Abstract
We have previously demonstrated that an acute dose of Δ9-tetrahydrocanninbinol (THC), administered prior to extinction learning, facilitates later recall of extinction learning and modulates the underlying neural circuitry, including the ventromedial prefrontal cortex (vmPFC), hippocampus (HPC), and amygdala (AMYG). It remains unknown whether THC-induced changes in fear-extinction neural circuitry can be detected following extinction learning, which may reflect ongoing processes involved consolidation of the extinction memory. To address this gap, we used a randomized, double-blind, placebo-controlled, between-subjects design to compare acute pharmacological effects of THC (7.5mg) vs. placebo (PBO) on post-extinction resting-state functional connectivity (RS-FC) within fear-extinction circuitry in 77 healthy adults (THC = 40; PBO = 37). RS-FC was examined between vmPFC, HPC, and AMYG using two complementary approaches: 1) static RS-FC (average correlation in ROI-ROI pairs across the entire scan); and 2) dynamic (i.e., time-varying) RS-FC (sliding window correlation time series’ variance). RS-FC was then linked to behavioral and brain measures of extinction recall. Compared to PBO, THC administration was associated with lower AMYG-HPC static RS-FC, but higher AMYG-vmPFC dynamic RS-FC. Lower AMYG-HPC static RS-FC was associated with higher HPC activation, as well as, better extinction recall. Moreover, lower AMYG-HPC static RS-FC following extinction learning mediated the link between THC administration and extinction recall. Post-extinction RSFC patterns may reflect sustained effects of THC on fear-extinction circuitry even in the absence of an overt task, and/or effects of ongoing processes that serve to strengthen the neural connections supporting the consolidation of the memory and better extinction recall.
Keywords: extinction, cannabinoid, resting-state functional connectivity, amygdala, hippocampus, ventromedial prefrontal cortex
1. Introduction
Recent human and nonhuman animal research shows that the endogenous cannabinoid (CB) system plays a key role in the modulation of emotional states and the extinction of aversive memories. Although type 1 cannabinoid receptors (CB1) are located throughout the brain, they are highly expressed in regions critical for the learning and retention of extinction memories, including the amygdala (AMYG), hippocampus (HPC), and ventromedial prefrontal cortex (vmPFC; Milad and Quirk, 2012). Antagonism of CB1 signaling, either by genetic or pharmacologic means, has been shown to lead to profound deficits in extinction learning in animals (Marsicano et al., 2002). In contrast, pharmacological activation of CB1 receptors, via agonists like Δ9tetrahydrocannabinol (THC), facilitates extinction learning and its later recall (Bitencourt et al., 2008; Varvel et al., 2007). Given the similarities between extinction processes and exposure-based psychotherapy, interventions that enhance the CB system during extinction have become a prime translational target for improving the treatment of fear-based disorders such as posttraumatic stress disorder (PTSD).
Using a double-blind, placebo-controlled between subjects design, we have previously demonstrated that pre-extinction administration of an acute dose of THC facilitates recall of extinction, in healthy adults (Rabinak et al, 2013). In a follow-up functional magnetic resonance imaging (fMRI) study in a separate group of healthy adults, we demonstrated that THC administration was associated with increased activity within HPC-vmPFC circuitry during extinction recall, relative to PBO (Rabinak et al., 2014). Increased HPC-vmPFC activation and connectivity, and increased vmPFC cortical thickness, is associated with better extinction recall in healthy individuals (Milad et al., 2007, 2005), likely through the suppression of conditioned fear responding via modulation of AMYG output (Quirk and Mueller, 2008). Thus, CB system modulators have emerged as a promising pharmacological means for enhancing extinction recall via modulation of HPC-AMYG-vmPFC circuitry (Rabinak and Phan, 2014).
The present study examines the effects of THC on fear-extinction neural circuitry during a rest period following extinction learning, to test for enduring effects of THC on functional connectivity. One previous study demonstrated that resting-state functional connectivity (RS-FC) between the AMYG and dorsomedial prefrontal cortex (dmPFC) is augmented following a Pavlovian fear conditioning session (Schultz et al., 2012). The authors suggested that the observed RS-FC patterns following a learning session may reflect an ongoing consolidation process, whereby a strengthening of neural connections supports the more permanent storage of the memory following the learning session (Schultz et al., 2012). Thus, given the observed effects of THC on later recall of extinction learning, we aimed to examine RS-FC patterns that may be similarly observed following extinction learning. Research in experimental animals suggests that consolidation of the extinction memory requires initiation of various molecular cascades during the post-learning period (Quirk and Mueller, 2008).
RS-FC is conventionally computed as the correlation in activity of discrete brain regions, calculated across the entire resting-state scan. The magnitude in correlation – so-called static RS-FC – has been used to index the stable strength of connections between brain regions (Friston, 2011). However, emerging research has evaluated dynamic RS-FC, which evaluates how relationships between brain regions change over time (Calhoun et al., 2014; Chang and Glover, 2010; Hutchison et al., 2013a; Marusak et al., 2016). Dynamic RS-FC can be investigated by measuring variability in the strength of functional connections (Allen et al., 2014; Barttfeld et al., 2015; Handwerker et al., 2012; Hindriks et al., 2016; Hutchison et al., 2013b; Marusak et al., 2016). Prior research shows that brain regions with shared functions (e.g., bilateral homologues) tend to show lower dynamic RS-FC relative to regions involved in more flexible processes (Meunier et al., 2009; Shen et al., 2015), and that variation in RS-FC dynamics is observed during different emotional states (Cribben et al., 2012) or arousal levels (Chang et al., 2013). Dynamic and static RS-FC can be viewed as complementary; providing unique information about the stable connectional properties of neural connections and time-varying patterns that emerge and dissolve over time (Calhoun et al., 2014).
The investigation of static and dynamic RS-FC may provide new insights into the effects of THC on fear extinction-related neural circuitry. Given our prior studies demonstrating that THC enhances extinction recall and augments recall-related activation within HPC-vmPFC circuitry (Rabinak et al., 2014, 2013), we predicted that THC (relative to PBO) would be associated with an increase in static RS-FC (reflecting a strengthening of neural connections) and a decrease in dynamic RS-FC (reflecting more congruent activity) following an extinction learning session, within fear-extinction neural circuitry.
Prior neuroimaging studies have demonstrated that higher activation within HPCvmPFC circuitry is associated with increased extinction recall (Milad et al, 2007), and we have shown that pre-extinction administration of THC enhances activation within HPC-vmPFC during recall of extinction learning 24 hours later (Rabinak et al., 2014). Pre-extinction administration of THC may facilitate later recall of extinction learning by augmenting the consolidation of extinction learning. Here, we tested whether patterns of RS-FC within fear-extinction circuitry at rest following an extinction learning session correlated with the neural markers of extinction recall we reported in Rabinak et al, 2014. We also tested for potential mediating effects of post-extinction RS-FC on the previously reported link between THC administration and activation in extinction-recall neural circuitry and extinction recall tested 24 hours later.
