Abstract
Preterm infants are at increased risk for patent ductus arteriosus (PDA). Prolonged exposure to PDA may be deleterious and has been associated with neonatal morbidity and mortality. Although the molecular mechanisms underlying regulation of postnatal ductus arteriosus closure are not fully understood, clinical experience and research trials have informed recent changes in PDA management strategies and refocused treatment strategies on smaller subsets of infants who require intervention. This review examines current diagnostic and management approaches to PDA in preterm neonates.
INTRODUCTION
The ductus arteriosus is a central vascular shunt connecting the pulmonary artery to the aorta, allowing oxygenated blood from the placenta to bypass the uninflated fetal lungs and enter the systemic circulation. Rapid closure of the ductus arteriosus after birth is essential for vascular transition to the mature, divided pattern of arteriovenous circulation. Failure of ductus arteriosus closure, termed patent ductus arteriosus (PDA), is primarily an affliction of prematurity, with the ductus remaining open at 7 days of age in up to 64% of infants born at 27 to 28 weeks’ gestation and 87% of infants born at 24 weeks. (1) There has been a shift in presentation and treatment of infants with PDA over the past 30 years. (2) (3) Before the use of antenatal corticosteroids, PDA was frequently found in premature infants of all gestational ages and was associated with respiratory distress syndrome. Treatment with indomethacin was standard, and studies evaluated the benefits of early (or even prophylactic) PDA ligation. (4)
With advances in ventilation strategies, use of antenatal corticosteroids and exogenous surfactant, and increased willingness to wait for spontaneous ductus arteriosus closure, today’s more mature preterm infants rarely require intervention for a ductus arteriosus. (5) Instead, PDA management is now focused on the most premature infants, in whom the ductus can be resistant to pharmacologic treatment. This review discusses the evolving indications for treatment of a PDA and the various treatment options available.
RISK FACTORS
The incidence of PDA is inversely associated with the degree of prematurity. Other factors associated with an increased risk of PDA in the premature infant include respiratory distress syndrome, high volume of intravenous fluids (>170 mL/kg per day) in the first week, sepsis, prolonged rupture of membranes, furosemide, male sex, and other contributors. (6) Evidence also shows that aminoglycoside antibiotics and certain antacids, frequently used in neonates, paradoxically increase the risk of a PDA. (7) Antenatal corticosteroids (8) and maternal hypertension (9) decrease the incidence of PDA. The effect of antenatal corticosteroids on closure of the ductus arteriosus is independent of their effect on lung maturation, and is most beneficial when corticosteroids are administered at least 24 hours before delivery. (10) Exogenous surfactant does not directly affect the ductus arteriosus, but can unmask a patent ductus by decreasing the pulmonary vascular resistance and thus allowing for increased left to right shunting. (1)
DIAGNOSIS
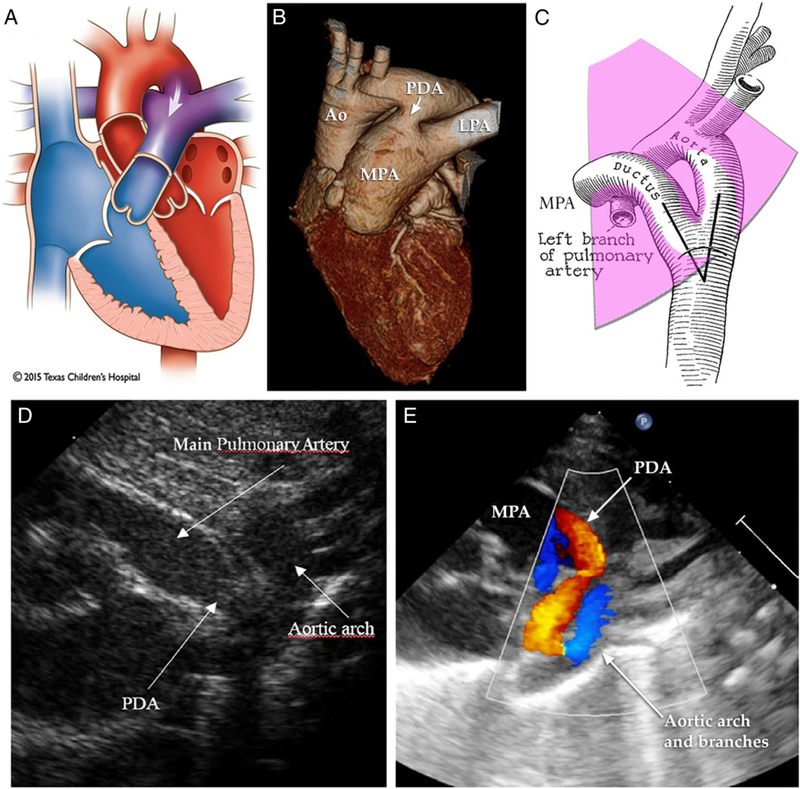
A consensus definition for hemodynamically significant PDA is lacking. The diagnosis is often suspected clinically, when an infant demonstrates signs of excessive shunting from the arterial to pulmonary circulation (Fig 1A and 1B):
Figure.
