Abstract
Background.
Traffic-related air pollution has been linked to multiple adverse pregnancy outcomes. However, few studies have examined pregnancy loss, targeting losses identified by hospital records, a large limitation as it does not capture events not reported to the medical system.
Methods.
We used a novel variation of the time-series design to determine the association, and identify the critical window of vulnerability, between week-to-week traffic-related air pollution and conceptions resulting in live births, using nitrogen dioxide (NO2) as a traffic emissions tracer. We used information from all live births recorded at Beth Israel Deaconess Medical Center in Boston, MA, USA (2000–2013) and all live births in Tel Aviv District, Israel (2010–2013).
Results:
In Boston (68,969 live births), the strongest association was during the 15th week of gestation; for every 10 ppb of NO2 increase during that week we observed a lower rate of live births (rate ratio [RR]=0.87; 95% confidence interval [CI]: 0.78–0.97), using live birth-identified conceptions to infer pregnancy losses. In the Tel Aviv District (95,053 live births), the strongest estimate was during the 16th gestational week gestation (RR=0.82; 95%CI: 0.76–0.90 per 10 ppb of NO2).
Conclusions:
Using weekly conceptions ending in live birth rather than identified pregnancy losses, we comprehensively analyzed the relationship between air pollution and all pregnancy loss throughout gestation. The observed results, with remarkable similarity in two independent locations, suggest that higher traffic-related air pollution levels are associated with pregnancy loss, with strongest estimates between the 10th and 20th gestational weeks.
Keywords: Air pollution, traffic, nitrogen dioxide, pregnancy loss, miscarriage
Introduction
A large number of studies have found air pollution to be associated with a multitude of adverse health effects.1–4 In addition, several studies have estimated adverse reproductive effects of exposure to air pollution including reduced birth weight, pre-term birth, and small for gestational age birthweight.5–12 These reproductive endpoints are relatively easy to measure and thus lend themselves to more feasible analyses of exposure-related effects. It is much more challenging, however, to study the impact of air pollution exposure on pregnancy loss as this outcome is much more difficult to identify.
A few studies have explored the relation between air pollutants and stillbirth – defined as loss of a fetus after 20 weeks of gestations – in part because these often are recorded in administrative data.13–18 Even fewer have tried to examine the relation between air pollutants and pregnancy loss at or before 20 weeks of gestation, typically in high exposure settings.19–23 Together, these studies suggest an association between air pollution and early pregnancy loss, although some are in very high exposure settings and all have some important limitations, such as small sample size or inappropriate statistical methods. Furthermore, all of these studies identified cases from hospital data. However, in the general population, pregnancy losses may not be documented in medical records. Many pregnancies may not even be recognized and may be lost before they come to medical attention. It is estimated that only 60%−70% of fertilized eggs result in a live birth.24,25 The incidence of early fetal loss is estimated to be 20%−30% among women planning a pregnancy,25,26 but approximately half of pregnancies are unintended.27,28
In the current study we focus on traffic-related air pollution, a major air pollution source, especially in urban environments, that has been associated specifically with adverse pregnancy outcomes in previous studies.29–32 To explore pregnancy loss in the general population, overcoming limitations of previous studies, we devised a novel approach using the outcome metric of live birth-identified conceptions in a variation of the time-series design. Using complete data on live births from hospitals in Boston, MA, USA and Tel Aviv District, Israel, we determined for each calendar week the number of conceptions that resulted in live births (live birth-identified conceptions) using data on gestational age at birth. We then related the number of live birth-identified conceptions with the traffic-related air pollution exposure in each subsequent gestational week. The expectation was that, after accounting for seasonal and long-term time trends, if higher nitrogen dioxide (NO2) exposure in a given week of gestation is related to pregnancy loss, then fewer live birth-identified conceptions would be observed coming from the corresponding week of conception.
Methods
Health Data
Boston, MA, USA.
We used all live births at the Beth Israel Deaconess Medical Center (BIDMC) in Boston from 1 January 2000 through 31 December 2013. We used clinical and administrative databases to extract relevant information, including the date of birth, gestational age (in weeks since date of last menstrual period) and maternal residence zip code. In the main analysis, we included pregnancies of women who lived in zip codes whose centroids were within 60 km the hospital. In sensitivity analyses to assess the impact of exposure measurement error we further restricted to pregnancies of women who lived in zip-codes whose centroids were within 20 km of the hospital. There were no exclusion criteria.
Tel Aviv District, Israel.
