Abstract
Purpose
We present a new method for analyzing relative corneal refractive power (RCRP) in children undergoing orthokeratology and explore its potential association to effective myopic control
Methods
A total of 55 children aged 8 to 12 years participated in the study. Axial growth was calculated as the difference in axial length before and 1 year after orthokeratology. Growth <0.30 mm was considered as effective control. Corneal topography was obtained before and 4 months after lens dispatch. The topography was divided into 36 10° slices and the maximal RCRP (mRCRP) in each was calculated and fitted into a model that integrated the effects of mean refractive power (M), corneal asymmetry (f1), and astigmatism (f2). The relationship between the probability of achieving effective control and the modulation of mRCRP was analyzed with logistic regression.
Results
A total of 45 subjects achieved effective control, but for 10 the treatment was ineffective. The M-values were not different between the groups. Modulations of mRCRP were significantly larger in the effective than the ineffective group (1.17 vs. 0.64 diopters [D] for f1, P = 0.02; 0.85 vs. 0.35 D for f2, P = 0.03). The probability to achieve effective control increased with modulation of mRCRP (P = 0.02). With a peak mRCRP > 4.5 D, a subject had an above 80% chance to achieve effective control.
Conclusions
The new method reveals that how the combination of spherical equivalent (SE), corneal asymmetry, and astigmatism determines modulation of the mRCRP and a large amplitude of modulation is associated with a higher probability of effective myopic control.
Translational Relevance
Our finding enables clinicians to estimate the outcome early and provides new insights to lens design.
Keywords: myopic progression, axial growth, orthokeratology lens, probability, relative corneal refractive power
Introduction
An orthokeratology lens is a rigid contact lens with a reverse geometry on its back surface.1,2 Overnight wear of the lens flattens the central cornea zone and increases the relative corneal refractive power (RCRP) in the periphery. This inverted pattern of corneal peripheral refraction induces relative peripheral myopic defocus on the retina, which is considered the working mechanism of the orthokeratology lenses.3–5 Orthokeratology lens has proven to be effective in slowing down myopic progression.6 In comparison with controls wearing a single-vision spectacle or soft contact lens, axial growth has been reduced as much as 32% to 63% per year in subjects of different ethnicities.7–11
The average changes in axial length for subjects wearing single vision spectacles range from 0.18 to 0.24 mm per half year, and 0.30 to 0.39 mm a year.7,8,12–15 With orthokeratology treatment, axial growth slows down further. Since the change in axial length is small, axial length usually is evaluated once, or at most twice a year, in clinic. Therefore, orthokeratology treatment requires long-term commitment from doctors and patients to see the effect become appraent.16 Ineffective control after substantial money and time invested often leads to great frustration on both sides. It is of paramount importance to estimate the probability that a patient would achieve effective control in myopic progression before treatment or early during its course. To this end, efforts have been made to investigate the correlations between axial growth and a variety of parameters at the baseline, including age, spherical equivalent (SE), corneal eccentricity, and corneal thickness.17–20 These measurements are nonspecific, indirect, and based on the assumption that their relationships to axial growth are linear, which often leads to contradictory findings and results that are hard to interpret by physicians.
A more specific and direct way is to look at the RCRP increase induced by the lens, which indicates the relative peripheral myopic defocus on the retina.21–23 Nevertheless, this method is weakened by oversimplification of the RCRP calculation, in which RCRPs are averaged across the entire cornea, assuming that it is equally distributed along different meridians. This oversimplification is questionable for several reasons. First, many patients undergoing orthokeratology treatment have corneal astigmatism, which is an uneven distribution of refractive power along the meridians by definition.24–26 Second, patients who receive orthokeratology treatment also often show a substantial amount of corneal asymmetry.27,28 Third, peripheral defocus varies across meridians on the retina of myopic children.29 If there is a threshold for peripheral myopic defocus to be effective as a slow-down sign for axial growth, then different amounts of RCRP increase are needed on different meridians to push the existing peripheral defocus over the threshold. Therefore, the spatial distribution of the RCRP is more informative and deserves additional examination.
We investigated whether the probability to achieve effective control of myopic progression is related to the spatial distribution of RCRP. Specifically, we developed a novel model that integrates the effects of mean SE, corneal asymmetry and corneal astigmatism into calculation of the RCRP spatial distribution. The model yields a single metric, maximal peripheral corneal relative power (mRCRP), which could be used to quantify the probability of achieving an effective control of myopic progression via a logistic regression-based analysis. Since the corneal shape usually stabilizes 1 month after lens wearing,30–33 our model potentially enables clinicians to know the outcome early in the course of orthokeratology lens treatment.
