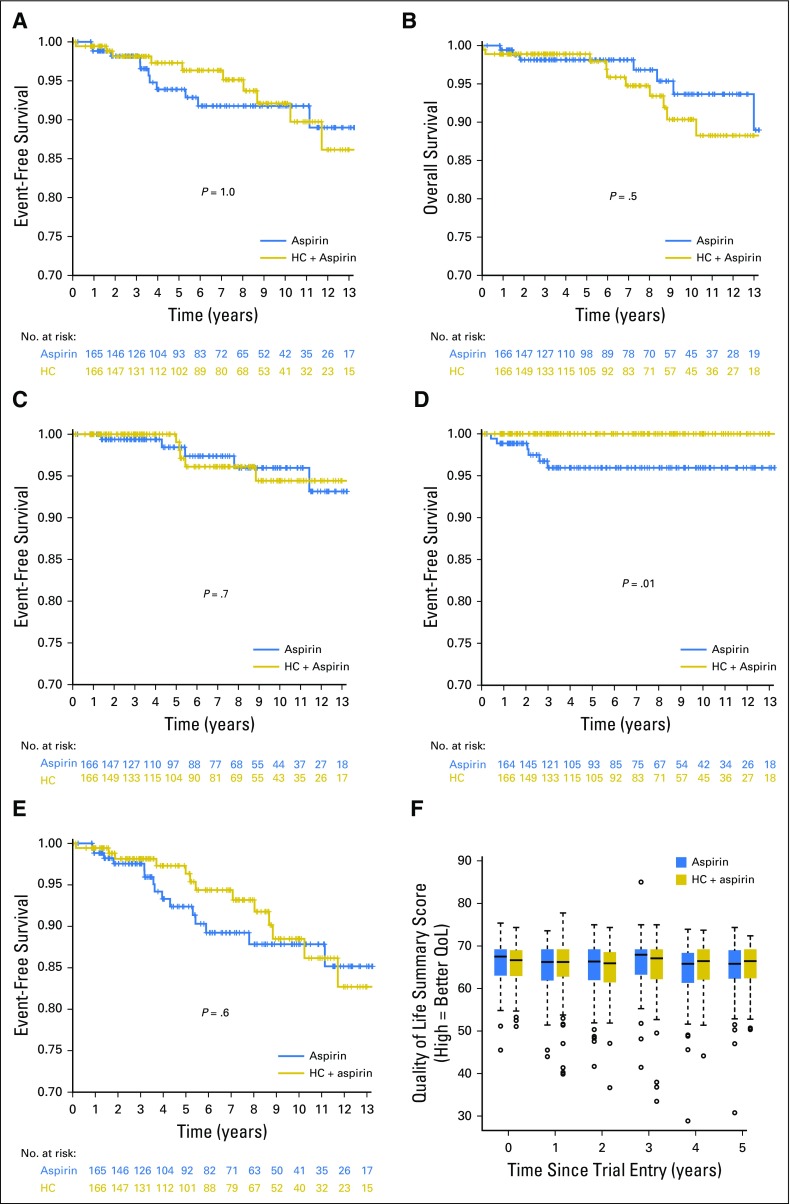
Numbers of vascular events and deaths are provided in Table 2. There was no significant difference between the two arms in the composite primary end point of time from random assignment to arterial or venous thrombosis, serious hemorrhage, or death from vascular causes (11 events in each arm; odds ratio [OR] for hydroxycarbamide plus aspirin compared with aspirin alone was 0.98 [95% CI, 0.42 to 2.25; P = 1.0; Fig 2A, Table 2). The incidence of these vascular events during follow-up was 0.93 per 100 patient-years (95% CI, 0.61 to 1.41 per 100 patient-years). There remained no significant difference in this end point in a prespecified secondary analysis, in which patients were censored on reaching the age of 60 years, because most would start cytoreduction at this age if they were not already receiving it (OR, 0.54; 95% CI, 0.19 to 1.53; P = .3). There were no significant associations between the rate of the primary end point and the presence of JAK2, CALR, or MPL mutations, age at trial entry, sex, or blood counts at diagnosis (hemoglobin, white cell count, platelet count; all P > .05; data not shown).
Table 2.