Evaluation of patent ductus arteriosus (PDA) with echocardiography. A, B. Left-to-right (L-R) shunt (indicated by arrows) via the ductus produces most of the physical signs and complications related to PDA. C–E.Short-axis andsuprasternal views reveal structural relationships and color Doppler flow patterns indicative of L-R shunt across the ductus arteriosus. Ao=aorta; LPA=left pulmonary artery; MPA=main pulmonary artery. Images adapted or reprinted with permission from Texas Children΄s Hospital,61)(62) and by Creative Commons license.(63)
Continuous or systolic murmur; note, a “silent” PDA may also occur when the ductus shunt is large enough that nonturbulent flow fails to generate a detectible murmur.
A low diastolic blood pressure (due to runoff into the ductus during diastole, more frequent in the most premature infants)
A wide pulse pressure (due to ductus runoff or steal)
Hypotension (especially in the most premature infants)
Bounding pulses
Increased serum creatinine concentration or oliguria
Hepatomegaly
Signs of pulmonary edema are often seen, including tachypnea, decreased oxygen saturation, and increasing respiratory support. Chest radiography can show stigmata of pulmonary edema. The neonatal cardiac output can increase as much as 25% in an effort to overcome ductus steal. (11)
Clinical suspicion should be confirmed with echocardiography (Table 1). Ductus size alone is inadequate to attribute hemodynamic significance. Although an absolute transductus diameter of more than 1.5 to 1.7 mm by color Doppler (Fig 1C and 1D) has been associated with increased propensity for hypoperfusion, the exact diameter at which hemodynamic significance will occur varies by patient and postnatal age, depending on body size and many other factors, including oxygen saturation, surfactant administration, and furosemide. (12) El Hajjar et al suggest that by normalizing to body weight, a ductus diameter of more than or equal to 1.4 mm/kg is a more sensitive measure. (13) A large left-to-right shunt suggests a hemodynamically significant shunt. However, the degree of shunting depends in large part on pulmonary vascular resistance. A large ductus may not demonstrate large left-to-right flow if pulmonary vascular resistance is increased. In contrast, a right-to-left shunt may indicate significant pulmonary hypertension; acute closure of the ductus in this scenario is not indicated, because it could result in worsening right heart failure. With a large left-to-right shunt, indirect signs of obligate pulmonary hypertension may often be seen; this generally is not a contraindication to closure of the ductus but rather a natural consequence of excessive pulmonary blood flow.
TABLE 1.
Echocardiographic Criteria Commonly Used to Diagnose PDA
| INDICATOR | SENSITIVITY | SPECIFICITY |
|---|---|---|
| LA/Ao ratio ≥1.5 (20) | 88% | 95% |
| LVO/SVC ratio >4 (19) | 92% | 91% |
| Ductus diameter ≥1.5 mm (64) [≥1.4 mm] (19)(20) | 95% (87%−92%) | 80% (61%−91%) |
| Ductus diameter >1.4 mm/kg (19) | 94% | 90% |
| End diastolic flow velocity in left PA ≥0.2 m/s | 82% | 83% |
| Increased left ventricular output >60 mL/kg per minute above baseline (65) | N/A | N/A |
| Transductal velocity ratio <1.8 (66) | N/A | N/A |
LA/Ao=left atrium to aortic ratio; LVO/SVC=left ventricular output to superior vena cava flow ratio; PA=pulmonary artery; N/A=not available.
Other echocardiographic criteria used to determine hemodynamic significance are the presence of reversal of forward flow in the descending aorta during diastole (indicating shunting through the PDA), and left atrial or ventricular dilation, which are the consequence of over-circulation of the pulmonary vascular bed and a chronic hyperdynamic state. Left-to-right flow across the intra-atrial septum is indicative of a large shunt. The ratio of the left ventricular output to superior vena cava flow is directly proportional to the ductus flow and, when greater than or equal to 4, may indicate hemodynamic significance. A left atrial to aortic root (LA/Ao) ratio is most sensitive when performed after day 1, and is considered abnormal if greater than 1.5. For comparison, the ductus is considered widely patent when the LA/Ao ratio is greater than 1.6, and closed when the ratio is 1.17. (14) If a cutoff of 1.4 is used, sensitivity of the LA/Ao ratio drops from 88% to between 50% and 79%. (14)(15)
PDA severity scores have been developed that combine various factors and can be used to predict higher likelihood of serious outcomes, such as death/chronic lung disease (CLD), periventricular leukomalacia, or necrotizing enterocolitis (NEC). The benefit of a scoring system is that it emphasizes the importance of examining multiple factors when determining when and whether to intervene for a PDA. Table 2 shows a comparison of 2 separate scoring systems. Although they use different factors, they are equivalent in defining a hemodynamically significant PDA and prediction of CLD or death. The El-Khuffash score correlates significantly with NEC and the Shaare Zedek score correlates significantly with periventricular leukomalacia. (16)
TABLE 2.