Methods
Patient Information
The study was conducted at the Eye Hospital of the Tianjin Medical University (TMU) between February 2016 and March 2017. The study protocol was in agreement with the tenets of the Declaration of Helsinki and was approved by the ethics committee of the TMU. The inclusion criteria were: age 8 to 12 years; no history of previous orthokeratology lens or contact lens wear; no ocular surface diseases that might affect refractive error; no systemic diseases that might affect refractive error; measures in wet refraction (compound tropicamide eye drops, 5 mg/mL, one drop every 5 minutes for 4 times): −5.50 ≤ SE ≤ −1.00 diopter (D), astigmatism ≤ 1.5 D, and anisometropia < 1.0 D; best corrected distance visual acuity better than 20/20; and no pharmaceutical history that might affect development of the visual system or refractive errors. A total of 60 subjects were enrolled in the study. Written consent was obtained from the subjects and their guardians after they were informed about the details of the study.
Lens Dispensing and Follow-Up Schedule
The orthokeratology lens used in this study had a 4-zone reverse geometry, with a nominal oxygen transmissibility (DK) of 127 × 10–11 (cm2/s; mL O2/ mL × mm Hg; ISO/Fatt). The lens diameter was 10.6 mm with optical zone diameter 6.0 mm, and the lens thickness was 0.22 mm. The lens fitting procedures strictly followed the recommendations from the lens manufacturer (Euclid Systems Corp., Herndon, VA). Throughout the course of the study, patients were requested to wear the lenses every night for 8 consecutive hours. The patients were scheduled for 1-day, 1-week, and monthly follow-up visits until 1 year after lens dispensing. On the day of the visit, the patients removed the lens before 8 AM To reduce diurnal variations, all visits were scheduled in the afternoon between 2 and 5 PM. At each visit, the fitting of the lens, visual acuity, and refraction were evaluated. Among the 60 initially enrolled subjects, 55 (25 boys and 30 girls; average age, 11.75 ± 2.2 years; range, 8–12 years) completed the 1-year follow-up. Three subjects dropped out due to inability to comply with the follow-up schedules and two stopped wearing the lens due to discomfort.
Axial Length Measurement
Axial length (distance between the anterior cornea surface and retinal pigment epithelium) was measured with a noncontact optical biometry (Lenstar 900; Haag-Streit AG, Switzerland) before (baseline) and 1 year after lens wearing. At each visit, a single examiner measured the axial length for three consecutive times and mean value was taken for data analysis. After 1 year, a subject with axial growth <0.3 mm was defined as having achieved effective control of myopic progression.
Corneal Topography and Corneal Power Profile
Corneal topography was obtained with Pentacam (Oculus, Wetzlar, Germany) at baseline and at each follow-up visit until 4 months after lens wear. The 4-month topography was taken as the representative post-orthokeratology topography. To compute the profile of the corneal power, the map of sagittal (axial) power was used (Fig. 1A). Starting from the nasal horizon, the map was divided into 36 sections in steps of 10° counterclockwise (Fig. 1B). Within each section, the data points were smoothed into a curve representing the power profile along the eccentricity (Fig. 1C). For each power profile, RCRP was calculated by subtracting the central corneal refractive power from the curve, and the maximal RCRP value (mRCRP) was identified (Fig. 1D). A total of 36 mRCRPs were obtained for each map and plotted along the direction of the sections (Fig. 1E).
Figure 1.
Data analysis. (A) An example corneal topography. (B) Division of the topography into 36 sections. (C) The profile of refractive power within each section was obtained by averaging the values along the eccentricity. (D) From each power profile, mRCRP was calculated by subtracting the central value from the peak value. (E) The mRCRPs vary in the 36 sections.
Modeling of the Modulation of mRCRP
Through Fourier transformation, a complex signal can be decomposed as a series of sine waves with different amplitudes and phases. We modeled the modulation of mRCRPs over the 360° as a sum of three components: mRCRP = M + F1 + F2, in which M is mean power, F1 is a sine wave running a cycle over the 360° [M*f1*sin(x + phase1)], and F2 is a sine wave running two cycles over the 360° ([M*f2*sin(2*x + phase2)]; Fig. 2). The association between model components and other parameters was analyzed with multiple linear regression (Table). M was significantly associated with the SE at 4 months. The f1 component was significantly associated with a corneal asymmetry that has one peak over the 360°. The f2 component was significantly associated with the corneal astigmatism that runs two cycles over 360°.
Figure 2.
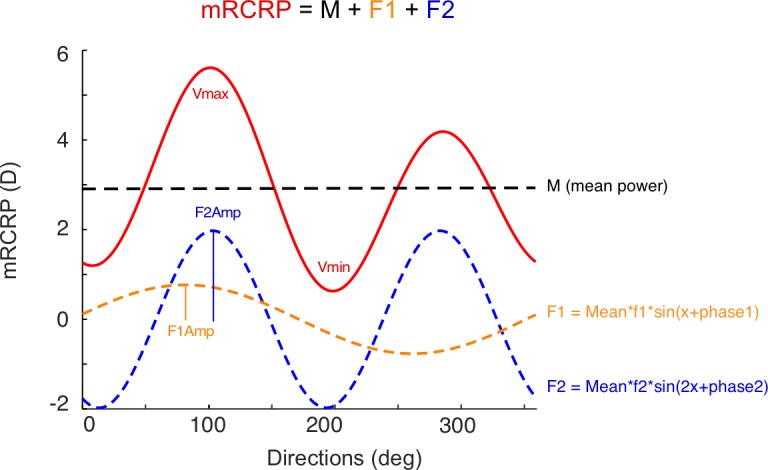
mRCRP is modeled as the sum of three components: mean value (mean, black), a sine wave with 1 cycle in 360° (F1, orange), and a sine wave with 2 cycles in 360° (F2, blue). F1Amp, amplitude of F1 component; F2Amp, amplitude of F2 component.