2. Materials and Methods
2.1. Participants.
Seventy-seven right-handed volunteers (43 female), recruited from the local community via print advertisements and flyers, participated in the study. To be eligible, volunteers had to be free of any current psychoactive medications, neurological or medical illness as confirmed by a physician evaluation, and any DSM-IV lifetime Axis I psychiatric disorders (including substance use disorders) as confirmed by the Structured Clinical Interview for DSM-IV (SCID-NP; First et al., 2002). Moreover, participants had a minimal history of marijuana use (limited to < 10 lifetime exposures) to limit prior THC exposure. No participant had used cannabis within the past 30 days, which was verified by a negative urine toxicology screen at time of study. No participant was a daily tobacco smoker. Given that estradiol has been shown to facilitate fear extinction (Milad et al., 2010; Zeidan et al., 2011), scanning for all female participants was performed about one week prior to menses onset (based on self-reports of last period and cycle length), while estrogen levels are low.
Following removal of subjects for data quality issues (e.g., movement > 3 mm; described below), the total sample was N = 75. Thirty-eight of the seventy-five participants were randomly assigned to the THC group and the other thirty-seven participants to the PBO group. Of note, data from twenty-five of the participants (THC = 11; PBO = 14) were collected at the University of Michigan (UM) and all of the UM participants in the present study were included in our previous study showing that pre-extinction administration of THC enhances activation within HPC-vmPFC during recall of extinction learning 24 hours later (Rabinak et al., 2014; subjects reported in Rabinak et al., 2013 were not included in the present study as this previous study included only behavioral measures and did not include a neuroimaging component). While Rabink et al., 2014 included behavioral and brain measures during recall of extinction learning following pre-extinction of THC, resting-state fMRI was not reported in the previous study. Data from the other fifty participants (THC = 27; PBO = 23) were collected at the University of Illinois at Chicago (UIC). Both of these samples have been previously described in our fMRI task-based studies (UIC sample, Gorka et al., 2016; UM sample, Rabinak et al., 2014). Of note, the study at UIC was designed to be a replication of the UM study, and thus procedures and scan parameters were similar across sites. Additionally, the THC group was composed of 14 males and 24 females, however a chisquared test yielded a non-significant association between drug group and sex (χ(1)2 = 1.068, p = 0.301). Subject demographics for each drug group, by study site, are detailed in Table 1. For both sites, participants were required to be between the ages of 21 and 45 to minimize developmental differences in brain maturation and cognitive function and ensure that participants were old enough to provide consent to receive a controlled substance. All participants gave written informed consent after explanation of the experimental protocol, as approved by the UM and the UIC Institutional Review Boards.
Table 1.
Subject demographics
| PBO (n=37) | THC (n=38) | |||||
|---|---|---|---|---|---|---|
| Age |
UM (n=14) |
UIC (n=23) |
Total |
UM (n=11) |
UIC (n=27) |
Total |
| 25.43 (5.05) | 25.83 (5.53) | 25.68 (5.29) | 23.91 (3.53) | 25.07 (3.99) | 24.74 (3.85) | |
| Gender (Male/Female) | 9/5 | 9/14 | 18/19 | 2/9 | 12/15 | 14/24 |
| Ethnicity | ||||||
| Hispanic or Latino | 0 | 2 | 2 | 1 | 10 | 11 |
| Not Hispanic or Latino | 14 | 21 | 35 | 12 | 17 | 29 |
| Race | ||||||
| American | 0 | 0 | 0 | 0 | 2 | 2 |
| Indian/Alaskan Native | ||||||
| Asian | 2 | 8 | 10 | 1 | 5 | 6 |
| Black/African | 1 | 1 | 2 | 0 | 3 | 3 |
| American | ||||||
| Native | 0 | 1 | 1 | 0 | 0 | 0 |
| Hawaiian/Pacific | ||||||
| Islander | ||||||
| White | 11 | 12 | 23 | 8 | 9 | 17 |
| More than 1 race | 0 | 1 | 1 | 2 | 7 | 9 |
| Unknown | 0 | 0 | 0 | 0 | 1 | 1 |
THC, Δ9-tetrahydrocannabinol; PBO, placebo; UM, University of Michigan; UIC, University of Illinois at Chicago; Age is given as mean (SD)
2.2. Procedure.
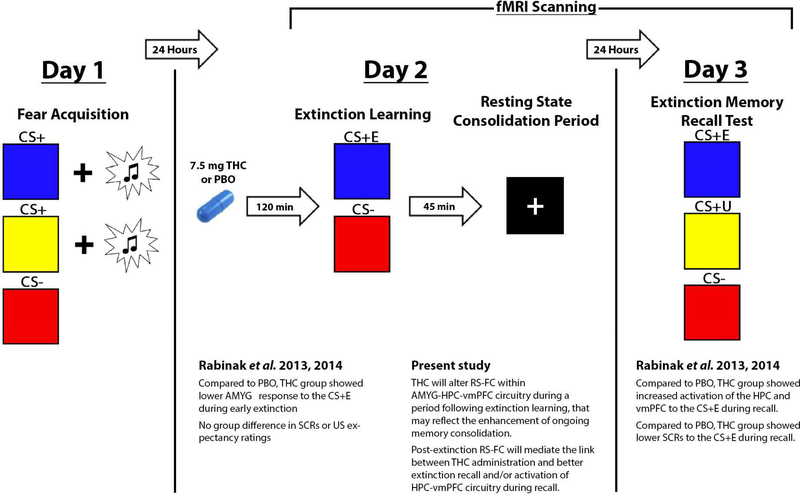
The present study reports on resting-state fMRI data collected on Day 2 as part of a three-day study (see Figure 1). The study used a double-blind, placebo-controlled, between-subject design as previously described (Rabinak et al., 2014). On Day 1, participants underwent a standard Pavlovian fear conditioning paradigm (Rabinak et al., 2014), which involved the pairing of two conditioned stimuli (CS+) with an unconditioned stimulus (US; aversive white noise burst). A third CS was presented but was never paired with the US (CS-). Twenty-four hours later, on Day 2, participants ingested an opaque gelatin capsule (size 00) with dextrose filler that contained either synthetic THC (Marinol; 7.5 mg; Solvay Pharmaceuticals, Marietta, GA) or dextrose alone (PBO) approximately 120 min prior to scanning. The dose of 7.5 mg is considered a low dose and was chosen because it has previously been used in other pharmaco-fMRI studies of emotion processing (Phan et al., 2008) and is the lowest effective dose that has been found to produce behavioral and subjective effects (Curran et al., 2002; Rabinak et al., 2013). During the scan, participants completed extinction learning, during which one CS+ and the CS- were presented but were not paired with the US. Following extinction learning, participants completed a RS-fMRI scan, to assess potential enduring effects of THC on fear-extinction neural circuitry following extinction learning. During the eyes-open RS-fMRI scan, participants were instructed to fixate on a white crosshair that was centrally projected against a black background and let their mind wander without falling asleep. Twenty-four hours later on Day 3, participants from the UM site underwent a test of extinction memory recall during fMRI scanning, during which the CS- as well as the previously extinguished CS+ (CS+E) and the unextinguished CS+ (CS+U) were presented in absence of the US. Of note, the study protocol was identical across both study locations during Days 1 and 2, with the exception that the duration of the RS-fMRI scan differed by sites (5 min, UIC; 8 min, UM).