We used all live births of women whose home address at the date of birth was in one of the cities that belong to the District of Tel Aviv (as defined by The Israeli Ministry of Health), from 11 April 2010 through 22 December 2013. We extracted all relevant information for these analyses (date of birth, gestational age, residential address) from a computerized database of all birth certificates.
Our analyses were approved by the Committee on Clinical Investigation of BIDMC, the Human Subjects Committee of the Harvard T.H. Chan School of Public Health, and the supreme Helsinki committee of the Israeli Ministry of Health.
Exposure Data
Boston, MA, USA.
NO2, a pollutant commonly used as a traffic emissions tracer,33 was measured at state urban monitoring sites in the Boston, MA, metropolitan area and data were obtained from the US Environmental Protection Agency’s (EPA) Air Quality System (AQS) database (https://www.epa.gov/aqs). During the study period, three to five monitors were active at each time. City-wide exposures were estimated by averaging daily data from all available sites, using data from Federal Reference Method (FRM) or Federal Equivalent Method (FEM) primary monitors. Temperature data were obtained from the Boston Logan International Airport weather station. We created weekly averages for both NO2 and temperature. All data were obtained starting in 1999, to align with the conceptions that resulted in births in 2000.
Tel Aviv District, Israel.
NO2 was estimated using an optimized dispersion model,34 developed for the central coastal area of Israel. The model is based on air pollution monitoring data and meteorologic records from 1997 through 2013 obtained from the Technion Center of Excellence in Exposure Science and Environmental Health’s air pollution monitoring database. The model includes these data along with proxies for traffic emissions (time-of-day specific traffic volumes obtained from an independent traffic assignment model) to produce half-hourly concentration maps of NO2 at 500500 m2 grid resolution. Model performance was assessed using leave-one-out cross-validation that yielded unbiased predicted compared to observed concentrations and a cross-validation R2=0.62. For the current study, we used these spatiotemporal model results to calculate weekly NO2 averages over the entire Tel Aviv District during 2009 – 2013. We obtained temperature data for Tel Aviv from the Israeli Meteorological Service website (https://ims.data.gov.il/he/ims/1).
Study Design and Outcome Definition
The total number of conceptions during each calendar week that result in a live birth (live birth-identified conceptions) is equal to the total number of conceptions in a given week minus all conceptions that are lost at some point during gestation (whether or not they are recognized as a pregnancy). Therefore, if traffic pollution affects either conceptions or pregnancy loss, there should be an association between traffic pollution and live birth-identified conceptions. An effect on conceptions would occur before the conception occurs; thus, associations between traffic pollution after conception and live birth-identified conceptions would suggest an effect on pregnancy loss. I.e., by using information on these conceptions and examining post-conception exposures, we are able to infer the association of traffic-related air pollution exposure during gestation and pregnancy loss. Motivated by this notion, we used a variation of a time-series design to assess whether week-to-week traffic-related air pollution, using NO2 as a tracer for traffic emissions, is associated with the number of live birth-identified conceptions to infer associations with pregnancy loss.
Using gestational age at birth and date of birth, we determined the week of the last menstrual period (LMP) for each pregnancy, with conception expected two weeks later. We then summed the number of live birth-identified conceptions that shared the same LMP week for each week during the study period separately for each location. In both study locations, we based gestational age on the best obstetric estimate, i.e. the date of the last menstrual period and/or ultrasound.
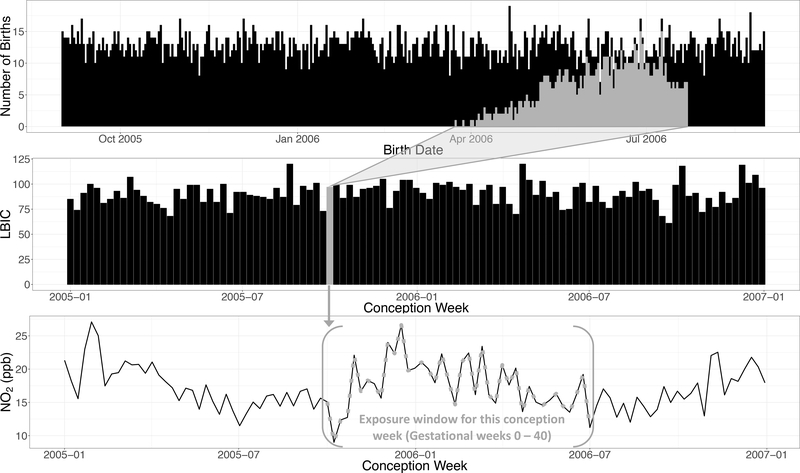
We then assigned to each LMP week the weekly exposures for the weeks of gestation that followed, from the LMP week itself (lag 0) to gestational week 40 (lag 40) (Figure 1). With this design, we investigated whether week-to-week NO2 variability over the course of pregnancy is associated with week-to-week changes in the number of live birth-identified conceptions, after accounting for seasonal and time trends. Thus, for instance, if NO2 is associated with pregnancy loss and if the NO2 exposure during the th week of gestation is harmful, one would expect fewer births coming from conceptions with an LMP week for which the NO2 exposure weeks later was higher than average.