Table.
The Model Components versus SE, Lens Decentration, and Astigmatism

Computation of Lens Decentration
To calculate the lens decentration, the difference map was calculated by subtracting the baseline map from the topography obtained at 4 months after orthokeratology. The points with changes ≥ 0.25 D were defined as the reverse zone, and the central points with changes <−0.25 D were defined as the central treatment zone. The points in between with values −0.25 to 0.25 D were defined as the transition zone and were fitted into a circle using a custom Matlab function (MathWorks, Natick, WA) to determine the center location.
Statistical Analysis
For each subject, only the right eye data were used for analysis. The normality of the data was tested with a Schapiro-Wilk test. Multiple linear regression was used to show how model components related to SE, lens decentration, astigmatism, and corneal asphericity (e). Ranksum tests were used to compare the model components between the effective and ineffective groups. Logistic regression was used to show how the probability of a subject achieving effective control varied with peak value of mRCRP. All analyses were performed using the R programming package (version 3.3.1).34 P < 0.05 was defined as statistically significant.
Results
General Information
At baseline, mean SE was −3.22 ± 1.06 D (−5.50 to −1.25 D). The uncorrected visual acuity for distance was 0.93 ± 0.47 logMAR, and the corrected visual acuity for distance was −0.03 ± 0.05 logMAR. One year after wearing the orthokeratology lens, the uncorrected distance visual acuity improved to 0.01 ± 0.02 logMAR (P < 0.0001), but the corrected distance visual acuity did not change significantly (−0.03 ± 0.05 logMAR, P = 0.892).
Spatial Profile of RCRP
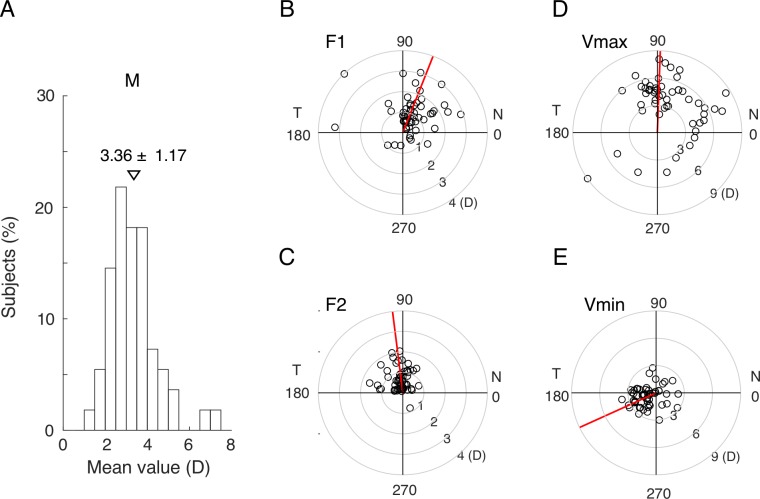
The three-components model fitted the spatial profiles of RCRP very well with a mean R2 value of 0.93 ± 0.06 (median 0.94). Mean power (M) was 3.36 ± 1.17 D (Fig. 3A). Average amplitude of F1 was 1.31 ± 0.85 D and pointed towards the superonasal direction (69.21°, Fig 3B). Average amplitude of F2 was 0.80 ± 0.51 D and pointed upwards (97.15°, Fig 3C). The combination of the three underlying components, M, F1, and F2, caused the mRCRPs to modulate over the 360° circle. Mean maximal value of mRCRPs (Vmax) was 5.22 ± 1.89 D and pointed upwards (88.53°, Fig. 3D). Mean minimal value of mRCRPs (Vmin) was 1.62 ± 1.09 D and pointed to the inferior temporal side (205°, Fig. 3E).
Figure 3.
The distribution of the parameters from the fitted model. (A) Histogram showing the distribution of mean power. (B) Amplitudes and phases of the F1 component. (C) Amplitudes and phases of the F2 component. (D) Amplitudes and phases of the Vmax. (E) Amplitudes and phases of the Vmin. The red line indicates the median phase.
