Abstract
Hypertrophic cardiomyopathy (HCM) is usually manifested by increased myofilament Ca2+ sensitivity, excessive contractility, and impaired relaxation. In contrast, dilated cardiomyopathy (DCM) originates from insufficient sarcomere contractility and reduced cardiac pump function, subsequently resulting in heart failure. The zebrafish has emerged as a new model of human cardiomyopathy with high-throughput screening, which will facilitate the discovery of novel genetic factors and the development of new therapies. Given the small hearts of zebrafish, better phenotyping tools are needed to discern different types of cardiomyopathy, such as HCM and DCM. This article reviews the existing models of cardiomyopathy, available morphologic and functional methods, and current understanding of the different types of cardiomyopathy in adult zebrafish.
Keywords: Cardiac Remodeling, Cardiomyopathy, Sarcomere, Zebrafish
1. Adult zebrafish: an emerging cardiomyopathy model
Cardiomyopathy (CM) manifests defective myocardium with impaired contractile function. CM can be ischemic or nonischemic. Nonischemic CM can be broadly divided into hypertrophic, restrictive, and dilated (HCM, RCM, and DCM, respectively). The categorization system is largely built on available phenotyping tools in human patients and is strengthened by studies in animal models. Consistent with the phenotypic differences of CMs, molecular etiology is also different among the different types. The first known HCM mutation in the myosin heavy-chain protein gene (MYH) was discovered almost 30 years ago (Geisterfer-Lowrance et al., 1990). Later studies provided further evidence that HCM is an autosomal dominant cardiac disease, most often caused by mutations in sarcomere genes (Maron et al., 2012). In contrast, DCM-linked genes were found to be responsible for various functions in the cell (Ahmad et al., 2005; Haas et al., 2015; Maron et al., 2012; Shih et al., 2015); 12% to 25% of DCM cases are associated with mutations in the titin gene (Haas et al., 2015; Herman et al., 2012). Existing gene panels for genetic assessment of DCM include as many as 111 genes (McNally and Mestroni, 2017). Besides causative genes, highly variable phenotypes are noted in probands harboring the same genetic mutation (Herman et al., 2012). A major contributing factor to phenotypic variation is different genetic background in each person. Unlike the causative genes, the identity of these genetic modifiers remains largely unknown.
The zebrafish (Danio rerio) has emerged as a new animal model to facilitate studies of the genetic basis of human CMs because of the following strong features: 1) diploid genome well conserved among vertebrates, 2) easy for gene editing, 3) easy for gene modifier and drug screens, 4) low cost, and 5) availability of both embryonic and adult models. Having a single ventricle and a single atrium with well-differentiated cardiomyocytes, zebrafish may be one of the simplest vertebrate models for human CMs. At the gene level, a recent study provides evidence to support the zebrafish as a relevant model for studying CM (Shih et al., 2015). Among about 51 known DCM-causative genes, homologues of 49 genes, or 96%, were found in zebrafish (Table 1) (Shih et al., 2015). Compared with rodent models, the simpler zebrafish model promises a much-increased throughput in structure-function studies for known CM genes. Established zebrafish models can benefit from chemical screens to search for novel therapeutics. More importantly, a forward genetic screen was recently established to identify new CM genes (Ding et al., 2016), opening the door to systematically identifying genetic factors for CM.
Table 1.
Human DCM-associated genes (N=49) with established zebrafish homologues, according to Shih et al. (2015)
| ABCC9 | CSRP3 | EYA4 | LDB3 | NEXN | SCN5A | TMPO |
| ACTC1 | DES | FHL2 | LMNA | PDLIM3 | SDHA | TNNC1 |
| ACTN2 | DMD | FKTN | MYBPC3 | PKP2 | SGCD | TNNI3 |
| ANKRD1 | DNAJC19 | GATAD1 | MYH6 | PLN | SYNE1 | TNNT2 |
| BAG3 | DSC2 | ILK | MYH7 | PSEN1 | SYNE2 | TPM1 |
| CAV3 | DSP | LAMA4 | MYPN | PSEN2 | TAZ | TTN |
| CRYAB | EMD | LAMP2 | NEBL | RBM20 | TCAP | VCL |
A major challenge for developing the adult zebrafish as a new animal model for CM is the lack of phenotyping tools, largely due to its small heart. This review analyzes the current status of the adult zebrafish CM models and reviews morphologic and functional methods and approaches in order to find distinct hallmarks of different types of CM in the zebrafish model.
2. Existing CM models in adult zebrafish
The first attempts to use zebrafish to model human CMs were reported in 2002, when mutations in ttn and tnnt were identified as causative genes for 2 embryonic lethal mutants identified from a forward genetic screen: pickwick and silent heart (Sehnert et al., 2002; Xu et al., 2002). Phenotypes in these embryonic models of CM mainly manifest as reduced ejection fraction, disrupted sarcomere structure, and decreased survival. As expected, many progressive pathogeneses in human CM cannot be fully recapitulated in these embryonic models.
2.1 Anemia-induced cardiac remodeling
The first adult CM model in zebrafish was tr265/tr265, which is caused by a band3 mutation that primarily affects erythroblast development (Paw et al., 2003). The chronic anemia imposes high-output stress to the heart, resulting in cardiac remodeling with hallmarks of human CM (Sun et al., 2009). Phenotypes include reduced survival, slowed heart rate, cardiomyocyte hypertrophy and hyperplasia, muscular disarray, and oncosis. A similar cardiac remodeling process can be induced by treating adult fish with phenylhydrazine (Sun et al., 2009). The progression of cardiac pump dysfunction induced by phenylhydrazine was recently characterized with high-frequency underwater echocardiography (Wang et al., 2017).
2.2 Doxorubicin-induced CM
CM (often followed by heart failure) is a frequent complication of anthracycline chemotherapy in patients with cancer (Talavera et al., 2015). We found that a single intraperitoneal bolus injection of doxorubicin in adult zebrafish is sufficient to induce CM (Ding et al., 2016). With use of light-sheet fluorescent imaging, 3-dimensional reconstruction, and classic echocardiography, ultrastructural changes in the hearts of the doxorubicin-induced cardiomyopathy model have been determined (Packard et al., 2017). Global cardiac injury suggested by reduced myocardial and endocardial volumes was identified on day 3 after injection. Days 30 and 60 after injection were characterized by ventricular remodeling and regeneration. Given its high efficiency, doxorubicin has been successfully used to stress zebrafish from an insertional cardiac mutant collection (ZIC) to identify genetic modifiers of CM (Ding et al., 2016).
2.3 Diabetes-induced CM
To model diabetic CM, Sun et al (2017) incubated adult fish in a solution containing 2% glucose for 32 weeks. Under hyperglycemic stress, zebrafish hearts manifest hypertrophy, apoptosis, myofibril loss, fetal gene reactivation, and severe arrhythmia. Hyperglycemia induced inhibition of glucose transporter 1 expression and increased ventricular volume. Diastolic dysfunction was noted at an early stage, and systolic dysfunction developed at a later stage (decompensational CM), findings consistent with those in patients with diabetes.
2.4 Heart regeneration models
Both ventricular section and cryoinjury can be used to remove part of the zebrafish heart. Unlike mammals, zebrafish are capable of completely regenerating a heart without any scar. One study found that almost complete functional and morphologic recovery of cryo-injured hearts can be achieved within 45 days after damage (Hein et al., 2015). A diphtheria toxin A–induced myocarditis also has been established as an inducible myocardial damage model (Wang et al., 2011; Wang et al., 2017). The zebrafish can survive after loss of more than 60% of the ventricular myocardium, recapitulating the situation of a severe heart attack in humans (Wang et al., 2011). Of note, these models were mainly used to study heart regeneration. Whether and how these stresses incur cardiac remodeling or CM remains to be studied. Of note, the impact of regeneration can be assessed in heart explants, which can be cultured for several weeks (Cao and Poss, 2016; Wang et al., 2015).