Figure 1.
An example of the study design (using simulated data). The top plot shows the daily number of births, i.e. the observable data. The middle plot shows the weekly counts of live-birth identified conceptions (LBIC), which are constructed by mapping the live births back to the conception week by subtracting gestational age from the date of birth. The bottom plot shows the weekly NO2 time-series. In gray, we highlight one example for the unit of analysis (weekly number of LBIC), from the daily births, to the NO2 exposure window of that LBIC (in gestational weeks).
Statistical Analysis
We employed a Poisson regression, using a quasi-likelihood to account for potential overdispersion in the outcome distribution given covariates, and fitted distributed lag models to estimate the time-varying association between pregnancy loss, using live birth-identified conceptions as a proxy, and NO2 exposure in each gestational week. The distributed lag model framework allows adjustment for exposures at other weeks while estimating the temporal trends of this association, assuming that it varies smoothly as a function of time.35–37 We modeled this smooth function using natural splines with 4 degrees of freedom (df). In this design, the unit of analysis is calendar week; confounders, thus, can only be population-level variables that vary from week to week in our population. Individual-level variables, such as maternal BMI, and smoking cannot act as confounders. To adjust for potential confounding by long-term and seasonal trends, we used natural splines with 4 df per year in Boston and 10 df total in Tel Aviv District. Finally, to adjust for potential confounding by temperature (week to week variation of which is associated with weekly NO2 levels, and has also been reported to be associated with some birth outcomes38,39) we included distributed non-linear lags; we used natural splines with 4 df for the time constraint, and natural splines of 3 df and 2 df for each lag in Boston and Tel Aviv District, respectively, to account for potential non-linear confounding within each gestational week. The df were selected based on the best-fitting model in each location separately, which was chosen using leave-one-out cross-validation. Finally, in both locations we used indicators for weeks that include location-specific holidays (yes/no).
To avoid missing births conceived in the same calendar week as births in the early weeks of the study period but born prior to the start of our birth data, and similarly missing births that occurred after the end of the study period from a conception week towards the end of the study duration,40 we repeated our analyses after excluding the first and last 36 calendar weeks of the conception week data from the time series analyses in both locations.
We repeated analyses in Boston and Tel Aviv District in separate models. All results are presented as rate ratios (RR) and 95% confidence intervals (CI) per 10 ppb increase of NO2 for comparability with other studies. For the statistical analyses, we used the R Statistical Software, version 3.3.1 (Foundation for Statistical Computing, Vienna, Austria).
Results
Overall, there were 68,969 live births in Boston over the 736 weeks of the study period there, and 95,053 live births in Tel Aviv District over the 191 weeks of the study period there. Summary statistics for the number of live birth-identified conceptions per week and the NO2 distributions at both locations can be found in Table 1. The traffic-related air pollution levels were similar in the two locations, with slightly higher and less variable NO2 concentrations in Boston (mean = 17.9 ppb, standard deviation (SD) = 4.3 ppb) vs. in Tel Aviv District (mean = 16.8 ppb, SD = 5.8 ppb).
Table 1.
Summary statistics at each location per week.