Number of Patients Reaching Principal Study End Points
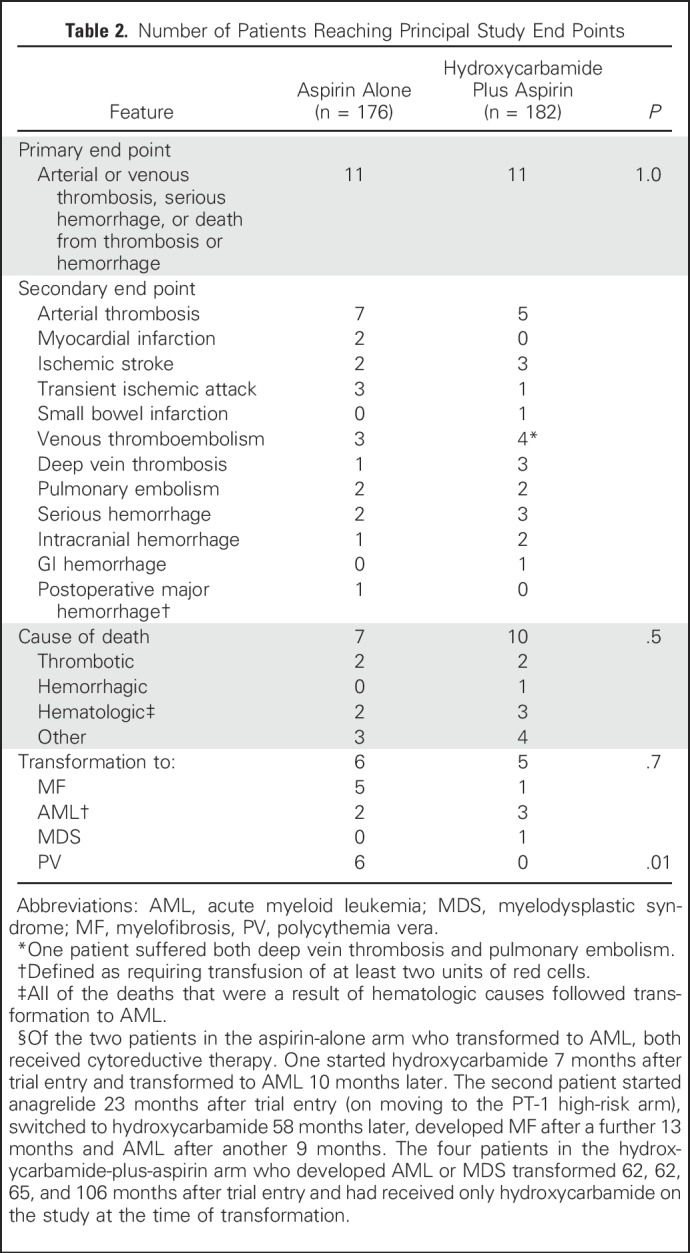
Fig 2.
Kaplan-Meier curves for the (A) primary composite end point of arterial or venous thrombosis, serious hemorrhage, or death from vascular causes; (B) overall survival; (C) composite end point of rate of transformation to myelofibrosis (MF), acute myeloid leukemia (AML), or myelodysplastic syndrome (MDS); (D) transformation to polycythemia vera (PV); (E) composite end point of any major disease-related complication (arterial thrombosis, venous thromboembolism, major hemorrhage, transformation to AML, MDS, MF, or death from any of these causes). (F) Box plots showing summary scores for the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 questionnaire, performed at baseline and annually for 5 years after study entry; there were no significant differences between the study arms at any time point. Box and whisker plot showing median and vertical whisker showing 2nd and 98th percentile. HC, hydroxycarbamide.
There were seven deaths during follow-up in the aspirin-alone arm and 10 in the hydroxycarbamide-plus-aspirin arm (Table 2), with no significant difference in overall survival between the arms (OR, 1.4; 95% CI, 0.54 to 3.61; P = .5; Fig 2B).