Axial Growth
At baseline, mean axial length was 24.81 ± 0.62 mm. One year after wearing the orthokeratology lenses, mean axial length was 25.00 ± 0.60 mm. The axial growth over 1 year's time is shown in Figure 4. The subjects were categorized as a groups showing clear (axial growth < 0.3 mm) and weak (axial growth ≥ 0.3 mm) myopic control effect. The choice of 0.3 mm as the criterion was based on the following reasons. First, the distribution of axial change in this study was not normal (P < 0.001), and the long tail on the right side indicated the existence of two subgroups, one with small and the other with large axial growth. A double Gaussian distribution was fitted into the data with one centered on the small and the other on the large axial growths. The 0.3 mm value was where the two Gausses intersected. Second, previous studies reported that the average changes in axial length for subjects wearing single vision spectacles ranged from 0.30 to 0.39 mm a year.7,8,12–15 Third, the effectiveness of a myopic control device is a relative measure compared to single vision spectacle lenses. A difference in axial length change range from 0 to 0.09 mm a year usually is considered as a weak effect.6 Therefore, the 0.30 mm value was chosen for the initial criterion for analysis.
Figure 4.
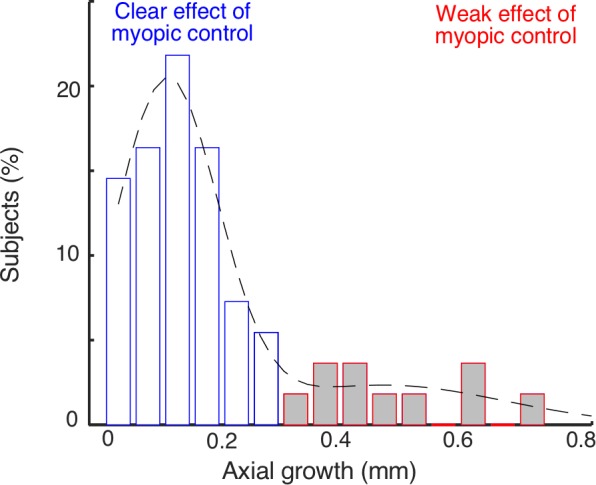
Axial growth after wearing orthokeratology lenses for 1 year. The blue and red bars represent the subjects showing clear (growth < 0.3 mm) and weak (growth ≥ 0.3 mm) myopic control effects in myopic progress, respectively. The dotted line indicates a fitted double Gaussian distribution.
Corneal Profile and Axial Growth
Figure 5 shows two examples to illustrate the relationship between mRCRP modulation and axial length growth. Larger axial growth (weak myopic control effect) was associated with a smaller modulation of the mRCRP (Fig. 5A), while smaller axial growth (clear myopic control effect) was associated with a larger modulation of mRCRP (Fig. 5B).
Figure 5.
Examples illustrating the relationship between mRCRP modulation and axial length growth. (A) A subject with smaller corneal mRCRP modulation and larger length growth. (B) A subject with larger modulation in mRCRP and smaller axial growth.
Therefore, for the next step, we looked at the association between axial growth and Vmax. We first separated the subjects into two groups, weak (red) versus clear (blue) myopic control effect groups (Fig. 6A). Mean mRCRP values (M) were not different between the two groups (P = 0.06, ranksum test, Fig. 6B). However, subjects in the weak myopic control effect group had a significantly smaller amplitude of F1 (0.64 vs. 1.17 D for the weak and clear groups, respectively, P = 0.03, ranksum test) or F2 (0.35 vs. 0.85D for the weak and clear groups, respectively, P = 0.02 ranksum test, Fig. 6C). In other words, the subjects in the weak myopic control effect group had a much smaller modulation of mRCRP over the 360°. Those in the clear myopia control effect group had a much larger modulation over the 360°.
Figure 6.
mRCRP modulation and axial length growth. (A) Scatter plot illustrating the relationship between Vmax and axial growth. Growths >0.3 mm (red) and <0.3 mm (blue) are considered weak and clear myopic control effects respectively. (B) There was no difference in mean mRCRP values between the weak and clear control effect groups. (C) Subjects in the weak effect group had significantly smaller amplitudes of F1 and F2. (D) Logistic regression showing the probability of having clear myopic control increases with the value of Vmax. (E) Threshold to have a >80% chance (blue line) or a <20% probability (red line) of achieving a clear control effect. (F) Logistic regression using different amounts of axial growth as the criteria of effective control. As along as the criterion is >0.25 mm, the logistic regressions are significant.
Logistic regression on all subjects' data (red and blue dots) showed that the probability of having an clear myopic control effect was tightly associated with increasing Vmax (P = 0.023, Fig. 6D). The proportion of subjects achieving a clear effect in the bins having Vmax values of 1–3, 3–5, 5–7, 7–9, and >9 D (white circles) fell nicely on the fitted logistic function. The threshold of Vmax to have a >80% chance of achieving clear control effect was 4.5 D and the threshold of Vmax to have a <20% chance of achieving clear control effect was 1.2 D (Fig. 6E). To see if this significant association was merely due to the selection of the cutoff criterion at 0.3 mm, multiple criterion values, from 0.10 to 0.30 in steps of 0.05 mm, were tested in logistic regressions (Fig. 6F). As along as the criterion was >0.25 mm, the logistic regressions became significant.