| Mean (SD) | Min | Q1a | Median | Q3a | Max | |
|---|---|---|---|---|---|---|
| BIDMCb, Boston, MA, USA (n = 736 weeks) | ||||||
| Number of LBICc | 88.7 (12.6) | 43.0 | 81.0 | 89.0 | 97.3 | 138.0 |
| NO2 (ppb) | 17.9 (4.3) | 9.0 | 14.6 | 17.4 | 20.8 | 33.2 |
| Temperature (°C) | 10.9 (8.9) | -10.9 | 3.3 | 11.1 | 19.0 | 28.7 |
| Tel Aviv District, Israel (n = 191 weeks) | ||||||
| Number of LBICc | 497.7 (36.1) | 413.0 | 472.0 | 499.0 | 524.0 | 583.0 |
| NO2 (ppb) | 16.8 (5.8) | 7.5 | 11.6 | 16.3 | 20.9 | 38.1 |
| Temperature (°C) | 22.0 (4.7) | 12.3 | 17.7 | 22.6 | 26.5 | 29.3 |
Q1: 25th percentile; Q3: 75th percentile
BIDMC: Beth Israel Deaconess Medical Center
LBIC: Live birth-identified conceptions
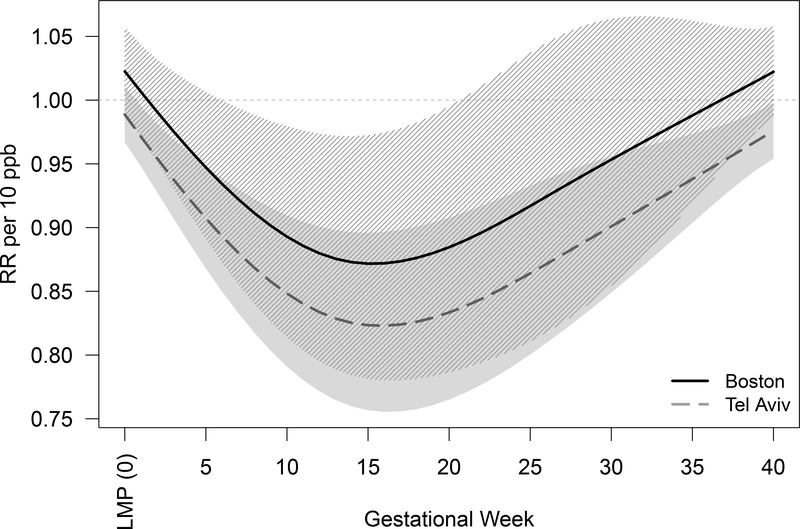
Figure 2 shows the association between NO2 across gestational weeks and the number of LBIC in Boston, MA, and Tel Aviv District, Israel. Similar trends were observed in both cities with strongest estimates between the 10th and 20th gestational weeks. In Boston, the strongest estimate was during the 15th week of gestation; for every 10 ppb of NO2 increase during that week, we observed a decreased rate in LBIC (RR = 0.87; 95% confidence interval [CI]: 0.78–0.97). We observed decreases in live birth-identified conceptions associated with NO2 exposure between gestational weeks 6 and 19. Similarly, in Tel Aviv District, the strongest estimate was during the 16th week of gestation; for every 10 ppb of NO2 increase during that week, the RR = 0.82 (95%CI: 0.76–0.90) in LBIC, with decreases after the first gestational week.
Figure 2.
Association between weekly NO2 levels over gestation and change in number of live births (solid black line for Boston and gray dashed line for Tel Aviv District). The shaded areas represent the 95% confidence intervals (striped black for Boston and solid gray for Tel Aviv District).
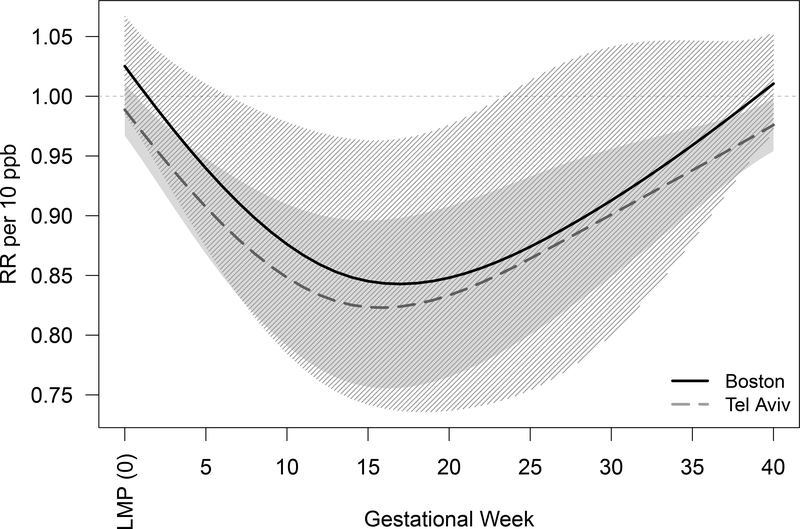
In a sensitivity analysis, we restricted the data in Boston to births of women whose residence zip code was within 20 km of BIDMC (median = 60 live birth-identified conceptions per week, interquartile range (IQR) = 54 – 67). In this analysis, we observed a stronger similarity with the results from Tel Aviv District (Figure 3). Specifically, the strongest association was observed for gestational week 17; per 10 ppb of NO2 exposure during that week of gestation, we observed a RR = 0.84 (95%CI: 0.74–0.96) in live birth-identified conceptions, with decreases between gestational weeks 7 and 23.