PDA Severity Scoring Systems (I6)
| SHAARE ZEDEK | EL-KHUFFASH |
|---|---|
| Ductus arteriosus diameter | Ductus arteriosus diameter |
| LA/Ao ratio | Gestation |
| Retrograde diastolic flow in the abdominal aorta | LVOa |
| Ductus arteriosus shunt flow pattern | Vmax across PDA (m/s) |
| Left ventricular late diastolic (a’) velocity |
LA/Ao=left atrium to aortic ratio; LVO=left ventricular output;
PDA=patent ductus arteriosus; Vmax=maximum flow velocity;
VTI=velocity time interval.
(Aortic cross-sectional area × VTI × heart rate) ÷ weight
Biomarkers
The N (amino)-terminal prohormone of B-type natriuretic peptide (NT-proBNP) and mature B-type natriuretic peptide (BNP) may be useful in detecting a hemodynamically significant PDA. BNP is secreted and released by the ventricular myocardium when under stress from either increased volume or pressure. NT-proBNP, BNP, and cardiac troponin rise with increase in echocardiographic markers of PDA, and decrease following closure of the ductus. Not enough studies have been conducted to recommend these bio-markers for routine use in clinical management of a PDA. (17) They are likely to be of most benefit when the diagnosis of PDA is confirmed with echocardiography, but serial echocardiography to determine hemodynamic significance is unavailable, (12) or to shorten a course of pharmacologic treatment for a ductus. Shin et al stopped treatment with ibuprofen in infants with a PDA when BNP concentration dropped to less than 600 pg/mL, resulting in fewer doses of ibuprofen in the group whose treatment was tailored based on BNP. (18)
Biosensors
Bioengineering and analytical techniques have also been developed to monitor or detect PDA in preterm infants. Various approaches include interpretation of pulse oximetry information (perfusion index, plethysmography), (19)(20)(21) interpretation of transthoracic electrical signals (impedance, cardiometry, velocimetry, bioreactance), (22)(23)(24)(25) regional oxygenation (near-infrared spectroscopy), (26) alterations in skin microcirculation (side stream dark-field imaging, reflectance spectrophotometry), (27)(28) resonance Raman spectroscopy (Lauren J. Ruoss, personal communication, May 2018), and others.
OUTCOME
In healthy full-term infants, the ductus arteriosus closes within 48 to 72 hours. In premature infants born weighing more than 1,000 g, the ductus closes spontaneously in 67% by day 7 and in 94% by discharge. (5) Overall, only 3% of infants weighing more than 1,000 g may require intervention for a PDA. (1)(5)
However, in extremely premature infants weighing less than 1,000 g at birth (extremely low birthweight), 57% to 69% will still have a PDA at 7 to 10 days of age. (5)(9) Of those that close, up to 30% will reopen and may then reclose, or go on to become hemodynamically significant and require pharmacologic or surgical closure. When left untreated, the median time to ductus closure in this population is 56 days. (5) Some infants undergo spontaneous ductus closure as late as 24 months of age. (29)(30) Although these infants only account for a small proportion of premature infants and have small PDAs at the time of discharge, (31) the recognition that PDA closure occurs late in some cases has prompted some investigators to completely withhold retreatment. (32)(33) Because hemodynamically significant PDA has been associated with intraventricular hemorrhage (IVH), pulmonary hemorrhage, NEC, CLD, (9) and death, more substantial studies are required before nonintervention approaches can be widely adopted.
TREATMENT
Some clinicians choose a prophylactic approach to indo-methacin treatment, with a goal of preventing IVH, PDA, and the adverse consequences that have been associated with PDA in extremely low-birthweight infants. (34) Despite successful reduction in short-term outcomes (IVH, pulmonary hemorrhage, hypotension, symptomatic PDA, need for ligation), long-term benefits are uncertain and infants may needlessly be exposed to a potentially harmful agent. (3)(35)(36)(37)(38)(39) On the other hand, NICUs with consistent, high-level use of a prophylactic strategy (40) or an echocardiography guided selective approach to prophylactic treatment (41) may have improved outcomes or less drug exposure. Well-designed studies are needed to clarify the risks/benefits of this approach.
The indications for treatment of a symptomatic PDA include respiratory compromise (eg, requiring persistent mechanical support), heart failure, or large left-to-right ductus shunt with evidence of hemodynamic compromise, such as reversal of flow in the descending aorta during diastole, oliguria or rising serum creatinine concentration, hypotension, or wide pulse pressure.
In patients of more than 1,000 g birthweight with few risk factors, a PDA can generally be successfully managed conservatively, with modest fluid restriction and use of positive end expiratory pressure to treat pulmonary edema. Certain diuretics, such as furosemide, can prevent a ductus from closing (42) and are not recommended in the first 1 to 3 weeks, when the greatest decrease in ductus diameter occurs spontaneously. Conservative measures also include avoidance of other drugs that promote ductus arteriosus relaxation (43) and proactive use of agents like caffeine that are associated with lower rates of PDA. (44)
In patients at higher risk of PDA, or who weigh less than 1,000 g at birth, conservative treatment is recommended before starting pharmacologic treatment. Treatment is generally not necessary in the first few days after birth when the pulmonary vascular resistance is still elevated. However, during the second week, treatment should be considered if conservative measures have failed to control pulmonary edema, or if there is cardiac or renal failure. After week 3, pharmacologic measures are less likely to be successful.