Multivariable logistic regression analyses showed that the factors significantly associated with odds of having an clear control in myopic progression were corneal asphericity (odds ratio [OR] = 8.81 × 10–6, P = 0.009) and Vmax (OR = 3.59, P = 0.026). The association with the amount of astigmatism was marginal (OR = 6.61, P = 0.063). The association with other parameters, including baseline axial length, baseline SE, and lens decentration after lens wearing, did not reach the significance level.
Corneal Profile and Axial Growth: Calculation Based on Mean Values
In the calculation of mRCRP, only the maximal value from each section was considered. To use all values in each section, RCRP was recalculated again as mean value of relative power in each section (mean RCRP). Its association with axial growth (Fig. 7) was similar to those found when using maximal values. Mean M values were not different between the two groups (P = 0.91, ranksum test, Fig 7B). Subjects in the weak control effect group had significantly smaller amplitudes of F1 (0.28 vs. 0.79 D for the weak and clear groups, respectively, P = 0.045, ranksum test) or F2 (0.21 D vs. 0.52 D, respectively, P = 0.03 ranksum test, Fig 7C). The probability of having a clear myopia control effect was tightly associated with increasing Vmax (P = 0.04, Fig 7D) and the threshold of Vmax to have a >80% chance of achieving clear control effect was 3.0 D (Fig. 7E). As along as the criterion was >0.25 mm, the logistic regressions became significant (Fig. 7F).
Figure 7.
Axial length growth and RCRP calculated from mean values in each section. (A) Scatter plot illustrating the relationship between Vmax and axial growth. Growth >0.25 mm (red) is considered a weak myopic control effect and growth smaller than 0.25 mm (blue) is considered a clear myopic control effect. (B) There was no difference in the M values between the weak and clear groups. (C) Subjects in the weak group had significantly smaller amplitudes of F1 and F2. (D) Logistic regression showing the probability of having clear myopic control increases with the value of Vmax. (E) Threshold to have a >80% chance of achieving clear control effect. (F) Logistic regression using different amounts of axial growth as the criteria of clear control. As along as the criterion was >0.25 mm, the logistic regressions are significant.
Stability of the Corneal Profile
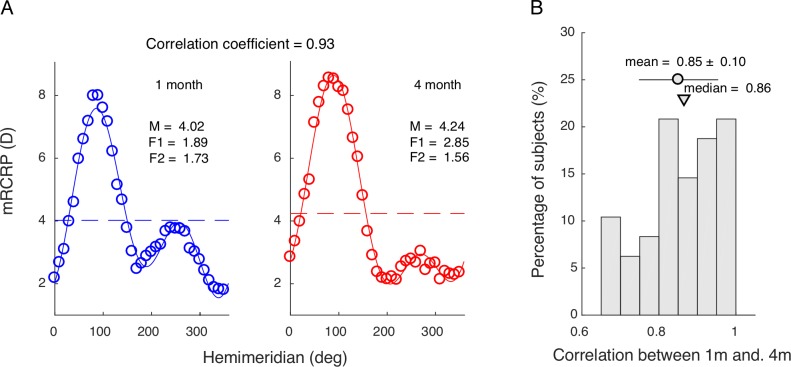
To show that corneal profile remained stable, cross-correlation was calculated for mRCRP obtained from 1 and 4 months after the initial lens dispatch (Fig. 8). The average coefficient was 0.85 ± 0.10.
Figure 8.
Stability of the corneal profile. (A) mRCRP calculated at 1 month (left) and 4 months (right) after the initial lens dispatch. (B) The distribution of correlation coefficient of between mRCRP of 1 and 4 months for all subjects. Circle represents mean value and triangle represents median value.
Discussion
We presented a new analysis method to quantify the modulation of mRCRP across different hemimeridians and how this modulation is related to the SE, asymmetry caused by lens decentration, and astigmatism. Moreover, we demonstrated that a clear myopic control effect is potentially related to a larger modulation of mRCRP.
Speculation on the Existence of a Threshold
Based on our findings, we speculated that a relative peripheral myopic defocus must exceed a certain threshold to be effective in slowing down myopic progression.35 As shown in Figure 6E, a peak mRCRP >4.5 D indicates an 80% chance to achieve an effective control. Mean and modulation are needed to push mRCRP to exceed this threshold. Subjects with an M >4.5 D are most likely to exceed the threshold (Fig. 9A). Subjects with an M value <1.2 D are least likely to exceed the threshold anywhere on the ring (Fig. 9B). For subjects with medium M values, the modulation of the mRCRP becomes critical in pushing mRCRP to exceed the threshold. A flat profile is less likely to have any portion of the ring exceeding the threshold level (Fig. 9C). On an uneven profile, the mRCRP was not evenly distributed (Fig. 9D). Some portions on the profile would have a really small mRCRP. As a consequence, other regions would have a much larger mRCRP that exceeds the threshold. Therefore, an uneven profile would increase the probability of having a certain region above the threshold and being effective in slowing down the axial growth.