Disease Transformation and All Disease-Related Complications
The rate of disease transformation was low in both arms (Table 2). In the aspirin-alone arm, five patients developed transformation to MF, two to AML (one after MF), and none transformed to MDS. In the hydroxycarbamide-plus-aspirin arm, one patient developed transformation to MF, three to AML, and one to MDS. There was no significant difference in the composite end point of rate of transformation to MF, AML, or MDS (OR, 0.79; 95% CI, 0.24 to 2.58; P = .7; Fig 2C). In addition, six patients in the aspirin-alone arm transformed to PV compared with none in the hydroxycarbamide arm (P = .01; Fig 2D), in keeping with the nonspecific effect of hydroxycarbamide in constraining erythropoiesis. A prespecified analysis for any major disease-related complication (ie, arterial thrombosis, venous thromboembolism, major hemorrhage, transformation to AML, MDS, or MF, or death from any of these causes) did not show any difference between the two arms (OR, 0.83; 95% CI, 0.41 to 1.7; P = .6; Fig 2E).
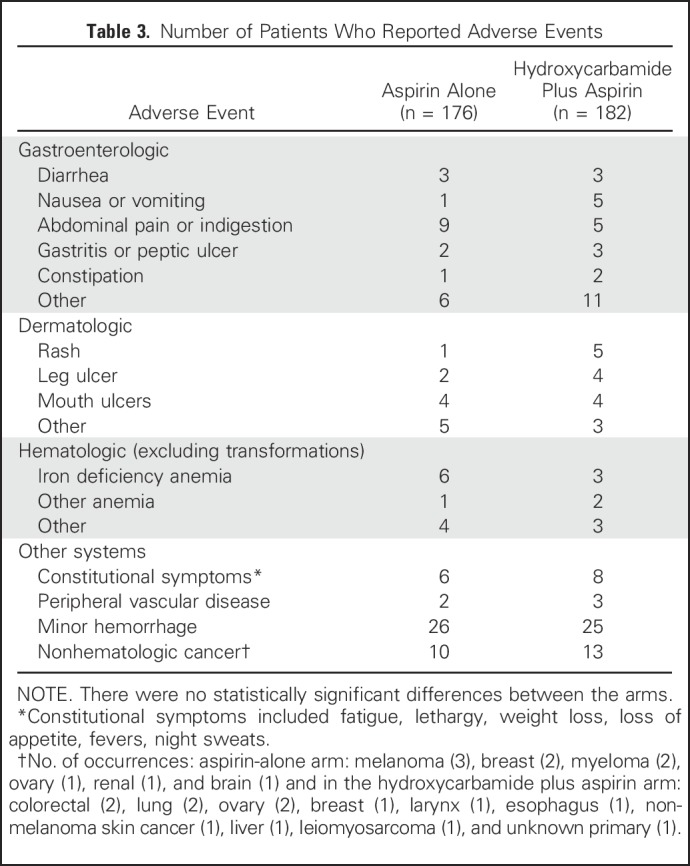
Adverse Events and Quality of Life
There were no significant differences between the arms of the study in the numbers of patients with specific adverse events, either when symptoms were considered individually or when grouped by system (eg, gastroenterologic, dermatologic). Selected adverse events are listed in Table 3. There were no significant differences in EORTC QLQ-C30 quality-of-life summary scores21 between the arms at any year after study entry (Fig 2F).
Table 3.
Number of Patients Who Reported Adverse Events
Impact of Treatment Changes
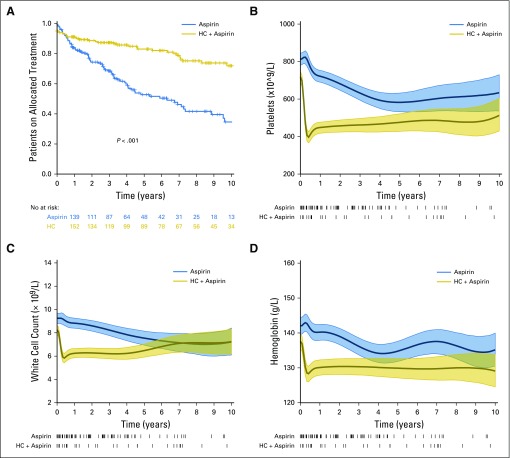
Because patients in the aspirin-alone arm were more likely to undergo a change in their allocation to cytoreductive therapy (P < .001; Figs 1 and 3A), we considered the possibility that this may have masked a difference between the arms. However the primary end point continued to show no difference between the arms in a post hoc analysis in which patients were censored at age 60 years or on the date of therapy initiation or change to their cytoreductive treatment (OR, 0.72; 95% CI, 0.16 to 3.19; P = .7). Moreover, hemoglobin levels, white cell counts, and platelet counts were all significantly lower in the hydroxycarbamide-plus-aspirin arm from early in the study, and CIs started to overlap only approximately 5 to 6 years after trial entry for platelet and white cell counts and 3 to 4 years after trial entry for hemoglobin (Fig 3B-D).