Early use of indomethacin to close a PDA soon after birth (day 1 2 of age), compared with waiting 3 to 4 days after diagnosis, decreases the risk for pulmonary hemorrhage and IVH, and need for ligation. Although PDA is associated with CLD, there is limited evidence to suggest that early pharmacologic closure improves incidence of CLD. (40)(45) Pharmacologic closure is associated with decreased pulmonary edema and improved alveolarization (in premature baboons). This may be due at least in part to a direct effect on the lung. Use of ibuprofen or indomethacin is associated with increased amiloride-sensitive alveolar epithelial sodium channels, increased lung water clearance, and improved lung compliance. (45)
PHARMACOLOGY
Three pharmacologic treatments are available to induce constriction of a PDA: indomethacin, ibuprofen, and acetaminophen (paracetamol) (Table 3). Indomethacin and ibuprofen are classic nonsteroidal anti-inflammatory drugs (NSAIDs), which nonselectively inhibit the cyclooxygenase enzymes, preventing the conversion of arachidonic acid to prostaglandins, which play a central role in maintaining ductus patency. Since 1976, indomethacin has been used to treat PDA in premature infants. Around the same time, ibuprofen was shown in lamb models to effect ductal closure but it was not widely used in human infants until the mid-1990s. Acetaminophen reduces prostaglandin synthesis by a different mode of action than most NSAIDs. Recent randomized clinical trials confirm its efficacy for PDA closure, though Food and Drug Administration approval for this indication is pending.
TABLE 3.
Common Dosing Regimens for Treatment of PDA
| MEDICATION | DOSING | CONTRAINDICATIONS | DRUG MONITORING |
|---|---|---|---|
| Acetaminophen | IV or PO: 7.5,10, or 15 mg/kg per dose every 6–8 hours for 3–7 days (length determined by echo) | •Liver failure | AST, ALT, GGT and acetaminophen level before 9th dose |
| Ibuprofen | IV: 20 mg/kg followed by 10 mg/kg 24 hours apart for total 3 doses | •Significant renal impairmenta | Urine output |
| •Necrotizing enterocolitis | |||
| PO: 10 mg/kg followed by 5 mg/kg 24 hours apart for total 3 doses | •Spontaneous intestinal perforation | ||
| •Thrombocytopeniab | |||
| Indomethacin | IV: 2–7 days of age: 0.2 mg/kg every 12 h for 3 doses, can be followed bya4th dose24 hours after the 3rd | •Significant renal impairmenta | Urine output |
| •Necrotizing enterocolitis | |||
| >7 days of age: 0.25 mg every 12 h for 3 doses, can be followed by a 4th dose 24 hours after the 3rd | •Spontaneous intestinal perforation | ||
| Enteraland rectalroutes not recommended | •Thrombocytopeniab |
ALT=alanine transaminase; AST=aspartate transaminase; echo=echocardiography; GGT=γ-glutamyl transferase; IV=intravenous; PDA=patent ductus arteriosus; PO=oral.
A hemodynamically significant PDA may cause renal impairment with oliguria and mild to modest increase in serum creatinine over baseline which is not a contraindication to pharmacologic treatment. Anuria or a significant increase in serum creatinine is a contraindication.
Platelets <50,000/μL (<50×109/L).
Indomethacin is administered intravenously. Enteral and rectal preparations are not recommended in infants because of increased risk for gastrointestinal bleeding.
In patients who receive a second course of indomethacin, only half will experience ductus closure. The odds of nonresponse to the second course of indomethacin are increased by 90% if there was nonresponse to the first course. Advancing gestational age appears to predict non-response to indomethacin. (46)
When studied head-to-head, indomethacin and ibuprofen have a similar efficacy (70%) for an initial course of 3 doses. Regardless of the treatment, there is about a 25% rate of reopening, especially in the most premature infants. Because of this high rate of reopening, some advocate a fourth dose of indomethacin, given 24 hours after the third dose.
Acetaminophen is speculated to decrease prostaglandin synthesis by interrupting prostaglandin synthesis at the peroxidase site of prostaglandin H2 synthetase (cyclooxygenase). Acetaminophen for treatment of PDA is associated with less elevation in serum creatinine concentration and oliguria compared to ibuprofen or indomethacin, and less elevation in bilirubin compared to ibuprofen. (47) Acetaminophen has been used for rescue therapy after failed response to indomethacin in extremely premature infants, resulting in 46% of infants having a smaller or closed ductus. (48) When used as primary treatment, the efficacy ranges from 70% to 81%. (49)(50) Efficacy appears to be affected by both gestational age and postnatal age, with improved efficacy noted when treatment was started within the first week. (51) In fact, many case reports describing the use of acetaminophen start treatment as early as 2 to 3 days of age, early enough that many patients may go on to experience complete closure. Ductus closure rates are lower for the 3-day course (56%). (49)
There is a range of reported treatment regimens for acetaminophen, from 7.5 mg to 10 or 15 mg/kg every 6 hours for 3 to 7 days. Acetaminophen can be given orally, at the same dosage and interval, with similar reported efficacy as the intravenous route. (49) Kessel et al (52) showed that paracetamol levels in infants of age 26 to 30 weeks treated with 15 mg/kg per day orally remained mostly within the recommended range of 10 to 20 mg/mL for analgesia before the fifth and ninth doses, with only 2 of 8 patients exceeding the desired range before the ninth dose.