2.5 Isoproterenol model of heart failure
In mammals, chronic isoproterenol (ISO) administration triggers cardiac remodeling in a healthy heart, which could lead to heart failure. A recent study tested whether embryonic zebrafish have essential components of the β-adrenergic signaling pathway and how fish embryos respond to ISO (Kossack et al., 2017). All dominant β-adrenoreceptor isoforms, as well as their elimination cofactors such as β-arrestin, showed robust expression in fish larvae at 3 days post-fertilization (dpf). The authors then tested chronic ISO exposure in both embryos and adult fish and found severe cardiac dysfunction in adults after 14 days of treatment. Phenotypes include declined systolic function, blunted ISO-induced positive inotropy, increased cell death, increased inflammation, and disturbed calcium handling, resemble phenotypes seen in mammals following chronic ISO treatment (Kossack et al., 2017).
2.6 Genetic models of CM
Several transgenic models of CM have been reported in adult zebrafish. A transgenic 2057del2 plakoglobin zebrafish was established as an arrhythmogenic CM model, manifested by enlarged hearts, cardiomyocytes with reduced sodium and potassium transmembrane currents, and prolonged action potentials (Asimaki et al., 2014). Another adult transgenic zebrafish model harboring a mutation in the sodium channel gene scn5a also had arrhythmogenic CM (Huttner et al., 2013). ERBB2 is a member of the receptor tyrosine kinase family, a part of the epidermal growth factor receptor complex, which has important roles in cardiogenesis. A transgenic erbb2 zebrafish heart manifests signs of the DCM phenotype, such as enlarged ventricle and reduced trabeculation (Reischauer et al., 2014). An enlarged heart was also noted in transgenic fish harboring a DCM-causative mutation in gatad1 (Yang et al., 2016). Besides transgenic models, cardiac phenotypes in adult fish containing mutations in sarcomeric genes have been defined. Myosin heavy-chain 6 (myh6hu423/+) has reduced atrial contractility and heart morphologic changes in juvenile and adult fish (Singleman and Holtzman, 2012). Severe systolic dysfunction has been detected in essential light-chain lazy susan (lazm647/+) mutants (Scheid et al., 2016).
Similar to the discovery of the TTN mutation as an embryonic CM model, adult models of CM could result from forward genetic screen, as exemplified by studies on dnajb6. Homozygous GBT411/dnajb6 mutants had enlarged hearts at 1 year (Ding et al., 2013), whereas heterozygous GBT411/dnajb6 mutants exerted a deleterious modifying effect on doxorubicin-induced cardiomyopathy. Later, mutations in DNAJB6 from human patients with DCM were identified, and, in transgenic fish, the disease-causing mutation also exerted deleterious modifying effects on doxorubicin-induced cardiomyopathy (Ding et al., 2016).
The recent discovery of 49 homologues of human genes related to DCM in zebrafish laid the foundation for systematically generating corresponding genetic CM models in adult zebrafish (Table 1) (Shih et al., 2015). The advent of genome-editing technology, such as those based on TALEN (transcription activator-like effector nucleases) and CRISPR/Cas9 (clustered, regularly interspaced, short palindromic repeats/protein-9 nuclease), can conveniently generate large numbers of knock-out/knock-in models. Thus, demands are increasing for the development of better phenotyping tools that can be used to discern different CM models in zebrafish.
3. Hallmarks of CM in adult zebrafish models
3.1 Cardiac structural remodeling
HCM cardiac phenotype in humans is characterized by severe asymmetric hypertrophy, increased left ventricular and septal wall thickness, and, therefore, higher left ventricular and heart mass. Findings are similar in HCM mouse models (Alves et al., 2014; Flenner et al., 2016; Martins et al., 2015; Prabhakar et al., 2001; Wang et al., 2012; Wilder et al., 2015; Yuan et al., 2015). The considerable thickening of the wall is accompanied by an increase in volume of interstitium and collagenous fibers (Prabhakar et al., 2001). Focally accentuated fibrosis may originate as a replacement fibrosis after a loss of cardiomyocytes (Kaltenbach et al., 1987). In contrast, normal or thinner walls of the left ventricle and chamber dilatation are common features of DCM (Bollen et al., 2017). In animal models, slightly enlarged hearts develop (Du et al., 2007; Ryba et al., 2017; Song et al., 2010), and ejection fraction is reduced (Song et al., 2010). DCM is often accompanied by muscle dystrophy and loss of myofibrils (Hoorntje et al., 2017; Makarenko et al., 2004). In mouse models, dilated hearts may be associated with heart failure (Du et al., 2007; Du et al., 2014), fibrosis, and enlarged cardiomyocytes (Burke et al., 2016; Du et al., 2007). The interstitial fibrosis and myofibrillar disorganization are common in HCM (Alves et al., 2014; Flenner et al., 2016; Kazmierczak et al., 2013; Li et al., 2013; Prabhakar et al., 2001; Schulz et al., 2013; Yuan et al., 2015) and also occur in DCM, but rarely (Vikhorev et al., 2017).
Because of the limited spatial resolution of noninvasive imaging technologies, cardiac remodeling in adult zebrafish is mainly characterized by quantifying the size of a dissected heart. For comparison of heart size among fish of different body sizes, the area of the ventricle can be normalized to either body length or body weight (Singleman and Holtzman, 2012). In the established zebrafish anemia model, the area of ventricle to body weight ratio is dramatically increased (Sun et al., 2009). Of note, the wall thickness is difficult to quantify in an adult zebrafish ventricle because of its highly trabeculated structure. Whether the thickness of the compact layer can be used as a surrogate for wall thickness remains unclear. Histologic analysis of sectioned hearts after staining with hematoxylin-eosin remains the standard for assessing heart structural remodeling at the organ level, but new state-of-the-art approaches in microscopy have been used to generate 3-dimensional structures of the zebrafish heart. Light-sheet microscopy has been used to reconstruct 3-dimensional structures (Packard et al., 2017), and the internal structure of the 3-dimensional model can be visualized with virtual reality (Ding et al., 2017).
3.2 Functional remodeling: diastolic and systolic dysfunction
CM is a disease of insufficient cardiac pump function due to defects in myocardium contractility. Contractility is a dynamic measure of the ability of the heart to contract and relax (Gwathmey et al., 1995). Pump function defects can be analyzed from ejection fraction, and impaired myocardium contractility is usually assessed from fractional shortening and velocities of contraction or relaxation. Unlike mammals, fish have a 2-chamber heart; nevertheless, the most important phases of the cardiac cycle are preserved. Systole and diastole both are divided into isovolumic and dynamic phases; dynamic phases (ejection and filling) can be also divided into fast and slow subphases (Lee et al., 2014).
Echocardiography in M-mode, pulsed-wave Doppler echocardiography, or magnetic resonance imaging technique can be used to obtain images of a beating heart in vivo. HCM is typically manifested as hypercontractility and diastolic dysfunction and DCM, as hypocontractility and systolic dysfunction. In most HCM hearts, ejection fraction is either normal or increased; fractional shortening may be reduced even if ejection fraction is normal (Alves et al., 2014; Flenner et al., 2016; Yuan et al., 2015). Mouse HCM models typically show increased left ventricular mass and decreased diastolic function (Alves et al., 2014; Schulz et al., 2013; Wilder et al., 2015). In some HCM models, contractile velocity may also be reduced (Alves et al., 2014). For diagnosis of DCM in the clinical setting, the following criteria are used: fractional shortening less than 25%, ejection fraction less than 45%, and left ventricular diameter more than 117%. Similarly, DCM hearts in animal models have increased end-diastolic volume, systolic dysfunction, and reduced ejection fraction (Du et al., 2007; Du et al., 2014).