Figure 3.
Sensitivity Analysis: Association between weekly NO2 levels over gestation and change in number of live births (solid black line for Boston and gray dashed line for Tel Aviv District). The shaded areas represent the 95% confidence intervals (striped black for Boston and solid gray for Tel Aviv). For Boston this analysis is restricted to births of women living within 20 km of BIDMC. For Tel Aviv District these are the same results as those presented in Figure 2.
Furthermore, when we excluded from analyses the first and last 36 calendar weeks of the time-series the results remained unchanged for BIDMC in Boston. However, in Tel Aviv District the results were more sensitive, as 40% of the observations were removed for this sensitivity analysis. Although the estimated RRs remained below 1, we detected no apparent differences in the lag-specific estimates, likely suggesting lack of power in this reduced dataset. These results are presented in eFigures 1 and 2.
Discussion
We employed a novel approach using the outcome metric of live birth-identified conceptions and a variation of a time-series design to investigate the association between traffic-related air pollution exposure during pregnancy, using NO2 as a traffic emissions tracer, and pregnancy loss, using data from two different locations, Boston and Tel Aviv District. To explore this association, we used changes in the number of live birth-identified conceptions to infer changes in pregnancy loss. Overall, we observed harmful effect estimates in both locations, with the strongest estimates for gestational weeks 15 – 17. Specifically, we observed a smaller number of conceptions that ended in live births for conception cohorts that experienced increased traffic-related air pollution exposure during the 15th – 17th week of gestation, suggesting that high exposure during this period may be particularly toxic to the fetus. It is important to recognize, though, that this does not necessarily mean the increased pregnancy losses occurred during these weeks; exposure in these weeks could have initiated a process that led to losses later in pregnancy. While live birth-identified conception also could be affected by effects on conception itself, the fact that we saw associations with NO2 after conception (week 2) and not at lags 0–2 (prior to and up to presumed conception) suggests that the associations were likely not related to effects on conception itself.
The notable similarity in the results across the two locations strengthens our confidence in our findings. The shape of the estimated associations across the gestational weeks is remarkably similar, but the effect estimates in Tel Aviv are slightly larger in absolute magnitude than in Boston. There are a couple of possible explanations for this difference. First, it is quite likely that the distributions of one or more potential effect modifiers across the two locations are different, which would contribute to effect estimate heterogeneity. Second, the exposure assessment was conducted differently, which could have different impacts on the effect estimates. In Tel Aviv, the use of spatio-temporal optimized dispersion NO2 prediction models that cover the entire study area might have captured the population exposures better.41,42 Indeed, when we restricted our population in Boston to births only to mothers within 20 km of BIDMC, in an effort to reduce exposure measurement error, the estimated associations increased in absolute magnitude and became even more similar to the estimates observed in Tel Aviv District. Finally, the larger variability in NO2 concentrations and the much larger number of LBIC per week in Tel Aviv District may explain the narrower confidence intervals in the latter, despite the longer study duration in Boston.