Fig 3.
Treatment changes and blood counts during follow-up. (A) Kaplan-Meier curves showing changes from the allocated trial treatment (ie, patients in the aspirin-alone arm who started cytoreduction and patients in the hydroxycarbamide [HC]-plus-aspirin arm who stopped hydroxycarbamide and/or started an alternative cytoreductive agent). (B) Platelet counts, (C) white cell counts, and (D) hemoglobin levels during the study; mean and 95% CIs are shown. Baseline counts were not significantly different between the two arms (Table 1). The high density of early blood counts and the rapid change after starting hydroxycarbamide means that the spline curves of blood count data generate the false impression of a difference between the two arms at baseline, which is in fact not present. Treatment changes are shown as ticks below the x-axis. HC, hydroxycarbamide.
DISCUSSION
Previous studies have shown that, compared with no cytoreduction or anagrelide, hydroxycarbamide reduces the risk of vascular events in patients with high-risk ET.8,9 We report the only (to the best of our knowledge) prospective, randomized study to have compared treatment with and without cytoreduction in ET patients who lack high-risk factors. Patients were recruited over a 15-year period, and the median duration of follow-up was more than 6 years, with a total follow-up of 2,373 patient years, making this study unique within the field.
This trial compared two treatment strategies in patients with ET age 40 to 59 years who lacked high-risk factors and showed that preemptive addition of hydroxycarbamide to aspirin did not reduce the risk of vascular events or myelofibrotic or leukemic transformation. Our data indicate that the risk of significant vascular events in this patient population is low and is not reduced by preemptive hydroxycarbamide. There was also no association between the primary end point and blood counts at diagnosis. This specific group of patients should therefore be treated without cytoreduction until another clinical indication arises.
It has been suggested that hydroxycarbamide may increase the risk of leukemic transformation,15 although this proposal remains controversial.3,17,18,23 Our results contribute the only data from a prospective, randomized trial comparing hydroxycarbamide with no cytoreduction in the myeloproliferative neoplasms in patients who lack high-risk features. Rates of transformation to MF, AML, and MDS were low, with no significant difference between the two arms over the course of the study. Hydroxycarbamide was also well tolerated; only 6% of patients allocated to the drug discontinued it as a result of adverse effects.
Limitations of this study reflect the fact that myeloproliferative neoplasms, most particularly ET, have a chronic course and so require long-term clinical trials. Such trials face inherent challenges, including predictable accumulation of changes to allocated treatment over time and the evolution of diagnostic criteria and risk stratification models. Long-term follow-up was important in this study because events can occur late in the disease, but long-term follow-up was reflected in changes in the risk profile of some patients during the study. Many patients in the aspirin-alone arm started cytoreduction during follow-up as a consequence of accepted clinical indications; similarly, some patients allocated to hydroxycarbamide plus aspirin changed or stopped cytoreduction during the study. Despite these changes, the primary end point showed no difference between the arms in a post hoc analysis with censoring at the time of treatment change, and blood counts remained significantly different between the arms for several years after trial entry. Treatment changes do, however, limit the conclusions that can be drawn about the long-term safety of hydroxycarbamide. A second limitation of the study is that a small number of patients were recruited at each center. We suspect that any selective recruitment was most likely to reflect the uncertain benefits and toxicities of hydroxycarbamide in this group of patients without previous disease complications, and we would not expect the generalizability of the results to be affected.