Pulsed-wave Doppler echocardiography allows for monitoring of passive (early, E-wave velocity) and active (atrial, A-wave velocity) ventricular filling; for cardiac phenotyping, the E/A ratio is used extensively. Abnormalities in the E/A ratio suggest an improper filling phase during the cardiac cycle and diastolic dysfunction or heart failure. For genetic HCM models, a lower E/A ratio may indicate diastolic dysfunction in the early phase, whereas in later phases, hearts may turn to a restrictive pattern and the E/A ratio may normalize or even increase, albeit with abnormally reduced filling times (Alves et al., 2014; Flenner et al., 2016; Li et al., 2013; Prabhakar et al., 2001). Findings on pulsed-wave Doppler echocardiography can be further used to obtain the myocardial performance index, a ratio of isovolumic contraction time plus isovolumic relaxation time to ejection time (Packard et al., 2017). This index reversely reflects a portion of the ejection phase interval in a cardiac cycle and may serve as an index of cardiac pump function; both systolic and diastolic dysfunction prolong the relaxation period, an effect that increases the myocardial performance index.
A recent comprehensive study of high-frequency echocardiography in zebrafish provided standardized instruction on this technology (Wang et al., 2017). Interestingly, peak E velocity gradually declined with age from 50 to 20 mm/s but peak A velocity reciprocally increased from 200 to 300 mm/s during the aging process; as a result, the E/A ratio gradually decreased. Myocardial damage with diphtheria toxin A induced chamber dilatation and reduced ejection fraction and other indices of ventricular contractility such as fractional area change, global longitudinal strain, and fractional shortening. Despite the preserved E/A ratio, there was clear evidence of diastolic dysfunction, suggested by substantial reduction in all velocity peaks. In another model, phenylhydrazine-treated fish showed generalized pallor and increases in ejection fraction and other indices of contractility. Accordingly, E-wave velocity was increased, and thus the E/A ratio was increased in these hypercontractile phenylhydrazine-treated hearts. However, treatment of fish with atenolol reduced the ejection fraction, fractional shortening, and fractional area change; isoproterenol had the opposite effects on these variables and also increased the E/A ratio (Hein et al., 2015). Doxorubicin-injected fish had an increased E/A ratio at 30 days after injury, and this normalized at 60 days after injury. These fish also had an increased myocardial performance index at 30 days after injury; a finding that can be interpreted as worsening global cardiac function (Packard et al., 2017).
These discrepancies of the E/A ratio change may originate from high variability in control (healthy) groups. Unlike in humans, the E/A ratio is less than 1 in zebrafish, a difference reflecting higher active versus passive filling (Lee et al., 2014). The E/A ratio is highly dependent on magnitudes of both E and A waves; hence, it should be considered in association with other cardiac performance indices. Substantial reduction in both velocity peaks may not be reflected by the ratio. In contrast, diminished filling phase velocities may suggest diastolic dysfunction.
3.3 Cardiac transcriptome analysis
Changes at the transcriptional level for molecular markers have been extensively used to indicate pathologic cardiac remodeling. Many of these marker genes are part of the fetal reprogramming genes (genes that are activated during cardiogenesis, become quiet at the adult stage, and are reactivated with pathologic stresses). Corresponding cardiac remodeling markers in zebrafish have been identified, including nppa and nppb that encode natriuretic peptides, myosin heavy-chain isoforms that shift their expression ratio during pathogenesis (vmhc/vmhcl), and calcium-handling pathway genes (pln, ryr2b, atp2a2, slc8a1a) that are downregulated during pathogenesis (Shih et al., 2015).
3.4 Swimming performance
A critical criterion for diagnosing human heart failure is to quantify exercise capacity, as measured by the treadmill exercise test. In zebrafish, physical capacity can be monitored with a swim tunnel respirometer and measured with the maximal swimming velocity. As expected, mutant fish with failing hearts cannot swim as fast as their wild-type siblings, as indicated by reduced maximal swimming velocity (Scheid et al., 2016; Sun et al., 2015; Wang et al., 2011; Zhang et al., 2009).
4. Phenotyping CM at cellular and subcellular levels
4.1 Single cardiomyocyte and intact muscle contractility
In general, hypertrophic hearts can be characterized by enlarged cardiomyocytes with a high width to length ratio (Alves et al., 2014; Flenner et al., 2016; Kazmierczak et al., 2013). In contrast, cardiomyocytes from DCM hearts are usually elongated (Xu et al., 2002). In the zebrafish doxorubicin model, reduced CM density in sectioned hearts and increased width of isolated single cardiomyocytes suggest cardiomyocyte hypertrophy (Ding et al., 2011).
At the cellular level, contractile function of single cardiomyocytes and intracellular Ca2+ handling can be analyzed experimentally. A general characteristic of a failing heart is that the twitch force and cytosolic Ca2+ transient of a membrane-intact cardiomyocyte are prolonged resulting in impaired relaxation; another feature of the muscle cells is a negative force-frequency relationship (Gwathmey et al., 1995). In some CM models, cardiomyocytes have normal twitch force and Ca2+ transient variables, but a compromised response to β-adrenergic stimulation, a suggestion of reduced functional reserve (Flenner et al., 2016; Wilkinson et al., 2015).
Cardiac remodeling at the cellular level can be quantified by staining of sectioned hearts. The border of cardiomyocytes can be stained with β-catenin for quantification of individual cell size. Nuclei of cardiomyocytes can be labeled by staining with an anti-Mef2 antibody, and the density of CM is used as an index of proliferation and to reflect cardiomyocyte size indirectly (Sun et al., 2009). Cardiomyocyte hypertrophy can be validated by culturing isolated single cardiomyocytes and then measuring the cell size directly (Ding et al., 2011). Cardiomyocyte proliferation can be quantified by staining sectioned heart with proliferating cell nuclear antigen; cardiomyocyte apoptosis can be quantified by staining sectioned heart with TUNEL (terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling) (Ding et al., 2016; Ding et al., 2011; Hoage et al., 2011; Sun et al., 2009; Yang et al., 2016). These assays are particularly important in zebrafish, because both hyperplasia (muscle growth by cell division) and hypertrophy (muscle growth by cell size) occur during adulthood (Palstra et al., 2010). These cellular assays have shown distinct types of CM modes in adult fish. In the anemia model, proliferation of cardiomyocytes is substantially increased, as quantified by proliferating cell nuclear antigen staining, increased apoptosis, and increased cardiomyocyte density at later stages. In contrast, cell proliferation is not activated in the doxorubicin-induced cardiomyopathy model, a finding is consistent with the DNA damaging property of doxorubicin. (Ding et al., 2011; Hoage et al., 2011; Sun et al., 2009).
We adopted 2 single-cell–based biophysical approaches in zebrafish (Bovo et al., 2013; Dvornikov et al., 2014). With these new approaches, one can analyze mechanically loaded or unloaded single zebrafish cardiomyocyte mechanics to study cell shortening, sarcomere length dynamics, quantify Ca2+ transient dynamics: diastolic and peak Ca2+ concentrations, or sarcoplasmic reticulum Ca2+ content. We found that twitch force of the zebrafish single ventricular cardiomyocyte is sigmoidally dependent on [Ca2+]o; the EC50 for twitch force ranged from 2 to 2.4 mM. These assays are anticipated to help uncover the unique pathogenesis of different CM models in adult zebrafish.
4.2 Myofilament calcium sensitivity
In human permeabilized cardiac samples from patients with HCM who have mutations in genes encoding myofilament proteins such as MYH7, MYBPC3, TNNT2, TNNI3, and TPM1, high myofilament Ca2+ sensitivity is detected (Sequeira et al., 2013). This increased sensitivity of myofilaments to Ca2+ in healthy cardiac tissues can be lowered with exogenous protein kinase A treatment. Besides tension, sensitivity of muscle adenosine triphosphatase activity to calcium is also increased (Warren et al., 2015). Increased myofilament Ca2+ sensitivity has been found in almost all mouse HCM-related permeabilized cardiac samples (Alves et al., 2014; Barefield et al., 2015; Gomes and Potter, 2004; Kazmierczak et al., 2013; Li et al., 2013; Messer et al., 2016; Messer et al., 2017; Muthu et al., 2014; Prabhakar et al., 2001; Schulz et al., 2013; Wang et al., 2012; Wilder et al., 2015; Yuan et al., 2015; Yumoto et al., 2005).