Treatment with acetaminophen can be associated with increase in serum concentration of liver enzymes in children and adults. This has been reported in preterm infants after as few as 4 doses of 15 mg/kg per day. Spontaneous resolution has been reported in all cases after the cessation of acetaminophen administration. Immaturity of the hepatic CYP enzymes responsible for acetaminophen metabolism may be protective against short-term toxicity in preterm infants; however, caution is warranted because liver injury is still possible. (53)
Whether or not to withhold enteral nutrition when a patient has a significant PDA or during pharmacologic treatment has long been a source of practice variability. A hemodynamically significant PDA can reduce forward blood flow to the superior mesenteric artery during diastole, and indomethacin acutely decreases gut blood flow. On the other hand, fasting is associated with intestinal mucosal atrophy which could increase the risk for NEC. A trial of premature infants being treated with indomethacin or ibuprofen and randomized to either receive 15 mL/kg of feeds per day or fast during treatment showed no difference in rates of NEC, and the patients randomized to continue feedings reached full feeds earlier than infants whose feeds were held. (54)
LIGATION
Surgical ligation is performed when a neonate has a hemo-dynamically significant PDA that results in cardiac dysfunction, renal failure, or respiratory failure. Ligation is typically performed with an open thoracic approach, and either using a metal clip or tying off the vessel. Intravascular approaches with placement of an occluding coil are available for patients weighing more than 5 kg. Trials are under way with products in development to accomplish catheter-based PDA closure in smaller patients. (55)
PDA ligation is associated with many adverse effects: vocal cord paralysis, postoperative hypotension, diaphragm paralysis, (56) bronchopulmonary dysplasia, and worse neurodevelopment. (57) Early PDA ligation is an independent risk factor for BPD (45) and worse neurodevelopment compared with ligation at a later age. (58) There does not appear to be increased risk of major complications when infants who have failed indomethacin treatment but do not have cardiopulmonary compromise are treated conservatively, rather than treated with ligation. (59) However, poor outcomes after PDA ligation may be overestimated by trial design, (60) suggesting that surgical approaches deserve full consideration for infants with refractory, symptomatic PDA.
CONCLUSION
PDA remains an important condition among premature infants born at less than 28 weeks’ gestation. Recent advances include the addition of acetaminophen (paracetamol) to the arsenal of available treatments, and further support for conservative management of the asymptomatic or mildly symptomatic PDA, before the consideration of pharmacologic treatment. Improvements are still needed regarding standardized echocardiographic criteria, optimal timing of treatment (when indicated), dosing regimens for acetaminophen, and development of endovascular occlusive devices for the smallest preterm infants.
Education Gaps.
It is important to recognize the risks for symptomatic patent ductus arteriosus. Infants born weighing more than 1,000 g or at more than 28 weeks’ gestation generally do not require pharmacologic or surgical intervention for patent ductus arteriosus.
Criteria that define patent ductus arteriosus pathophysiology need to be identified. Size of the ductus arteriosus alone is inadequate as a determinant when deciding whether to intervene pharmacologically or surgically. Criteria such as gestational age, postnatal age, and markers of hemodynamic significance, including degree of respiratory support, presence of oliguria, and other echocardiographic indicators, should be considered.
Objectives
After completing this article, readers should be able to:
Explain the efficacy and associated side effects of the 3 pharmacologic agents (acetaminophen, ibuprofen, and indomethacin) used to treat a hemodynamically significant patent ductus arteriosus (PDA).
Identify clinical signs of a hemodynamically significant PDA.
Identify known risk factors for PDA in the preterm population.
Describe the characteristic echocardiographic features associated with a PDA and their sensitivity and specificity.
American Board of Pediatrics Neonatal-Perinatal Content Specifications
Recognize the clinical features of a preterm neonate with a patent ductus arteriosus.
Recognize the laboratory, imaging, and other diagnostic features of a preterm neonate with a patent ductus arteriosus.
Formulate a differential diagnosis of a preterm neonate with a patent ductus arteriosus.
Know the evaluation and medical and/or surgical management and associated potential complications or adverse effects of such management for a preterm neonate with a patent ductus arteriosus.