Figure 1.
Experiment overview, results of previous findings from a behavioral study in healthy adults (Rabinak et al., 2013) and a neuroimaging study in a separate group of health adults (Rabinak et al., 2014), and present study aims. Of note, all of the UM participants in the current study were studied in Rabinak et al., 2014. THC, Δ9tetrahydrocannabinol; PBO, placebo; AMYG, amygdala; HPC, hippocampus; vmPFC, ventromedial prefrontal cortex; CS+E, previously extinguished conditioned stimulus; CS+U, previously unextinguished conditioned stimulus; CS-, safety cue (i.e., conditioned stimulus never paired with the US); US, unconditioned stimulus.
2.3. Subjective Drug Effects.
At six time points throughout the Day 2 visit (baseline and 30, 60, 90, 180, and 240 min post-dose; see Figure S1), participants completed a Likertstyle questionnaire that assessed drug effects (0–4, where 0 = ‘No Drug Effect At All’ and 4 = ‘Very Strong Drug Effect’). Participants also were asked to guess whether they received the PBO (coded 0) or the active drug (THC, coded 1). Importantly, participant guess of capsule contents was not associated with actual capsule contents (χ(1)2 = 0.36, p = 0.55), suggesting that blinding was effective. Associations between median ratings of drug effect, median participant guesses of capsule contents, and THC/PBO group are presented in the Supplemental Material, using nonparametric correlation coefficients (Kendall’s Tau-b). Nonparametric coefficients (Kendall’s Tau-b) between median ratings of drug effect and dynamic and static connectivity measures were also calculated to explore potential relationships between subjective drug effect ratings and RS-FC patterns. These results are presented in the Supplemental Material.
2.4. Functional Imaging Acquisition.
FMRI scanning at UM Functional MRI Laboratory was performed on a 3T GE Signa System (General Electric Healthcare; Waukesha, WI) using a standard single-channel radiofrequency coil. Whole-brain functional images (i.e., blood oxygen level-dependent [BOLD]) were collected from 43 axial, 3 mm thick slices using a T2*-sensitive gradient echo reverse spiral acquisition sequence (repetition time, 2000 ms; echo time, 30 ms; 64 × 64 matrix; 220 mm field of view; flip angle, 90; 240 volumes), optimized to minimize susceptibility artifacts (signal loss) in the medial temporal lobe (Stenger et al., 2000).
FMRI scanning at the Center for Magnetic Resonance Research at UIC was performed on a 3T GE MR 750 System (General Electric Healthcare; Waukesha, WI) using an 8-channel phased-array radio frequency head coil. Whole-brain functional images were collected from 44 axial, 3mm thick slices using a standard T2*-sensitive gradient-echo echoplanar imaging (EPI) sequence was used (repetition time, 2000 ms; echo time, 22.2 ms; 64 × 64 matrix; 220 mm field of view; flip angle, 90; 152 volumes). At both sites, a high-resolution T1-weighted anatomical image was acquired prior to functional scanning for normalization and coregistration of functional data.
2.5. Functional Imaging Preprocessing.
Data from seventy-five participants met criteria for high quality and scan stability with minimum motion correction and were subsequently included in the RS-FC analyses (< 3 mm displacement in any one direction). The first three volumes were discarded to allow for T1 equilibration effects, with preprocessing of functional images integrated from Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, London; www.fil.ion.ucl.ac.uk/spm/software/spm8). In brief, images were slice time corrected, realigned, co-registered to the T1-weighted image in Montreal Neurological Institute (MNI) space, re-sampled to 2 mm3 voxels, and smoothed using a 6-mm Gaussian kernel. Images were not censured for high motion frames in order to preserve the temporal integrity of the data for the subsequent sliding-window dynamic RSFC analysis (see Allen et al., 2014).
Using AFNI’s 3dTproject (Cox, 1996) and FSL (Jenkinson et al., 2002; Woolrich et al., 2009) eight nuisance covariates (white matter, cerebrospinal fluid, and the six movement parameters) were linearly regressed from the time series and frequencies below 0.01 and above 0.15 Hz were removed. Head motion was assessed in the dimensions of x, y, and z through translation and rotation measures using SPM8, and there were no significant between group differences in estimates of mean or maximum framewise displacement (all p’s > 0.50). Following recommendations for multi-site fMRI studies (Glover et al., 2013), we: (i) include site, as well as gender and subject motion, as a covariates in group analyses, and (ii) perform parallel analyses where the two sites are considered independently rather than combined (i.e., replication; see Supplemental Material). Previous test-retest RS-FC studies have found that site and scanner manufacturer are not significant sources of variability (e.g., Noble et al., 2017; Zou et al., 2005). Rather, between-subjects effects and magnetic field strength represent significant sources of variability (Noble et al., 2017; Zou et al., 2005). Interactions between all covariates and drug group were explored but were dropped from the model after they were found to be non-significant (all p’s > 0.20).
As previously reported in Rabinak et al., 2014, extinction recall fMRI data from the UM participants were processed and analyzed using Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, London; www.fil.ion.ucl.ac.uk/spm/software/spm8). In brief, images were slice time corrected, realigned, co-registered their T1-weighted image, normalized to Montreal Neurological Institute (MNI) space, re-sampled to 2 mm3 voxels, and smoothed with a 6 mm Gaussian kernel.