In various transgenic and mutant mouse DCM models, myofilament Ca2+ desensitization of tension and adenosine triphosphatase activity is frequently reported (Cheng et al., 2016; Du et al., 2007; Du et al., 2014; Karam et al., 2011; Morimoto et al., 2002; Ryba et al., 2017; Utter et al., 2015); however, myofilament Ca2+ sensitivity may be increased (Song et al., 2010; Vikhorev et al., 2014) or unchanged (Huang et al., 2015; Memo et al., 2013). Sometimes, it is difficult to find consistent changes in myofilament Ca2+ sensitivity in myocardium carrying DCM mutations: here changes in myofilament Ca2+ sensitivity are not affected by the level of troponin I phosphorylation, suggesting that changes in Ca2+ sensitivity and troponin I phosphorylation are uncoupled (Memo et al., 2013). Another variable suggestive of increased myofilament Ca2+ sensitivity that can be easily measured in myocardium is diastolic sarcomere length. This parameter is often reduced, likely due to background cross-bridge cycling in the Ca2+-sensitized hearts (Flenner et al., 2016).
We have studied permeabilized (skinned) preparations (cells or single myofibrils) to assess myofilament Ca2+-induced force dynamics. We found that single ventricular cardiomyocytes are similar to single myofibrils dimensionally; thus single skinned cardiomyocytes can be also attached to the glass microtools and their tension can be accurately measured. Myofilament Ca2+ sensitivity in zebrafish cardiac muscle is similar to that in mammals, namely, pCa50 ranges from 5.4 to 5.7. Likewise, the Hill coefficient, a measure of cooperativity, values range from 1 to 2 (Dvornikov et al., 2014; Iorga et al., 2011).
4.3 Length-dependent activation
Myofilament length-dependent activation (LDA), a molecular mechanism that increases myofilament calcium sensitivity upon stretch, underlying the Frank-Starling law of the heart, has been extensively studied in HCM cardiac tissues. LDA was reduced in HCM myocardium (Barefield et al., 2015; Flenner et al., 2016; Li et al., 2013; Mickelson and Chandra, 2017; Prabhakar et al., 2001; Sequeira et al., 2013). Moreover, some DCM human samples also had increased Ca2+ sensitivity and reduced LDA compared with controls, although in some samples LDA was preserved (Bollen et al., 2017; Vikhorev et al., 2014). From these data, it can be speculated that, with maximal Ca2+ sensitization, the sensitivity of cardiac troponin C to calcium cannot be further increased in the stretched myocardium, and LDA is blunted. Likely, this is not the only mechanism, because in our recent study in human skinned cardiac samples and whole troponin complex exchange with RCM mutation cTnI-R145W, we did not find a substantial decrease in myofilament LDA despite a severe leftward shift of the force-pCa curve (Dvornikov et al., 2016).
Likewise, we found a robust myofilament LDA in both zebrafish membrane intact and skinned cardiomyocytes (Dvornikov et al., 2014), concluding that LDA is a general property of the cardiac sarcomere also inherent in teleost fish. We also found linear relationships between force and sarcomere length in zebrafish, in contrast to curvilinear relations in mammals. Whether LDA is affected in fish with CM remains to be further investigated.
4.4 Maximal isometric tension
An isometric tension generated by the sarcomere at a certain length on maximal activation (Tmax) or force (Fmax) is a dynamic variable that may reflect the state of the thin filament activation, thick filament regulation (crossbridge recruitment), and thin-thick filament interaction (crossbridge cycling, cooperativity). In a simplified 2-state model, Tmax is directly related to the number of turning-over force-generating crossbridges (Belus et al., 2008; Brenner, 1988; Rice and de Tombe, 2004). Alternatively, tension generated by myofibrils is a dynamic variable also related to the kinetics of crossbridge cycling, namely, rate of crossbridge turnover: f / (f + g), where f is a rate of crossbridge formation, and g is a rate of detachment (Brenner, 1988). Indeed, reduced Tmax will result in hypocontractility (ie, at a given amount of activator Ca2+, cardiomyocyte will be less contractile).
Tmax is usually reduced in cardiac skinned fibers or cells from various animal HCM models (Barefield et al., 2015; Belus et al., 2008; Flenner et al., 2016; Kazmierczak et al., 2013; Sequeira et al., 2013; Yuan et al., 2015). In only a few cases, Tmax was unaffected or increased in mutants (Dvornikov et al., 2016; Prabhakar et al., 2001). Tmax was not diminished in human DCM samples, except in LMNA, where Tmax was reduced. Decreased Tmax might be related to decreased myofibril density in LMNA mutant hearts (Bollen et al., 2017; Hoorntje et al., 2017). In a study describing contractile function in myofibrils from human cardiac samples with missense mutations in TNNI3, TNNC1, MYH7, and three TTNtv mutations, Tmax did not differ between groups (Vikhorev et al., 2017). In various DCM models, Tmax may be unaffected (Du et al., 2007; Karam et al., 2011; Morimoto et al., 2002; Song et al., 2010; Vikhorev et al., 2014) or reduced (Cheng et al., 2016; Edes et al., 2008; Hoorntje et al., 2017; Huang et al., 2015; Ryba et al., 2017; Utter et al., 2015). Interestingly, if one normalizes tension by myofibril density, Tmax is no longer reduced, a suggestion that myofibril density is a pivotal cause of reduced contractility in DCM hearts (Hoorntje et al., 2017). In a human HCM β-myosin mutation R403Q sample, reduced Tmax was speculated to originate from the accelerated crossbridge detachment rate g and faster crossbridge turnover (Belus et al., 2008).
In zebrafish skinned cardiomyocytes and single myofibrils, we found Tmax to be 30 to 35 kPa (Dvornikov et al., 2014); in another study in adult cardiac myofibrils, it was about 50 kPa, a value less than in somite myofibrils (Iorga et al., 2011). In conclusion, zebrafish permeabilized myocardium has maximal isometric tension and force-calcium (force-pCa) relationships that are similar to those in mammalian preparations.
4.5 Cardiac muscle activation and relaxation kinetics
Applying a rapid solution-switching technique to ultrathin permeabilized fiber attached to glass microtools allows for measurement of fast activation and relaxation kinetics (Belus et al., 2008; de Tombe and Stienen, 2007; Dvornikov et al., 2014; Dvornikov et al., 2016; Poggesi et al., 2005). Opposite changes in variables of kinetics of myofibril activation and relaxation in various genetic CM models and also human CM samples have been reported. However, in general, single myofibril relaxation kinetics were slower in HCM and RCM (and some DCM) preparations (Cheng et al., 2016; Dvornikov et al., 2016; Flenner et al., 2016; Vikhorev et al., 2014). Myofibril relaxation kinetics can be assessed by measurement of rates of 2 distinct phases: slow linear and fast exponential phases (with rates kREL and kLIN, respectively). The kLIN is limited mainly by the rate of crossbridge detachment g. Interestingly, the increased constant g in β-myosin R403Q conditioned higher kLIN with faster relaxation. This finding is apparently inconsistent with clinical evidence of diastolic dysfunction in patients with HCM (Belus et al., 2008).
According to the simple 2-state model, the rate of isometric force redevelopment (kTR) is a function of crossbridge turnover (f + g) (Brenner, 1988). Activation rate (kACT) is a variable similar to kTR (Poggesi et al., 2005). The force-generating rate constant f is dependent on the concentration of activator Ca2+, whereas crossbridge detachment constant g is time-limiting (f ≫ g) and activation independent (Brenner, 1988; de Tombe et al., 2007; de Tombe and Stienen, 2007; Poggesi et al., 2005). Rate kTR is reduced in CM hearts (Edes et al., 2008), or it may be increased (Belus et al., 2008; Cheng et al., 2016). Some mutations, for example, β-myosin R403Q, may intrinsically accelerate crossbridge turnover because of a faster crossbridge detachment rate g (Belus et al., 2008). This increase, in turn, generates higher energy costs and a higher adenosine triphosphatase activity to tension ratio, which is common for failing hearts (Belus et al., 2008; de Tombe and Stienen, 2007). Crossbridge turnover may also change as a result of replacement of 1 myosin heavy-chain isoform with another one (ie, due to fetal gene response). To explain the discrepancy in the literature, we suggest a high sensitivity of the myofibril activation and relaxation kinetics on the disease stage and phasic activation of the fetal gene expression program.