ABBREVIATIONS
- BNP
B-type natriuretic peptide
- CLD
chronic lung disease
- IVH
intraventricular hemorrhage
- LA/Ao
left atrium to aortic ratio
- LVO/SVC
left ventricular output to superior vena cava flow ratio
- NEC
necrotizing enterocolitis
- NSAIDs
nonsteroidal anti-inflammatory drugs
- NT-proBNP
N (amino)-terminal prohormone of B-type natriuretic peptide
- PDA
patent ductus arteriosus
Footnotes
AUTHOR DISCLOSURE
Dr Gillam-Krakauer is supported, in part, by the Katherine Dodd faculty scholars program from the Department of Pediatrics, Vanderbilt University Medical Center. Dr Reese has disclosed an NIH award (HL128386). This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
References
- 1.Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36(2):123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Khuffash A, Weisz DE, McNamara PJ. Reflections of the changes in patent ductus arteriosus management during the last 10 years. Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F474–F478 [DOI] [PubMed] [Google Scholar]
- 3.Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J Perinatol. 2010;30(4):241–252 [DOI] [PubMed] [Google Scholar]
- 4.Mosalli R, Alfaleh K. Prophylactic surgical ligation of patent ductus arteriosus for prevention of mortality and morbidity in extremely low birth weight infants. Cochrane Database Syst Rev. 2008;(1): CD006181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemerofsky SL, Parravicini E, Bateman D, Kleinman C, Polin RA, Lorenz JM. The ductus arteriosus rarely requires treatment in infants >1000 grams. Am J Perinatol. 2008;25(10):661–666 [DOI] [PubMed] [Google Scholar]
- 6.Gillam-Krakauer M, Cotton RB, Reese J. Patent ductus arteriosus. In: Feld LG, Mahan JD, Lorenz JM, Seigel WM, eds. Succinct Pediatrics: Evaluation and Management for Newborn, Genetic, Neurologic, and Developmental-Behavioral Disorders. Elk Grove Village, IL: American Academy of Pediatrics; 2017:137–150 [Google Scholar]
- 7.Vucovich MM, Cotton RB, Shelton EL, et al. Aminoglycoside-mediated relaxation of the ductus arteriosus in sepsis-associated PDA. Am J Physiol Heart Circ Physiol. 2014;307(5):H732–H740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chorne N, Jegatheesan P, Lin E, Shi R, Clyman RI. Risk factors for persistent ductus arteriosus patency during indomethacin treatment. J Pediatr. 2007;151(6):629–634 [DOI] [PubMed] [Google Scholar]
- 9.Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117(4):1113–1121 [DOI] [PubMed] [Google Scholar]
- 10.Clyman RI, Ballard PL, Sniderman S, et al. Prenatal administration of betamethasone for prevention of patient ductus arteriosus. J Pediatr. 1981;98(1):123–126 [DOI] [PubMed] [Google Scholar]
- 11.Alverson DC, Eldridge MW, Johnson JD, et al. Effect of patent ductus arteriosus on left ventricular output in premature infants. J Pediatr. 1983;102(5):754–757 [DOI] [PubMed] [Google Scholar]
- 12.Sehgal A, McNamara PJ. The ductus arteriosus: a refined approach! Semin Perinatol. 2012;36(2):105–113 [DOI] [PubMed] [Google Scholar]
- 13.El Hajjar M, Vaksmann G, Rakza T, Kongolo G, Storme L. Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F419–F422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyer P, Evans N. Re-evaluation of the left atrial to aortic root ratio as a marker of patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 1994;70(2):F112–F117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson GL, Breart GL, Gewitz MH, et al. Echocardiographic characteristics of premature infants with patent ductus arteriosus. Pediatrics. 1983;72(6):864–871 [PubMed] [Google Scholar]
- 16.Fink D, El-Khuffash A, McNamara PJ, Nitzan I, Hammerman C. Tale of two patent ductus arteriosus severity scores: Similarities and differences. Am J Perinatol. 2018;35(1):55–58 [DOI] [PubMed] [Google Scholar]
- 17.Weisz DE, McNamara PJ, El-Khuffash A. Cardiac biomarkers and haemodynamically significant patent ductus arteriosus in preterm infants. Early Hum Dev. 2017;105:41–47 [DOI] [PubMed] [Google Scholar]
- 18.Shin J, Lee EH, Lee JH, Choi BM, Hong YS. Individualized ibuprofen treatment using serial B-type natriuretic peptide measurement for symptomatic patent ductus arteriosus in very preterm infants. Korean J Pediatr. 2017;60(6):175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goudjil S, Imestouren F, Armougon A, et al. Noninvasive technique for the diagnosis of patent ductus arteriosus in premature infants by analyzing pulse wave phases on photoplethysmography signals measured in the right hand and the left foot. PLoS One. 2014;9(6):e98763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khositseth A, Muangyod N, Nuntnarumit P. Perfusion index as a diagnostic tool for patent ductus arteriosus in preterm infants. Neonatology. 2013;104(4):250–254 [DOI] [PubMed] [Google Scholar]