Overall, our findings agree with the few studies that previously investigated the association between in-utero air pollution exposure and hospital-identified stillbirth. For instance, in a study using data from New Jersey, Faiz et al.15 reported a 16% and 13% increased risk of stillbirth per 10 ppb of average NO2 exposure during the first and second trimesters, respectively. Similarly, Green et al.16 reported an 18% increase in risk of spontaneous abortion comparing the 90th and 75th percentiles of daily maximum traffic at the maternal residence during pregnancy, with stronger estimates among African Americans and non-smokers. However, a case–control study in Taiwan found no evidence of an association between NO2 exposure and stillbirth.17 It should be noted, however, that all of these studies relied on medical records for the identification of fetal loss, and it is likely, therefore, that they might have not captured all the events and possibly a non-random set of the events. Moreover, none of the existing studies, to our knowledge, has explored the potential critical window of air pollution exposure at a finer resolution than trimesters, which can induce bias in effect estimates.43 A recent study examining in-utero exposure to residential fine particles (PM2.5) and cord blood telomere length at birth, however, also used distributed lag models and reported harmful associations for gestational weeks 12–25.44
Biological plausibility supports our findings of stronger associations early in the second trimester. Maternal arterial blood flow to the placenta is not fully established until the 10th to 12th gestational week, i.e. 10–12 weeks post-LMP, resulting in an oxidative stress burst during this period.45 The first trimester uterine oxygen gradient thus exerts a regulatory effect on the placenta protecting the developing embryo against oxygen free radicals and reactive oxygen species.46 Gestational weeks 10 – 14 are a transitional period, during which there is a gradual rise in intraplacental partial pressure of oxygen, which becomes more stabilized after approximately week 16,47 and higher overall during the second half of the pregnancy.46,48 Thus, it is likely that during this transitional period the fetus may be especially vulnerable to increases in maternal oxidative stress, which has consistent associations with air pollution exposure.49–51
With this study design, we were able to overcome many of the limitations of previous—mostly cohort—studies, including potential residual confounding and reliance on medical record documentation of spontaneous abortions. Nevertheless, our findings should be interpreted in light of our limitations. As with all observational studies, we cannot completely eliminate the possibility of residual confounding. However, using a time-series design, residual confounding would have to be induced by a population-level variable that varies from week to week, is associated both with week-to-week conceptions ending in live birth and week-to-week traffic-related air pollution, and is independent of long-term and seasonal trends and temperature. It would also have to be the same in Boston and Tel Aviv. Thus, this possibility is quite limited. Second, as is the case in all studies of ambient air pollution, our study is susceptible to exposure measurement error, which may have biased our estimates towards the null, especially in Boston where city-average exposure was estimated based on concentrations measured at monitoring stations.41,42 In fact, we observed stronger estimates when we restricted the population to women within 20 km, instead of 60 km, of BIDMC. Another source of potential exposure measurement error is the estimation of conception week. Presumably the timing of any effect of traffic-related air pollution is related to fetal age, rather than weeks from LMP. Given that the exact date of conception is not known, this could create some error in our exposure timing that would likely reduce the effect estimate for any given gestational age week. Furthermore, in Boston, we used complete birth records from only one hospital and not from all hospitals in the city. While this could hinder the generalizability of those findings, the fact that we saw very similar results in a completely different location—and one where all births in the region’s population were included—strengthens the generalizability of our findings. The current analysis did not assess potential effect modification of the relationship between NO2 and live birth-identified conception because of lack of information on modifiers common to both locations. Future research to identify potential effect modifiers and vulnerable subgroups would be of great interest.
In conclusion, we used an innovative method to explore the association between traffic-related air pollution and pregnancy loss that overcomes many limitations that other studies have previously faced and allowed us to explore critical windows of vulnerability in detail. Our findings suggest a harmful association between exposure to traffic pollution early in the second trimester of pregnancy and pregnancy loss. The consistency of our results across two very different locations, Boston and Tel Aviv District, strengthens this evidence further and increases the generalizability of our findings.
Supplementary Material
Acknowledgments
This work grew out of work related to specific aims #1 & 2 of grant R21 ES026900 to Dr. Weisskopf from NIEHS. This work was also supported by grants T32 ES007069, P30 ES000002, and P30 ES009089 from NIEHS. This publication was partially made possible by USEPA grant RD-835872. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Data and Code availability:
All data used and computing code developed for the analyses described in this paper can be available upon request, subject to the access requirements of the institutions that provided the data. Due to identifiability concerns, only weekly counts of live birth- identified conceptions can be available both for Tel Aviv District, Israel, and the Beth Israel Deaconess Medical Center in Boston, MA. For Tel Aviv District, weekly district-wide NO2 averages can also be available upon request. For Boston, all NO2 data were downloaded from the US Environmental Protection Agency’s Air Quality System (AQS) database (https://www.epa.gov/aqs). The weekly city-wide averages of NO2 that we used for our analyses can also be shared, however, to ensure full reproducibility.
Conflict of interest:
Nothing to disclose.