Myofilament kinetics in zebrafish is, on average, faster than in mammals; therefore, temperature in the experimental bath is usually reduced from 15°C (for mammals) to 10°C (for fish). With these conditions, variables of activation and relaxation are very similar to those in mammalian models (Dvornikov et al., 2014; Iorga et al., 2011). As in mammals, we found a sigmoidal dependence between kACT and activator [Ca2+], accelerating at higher free [Ca2+] (Poggesi et al., 2005). Also, as in mammalian myocardium, force relaxation showed a biphasic behavior (Poggesi et al., 2005) resulting in 2 different rates: a linear and an exponential phase. Both the linear relaxation rate and the duration of the linear phase were independent of the level of activation. Interestingly, the exponential phase was Ca2+-sensitive, accelerating at lower activation. Accumulated experimental data from our studies and other studies (Dvornikov et al., 2014; Iorga et al., 2011) strongly suggest that zebrafish share sarcomeric properties similar to those of known vertebrate species, a characteristic that lays the foundation for use of this attractive model to study sarcomere dysfunction and cardiomyopathies.
4.6 Passive stiffness
Another variable that can be documented in HCM skinned myocardium is increased passive stiffness. Enhanced passive stiffness may originate from interstitial collagen deposition or a shift in the average titin isoform length in the sarcomere (Kazmierczak et al., 2013; Yuan et al., 2015). In permeabilized myofibril thin preparations, extracellular collagen should not affect the result. Interestingly, human DCM cardiac myofibrils with titin truncated mutations have reduced passive stiffness, as shown by an attenuated Young modulus (Vikhorev et al., 2017). The reduced passive stiffness in DCM hearts may be due to a titin isoform switch (Makarenko et al., 2004). However, DCM hearts do not always have such compliance: increased passive stiffness has also been documented at late-stage DCM, a suggestion of a restrictive phenotype (Edes et al., 2008). Typically, reduction of diastolic stress in DCM hearts is accompanied by increased passive stress (Wu et al., 2002). As a measure of passive properties of the sarcomere, slack sarcomere length (SL) can also be recorded during a slack test.
Passive properties of zebrafish skinned cardiac muscle preparations have also been studied (Dvornikov et al., 2014; Iorga et al., 2011). According to the existing data, they are very similar to mammalian properties; however, there are some discrepancies. The passive tension-length relationships are not linear in mammals: myofibrils are compliant at SL less than 2.3 μm (shallow relation), and they become very stiff (steep relation) at SL more than 2.3 μm. The relationship is similar in zebrafish, except that the transition is shifted to the higher SL values: we found that these relationships were mostly linear at SL less than 2.4 μm. This quasi linearity was observed at 20% cell length stretch in intact cells and at 15% to 16% cell length stretch in skinned myofibrils (associated with SL about 2.4 μm) (Dvornikov et al., 2014; Iorga et al., 2011).
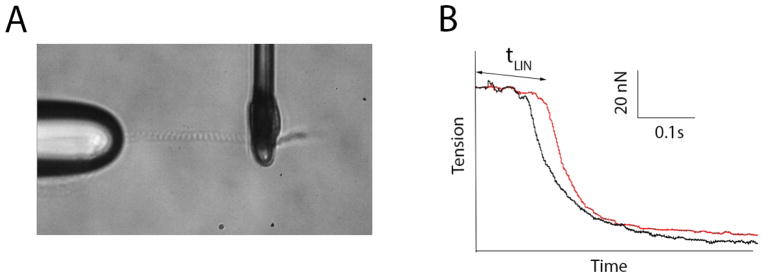
In summary, the HCM phenotype at the level of sarcomere can be characterized by increased Ca2+ sensitivity and diastolic dysfunction; alternatively, in general, DCM manifests a hypocontractile phenotype. However, at the level of sarcomere, maximal tension development may be reduced in both HCM and DCM; this reduction may also occur because of myofibril loss. Passive stiffness of myocardium can be decreased or increased depending on the stage of disease. As for zebrafish, there are no data on contractility and its kinetics in skinned cardiomyocyte or single myofibrils obtained from fish hearts carrying CM-linked mutations. Single permeabilized cell and single myofibril mechanic techniques have been recently adopted for zebrafish (Dvornikov et al., 2014; Iorga et al., 2011), opening new opportunities for investigators in the zebrafish field. Figure 1 depicts how the single myofibril technique can help in phenotyping CM. A single myofibril (or several myofibrils) can be attached to glass microtools; then, alternating solution currents with pCa = 9 (relaxing) and pCa = 4 (activation) are applied (Figure 1A).
Figure 1.
Assessment of sarcomere function in zebrafish heart. A, A single myofibril attached to glass microtools. B, Traces from the preliminary experiment showing increased relaxation time tLIN in mybpc3−/ − mutant fish (in red).
In a preliminary experiment, the activation traces from one mybpc3−/− mutant fish showed impaired relaxation kinetics, namely, dramatically prolonged time (tLIN) of the linear phase of relaxation (Figure 1B). This result, however, needs to be validated by more experiments. Impaired relaxation at the level of the sarcomere suggests that the primary defect linked to this mutation is present, despite an absence of a distinct phenotype at the organ level; impaired relaxation might be compensated at a higher level of organization. To study these primary defects of mutations and their compensation, the structure-function relationship in sarcomere proteins, scientific reductionism requires a primitive model system such as zebrafish.
5. Limitations of the zebrafish model
5.1 High capability for regeneration and unique myocardial morphologic features
Unlike mammals, zebrafish undergo continued growth during their life span (Cao and Poss, 2016; Palstra et al., 2010; Wang et al., 2015; Yu et al., 2010). A zebrafish heart is able to fully regenerate within several days, even when more than 60% of cells in the heart are depleted (Wang et al., 2011). This high regeneration capacity may compromise the use of zebrafish as a faithful model for human CM: pathologic phenotypes could be masked by its high regenerative capacity (Wang et al., 2011). However, this regeneration is not always the case, as indicated by the doxorubicin-induced CM model. Cardiomyocyte proliferation is not activated in this model, probably because of the antiproliferation activity of doxorubicin (Ding et al., 2011; Packard et al., 2017). Conversely, zebrafish can be an advantageous model to understand how cardiac regeneration can be leveraged to help the heart deal with biomechanical stresses. In addition to the reduced number of chambers in a zebrafish heart, another discrepancy between mammalian and fish heart is that the atrium in zebrafish has a large volume and also is more active during the cardiac cycle. Therefore, the E/A ratio in fish is less than 1, suggestive of higher active filling (A wave) than passive filling (E wave) in zebrafish (Lee et al., 2014). Another difference may be higher trabeculation of the ventricular myocardium, which may complicate our interpretation of how the zebrafish heart responds to pathologic processes and remodeling.
5.2 Specificity of cyclic adenosine monophosphate–dependent pathway in fish
Failing hearts may show a blunted response to neurohumoral regulation. A blunted adrenergic response helps the diseased heart to save energy. According to one hypothesis, myofilament Ca2+ sensitivity in DCM hearts is uncoupled from protein kinase A phosphorylation of the N-terminal domain of the cardiac isoform of troponin I (Memo et al., 2013; Messer and Marston, 2014; Song et al., 2010; Vikhorev et al., 2014). Indeed, uncoupling between adrenergic stimulation and subsequent positive inotropic (and other) muscle responses is a common feature of failed hearts. As a sequential event, protein kinase A/adrenergic response is usually blunted in diseased hearts (Dvornikov et al., 2016; Flenner et al., 2016; Memo et al., 2013; Messer et al., 2016; Messer et al., 2017; Messer and Marston, 2014; Song et al., 2010; Song et al., 2013; Vikhorev et al., 2017; Vikhorev et al., 2014; Wilkinson et al., 2015). Together with blunted LDA, CM hearts are not sensitive to either increased preload or β-adrenergic stimulation; both may radically reduce cardiac reserve.