Diagnostic criteria for ET have been refined many times over the 15-year recruitment period of this study.11,22,24 Nonetheless, a high degree of concordance with current diagnostic criteria was observed on central review of diagnostic material. Concerning risk stratification, the platelet threshold that defines high-risk disease has changed from 1,000 × 109/L to 1,500 × 109/L,11,12 and the inclusion criteria of the study were modified accordingly. The recently proposed International Prognostic Score of Thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis) system suggests that patients with ET who are younger than age 60 years and who lack a prior thrombotic history could be subdivided into very-low-risk and low-risk groups according to the absence and presence, respectively, of a JAK2 mutation.25 It has been proposed that patients with a very low IPSET-thrombosis score might not require aspirin therapy,26 but this was not tested in our study. We did not identify a difference in vascular events between JAK2-mutated and -unmutated patients, in contrast to other studies,27,28 perhaps reflecting the low number of events and that molecular data were not available for all patients. Our data do not support preemptive cytoreduction, even in the subgroup of JAK2-mutated patients, in the absence of high-risk factors.
In conclusion, preemptive addition of hydroxycarbamide to aspirin did not reduce the risk of vascular events, myelofibrotic progression, or leukemic transformation in ET patients age 40 to 59 years who lacked high-risk factors for thrombosis or extreme thrombocytosis. Patients age 40 to 59 years without other clinical indications for treatment (such as previous thrombosis or hemorrhage) who have a platelet count < 1,500 × 109/L should not receive cytoreductive therapy.
ACKNOWLEDGMENT
We thank the clinicians and patients who participated in the study. Full details of participating sites and clinicians are listed in the Data Supplement.
Footnotes
Supported by the Medical Research Council, United Kingdom, Cancer Research UK, the French National Cancer Institute, Bloodwise, Wellcome Trust, the Kay Kendall Leukaemia Fund, and the Leukemia and Lymphoma Society of America.
Presented at the 59th American Society of Hematology Annual Meeting and Exposition, Atlanta, GA, December 9-12, 2017.
Clinical trial information: NCT00175838.
See accompanying Oncology Grand Rounds on page 3349
AUTHOR CONTRIBUTIONS
Conception and design: Peter J. Campbell, Georgina Buck, Keith Wheatley, Mary Frances McMullin, Anthony R. Green, Claire N. Harrison
Collection and assembly of data: Anna L. Godfrey, Peter J. Campbell, Cathy MacLean, Georgina Buck, Julia Cook, Julie Temple, Bridget S. Wilkins, Jyoti Nangalia, Jacob Grinfeld, Cecily Forsyth, Jean-Jacques Kiladjian, Anthony R. Green, Claire N. Harrison
Data analysis and interpretation: Anna L. Godfrey, Peter J. Campbell, Georgina Buck, Keith Wheatley, Jyoti Nangalia, Jacob Grinfeld, Jean-Jacques Kiladjian, Anthony R. Green, Claire N. Harrison
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Hydroxycarbamide Plus Aspirin Versus Aspirin Alone in Patients With Essential Thrombocythemia Age 40 to 59 Years Without High-Risk Features
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Anna L. Godfrey
Travel, Accommodations, Expenses: Novartis Pharmaceuticals UK
Peter J. Campbell
No relationship to disclose
Cathy MacLean
No relationship to disclose
Georgina Buck
No relationship to disclose
Julia Cook
Employment: Amgen (I)
Stock or Other Ownership: Amgen (I)
Julie Temple
Employment: Dermal Laboratories
Bridget S. Wilkins
Honoraria: Novartis
Travel, Accommodations, Expenses: Novartis
Keith Wheatley
Research Funding: Novartis (Inst)
Jyoti Nangalia
No relationship to disclose
Jacob Grinfeld
No relationship to disclose
Mary Frances McMullin
Honoraria: Novartis, Celgene
Consulting or Advisory Role: Novartis, Italpharma
Travel, Accommodations, Expenses: Novartis, Celgene
Cecily Forsyth
Honoraria: Novartis, Celgene, Roche, Amgen, Bristol Myers Squibb, Alexion Pharmaceuticals
Consulting or Advisory Role: Celgene, Novartis, Amgen, Roche
Jean-Jacques Kiladjian
Consulting or Advisory Role: Novartis, AOP Orphan Pharmaceuticals, Celgene
Research Funding: Novartis (Inst), AOP Orphan Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Gilead Sciences
Anthony R. Green
No relationship to disclose
Claire N. Harrison
Honoraria: Gilead Sciences, Celgene, Novartis, Shire, CTI BioPharma, AOP Orphan Pharmaceuticals, Genentech
Speakers’ Bureau: Novartis, CTI BioPharma, Shire, Gilead Sciences, Incyte
Research Funding: Novartis (Inst)
REFERENCES