2.6. Resting-State Functional Connectivity Analysis.
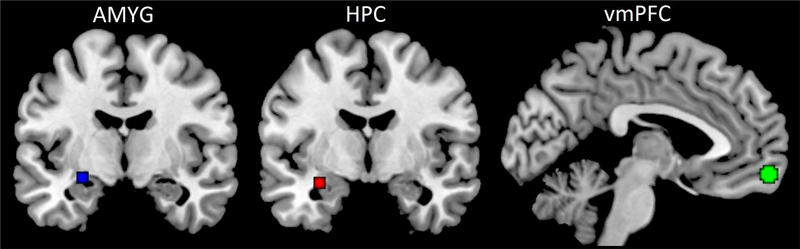
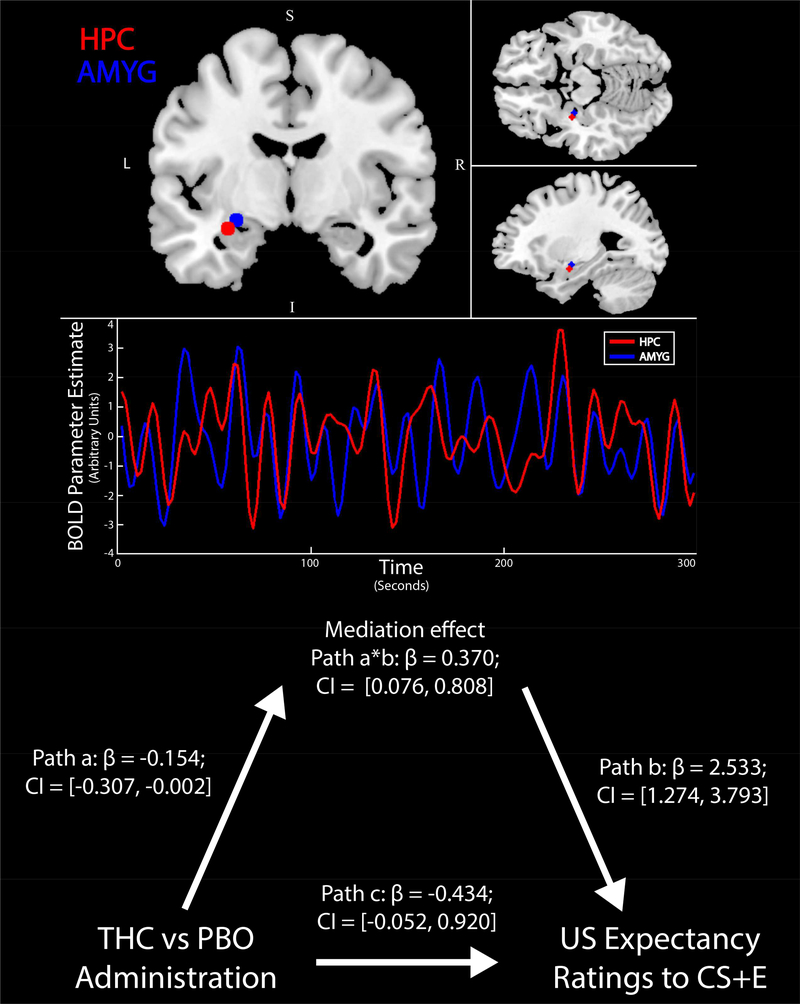
RS-FC within fear extinction-related neural circuitry was assessed using a region-of-interest to region-of-interest (ROI-ROI) approach. Seed regions in the left AMYG, left HPC, and vmPFC were defined functionally based on 3 and 5 mm spheres centered on peak activation coordinates identified in our prior research for effects of THC (relative to PBO) during extinction learning or recall (Figure 2; Rabinak et al, 2014). Of note, the vmPFC seed was 5 mm in radius while AMYG and HPC seeds were 3 mm in radius to ensure no overlapping voxels were contained in the ROIs. Similar results were obtained when using 5 mm radii ROIs for all regions. These results are presented in the Supplemental Material (Figures S1 and S2).
Figure 2.
Brain regions of interest involved in extinction recall. Seed regions in the hippocampus (HPC, blue), amygdala (AMYG, red), and ventromedial prefrontal cortex (vmPFC, green).
AFNI’s 3dmaskave was then used to extract the averaged time series of each of the seed ROIs from the preprocessed, nuisance regressed, and filtered images. Static RS-FC and dynamic RS-FC between ROIs was calculated to characterize the stable and the fluid properties of RS-FC, respectively. Static RS-FC was measured by computing ROI-ROI correlations across the entire scan. Dynamic RS-FC was measured by subjecting the extracted time series to a sliding-window analysis (Allen et al., 2014; Barttfeld et al., 2015; Handwerker et al., 2012; Hindriks et al., 2016; Hutchison et al., 2013b; Marusak et al., 2016), in which Pearson’s R correlations were calculated using a window size of 25 TRs (50 seconds) at 1 TR increments (96% overlap). This resulted in 125 or 213 correlation values (UIC or UM, respectively) for each of the 3 ROI-ROI pairs.
Both static and dynamic RS-FC measures were then z-scored and the standardized values were used for between-group statistical testing. Of note, one sample t-tests were performed using the unstandardized static and dynamic RS-FC measures, as z-scores would be inappropriate due to their centering at 0. Group differences in static and dynamic RS-FC measures were conducted via general linear models in IBM’s SPSS 25 (SPSS Inc., Chicago, IL). Gender, site, and mean framewise displacement were all included in the model to control for their effect, and significance is reported at p < 0.05 (two-tailed).
2.7. Association between RS-FC Following Extinction Learning and Brain Activation during Subsequent Recall of Extinction Learning.
As reported in Rabinak et al, 2014, we extracted BOLD signal responses from the UM participants (parameter estimates, βweights in arbitrary units [a.u.] of activation), averaged across all voxels within a 5-mm radius sphere surrounding the peak activations within the vmPFC and HPC ROIs from CS+E condition during extinction recall. Then, we correlated individual participant measures of static and dynamic RS-FC with magnitude of HPC and vmPFC activation to the CS+E during extinction recall reported in Rabinak et al., 2014. Kendall bivariate correlations were conducted in SPSS and significance is reported at p < 0.05 (two-tailed).
2.8. Association between RS-FC Following Extinction Learning and Subsequent Extinction Recall Success.
During recall of extinction learning, conditioned fear was evaluated by two distinct, but complementary measures: (1) skin conductance responses (SCRs) and (2) subjective ratings of US expectancy, following (Milad et al., 2007; Rabinak et al., 2014). SCRs were measured for each CS presentation and extinction recall success was calculated by comparing SCRs to the CS+E to those for the CS+U, with higher numbers indicating poorer extinction recall. For expectancy ratings, prior to each CS presentation participants were asked to rate their expectancy that the US would occur on a 5-point scale (“Will you hear a loud noise burst?”: 1 = Definitely not; 3 = Unsure; 5 = Definitely). Higher expectancy for CS+E trials indicates poorer extinction recall. As described above, we correlated individual participant measures of static and dynamic RS-FC with SCRs and US expectancy ratings reported in Rabinak et al, 2014. Kendall bivariate correlations were conducted in SPSS and significance is reported at p < 0.05 (two-tailed).