- 21.Vidal M, Ferragu F, Durand S, Baleine J, Batista-Novais AR, Cambonie G. Perfusion index and its dynamic changes in preterm neonates with patent ductus arteriosus. Acta Paediatr. 2013;102(4):373–378 [DOI] [PubMed] [Google Scholar]
- 22.Cotton RB, Lindstrom DP, Olsson T, et al. Impedance cardiographic assessment of symptomatic patent ductus arteriosus. J Pediatr. 1980;96(4):711–715 [DOI] [PubMed] [Google Scholar]
- 23.Lien R, Hsu KH, Chu JJ, Chang YS. Hemodynamic alterations recorded by electrical cardiometry during ligation of ductus arteriosus in preterm infants. Eur J Pediatr. 2015;174(4):543–550 [DOI] [PubMed] [Google Scholar]
- 24.Torigoe T, Sato S, Nagayama Y, Sato T, Yamazaki H. Influence of patent ductus arteriosus and ventilators on electrical velocimetry for measuring cardiac output in very-low/low birth weight infants. J Perinatol. 2015;35(7):485–489 [DOI] [PubMed] [Google Scholar]
- 25.Weisz DE, Jain A, Ting J, McNamara PJ, El-Khuffash A. Noninvasive cardiac output monitoring in preterm infants undergoing patent ductus arteriosus ligation: a comparison with echocardiography. Neonatology. 2014;106(4):330–336 [DOI] [PubMed] [Google Scholar]
- 26.Underwood MA, Milstein JM, Sherman MP. Near-infrared spectroscopy as a screening tool for patent ductus arteriosus in extremely low birth weight infants. Neonatology. 2007;91(2):134–139 [DOI] [PubMed] [Google Scholar]
- 27.De Felice C, Mazzieri S, Pellegrino M, et al. Skin reflectance changes in preterm infants with patent ductus arteriosus. Early Hum Dev. 2004;78(1):45–51 [DOI] [PubMed] [Google Scholar]
- 28.Hiedl S, Schwepcke A, Weber F, Genzel-Boroviczeny O. Microcirculation in preterm infants: profound effects of patent ductus arteriosus. J Pediatr. 2010;156(2):191–196 [DOI] [PubMed] [Google Scholar]
- 29.Herrman K, Bose C, Lewis K, Laughon M. Spontaneous closure of the patent ductus arteriosus in very low birth weight infants following discharge from the neonatal unit. Arch Dis Child Fetal Neonatal Ed. 2009;94(1):F48–F50 [DOI] [PubMed] [Google Scholar]
- 30.Weber SC, Weiss K, Bührer C, Hansmann G, Koehne P, Sallmon H. Natural history of patent ductus arteriosus in very low birth weight infants after discharge. J Pediatr. 2015;167(5):1149–1151 [DOI] [PubMed] [Google Scholar]
- 31.Reese J, Laughon MM. The patent ductus arteriosus problem: Infants who still need treatment. J Pediatr. 2015;167(5):954–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohamed MA, El-Dib M, Alqahtani S, Alyami K, Ibrahim AN, Aly H. Patent ductus arteriosus in premature infants: to treat or not to treat? J Perinatol. 2017;37(6):652–657 [DOI] [PubMed] [Google Scholar]
- 33.Sung SI, Chang YS, Chun JY, et al. Mandatory closure versus nonintervention for patent ductus arteriosus in very preterm infants. J Pediatr. 2016;177:66–71.e61 [DOI] [PubMed] [Google Scholar]
- 34.Reese J, Shelton EL, Slaughter JC, McNamara PJ. Prophylactic indomethacin revisited. J Pediatr. 2017;186:11–14.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ment LR, Vohr BR, Makuch RW, et al. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr. 2004;145(6):832–834 [DOI] [PubMed] [Google Scholar]
- 36.Laughon MM, Simmons MA, Bose CL. Patency of the ductus arteriosus in the premature infant: is it pathologic? Should it be treated? Curr Opin Pediatr. 2004;16(2):146–151 [DOI] [PubMed] [Google Scholar]
- 37.Liebowitz M, Clyman RI. Prophylactic indomethacin compared with delayed conservative management of the patent ductus arteriosus in extremely preterm infants: effects on neonatal outcomes. J Pediatr. 2017;187:119–126.e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt B, Davis P, Moddemann D, et al. ; Trial of Indomethacin Prophylaxis in Preterms Investigators. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344(26):1966–1972 [DOI] [PubMed] [Google Scholar]
- 39.Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010; (7):CD000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen EA, Dysart KC, Gantz MG, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association between use of prophylactic indomethacin and the risk for bronchopulmonary dysplasia in extremely preterm infants. J Pediatr. 2017;186:34–40.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014;99(2):F99–F104 [DOI] [PubMed] [Google Scholar]
- 42.Cotton R, Suarez S, Reese J. Unexpected extra-renal effects of loop diuretics in the preterm neonate. Acta Paediatr. 2012;101(8):835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reese J, Veldman A, Shah L, Vucovich M, Cotton RB. Inadvertent relaxation of the ductus arteriosus by pharmacologic agents that are commonly used in the neonatal period. Semin Perinatol. 2010;34(3):222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt B, Roberts RS, Davis P, et al. ; Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–2121 [DOI] [PubMed] [Google Scholar]