Importantly, zebrafish cardiac troponin I lacks the N-terminal domain (Fu et al., 2009). These double serine motifs are usually phosphorylated by protein kinase A in mammals (Bovo et al., 2013; Dvornikov et al., 2016; Memo et al., 2013). Therefore, this mechanism is not applicable in zebrafish; how protein kinase A regulates myofilament Ca2+ sensitivity and the prominent lusitropic response in zebrafish is still unclear. According to our unpublished data, zebrafish hearts show both inotropic and lusitropic responses to the adrenergic agent isoproterenol. Moreover, our study also found a different calcium-induced calcium-release mechanism in response to forskolin, an activator of the cyclic adenosine monophosphate–dependent pathway (Bovo et al., 2013). Moreover, the slow force stretch response that is related to calcium handling is also not found in zebrafish (Dvornikov et al., 2014). Therefore, experimenters need to be cautious when use teleost fish models for the direct study of cyclic adenosine monophosphate–dependent signaling and, related to that pathway, calcium-induced calcium-release, known to be important in the pathogenesis of CM.
6. Perspectives for future studies
Although the current phenotypic studies of CM models in adult zebrafish clearly indicate that different types of CM can be modeled, it remains challenging to confidently categorize each model into HCM, RCM, and DCM, as has been done in mammals. In the next several years, many new CM models will be generated with genome-editing technology, including those for known HCM-DCM-, and RCM-causative genes in mammals (Table 1). Detailed phenotypic analysis of these genetic models will significantly advance our understanding in zebrafish. Characteristic pathologic phenotypes for each type of CM that will be studied in these fish models are summarized in Table 2. DCM is usually related to only reduced contractility, whereas HCM is also correlated with relaxation problems; RCM is likely characterized by only a relaxation problem. In titin mutants, primary defects are thought to stem from stiffness changes. Loss of myofibrils might occur in both DCM and HCM, which could be a primary defect in the DCM model (eg, in diabetes or various toxicity models) but, rather, a sequential event in HCM models (Table 2). We recommend study of Ca2+ sensitivity and contractility defects linked to CM in a primitive zebrafish model, in which minimal compensatory or secondary effects can be observed.
Table 2.
Phenotypes and possible pathogeneses of sarcomere gene–related cardiomyopathiesa
| DCM | HCM | RCM | |
|---|---|---|---|
| ↓Contractility | ↑(or↓?) Contractility | ↑Ca2+ sensitivity | ↑Ca2+ sensitivity |
| Volume overload | Pressure overload | ↓Relaxation | ↓Relaxation |
| Hypertrophy (eccentric) | Hypertrophy (asymmetric) | No hypertrophy No compensation |
|
| ↓Myocardial stiffness | ↑Myocardial stiffness | ||
| ↑N2BA/N2B; ↑SLsl | ↓N2BA/N2B; ↓SLsl | ||
| Loss of myofibrils | Ischemia, loss of myofibrils, fibrosis | ||
| ↓Relaxation | OFT obstruction | ||
Possible primary defects are underlined. The most important compensatory effects are in boldfaced. Possible secondary effects are italicized. Note that the primary defect for titin mutations may be myocardial stiffness or N2BA/N2B ratio.
Abbreviations: DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; N2BA and N2B, long and short cardiac isoforms of titin, respectively; OFT, outflow tract; RCM, restrictive cardiomyopathy; SLsl, slack sarcomere length.
Zebrafish have been traditionally considered a strong gene discovery platform but less advantageous in terms of phenotyping. As described in this review, phenotyping tools that are being actively developed in adult zebrafish will considerably advance this emerging vertebrate model for mechanistic studies of CM. Using the adult zebrafish to define and discern different types of CM, the model has the potential to contribute substantially to the development and implementation of patient individualized treatment strategies aimed to combat CM and heart failure.
Acknowledgments
The authors acknowledge financial support from the National Institutes of Health (NIH) grants NIH R01 HL81753, R01 HL107304 (to Xu X.), NIH P01 HL62426 (project 3) and NIH R01 HL75494 (to P.P.d.T.), and Mayo Foundation (to Xu X.).
Abbreviations
- CM
cardiomyopathy
- DCM
dilated cardiomyopathy
- E/A
early, E-wave velocity
- HCM
hypertrophic cardiomyopathy
- ISO
isoproterenol
- LDA
length-dependent activation
- RCM
restrictive cardiomyopathy
- SL
sarcomere length
- Tmax
isometric tension on maximal activation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet. 2005;6:185–216. doi: 10.1146/annurev.genom.6.080604.162132. [DOI] [PubMed] [Google Scholar]
- Alves ML, Dias FAL, Gaffin RD, Simon JN, Montminy EM, Biesiadecki BJ, Hinken AC, Warren CM, Utter MS, Davis RTR, Sakthivel S, Robbins J, Wieczorek DF, Solaro RJ, Wolska BM. Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ Cardiovasc Genet. 2014;7:132–143. doi: 10.1161/CIRCGENETICS.113.000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, Wu JC, MacRae CA, Kleber AG, Saffitz JE. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med. 2014;6:240ra74. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefield D, Kumar M, Gorham J, Seidman JG, Seidman CE, de Tombe PP, Sadayappan S. Haploinsufficiency of MYBPC3 exacerbates the development of hypertrophic cardiomyopathy in heterozygous mice. J Mol Cell Cardiol. 2015;79:234–43. doi: 10.1016/j.yjmcc.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belus A, Piroddi N, Scellini B, Tesi C, D’Amati G, Girolami F, Yacoub M, Cecchi F, Olivotto I, Poggesi C. The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. J Physiol. 2008;586:3639–44. doi: 10.1113/jphysiol.2008.155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen IAE, Schuldt M, Harakalova M, Vink A, Asselbergs FW, Pinto JR, Kruger M, Kuster DWD, van der Velden J. Genotype-specific pathogenic effects in human dilated cardiomyopathy. J Physiol. 2017;595:4677–4693. doi: 10.1113/JP274145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovo E, Dvornikov AV, Mazurek SR, de Tombe PP, Zima AV. Mechanisms of Ca2+ handling in zebrafish ventricular myocytes. Pflugers Archiv-European Journal of Physiology. 2013;465:1775–1784. doi: 10.1007/s00424-013-1312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988;85:3265–9. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MA, Chang S, Wakimoto H, Gorham JM, Conner DA, Christodoulou DC, Parfenov MG, DePalma SR, Eminaga S, Konno T, Seidman JG, Seidman CE. Molecular profiling of dilated cardiomyopathy that progresses to heart failure. JCI Insight. 2016:1. doi: 10.1172/jci.insight.86898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Poss KD. Explant culture of adult zebrafish hearts for epicardial regeneration studies. Nat Protoc. 2016;11:872–81. doi: 10.1038/nprot.2016.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Hogarth KA, O’Sullivan ML, Regnier M, Pyle WG. 2-Deoxyadenosine triphosphate restores the contractile function of cardiac myofibril from adult dogs with naturally occurring dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2016;310:H80–91. doi: 10.1152/ajpheart.00530.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tombe PP, Belus A, Piroddi N, Scellini B, Walker JS, Martin AF, Tesi C, Poggesi C. Myofilament calcium sensitivity does not affect cross-bridge activation-relaxation kinetics. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1129–36. doi: 10.1152/ajpregu.00630.2006. [DOI] [PubMed] [Google Scholar]