2.9. Meditation Analyses.
For brain and behavioral (i.e., SCRs, US expectancy ratings) measures during recall that were significantly associated with post-extinction RS-FC, we subsequently performed meditation analyses. In particular, mediation analyses tested whether the link between THC administration and next-day extinction recall was meditated by post-extinction RS-FC patterns. Mediation analyses were conducted using the SPSS v25 PROCESS v3.0 macro, which applies a bootstrapping approach (N = 5,000 bootstrap resamples) and a 95% confidence interval to evaluate indirect effects (see Hayes and Preacher, 2014).
3. Results
3.1. Effects of THC on RS-FC Following Extinction Learning.
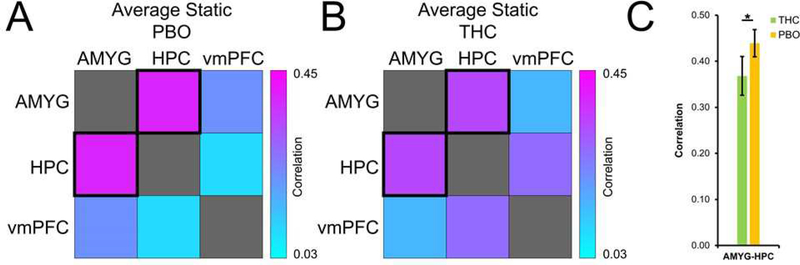
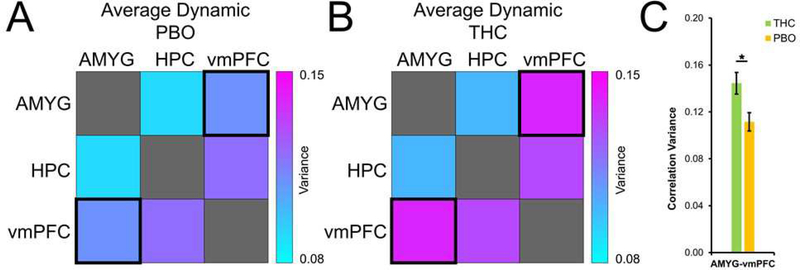
Compared to PBO, THC was associated with lower static RS-FC between the AMYG and HPC (B = −.436; p = 0.027; Figure 3), suggesting reduced amplitude in neural interactions between AMYG and HPC. However, relative to PBO, THC was associated with higher dynamic functional connectivity between the AMYG and vmPFC (B = .664; p = 0.004; Figure 4), indicating greater time-dependent changes in connectivity within AMYG-vmPFC circuitry. Taken together, these data suggest that THC administration is associated with reduction in strength of positive static AMYG-HPC coupling, as well as an increase in flexibility in AMYG-vmPFC interactions. For description of patterns of post-extinction RS-FC across the sample, see the Supplemental Material.
Figure 3.
Effects of THC on static resting-state functional connectivity of fear-extinction circuitry. Correlation between fear-extinction regions of interest in THC (A) and PBO (B) groups, and significant group differences (C). THC, Δ9-tetrahydrocannabinol (green); PBO, placebo (orange); AMYG, amygdala; HPC, hippocampus; vmPFC, ventromedial prefrontal cortex
Figure 4.
Effects of THC on dynamic resting-state functional connectivity of fear-extinction circuitry. Variance in sliding-window correlation values between fear-extinction regions of interest in THC (A) and PBO (B) groups, and significant group differences (C). THC, Δ9-tetrahydrocannabinol (green); PBO, placebo (orange); AMYG, amygdala; HPC, hippocampus; vmPFC, ventromedial prefrontal cortex
3.2. Association between RS-FC Following Extinction Learning and Brain Activation during Subsequent Recall of Extinction Learning.
Next, we tested whether post-extinction learning RS-FC patterns in fear-extinction circuitry were associated with HPC or vmPFC activation during recall of extinction learning. We found that, across the UM sample, lower static RS-FC between AMYG and HPC following extinction learning was associated with higher HPC activation to the CS+E during recall of extinction learning 24 hours later (Tau-b(21) = −0.381, p = 0.016; see Figure 5A). These results suggest that THC-related effects on post-extinction RS-FC (i.e., lower AMYG-HPC static RS-FC) may facilitate increased activation of extinction recall neural circuitry. Given that AMYGHPC RS-FC was influenced by THC administration, we tested the potential mediating effect of post-extinction AMYG-HPC RS-FC on the link between pre-extinction THC administration and HPC activation during the test of extinction recall, 24 hours later. The indirect effects were not significant (β = 0.04, SE = 0.07, lower limit confidence interval [LLCI] = −0.13, upper limit confidence interval [ULCI] = 0.17). There was no association between both static or dynamic post-extinction RS-FC and vmPFC activation during extinction recall.
Figure 5.
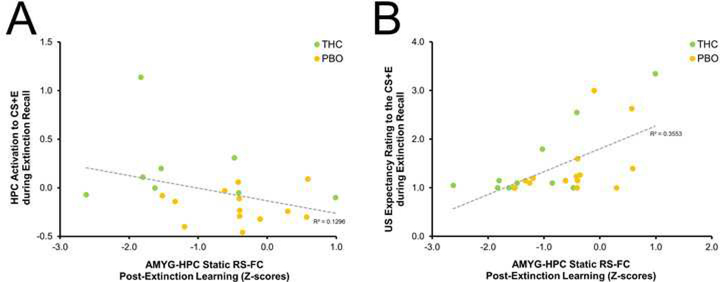
Correlation between resting-state functional connectivity and brain and behavioral measures during recall of extinction learning. Negative association between AMYG-HPC static RS-FC connectivity and HPC activation to the CS+E during recall of extinction learning (A). Positive correlation between AMYG-HPC static RS-FC connectivity and US expectancy ratings to the CS+E during recall of extinction learning (B). THC, Δ9-tetrahydrocannabinol (green); PBO, placebo (orange); AMYG, amygdala; HPC, hippocampus; CS+E, extinguished conditioned stimulus; US, unconditioned stimulus; RS-FC, resting-state functional connectivity
3.3. Association between RS-FC Following Extinction Learning and Subsequent Extinction Recall Success.
Next, we tested whether post-extinction learning RS-FC patterns in fear-extinction circuitry were associated with physiological or behavioral measures of extinction recall success, per SCRs and US expectancy ratings. We found that, across the UM sample, lower static RS-FC between AMYG and vmPFC following extinction learning was associated with lower SCRs to the CS+E (vs. CS+U) during recall of extinction learning 24 hours later (Tau-b(30) = 0.255, p = 0.048). Additionally, we found that, across the UM sample, lower static RS-FC between AMYG and HPC following extinction learning was associated with lower US expectancy ratings to the CS+E during recall of extinction learning 24 hours later (Tau-b(25) = 0.478, p = 0.001; see Figure 5B). Given that AMYG-HPC RS-FC was influenced by THC administration, we tested the potential mediating effect of post-extinction AMYG-HPC RS-FC on the link between pre-extinction THC administration and next-day extinction recall, as measured via US expectancy ratings. Mediation analyses indicated a significant indirect effect from THC to US expectancy ratings to the CS+E during recall of extinction learning via post-extinction AMYG-HPC RS-FC (β = −0.390, SE = 0.192, LLCI = −0.754, ULCI = −0.007; see Figure 6).