- 1.[No authors listed]: Polycythemia vera: The natural history of 1213 patients followed for 20 years: Gruppo Italiano Studio Policitemia. Ann Intern Med 123:656-664, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Cortelazzo S, Viero P, Finazzi G, et al. : Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol 8:556-562, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Marchioli R, Finazzi G, Landolfi R, et al. : Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 23:2224-2232, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Barbui T, Finazzi G, Carobbio A, et al. : Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 120:5128-5133, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Campbell PJ, MacLean C, Beer PA, et al. : Correlation of blood counts with vascular complications in essential thrombocythemia: Analysis of the prospective PT1 cohort. Blood 120:1409-1411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besses C, Cervantes F, Pereira A, et al. : Major vascular complications in essential thrombocythemia: A study of the predictive factors in a series of 148 patients. Leukemia 13:150-154, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Carobbio A, Antonioli E, Guglielmelli P, et al. : Leukocytosis and risk stratification assessment in essential thrombocythemia. J Clin Oncol 26:2732-2736, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Harrison CN, Campbell PJ, Buck G, et al. : Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 353:33-45, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Cortelazzo S, Finazzi G, Ruggeri M, et al. : Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 332:1132-1136, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Landolfi R, Marchioli R, Kutti J, et al. : Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 350:114-124, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Harrison CN, Bareford D, Butt N, et al. : Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br J Haematol 149:352-375, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Barbui T, Barosi G, Birgegard G, et al. : Philadelphia-negative classical myeloproliferative neoplasms: Critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 29:761-770, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggeri M, Finazzi G, Tosetto A, et al. : No treatment for low-risk thrombocythaemia: Results from a prospective study. Br J Haematol 103:772-777, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Larrán A, Pereira A, Guglielmelli P, et al. : Antiplatelet therapy versus observation in low-risk essential thrombocythemia with a CALR mutation. Haematologica 101:926-931, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterkers Y, Preudhomme C, Laï JL, et al. : Acute myeloid leukemia and myelodysplastic syndromes following essential thrombocythemia treated with hydroxyurea: High proportion of cases with 17p deletion. Blood 91:616-622, 1998 [PubMed] [Google Scholar]
- 16.Hansen IO, Sørensen AL, Hasselbalch HC: Second malignancies in hydroxyurea and interferon-treated Philadelphia-negative myeloproliferative neoplasms. Eur J Haematol 98:75-84, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Björkholm M, Derolf AR, Hultcrantz M, et al. : Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 29:2410-2415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tefferi A, Rumi E, Finazzi G, et al. : Survival and prognosis among 1545 patients with contemporary polycythemia vera: An international study. Leukemia 27:1874-1881, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budde U, van Genderen PJ: Acquired von Willebrand disease in patients with high platelet counts. Semin Thromb Hemost 23:425-431, 1997 [DOI] [PubMed] [Google Scholar]
- 20.White SJ, Freedman LS: Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer 37:849-857, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giesinger JM, Kieffer JM, Fayers PM, et al. : Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol 69:79-88, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Swerdlow SH, Campo E, Harris NL, et al. (eds): WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France, IARC Press, 2008 [Google Scholar]
- 23.Hanft VN, Fruchtman SR, Pickens CV, et al. : Acquired DNA mutations associated with in vivo hydroxyurea exposure. Blood 95:3589-3593, 2000 [PubMed] [Google Scholar]
- 24.Vardiman JW, Harris NL, Brunning RD: The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100:2292-2302, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Haider M, Gangat N, Lasho T, et al. : Validation of the revised International Prognostic Score of Thrombosis for Essential Thrombocythemia (IPSET-thrombosis) in 585 Mayo Clinic patients. Am J Hematol 91:390-394, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. : Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 5:e369, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rumi E, Pietra D, Ferretti V, et al. : JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 123:1544-1551, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotunno G, Mannarelli C, Guglielmelli P, et al. : Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 123:1552-1555, 2014 [DOI] [PubMed] [Google Scholar]