References
- 1.Di Q, Wang Y, Zanobetti A, et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017;376(26):2513–2522. doi:10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominici F, Peng R, Bell ML, Pham L, McDermott a. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama. 2006;295(10):1127–1134. doi:10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, et al. Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ Health Perspect. 2016;124(1):23–29. doi:10.1289/ehp.1408973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kioumourtzoglou M-A, Schwartz J, James P, Dominici F, Zanobetti A. PM2.5 and mortality in 207 US cities. Epidemiology. 2015;27(March):1. doi:10.1097/EDE.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen M, Giorgis-Allemand L, Bernard C, et al. Ambient air pollution and low birthweight: A European cohort study (ESCAPE). Lancet Respir Med. 2013;1(9):695–704. doi:10.1016/S2213-2600(13)70192-9. [DOI] [PubMed] [Google Scholar]
- 6.Shah PS, Balkhair T. Air pollution and birth outcomes: A systematic review. Environ Int. 2011;37(2):498–516. doi:10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Slama R, Darrow L, Parker J, et al. Meeting report: Atmospheric pollution and human reproduction. In: Environmental Health Perspectives. Vol 116; 2008:791–798. doi:10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113(4):375–382. doi:10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ Res. 2012;117:100–111. doi:10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Pereira G, Bell ML, Lee HJ, Koutrakis P, Belanger K. Sources of fine particulate matter and risk of preterm birth in connecticut, 2000–2006: A longitudinal study. Environ Health Perspect. 2014;122(10):1117–1122. doi:10.1289/ehp.1307741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang HH, Reich BJ, Miranda ML. Time-to-event analysis of fine particle air pollution and preterm birth: Results from North Carolina, 2001–2005. Am J Epidemiol. 2012;175(2):91–98. doi:10.1093/aje/kwr403. [DOI] [PubMed] [Google Scholar]
- 12.Hao H, Chang HH, Holmes HA, et al. Air pollution and preterm birth in the U.S. State of Georgia (2002–2006): Associations with concentrations of 11 ambient air pollutants estimated by combining community multiscale air quality model (CMAQ) simulations with stationary monitor measurements. Environ Health Perspect. 2016;124(6):875–880. doi:10.1289/ehp.1409651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Medeiros APP, Gouveia N, Machado RPP, et al. Traffic-Related Air Pollution and Perinatal Mortality: A Case–Control Study. Environ Health Perspect. 2009;117(1):127–132. doi:10.1289/ehp.11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFranco E, Hall E, Hossain M, et al. Air pollution and stillbirth risk: Exposure to airborne particulate matter during pregnancy is associated with fetal death. PLoS One. 2015;10(3). doi:10.1371/journal.pone.0120594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faiz AS, Rhoads GG, Demissie K, Kruse L, Lin Y, Rich DQ. Ambient air pollution and the risk of stillbirth. Am J Epidemiol. 2012;176(4):308–316. doi:10.1093/aje/kws029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green RS, Malig B, Windham GC, Fenster L, Ostro B, Swan S. Residential exposure to traffic and spontaneous abortion. Environ Health Perspect. 2009;117(12):1939–1944. doi:10.1289/ehp.0900943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang B-F, Lee YL, Jaakkola JJK. Air pollution and stillbirth: a population-based case–control study in Taiwan. Environ Health Perspect. 2011;119(9):1345–1349. doi:10.1289/ehp.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira LAA, Loomis D, Conceição GMS, et al. Association between air pollution and intrauterine mortality in Sao Paulo, Brazil. Environ Health Perspect. 1998;106(6):325–329. doi:10.2307/3434038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enkhmaa D, Warburton N, Javzandulam B, et al. Seasonal ambient air pollution correlates strongly with spontaneous abortion in Mongolia. BMC Pregnancy Childbirth. 2014;14(1):146. doi:10.1186/1471-2393-14-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou HY, Wang D, Zou XP, Yang ZH, Li TC, Chen YQ. Does ambient air pollutants increase the risk of fetal loss? A case–control study. Arch Gynecol Obstet. 2014;289(2):285–291. doi:10.1007/s00404-013-2962-1. [DOI] [PubMed] [Google Scholar]
- 21.Hemminki K, Niemi ML. Community study of spontaneous abortions: relation to occupation and air pollution by sulfur dioxide, hydrogen sulfide, and carbon disulfide. Int Arch Occup Environ Health. 1982;51(1):55–63. doi:10.1007/BF00378410. [DOI] [PubMed] [Google Scholar]
- 22.Perin PM, Maluf M, Czeresnia CE, Nicolosi Foltran Januario DA, Nascimento Saldiva PH. Impact of short-term preconceptional exposure to particulate air pollution on treatment outcome in couples undergoing in vitro fertilization and embryo transfer (IVF/ET). J Assist Reprod Genet. 2010;27:371–382. doi:10.1007/s10815-010-9419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moridi M, Ziaei S, Kazemnejad A. Exposure to ambient air pollutants and spontaneous abortion. J Obstet Gynaecol Res. 2014;40:743–748. doi:10.1111/jog.12231. [DOI] [PubMed] [Google Scholar]