Figure 6.
Mediating effect of post-extinction AMYG-HPC RS-FC on the link between pre-extinction THC administration and next-day extinction recall, as measured via US expectancy ratings to the CS+E. Path a, significant impact of THC on AMYG-HPC RSFC; Path b, significant correlation of AMYG-HPC RS-FC and next-day CS+E expectancy ratings; Path a*b, significant indirect impact of THC on next-day CS+E expectancy ratings; Path c, non-significant direct impact of THC on next-day CS+E expectancy ratings. THC, Δ9-tetrahydrocannabinol; PBO, placebo; AMYG, amygdala; HPC, hippocampus; CS+E, extinguished conditioned stimulus; US, unconditioned stimulus
4. Discussion
We have previously shown that pre-extinction administration of THC facilitates later extinction recall and enhances activation within HPC-vmPFC circuitry (Rabinak et al., 2014, 2013), regions critical for the successful recall of extinction learning. Here, we tested for effects of THC administration on RS-FC within fear-extinction neural circuitry during the “offline” period that follows an extinction learning session, a time during which processes underlying consolidation of the extinction memory (e.g., reactivation of neuronal ensembles supporting extinction learning) are thought to be occurring (McGaugh, 2000). Consistent with prior studies demonstrating that static and dynamic RS-FC patterns often have an inverse relationship (e.g., Chang and Glover, 2010), we found that THC was associated with enhanced dynamic but reduced static RS-FC within fear-extinction neural circuitry. Specifically, relative to PBO, THC administration was associated with higher dynamic RS-FC between the AMYG and vmPFC, and lower static RS-FC between the AMYG and HPC. These patterns may reflect the neural signature of THC effects on RS-FC, during the possible consolidation of the extinction memory. In support of this notion, we found that lower AMYG-HPC static RS-FC following extinction learning was associated with higher HPC activation to the previously extinguished cue (CS+E) during recall of extinction learning 24 hours later. Additionally, we found that lower AMYG-HPC static RS-FC following extinction learning mediated the link between THC administration and lower US expectancy ratings to the CS+E during recall of extinction learning 24 hours later. This suggests that THC administration reduces the strength of RS-FC in extinction-related neural circuitry during a time period in which the consolidation of the extinction memory is thought to be occurring. Further, post-extinction RS-FC explained the previously reported link between THC administration and later recall of extinction learning (Rabinak et al., 2014).
Our data suggest that THC affects the static and dynamic interactions between brain regions shown to underlie the recall of extinction learning, even in the absence of an overt task. Evidence of an “active rest” period is not surprising, as ongoing processes observed in the brain’s resting state may serve to consolidate memory traces and/or potentiate action. Indeed, experimental animal models have demonstrated that pharmacological augmentation of activity in AMYG-vmPFC-HPC circuitry during the post-extinction period augments extinction recall, whereas blockade (via, for example, anisomycin) impairs later recall (Quirk and Mueller, 2008). Here, we found that THC administration increased AMYG-vmPFC dynamic connectivity, which may reflect greater flexibility in a circuitry known to be critical for the top-down regulation of fear extinction-related neural responding (Quirk and Mueller, 2008). Previous rodent and human lesion studies implicate the basolateral AMYG and the infralimbic cortex (the homolog of the human vmPFC) in the consolidation of extinction memories (see review by Quirk and Mueller, 2008). For example, protein synthesis in the basolateral AMYG appears to be necessary for stabilizing extinction memories, and post-extinction facilitation of AMYG activity (via the GABA-A antagonist bicuculline) improves extinction ability (Quirk and Mueller, 2008). In humans, lesions in the vmPFC do not interfere with the ability to extinguish conditioned fear within session, but result in a deficit in retrieving the extinction memory the following day (Quirk and Mueller, 2008). In addition, studies using a variety of pharmacological agents indicate that consolidation of extinction involves initiation of molecular cascades during the post-extinction period in the vmPFC. The vmPFC has direct inhibitory connections with the intercalated cells of the AMYG, which serve to dampen AMYG output (Amano et al., 2010; Amir et al., 2011). Therefore, high AMYG-vmPFC dynamic RS-FC may reflect a functional pattern that supports the consolidation of the extinction memory. Consistent with this interpretation, one study found that individuals with higher AMYG-vmPFC static RS-FC following a fear reminder showed better extinction recall (Feng et al., 2016). The authors suggested that increased communication between these regions may reflect more vmPFC-based inhibitory signaling to control the expression of the fear memory trace within the AMYG, thus improving extinction (Feng et al., 2016).
In addition to an increase in AMYG-vmPFC dynamic RS-FC, we found that THC administration was associated with a decrease in static AMYG-HPC connectivity. Importantly, post-extinction AMYG-HPC RS-FC mediated the link between THC administration and better extinction recall the next day. These results provide a potential mechanism underlying the previously reported findings from our group and others that THC administration enhances extinction recall. Interestingly, it was weaker strength of AMYG-HPC connectivity during the post-extinction period that predicted better recall. One previous fMRI study in healthy adults found that stronger increases in AMYG-HPC RS-FC after fear conditioning predicted spontaneous recovery of fear the next day, and that these increases persisted after extinction learning (Hermans et al., 2017). Electrophysiological studies in experimental animals have demonstrated that coordinated activity between the AMYG and HPC increases after fear conditioning (Seidenbecher et al., 2003). Here, we found that THC administration reduced AMYG-HPC RS-FC during the post-extinction period, which may help to explain the lower expression of fear the next day. Future studies that also include a fear renewal session may help to understand whether THC affects the consolidation of the extinction memory and/or the reconsolidation of the fear memory.