- de Tombe PP, Stienen GJ. Impact of temperature on cross-bridge cycling kinetics in rat myocardium. J Physiol. 2007;584:591–600. doi: 10.1113/jphysiol.2007.138693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Abiri A, Abiri P, Li S, Chang CC, Baek KI, Hsu JJ, Sideris E, Li Y, Lee J, Segura T, Nguyen TP, Bui A, Sevag Packard RR, Fei P, Hsiai TK. Integrating light-sheet imaging with virtual reality to recapitulate developmental cardiac mechanics. JCI Insight. 2017:2. doi: 10.1172/jci.insight.97180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Liu W, Deng Y, Jomok B, Yang J, Huang W, Clark KJ, Zhong TP, Lin X, Ekker SC, Xu X. Trapping cardiac recessive mutants via expression-based insertional mutagenesis screening. Circ Res. 2013;112:606–17. doi: 10.1161/CIRCRESAHA.112.300603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Long PA, Bos JM, Shih YH, Ma X, Sundsbak RS, Chen J, Jiang Y, Zhao L, Hu X, Wang J, Shi Y, Ackerman MJ, Lin X, Ekker SC, Redfield MM, Olson TM, Xu X. A modifier screen identifies DNAJB6 as a cardiomyopathy susceptibility gene. JCI Insight. 2016:1. doi: 10.1172/jci.insight.88797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Sun X, Huang W, Hoage T, Redfield M, Kushwaha S, Sivasubbu S, Lin X, Ekker S, Xu X. Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circ Res. 2011;109:658–69. doi: 10.1161/CIRCRESAHA.111.248260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, Lu QW, Wang YY, Zhan DY, Mochizuki M, Kita S, Miwa Y, Takahashi-Yanaga F, Iwamoto T, Ohtsuki I, Sasaguri T. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185–94. doi: 10.1161/CIRCRESAHA.106.146670. [DOI] [PubMed] [Google Scholar]
- Du CK, Zhan DY, Morimoto S. In vivo effects of propyl gallate, a novel Ca(2+) sensitizer, in a mouse model of dilated cardiomyopathy caused by cardiac troponin T mutation. Life Sci. 2014;109:15–9. doi: 10.1016/j.lfs.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Dvornikov AV, Dewan S, Alekhina OV, Pickett FB, de Tombe PP. Novel approaches to determine contractile function of the isolated adult zebrafish ventricular cardiac myocyte. Journal of Physiology-London. 2014;592:1949–1956. doi: 10.1113/jphysiol.2014.270678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvornikov AV, Smolin N, Zhang M, Martin JL, Robia SL, de Tombe PP. Restrictive Cardiomyopathy Troponin I R145W Mutation Does Not Perturb Myofilament Length-dependent Activation in Human Cardiac Sarcomeres. J Biol Chem. 2016;291:21817–21828. doi: 10.1074/jbc.M116.746172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edes IF, Toth A, Csanyi G, Lomnicka M, Chlopicki S, Edes I, Papp Z. Late-stage alterations in myofibrillar contractile function in a transgenic mouse model of dilated cardiomyopathy (Tgalphaq*44) J Mol Cell Cardiol. 2008;45:363–72. doi: 10.1016/j.yjmcc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Flenner F, Friedrich FW, Ungeheuer N, Christ T, Geertz B, Reischmann S, Wagner S, Stathopoulou K, Sohren KD, Weinberger F, Schwedhelm E, Cuello F, Maier LS, Eschenhagen T, Carrier L. Ranolazine antagonizes catecholamine-induced dysfunction in isolated cardiomyocytes, but lacks long-term therapeutic effects in vivo in a mouse model of hypertrophic cardiomyopathy. Cardiovasc Res. 2016;109:90–102. doi: 10.1093/cvr/cvv247. [DOI] [PubMed] [Google Scholar]
- Fu CY, Lee HC, Tsai HJ. The molecular structures and expression patterns of zebrafish troponin I genes. Gene Expr Patterns. 2009;9:348–56. doi: 10.1016/j.gep.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- Gomes AV, Potter JD. Cellular and molecular aspects of familial hypertrophic cardiomyopathy caused by mutations in the cardiac troponin I gene. Mol Cell Biochem. 2004;263:99–114. doi: 10.1023/B:MCBI.0000041852.42291.aa. [DOI] [PubMed] [Google Scholar]
- Gwathmey JK, Liao R, Helm PA, Thaiyananthan G, Hajjar RJ. Is contractility depressed in the failing human heart? Cardiovasc Drugs Ther. 1995;9:581–7. doi: 10.1007/BF00878090. [DOI] [PubMed] [Google Scholar]
- Haas J, Frese KS, Peil B, Kloos W, Keller A, Nietsch R, Feng Z, Muller S, Kayvanpour E, Vogel B, Sedaghat-Hamedani F, Lim WK, Zhao X, Fradkin D, Kohler D, Fischer S, Franke J, Marquart S, Barb I, Li DT, Amr A, Ehlermann P, Mereles D, Weis T, Hassel S, Kremer A, King V, Wirsz E, Isnard R, Komajda M, Serio A, Grasso M, Syrris P, Wicks E, Plagnol V, Lopes L, Gadgaard T, Eiskjaer H, Jorgensen M, Garcia-Giustiniani D, Ortiz-Genga M, Crespo-Leiro MG, Deprez RH, Christiaans I, van Rijsingen IA, Wilde AA, Waldenstrom A, Bolognesi M, Bellazzi R, Morner S, Bermejo JL, Monserrat L, Villard E, Mogensen J, Pinto YM, Charron P, Elliott P, Arbustini E, Katus HA, Meder B. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur Heart J. 2015;36:1123–35a. doi: 10.1093/eurheartj/ehu301. [DOI] [PubMed] [Google Scholar]
- Hein SJ, Lehmann LH, Kossack M, Juergensen L, Fuchs D, Katus HA, Hassel D. Advanced echocardiography in adult zebrafish reveals delayed recovery of heart function after myocardial cryoinjury. PLoS One. 2015;10:e0122665. doi: 10.1371/journal.pone.0122665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJ, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–28. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoage T, Sun X, Xu X. Functions of the Wnt/beta-catenin pathway in an anemia-induced zebrafish model of cardiomyopathy are location dependent. Biochem Biophys Res Commun. 2011;415:490–6. doi: 10.1016/j.bbrc.2011.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorntje ET, Bollen IA, Barge-Schaapveld DQ, van Tienen FH, Te Meerman GJ, Jansweijer JA, van Essen AJ, Volders PG, Constantinescu AA, van den Akker PC, van Spaendonck-Zwarts KY, Oldenburg RA, Marcelis CL, van der Smagt JJ, Hennekam EA, Vink A, Bootsma M, Aten E, Wilde AA, van den Wijngaard A, Broers JL, Jongbloed JD, van der Velden J, van den Berg MP, van Tintelen JP. Lamin A/C-Related Cardiac Disease: Late Onset With a Variable and Mild Phenotype in a Large Cohort of Patients With the Lamin A/C p.(Arg331Gln) Founder Mutation. Circ Cardiovasc Genet. 2017:10. doi: 10.1161/CIRCGENETICS.116.001631. [DOI] [PubMed] [Google Scholar]
- Huang W, Liang J, Yuan CC, Kazmierczak K, Zhou Z, Morales A, McBride KL, Fitzgerald-Butt SM, Hershberger RE, Szczesna-Cordary D. Novel familial dilated cardiomyopathy mutation in MYL2 affects the structure and function of myosin regulatory light chain. FEBS J. 2015;282:2379–93. doi: 10.1111/febs.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner IG, Trivedi G, Jacoby A, Mann SA, Vandenberg JI, Fatkin D. A transgenic zebrafish model of a human cardiac sodium channel mutation exhibits bradycardia, conduction-system abnormalities and early death. J Mol Cell Cardiol. 2013;61:123–32. doi: 10.1016/j.yjmcc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Iorga B, Neacsu CD, Neiss WF, Wagener R, Paulsson M, Stehle R, Pfitzer G. Micromechanical function of myofibrils isolated from skeletal and cardiac muscles of the zebrafish. J Gen Physiol. 2011;137:255–70. doi: 10.1085/jgp.201010568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach M, Hopf R, Kunkel B. New Aspects of Hypertrophic Cardiomyopathy - Morphology, Mechanisms and Therapy - Overview. Zeitschrift Fur Kardiologie. 1987;76:R5–R6. [PubMed] [Google Scholar]