- 24.Chard T 11 Frequency of implantation and early pregnancy loss in natural cycles. Baillieres Clin Obstet Gynaecol. 1991;5(1):179–189. doi:10.1016/S0950-3552(05)80077-X. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of Early Loss of Pregnancy. N Engl J Med. 1988;319(4):189–194. doi:10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 26.Bonde JPE, Hjollund NHI, Jensen TK, et al. A follow-up study of environmental and biologic determinants of fertility among 430 danish first-pregnancy planners: Design and methods. Reprod Toxicol. 1998;12(1):19–27. doi:10.1016/S0890-6238(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 27.Finer LB, Zolna MR. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. 2011;84(5):478–485. doi:10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finer LB, Zolna MR. Declines in Unintended Pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852. doi:10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Wilhelm M, Chung J, Ritz B. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environ Res. 2011;111(5):685–692. doi:10.1016/j.envres.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Hooven EH; Jaddoe VW; de Kluizenaar Y; Hofman A; Mackenbach JP; Steegers EA; Miedema HM; Pierik FH., Pierik van den HEJV de KYHAMJSEMH. Residential traffic exposure and pregnancy-related outcomes: a prospective birth cohort study. Environ Health Perspect. 2009;8(2009):59. doi:10.1186/1476-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slama R, Morgestern V, Cyrys J, et al. Traffic-related atmospheric pollutants levels during pregnancy and offspring’s term birth weight: A study relying on a land-use regression exposure model. Environ Health Perspect. 2007;115(9):1283–1292. doi:10.1289/ehp.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaskins AJ, Hart JE, Mínguez-Alarcón L, et al. Residential proximity to major roadways and traffic in relation to outcomes of in vitro fertilization. Environ Int. 2018;115:239–246. doi:10.1016/j.envint.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seinfeld JH, Pandis SN. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; 2006.
- 34.Yuval, Bekhor S, Broday DM Data-driven nonlinear optimisation of a simple air pollution dispersion model generating high resolution spatiotemporal exposure. Atmos Environ. 2013;30(79):261–270. [Google Scholar]
- 35.Gasparrini A Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw. 2011;43(8). [PMC free article] [PubMed] [Google Scholar]
- 36.Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Stat Med. 2010;29:2224–2234. doi:10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanobetti A, Wand MP, Schwartz J, Ryan LM. Generalized additive distributed lag models: quantifying mortality displacement. Biostatistics. 2000;1(3):279–292. [DOI] [PubMed] [Google Scholar]
- 38.Kloog I, Melly SJ, Coull BA, Nordio F, Schwartz JD. Using satellite-based spatiotemporal resolved air temperature exposure to study the association between ambient air temperature and birth outcomes in Massachusetts. Environ Health Perspect. 2015;123(10):1053–1058. doi:10.1289/ehp.1308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha S, Liu D, Zhu Y, et al. Ambient Temperature and Stillbirth: A Multi-Center Retrospective Cohort Study. Environ Health Perspect. 2017;125(6). doi:10.1289/EHP945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strand LB, Barnett AG, Tong S. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol. 2011;11. doi:10.1186/1471-2288-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: Concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi:10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kioumourtzoglou M- A, Spiegelman D, Szpiro AA, et al. Exposure measurement error in PM2.5 health effects studies: A pooled analysis of eight personal exposure validation studies. Environ Heal. 2014;13(1):2. doi:10.1186/1476-069X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. Potential for bias when estimating critical windows to air pollution in children’s health. Am J Epidemiol. 2017;In Press. doi:10.1093/aje/kwx184/3860092/Potential-for-Bias-When-Estimating-Critical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martens DS, Cox B, Janssen BG, et al. Prenatal Air Pollution and Newborns’ Predisposition to Accelerated Biological Aging. JAMA Pediatr. 2017. doi:10.1001/jamapediatrics.2017.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: A possible factor in human early pregnancy failure. Am J Pathol. 2000;157(6):2111–2122. doi:10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12(6):747–755. doi:10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol. 2001;184(5):998–1003. doi:10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- 48.Burton GJ, Jauniaux E. Placental oxidative stress: From miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11(6):342–352. doi:10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003;60(8):612–616. doi:10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: A review. Eur Respir J. 2008;31(1):179–196. doi:10.1183/09031936.00128106. [DOI] [PubMed] [Google Scholar]
- 51.Laumbach RJ, Kipen HM. Acute effects of motor vehicle traffic-related air pollution exposures on measures of oxidative stress in human airways. In: Annals of the New York Academy of Sciences. Vol 1203 ; 2010:107–112. doi:10.1111/j.1749-6632.2010.05604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.