Importantly, deficits in extinction recall and reduced activation within HPC-vmPFC circuitry have been demonstrated in patients with PTSD relative to trauma-exposed controls (Garfinkel et al., 2014; Milad et al., 2009). Thus, interventions that regulate fear-extinction circuitry, such as THC and other CB modulators, have emerged as a promising therapeutic modality for improving the consolidation and/or later recall of extinction learning among patients with PTSD (Rabinak and Phan, 2014). Identifying the effects of THC on fear-extinction circuitry in healthy individuals is a critical first step towards understanding therapeutic potential. THC-related changes in fear-extinction circuitry at rest may reflect processes involved in the consolidation of the extinction memory and/or sustained effects of the drug on RS-FC. Further, one recent study demonstrated that dynamic RS-FC was a better predictor of PTSD diagnosis than static RS-FC, which suggests that the “ease with which brain regions engage or disengage with other regions may be more sensitive to underlying pathology than the strength with which they are engaged” (Jin et al., 2017). Importantly, in that study PTSD was associated with a reduction in whole-brain dynamic RS-FC, which the authors interpreted as reflecting a compromised ability to adjust behaviors and thoughts to changing environmental conditions (Jin et al., 2017). Of note, in the present study we found that THC is associated with an increase in dynamic RS-FC between the AMYG and vmPFC, suggesting that THC may help to normalize reductions in dynamic RS-FC.
Limitations of this study warrant mention. The study was designed so that peak drug effects occurred during extinction learning. However, the drug was still on board during the RS-FC scan. Therefore, given that consolidation may begin within minutes of learning (e.g., McGaugh, 2000), it is difficult to disentangle whether observed effects on RS-FC are due to effects of the active drug on post-extinction RS-FC, or whether observed effects would be present sans extinction. Another comparison group is needed, who did not undergo extinction learning. However, previous studies have shown that RS-FC is altered following fear-related learning tasks in healthy volunteers, and that RS-FC metrics are related to learning and behavioral performance (e.g., Schultz et al., 2012). Indeed, here we found that THC administration was associated with alterations in post-extinction RS-FC, and that patterns of RS-FC mediated the link between THC administration and extinction recall, as measured 24 hours later. Another limitation is that our study was in healthy individuals; THC-related effects on fear-extinction circuitry may differ in patient groups with known functional deficits within this circuitry (i.e., PTSD). Moreover, given that investigation of dynamic RS-FC measures is relatively recent as compared to static RS-FC, less is known about their stability, reproducibility, and their underlying physiological basis. However, several independent groups have reported reliably recurring patterns at different timescales (see review by Chen et al, 2017), and one recent study using 7,500 RS-FC fMRI datasets found that dynamic RS-FC patterns are reproducible (Abrol et al., 2017). Further, concurrent fMRI and electrophysiology studies suggest that dynamic RS-FC may have a neurophysiological origin (e.g., Allen et al, 2018). Nonetheless, dynamic RS-FC is a growing research area, and many questions remain in terms of methodological choices and interpretation.
5. Conclusions
In this work, we documented the effects of THC on both static and dynamic RS-FC within fear-extinction neural circuitry during a rest period following extinction learning in healthy individuals. We also linked post-extinction RS-FC to activation of extinction recall circuitry tested 24 hours later, and our data suggest that post-extinction RS-FC patterns mediate the link between THC administration and better recall of extinction learning. The motivation for this study stems from prior evidence from our group and others that THC augments fear-extinction neural circuitry during extinction learning and its later recall, and that PTSD and anxiety disorders are characterized by extinction recall deficits and reduced activation within fear-extinction neural circuitry. Although further study is needed in patient groups, our findings add to the growing body of literature on the effects of CBs on fear-extinction neural circuitry, and their potential therapeutic relevance.
Supplementary Material
Highlights.
Pre-extinction THC administration has been shown to enhance extinction recall
THC altered static and dynamic RS-FC during a post-extinction rest period
RS-FC mediated the link between pre-extinction THC and next-day recall
RS-FC effects may reflect processes supporting consolidation of extinction learning
ACKNOWLEDGEMENTS
This material is based on work supported by a grant from the National Center for Research Resources (UL1RR024986 and UL1RR029879) and from the National Institute of Mental Health (1R21MH093917) awarded to KLP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors declare no conflict of interest. We would like to thank Shoko Mori, Maryssa Lyons, and Mike Angstadt for their assistance with data collection at the University of Michigan, Donald McNair for his assistance with data collection at the University of Illinois at Chicago, and Brian Silverstein for his input on data analysis in earlier versions of this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrol A, Damaraju E, Miller RL, Stephen JM, Claus ED, Mayer AR, Calhoun VD, 2017. Replicability of time-varying connectivity patterns in large resting state fMRI samples. Neuroimage 163, 160–176. https://doi.org/10.1016/j.neuroimage.2017.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Eichele T, Wu L, Calhoun VD, 2018. EEG Signatures of Dynamic Functional Network Connectivity States. Brain Topogr. 31, 101–116. https://doi.org/10.1007/s10548-017-0546-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD, 2014. Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex 24, 663–676. https://doi.org/10.1093/cercor/bhs352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Paré D, 2010. Synaptic correlates of fear extinction in the amygdala. Nat. Neurosci 13, 489–494. https://doi.org/10.1038/nn.2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A, Amano T, Pare D, 2011. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J. Neurophysiol 105, 3054–3066. https://doi.org/10.1152/jn.00136.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S, 2015. Signature of consciousness in the dynamics of resting-state brain activity. Proc. Natl. Acad. Sci 112, 887–892. https://doi.org/10.1073/pnas.1418031112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN, 2008. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur. Neuropsychopharmacol 18, 849–859. https://doi.org/10.1016/j.euroneuro.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, Adali T, 2014. The Chronnectome: Time-Varying Connectivity Networks as the Next Frontier in fMRI Data Discovery. Neuron https://doi.org/10.1016/j.neuron.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH, 2010. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50, 81–98. https://doi.org/10.1016/j.neuroimage.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Liu Z, Chen MC, Liu X, Duyn JH, 2013. EEG correlates of time-varying BOLD functional connectivity. Neuroimage 72, 227–236. https://doi.org/10.1016/j.neuroimage.2013.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JE, Rubinov M, Chang C, 2017. Methods and Considerations for Dynamic Analysis of Functional MR Imaging Data. Neuroimaging Clin. N. Am https://doi.org/10.1016/j.nic.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cribben I, Haraldsdottir R, Atlas LY, Wager TD, Lindquist MA, 2012. Dynamic connectivity regression: Determining state-related changes in brain connectivity. Neuroimage 61, 907–920. https://doi.org/10.1016/j.neuroimage.2012.03.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran VH, Brignell C, Fletcher S, Middleton P, Henry J, 2002. Cognitive and subjective dose-response effects of acute oral Δ9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl). 164, 61–70. https://doi.org/10.1007/s00213-002-1169-0 [DOI] [PubMed] [Google Scholar]