- 45.Clyman RI. The role of patent ductus arteriosus and its treatments in the development of bronchopulmonary dysplasia. Semin Perinatol. 2013;37(2):102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Louis D, Wong C, Ye XY, McNamara PJ, Jain A. Factors associated with non-response to second course indomethacin for PDA treatment in preterm neonates. J Matern Fetal Neonatal Med. 2018; 31(11):1407–1414 [DOI] [PubMed] [Google Scholar]
- 47.El-Mashad AE, El-Mahdy H, El Amrousy D, Elgendy M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. Eur J Pediatr. 2017;176(2):233–240 [DOI] [PubMed] [Google Scholar]
- 48.Weisz DE, Martins FF, Nield LE, El-Khuffash A, Jain A, McNamara PJ. Acetaminophen to avoid surgical ligation in extremely low gestational age neonates with persistent hemodynamically significant patent ductus arteriosus. J Perinatol. 2016;36(8):649–653 [DOI] [PubMed] [Google Scholar]
- 49.Dang D, Wang D, Zhang C, Zhou W, Zhou Q, Wu H. Comparison of oral paracetamol versus ibuprofen in premature infants with patent ductus arteriosus: a randomized controlled trial. PLoS One. 2013;8(11):e77888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le J, Gales MA, Gales BJ. Acetaminophen for patent ductus arteriosus. Ann Pharmacother. 2015;49(2):241–246 [DOI] [PubMed] [Google Scholar]
- 51.Terrin G, Conte F, Scipione A, et al. Efficacy of paracetamol for the treatment of patent ductus arteriosus in preterm neonates. Ital J Pediatr. 2014;40(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kessel I, Waisman D, Lavie-Nevo K, Golzman M, Lorber A, Rotschild A. Paracetamol effectiveness, safety and blood level monitoring during patent ductus arteriosus closure: a case series. J Matern Fetal Neonatal Med. 2014;27(16):1719–1721 [DOI] [PubMed] [Google Scholar]
- 53.Walls L, Baker CF, Sarkar S. Acetaminophen-induced hepatic failure with encephalopathy in a newborn. J Perinatol. 2007;27(2):133–135 [DOI] [PubMed] [Google Scholar]
- 54.Clyman R, Wickremasinghe A, Jhaveri N, et al. ; Ductus Arteriosus Feed or Fast with Indomethacin or Ibuprofen (DAFFII) Investigators. Enteral feeding during indomethacin and ibuprofen treatment of a patent ductus arteriosus. J Pediatr. 2013;163(2):406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Backes CH, Rivera BK, Bridge JA, et al. Percutaneous patent ductus arteriosus (PDA) closure during infancy: A meta-analysis. Pediatrics. 2017;139(2):e20162927. [DOI] [PubMed] [Google Scholar]
- 56.Hsu KH, Chiang MC, Lien R, et al. Diaphragmatic paralysis among very low birth weight infants following ligation for patent ductus arteriosus. Eur J Pediatr. 2012;171(11):1639–1644 [DOI] [PubMed] [Google Scholar]
- 57.Pros Noori S. and cons of patent ductus arteriosus ligation: hemodynamic changes and other morbidities after patent ductus arteriosus ligation. Semin Perinatol. 2012;36(2):139–145 [DOI] [PubMed] [Google Scholar]
- 58.Wickremasinghe AC, Rogers EE, Piecuch RE, et al. Neurodevelopmental outcomes following two different treatment approaches (early ligation and selective ligation) for patent ductus arteriosus. J Pediatr. 2012;161(6):1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010;157(3):381–387.e381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weisz DE, Mirea L, Rosenberg E, et al. Association of patent ductus arteriosus ligation with death or neurodevelopmental impairment among extremely preterm infants. JAMA Pediatr. 2017;171(5):443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicol ED, Gatzoulis M, Padley SP, Rubens M. Assessment of adult congenital heart disease with multi-detector computed tomography: beyond coronary lumenography. Clin Radiol. 2007;62(6):518–527 [DOI] [PubMed] [Google Scholar]
- 62.Mancini AJ. A study of the angle formed by the ductus arteriosus with the descending thoracic aorta. Anat Rec. 1951;109(3):535–539 [DOI] [PubMed] [Google Scholar]
- 63.Arlettaz R Echocardiographic evaluation of patent ductus arteriosus in preterm infants. Front Pediatr. 2017;5:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kluckow M, Evans N. Early echocardiographic prediction of symptomatic patent ductus arteriosus in preterm infants undergoing mechanical ventilation. J Pediatr. 1995;127(5):774–779 [DOI] [PubMed] [Google Scholar]
- 65.Walther FJ, Kim DH, Ebrahimi M, Siassi B. Pulsed Doppler measurement of left ventricular output as early predictor of symptomatic patent ductus arteriosus in very preterm infants. Biol Neonate. 1989;56(3):121–128 [DOI] [PubMed] [Google Scholar]
- 66.Davies MW, Betheras FR, Swaminathan M. A preliminary study of the application of the transductal velocity ratio for assessing persistent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2000;82(3):F195–F199 [DOI] [PMC free article] [PubMed] [Google Scholar]