Figure 9.
The threshold and three-component model. (A) High M leads to a high probability of achieving effective control whether the ring is flat or uneven. (B) Low M leads to a low probability of achieving effective control whether the ring is flat or uneven. (C) Medium M combined with a flat ring has a low probability of achieving effective control. (D) Medium M combined with an uneven ring increases the probability of achieving effective control. EC, effective control of myopic progression.
The Advantage of Our Model
Because our model breaks down the modulation of mRCRP as the combination of a SE asymmetry caused by lens decentration and astigmatism, it helps to explain the contradictory reports on axial growth and initial refractive condition. The existing results about the relationship between initial SE and axial elongation in orthokeratology treatment are contradictory. While some studies showed that initial SE is negatively correlated with axial elongation,12,19,36 others reported a lack of association.8,9,37 A closer look showed that the ranges of initial SEs are different in those studies. In studies that report a lack of association, the subjects' initial SEs are in a limited middle range, mostly between −1 and −4 D.8,37 In studies that reported a significant association, the subjects' initial SEs often went beyond this range.9,19,36 Our model provided a potential explanation for this apparent contradiction. As shown in the Table, mean power (M) was significantly correlated with initial SEs. Subjects with an initial SE > −4.5 D are similar to the one shown in Figure 9A, which has a large M and is likely to achieve an effective control. Subjects with initial SE < −1 D are similar to the one shown in Figure 9B, which has a small M value and is most likely to have an ineffective myopic control. In linear regression, those patients are data points at the two extremes. With them included, a significant slope is more likely to be established. With those extreme values excluded from analysis, the reported significant association simply disappears.12,19,36 Subjects with initial SEs in the middle range are similar those shown in Figures 9C and 9D, in which the modulation of the mRCRP interferes with the effect of SE. That might explain the lack of a significant correlation between SE and axial growth in previous studies. Patients with a middle range of initial SE make up the majority of the clinical visits for orthokeratology. Therefore, it is of great clinical significance to show that the modulation of the mRCRP increases the chances for those patients to have an effective myopic control.
Limitations of Our Study
One caution in interpretation of our data is that we did not directly measure the relative peripheral defocus on the retina in the patients. We measured the relative corneal peripheral refractive powers. Fortunately, previous studies have suggested a very good correlation between the RCRP and induced relative defocus on the retina.38,39 Changes in mean relative peripheral defocus induced by orthokeratology were significantly correlated with axial myopia at baseline.39 In peripheral refraction for 30° and 35°, the amount of myopia induced has an almost one-to-one relationship with the amount of baseline SE to be corrected.38 The other limitation of our study is the lack of a control group. Although, the axial growth data for subjects wearing single vision spectacles could be found in existing literatures, control data collected from the same population would offer more convincing evidence. The third limitation of our study is the relative small sample size. With a larger sample size, we speculated that the logistic regressions might become significant even as the criteria <0.25 are chosen.
Agreement with Previous Studies on Corneal Asymmetry
Our findings agreed very well with those of two previous studies. Hiraoka et al.40 reported that the slowing down of myopic progression is significantly associated with increased coma found in those patients. Coma represents the corneal asymmetry of higher order wave front aberrations. The f1 component in our model represents the asymmetry found in mRCRP, a sine wave with one peak over the 360°. Moreover, our study specifically indicated where this asymmetry might have originated, since it is significantly related to the decentration of the orthokeratology lenses (Table). Kang and Swarbrick29 reported that, on the vertical meridian of myopes, there are pre-existing physiologic myopic defocus that are beneficial in slowing down the myopic progression. Our results dovetailed well with those findings. Those tested children all had larger refractive powers on the vertical meridian. Interestingly, the overall direction of the Vmax is found on the vertical semimeridians (Fig. 3D). In combination with the existing myopic defocus, this greatly increases the chance of the superior region to have a Vmax greater than the threshold and to be effective in myopia control. The other reason for the Vmax to be located on the vertical meridian is that the decentration of the lens was often in the inferior and temporal quadrants.40,41 Therefore, the superior quadrant has less pressure and would allow more epithelium migrating towards that region to have larger mRCRP values.
Potential Clinical Implications
Our findings provided two clinical implications. First, we can predict the treatment outcome much earlier since the corneal topography map usually stabilizes 1 month after the initiation of lens use. In some corneal topography systems, such as Medmont, it is easy to obtain the peak mRCRP by putting the section line around the vertical meridian and reading the values of corneal refractive power. A value <1.2 D would indicate a small chance and a value >4.5 D would indicate a high chance of achieving an effective control of myopic progression. Accordingly, the lens could be adjusted or alternative management could be planned. Second, this finding may provide new insight into lens design. The reverse curve could be made uneven to increase the modulation of mRCRP.