- Feng P, Zheng Y, Feng T, 2016. Resting-state functional connectivity between amygdala and the ventromedial prefrontal cortex following fear reminder predicts fear extinction. Soc. Cogn. Affect. Neurosci 11, 991–1001. https://doi.org/10.1093/scan/nsw031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version – Non-patient Edition (SCID-I/NP). Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Friston KJ, 2011. Functional and Effective Connectivity: A Review. Brain Connect. 1, 13–36. https://doi.org/10.1089/brain.2011.0008 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I, 2014. Impaired Contextual Modulation of Memories in PTSD: An fMRI and Psychophysiological Study of Extinction Retention and Fear Renewal. J. Neurosci 34, 13435–13443. https://doi.org/10.1523/JNEUROSCI.4287-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Mueller B.a, Turner J. a, Van TGM, Liu TT, Greve DN, Voyvodic JT, Brown GG, Keator DB, Calhoun VD, 2013. Function Biomedical Informatics Research Network Recommendations for Prospective Multi-Center Functional Magnetic Resonance Imaging Studies. J Magn Reson Imaging 36, 39–54. https://doi.org/10.1002/jmri.23572.Function [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Phan KL, Lyons M, Mori S, Angstadt M, Rabinak CA, 2016. Cannabinoid Modulation of Frontolimbic Activation and Connectivity during Volitional Regulation of Negative Affect. Neuropsychopharmacology 41 https://doi.org/10.1038/npp.2015.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Roopchansingh V, Gonzalez-Castillo J, Bandettini PA, 2012. Periodic changes in fMRI connectivity. Neuroimage 63, 1712–1719. https://doi.org/10.1016/j.neuroimage.2012.06.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Preacher KJ, 2014. Statistical mediation analysis with a multicategorical independent variable. Br. J. Math. Stat. Psychol 67, 451–470. https://doi.org/10.1111/bmsp.12028 [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Kanen JW, Tambini A, Fernández G, Davachi L, Phelps EA, 2017. Persistence of Amygdala-Hippocampal Connectivity and Multi-Voxel Correlation Structures During Awake Rest After Fear Learning Predicts Long-Term Expression of Fear. Cereb. Cortex 27, 3028–3041. https://doi.org/10.1093/cercor/bhw145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindriks R, Adhikari MH, Murayama Y, Ganzetti M, Mantini D, Logothetis NK, Deco G, 2016. Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage 127, 242–256. https://doi.org/10.1016/j.neuroimage.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C, 2013a. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 80, 360–378. https://doi.org/10.1016/j.neuroimage.2013.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS, 2013b. Restingstate networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum. Brain Mapp 34, 2154–2177. https://doi.org/10.1002/hbm.22058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WJenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jin C, Jia H, Lanka P, Rangaprakash D, Li L, Liu T, Hu X, Deshpande G, 2017. Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Hum. Brain Mapp 38, 4479–4496. https://doi.org/10.1002/hbm.23676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B, 2002. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418, 530–534. https://doi.org/10.1038/nature00839 [DOI] [PubMed] [Google Scholar]
- Marusak HA, Calhoun VD, Brown S, Crespo LM, Sala-Hamrick K, Gotlib IH, Thomason ME, 2016. Dynamic functional connectivity of neurocognitive networks in children. Hum. Brain Mapp https://doi.org/10.1002/hbm.23346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, 2000. Memory--a Century of Consolidation. Science (80-. ). 287, 248–251. https://doi.org/10.1126/science.287.5451.248 [DOI] [PubMed] [Google Scholar]
- Meunier D, Lambiotte R, Fornito A, Ersche KD, Bullmore ET, 2009. Hierarchical modularity in human brain functional networks. Front. Neuroinform 3, 37 https://doi.org/10.3389/neuro.11.037.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL, 2009. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol. Psychiatry 66, 1075–1082. https://doi.org/10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL, 2005. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc. Natl. Acad. Sci. U. S. A 102, 10706–10711. https://doi.org/10.1073/pnas.0502441102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, 2012. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 63, 129–151. https://doi.org/10.1146/annurev.psych.121208.131631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL, 2007. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol. Psychiatry 62, 446–454. https://doi.org/10.1016/j.biopsych.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM, 2010. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience 168, 652–658. https://doi.org/10.1016/j.neuroscience.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S, Scheinost D, Finn ES, Shen X, Papademetris X, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Olvet DM, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Thermenos H, Tsuang MT, van Erp TGM, Walker EF, Hamann S, Woods SW, Cannon TD, Constable RT, 2017. Multisite reliability of MR-based functional connectivity. Neuroimage 146, 959–970. https://doi.org/10.1016/j.neuroimage.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H, 2008. Cannabinoid Modulation of Amygdala Reactivity to Social Signals of Threat in Humans. J. Neurosci 28, 2313–2319. https://doi.org/10.1523/JNEUROSCI.5603-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D, 2008. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72. https://doi.org/10.1038/sj.npp.1301555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak C, Phan K, 2014. Cannabinoid Modulation of Fear Extinction Brain Circuits: A Novel Target to Advance Anxiety Treatment . Curr. Pharm. Des. 20, 2212–2217. https://doi.org/10.2174/13816128113199990437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Lyons M, Mori S, Milad MR, Liberzon I, Luan Phan K, 2014. Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiol. Learn. Mem 113, 125–134. https://doi.org/10.1016/j.nlm.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, Phan KL, 2013. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology 64, 396–402. https://doi.org/10.1016/j.neuropharm.2012.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz DH, Balderston NL, Helmstetter FJ, 2012. Resting-state connectivity of the amygdala is altered following Pavlovian fear conditioning. Front. Hum. Neurosci 6. https://doi.org/10.3389/fnhum.2012.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape H-C, 2003. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science 301, 846–50. https://doi.org/10.1126/science.1085818 [DOI] [PubMed] [Google Scholar]
- Shen K, Hutchison RM, Bezgin G, Everling S, McIntosh AR, 2015. Network Structure Shapes Spontaneous Functional Connectivity Dynamics. J. Neurosci 35, 5579–5588. https://doi.org/10.1523/JNEUROSCI.4903-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger VA, Boada FE, Noll DC, 2000. Three-dimensional tailored RF pulses for the reduction of susceptibility artifacts in T2/(*)-weighted functional MRI. Magn. Reson. Med 44, 525–531. https://doi.org/10.1002/1522-2594(200010)44:4<525::AID-MRM5>3.0.CO;2-L [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH, 2007. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology 32, 1032–1041. https://doi.org/10.1038/sj.npp.1301224 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM, 2009. Bayesian analysis of neuroimaging data in FSL. Neuroimage 45, S173–86. https://doi.org/10.1016/j.neuroimage.2008.10.055 [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR, 2011. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol. Psychiatry 70, 920–927. https://doi.org/10.1016/j.biopsych.2011.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou KH, Greve DN, Wang M, Pieper SD, Warfield SK, White NS, Manandhar S, Brown GG, Vangel MG, Kikinis R, Wells WM, 2005. Reproducibility of functional MR imaging: preliminary results of prospective multi-institutional study performed by Biomedical Informatics Research Network. Radiology. https://doi.org/10.1148/radiol.2373041630 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.