Conclusion
Our method, which breaks down the modulation of mRCRP into the combination of SE, corneal asymmetry, and astigmatism, offers a new approach to search for the potential mechanisms leading to an effective myopic control.
Acknowledgment
Supported by the National Natural Science Foundation of China, Grant No. 81770901; and the Tianjin Science and Technology Commission, Grant No. 17ZXHLSY00070.
Disclosure: J. Wang, None; D. Yang, None; H. Bi, None; B. Du, None; W. Lin, None; T. Gu, None; B. Zhang, None; R. Wei, None
References
- 1.Jessen GN. Contact lenses as a therapeutic device. Am J Optom Arch Am Acad Optom. 1964;41:429–435. doi: 10.1097/00006324-196407000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Swarbrick HA. Orthokeratology review and update. Clin Exp Optom. 2006;89:124–143. doi: 10.1111/j.1444-0938.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- 3.Charman WN, Mountford J, Atchison DA, Markwell EL. Peripheral refraction in orthokeratology patients. Optom Vis Sci. 2006;83:641–648. doi: 10.1097/01.opx.0000232840.66716.af. [DOI] [PubMed] [Google Scholar]
- 4.Kang P, Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optom Vis Sci. 2011;88:476–482. doi: 10.1097/OPX.0b013e31820f16fb. [DOI] [PubMed] [Google Scholar]
- 5.Smith EL., III Prentice Award Lecture 2010: a case for peripheral optical treatment strategies for myopia. Optom Vis Sci. 2011;88:1029–1044. doi: 10.1097/OPX.0b013e3182279cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123:697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Charm J, Cho P. High myopia-partial reduction ortho-k: a 2-year randomized study. Optom Vis Sci. 2013;90:530–539. doi: 10.1097/OPX.0b013e318293657d. [DOI] [PubMed] [Google Scholar]
- 8.Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53:7077–7085. doi: 10.1167/iovs.12-10565. [DOI] [PubMed] [Google Scholar]
- 9.He M, Du Y, Liu Q, et al. Effects of orthokeratology on the progression of low to moderate myopia in Chinese children. BMC Ophthalmol. 2016;16:126. doi: 10.1186/s12886-016-0302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R, Sugimoto K. Long-term efficacy of orthokeratology contact lens wear in controlling the progression of childhood myopia. Curr Eye Res. 2017;42:713–720. doi: 10.1080/02713683.2016.1221979. [DOI] [PubMed] [Google Scholar]
- 11.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: a comparison of vision-related quality-of-life measures between orthokeratology contact lenses and single-vision spectacles. Eye Contact Lens. 2013;39:153–157. doi: 10.1097/ICL.0b013e31827a0241. [DOI] [PubMed] [Google Scholar]
- 12.Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30:71–80. doi: 10.1080/02713680590907256. [DOI] [PubMed] [Google Scholar]
- 13.Zhu MJ, Feng HY, He XG, Zou HD, Zhu JF. The control effect of orthokeratology on axial length elongation in Chinese children with myopia. BMC Ophthalmol. 2014;14:141. doi: 10.1186/1471-2415-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Cheung SW, Cho P. Myopia control using toric orthokeratology (TO-SEE study) Invest Ophthalmol Vis Sci. 2013;54:6510–6517. doi: 10.1167/iovs.13-12527. [DOI] [PubMed] [Google Scholar]
- 15.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: refractive and biometric changes. Invest Ophthalmol Vis Sci. 2012;53:5060–5065. doi: 10.1167/iovs.11-8005. [DOI] [PubMed] [Google Scholar]
- 16.Li SM, Kang MT, Wu SS, et al. Efficacy, safety and acceptability of orthokeratology on slowing axial elongation in myopic children by meta-analysis. Curr Eye Res. 2016;41:600–608. doi: 10.3109/02713683.2015.1050743. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Li Z, Zeng J. A review of the potential factors influencing myopia progression in children using orthokeratology. Asia Pac J Ophthalmol (Phila) 2016;5:429–433. doi: 10.1097/APO.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 18.Kong Q, Guo J, Zhou J, Zhang Y, Dou X. Factors determining effective orthokeratology treatment for controlling juvenile myopia progression. Iran J Public Health. 2017;46:1217–1222. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B, Naidu RK, Qu X. Factors related to axial length elongation and myopia progression in orthokeratology practice. PLoS One. 2017;12:e0175913. doi: 10.1371/journal.pone.0175913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Factors preventing myopia progression with orthokeratology correction. Optom Vis Sci. 2013;90:1225–1236. doi: 10.1097/OPX.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 21.Lee EJ, Lim DH, Chung TY, Hyun J, Han J. Association of axial length growth and topographic change in orthokeratology. Eye Contact Lens. 2018;44:292–298. doi: 10.1097/ICL.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 22.Zhong Y, Chen Z, Xue F, Miao H, Zhou X. Central and peripheral corneal power change in myopic orthokeratology and its relationship with 2-year axial length change. Invest Ophthalmol Vis Sci. 2015;56:4514–4519. doi: 10.1167/iovs.14-13935. [DOI] [PubMed] [Google Scholar]
- 23.Zhong Y, Chen Z, Xue F, Zhou J, Niu L, Zhou X. Corneal power change is predictive of myopia progression in orthokeratology. Optom Vis Sci. 2014;91:404–411. doi: 10.1097/OPX.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 24.Mountford J, Pesudovs K. An analysis of the astigmatic changes induced by accelerated orthokeratology. Clin Exp Optom. 2002;85:284–293. doi: 10.1111/j.1444-0938.2002.tb03084.x. [DOI] [PubMed] [Google Scholar]
- 25.Hiraoka T, Furuya A, Matsumoto Y, et al. Quantitative evaluation of regular and irregular corneal astigmatism in patients having overnight orthokeratology. J Cataract Refract Surg. 2004;30:1425–1429. doi: 10.1016/j.jcrs.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 26.Sorbara L, Fonn D, Simpson T, Lu F, Kort R. Reduction of myopia from corneal refractive therapy. Optom Vis Sci. 2005;82:512–518. doi: 10.1097/01.opx.0000166772.68413.0e. [DOI] [PubMed] [Google Scholar]
- 27.Hiraoka T, Kakita T, Okamoto F, Oshika T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology. 2015;122:93–100. doi: 10.1016/j.ophtha.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 28.Maseedupally V, Gifford P, Lum E, Swarbrick H. Central and paracentral corneal curvature changes during orthokeratology. Optom Vis Sci. 2013;90:1249–1258. doi: 10.1097/OPX.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 29.Kang P, Swarbrick H. New perspective on myopia control with orthokeratology. Optom Vis Sci. 2016;93:497–503. doi: 10.1097/OPX.0000000000000826. [DOI] [PubMed] [Google Scholar]
- 30.Hiraoka T, Okamoto C, Ishii Y, Kakita T, Okamoto F, Oshika T. Time course of changes in ocular higher-order aberrations and contrast sensitivity after overnight orthokeratology. Invest Ophthalmol Vis Sci. 2008;49:4314–4320. doi: 10.1167/iovs.07-1586. [DOI] [PubMed] [Google Scholar]
- 31.Kang P, Swarbrick H. Time course of the effects of orthokeratology on peripheral refraction and corneal topography. Ophthalmic Physiol Opt. 2013;33:277–282. doi: 10.1111/opo.12027. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Dong P, Liu H. Effect of overnight wear orthokeratology lenses on corneal shape and tears. Eye Contact Lens. 2018;44:304–307. doi: 10.1097/ICL.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 33.Alharbi A, Swarbrick HA. The effects of overnight orthokeratology lens wear on corneal thickness. Invest Ophthalmol Vis Sci. 2003;44:2518–2523. doi: 10.1167/iovs.02-0680. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team. R Foundation for Statistical Computing. Vienna, Austria: (2013). R: a language and environment for statistical computing. [Google Scholar]
- 35.Berntsen DA, Barr CD, Mutti DO, Zadnik K. Peripheral defocus and myopia progression in myopic children randomly assigned to wear single vision and progressive addition lenses. Invest Ophthalmol Vis Sci. 2013;54:5761–5770. doi: 10.1167/iovs.13-11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakita T, Hiraoka T, Oshika T. Influence of overnight orthokeratology on axial elongation in childhood myopia. Invest Ophthalmol Vis Sci. 2011;52:2170–2174. doi: 10.1167/iovs.10-5485. [DOI] [PubMed] [Google Scholar]
- 37.Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci. 2012;53:3913–3919. doi: 10.1167/iovs.11-8453. [DOI] [PubMed] [Google Scholar]
- 38.Queiros A, Gonzalez-Meijome JM, Jorge J, Villa-Collar C, Gutierrez AR. Peripheral refraction in myopic patients after orthokeratology. Optom Vis Sci. 2010;87:323–329. doi: 10.1097/OPX.0b013e3181d951f7. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Meijome JM, Faria-Ribeiro MA, Lopes-Ferreira DP, Fernandes P, Carracedo G, Queiros A. Changes in peripheral refractive profile after orthokeratology for different degrees of myopia. Curr Eye Res. 2016;41:199–207. doi: 10.3109/02713683.2015.1009634. [DOI] [PubMed] [Google Scholar]
- 40.Hiraoka T, Mihashi T, Okamoto C, Okamoto F, Hirohara Y, Oshika T. Influence of induced decentered orthokeratology lens on ocular higher-order wavefront aberrations and contrast sensitivity function. J Cataract Refract Surg. 2009;35:1918–1926. doi: 10.1016/j.jcrs.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Chen Z, Xue F, Zhou J, et al. Prediction of orthokeratology lens decentration with corneal elevation. Optom Vis Sci. 2017;94:903–907. doi: 10.1097/OPX.0000000000001109. [DOI] [PubMed] [Google Scholar]