- Karam CN, Warren CM, Rajan S, de Tombe PP, Wieczorek DF, Solaro RJ. Expression of tropomyosin-kappa induces dilated cardiomyopathy and depresses cardiac myofilament tension by mechanisms involving cross-bridge dependent activation and altered tropomyosin phosphorylation. J Muscle Res Cell Motil. 2011;31:315–22. doi: 10.1007/s10974-010-9237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak K, Paulino EC, Huang W, Muthu P, Liang J, Yuan CC, Rojas AI, Hare JM, Szczesna-Cordary D. Discrete effects of A57G-myosin essential light chain mutation associated with familial hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2013;305:H575–89. doi: 10.1152/ajpheart.00107.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossack M, Hein S, Juergensen L, Siragusa M, Benz A, Katus HA, Most P, Hassel D. Induction of cardiac dysfunction in developing and adult zebrafish by chronic isoproterenol stimulation. J Mol Cell Cardiol. 2017;108:95–105. doi: 10.1016/j.yjmcc.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Lee J, Cao H, Kang BJ, Jen N, Yu F, Lee CA, Fei P, Park J, Bohlool S, Lash-Rosenberg L, Shung KK, Hsiai TK. Hemodynamics and ventricular function in a zebrafish model of injury and repair. Zebrafish. 2014;11:447–54. doi: 10.1089/zeb.2014.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AY, Stevens CM, Liang B, Rayani K, Little S, Davis J, Tibbits GF. Familial hypertrophic cardiomyopathy related cardiac troponin C L29Q mutation alters length-dependent activation and functional effects of phosphomimetic troponin I*. PLoS One. 2013;8:e79363. doi: 10.1371/journal.pone.0079363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–16. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol. 2012;60:705–15. doi: 10.1016/j.jacc.2012.02.068. [DOI] [PubMed] [Google Scholar]
- Martins AS, Parvatiyar MS, Feng HZ, Bos JM, Gonzalez-Martinez D, Vukmirovic M, Turna RS, Sanchez-Gonzalez MA, Badger CD, Zorio DAR, Singh RK, Wang Y, Jin JP, Ackerman MJ, Pinto JR. In Vivo Analysis of Troponin C Knock-In (A8V) Mice: Evidence that TNNC1 Is a Hypertrophic Cardiomyopathy Susceptibility Gene. Circ Cardiovasc Genet. 2015;8:653–664. doi: 10.1161/CIRCGENETICS.114.000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally EM, Mestroni L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ Res. 2017;121:731–748. doi: 10.1161/CIRCRESAHA.116.309396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memo M, Leung MC, Ward DG, dos Remedios C, Morimoto S, Zhang L, Ravenscroft G, McNamara E, Nowak KJ, Marston SB, Messer AE. Familial dilated cardiomyopathy mutations uncouple troponin I phosphorylation from changes in myofibrillar Ca(2)(+) sensitivity. Cardiovasc Res. 2013;99:65–73. doi: 10.1093/cvr/cvt071. [DOI] [PubMed] [Google Scholar]
- Messer AE, Bayliss CR, El-Mezgueldi M, Redwood CS, Ward DG, Leung MC, Papadaki M, Dos Remedios C, Marston SB. Mutations in troponin T associated with Hypertrophic Cardiomyopathy increase Ca(2+)-sensitivity and suppress the modulation of Ca(2+)-sensitivity by troponin I phosphorylation. Arch Biochem Biophys. 2016;601:113–20. doi: 10.1016/j.abb.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer AE, Chan J, Daley A, Copeland O, Marston SB, Connolly DJ. Investigations into the Sarcomeric Protein and Ca2+-Regulation Abnormalities Underlying Hypertrophic Cardiomyopathy in Cats (Felix catus) Front Physiol. 2017;8:348. doi: 10.3389/fphys.2017.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer AE, Marston SB. Investigating the role of uncoupling of troponin I phosphorylation from changes in myofibrillar Ca(2+)-sensitivity in the pathogenesis of cardiomyopathy. Front Physiol. 2014;5:315. doi: 10.3389/fphys.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson AV, Chandra M. Hypertrophic cardiomyopathy mutation in cardiac troponin T (R95H) attenuates length-dependent activation in guinea pig cardiac muscle fibers. Am J Physiol Heart Circ Physiol. 2017;313:H1180–H1189. doi: 10.1152/ajpheart.00369.2017. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Lu QW, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M, Sasaguri T, Ohtsuki I. Ca(2+)-desensitizing effect of a deletion mutation Delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:913–8. doi: 10.1073/pnas.022628899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu P, Liang J, Schmidt W, Moore JR, Szczesna-Cordary D. In vitro rescue study of a malignant familial hypertrophic cardiomyopathy phenotype by pseudo-phosphorylation of myosin regulatory light chain. Arch Biochem Biophys. 2014;552–553:29–39. doi: 10.1016/j.abb.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard RRS, Baek KI, Beebe T, Jen N, Ding Y, Shi F, Fei P, Kang BJ, Chen PH, Gau J, Chen M, Tang JY, Shih YH, Ding Y, Li D, Xu X, Hsiai TK. Automated Segmentation of Light-Sheet Fluorescent Imaging to Characterize Experimental Doxorubicin-Induced Cardiac Injury and Repair. Sci Rep. 2017;7:8603. doi: 10.1038/s41598-017-09152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palstra AP, Tudorache C, Rovira M, Brittijn SA, Burgerhout E, van den Thillart GE, Spaink HP, Planas JV. Establishing zebrafish as a novel exercise model: swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS One. 2010;5:e14483. doi: 10.1371/journal.pone.0014483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paw BH, Davidson AJ, Zhou Y, Li R, Pratt SJ, Lee C, Trede NS, Brownlie A, Donovan A, Liao EC, Ziai JM, Drejer AH, Guo W, Kim CH, Gwynn B, Peters LL, Chernova MN, Alper SL, Zapata A, Wickramasinghe SN, Lee MJ, Lux SE, Fritz A, Postlethwait JH, Zon LI. Cell-specific mitotic defect and dyserythropoiesis associated with erythroid band 3 deficiency. Nat Genet. 2003;34:59–64. doi: 10.1038/ng1137. [DOI] [PubMed] [Google Scholar]
- Poggesi C, Tesi C, Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflugers Arch. 2005;449:505–17. doi: 10.1007/s00424-004-1363-5. [DOI] [PubMed] [Google Scholar]
- Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro RJ, Wieczorek DF. A familial hypertrophic cardiomyopathy alpha-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol. 2001;33:1815–28. doi: 10.1006/jmcc.2001.1445. [DOI] [PubMed] [Google Scholar]
- Reischauer S, Arnaout R, Ramadass R, Stainier DY. Actin binding GFP allows 4D in vivo imaging of myofilament dynamics in the zebrafish heart and the identification of Erbb2 signaling as a remodeling factor of myofibril architecture. Circ Res. 2014;115:845–56. doi: 10.1161/CIRCRESAHA.115.304356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JJ, de Tombe PP. Approaches to modeling crossbridges and calcium-dependent activation in cardiac muscle. Prog Biophys Mol Biol. 2004;85:179–95. doi: 10.1016/j.pbiomolbio.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Ryba DM, Li J, Cowan CL, Russell B, Wolska BM, Solaro RJ. Long-Term Biased beta-Arrestin Signaling Improves Cardiac Structure and Function in Dilated Cardiomyopathy. Circulation. 2017;135:1056–1070. doi: 10.1161/CIRCULATIONAHA.116.024482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid LM, Mosqueira M, Hein S, Kossack M, Juergensen L, Mueller M, Meder B, Fink RH, Katus HA, Hassel D. Essential light chain S195 phosphorylation is required for cardiac adaptation under physical stress. Cardiovasc Res. 2016;111:44–55. doi: 10.1093/cvr/cvw066. [DOI] [PubMed] [Google Scholar]
- Schulz EM, Wilder T, Chowdhury SA, Sheikh HN, Wolska BM, Solaro RJ, Wieczorek DF. Decreasing tropomyosin phosphorylation rescues tropomyosin-induced familial hypertrophic cardiomyopathy. J Biol Chem. 2013;288:28925–35. doi: 10.1074/jbc.M113.466466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet. 2002;31:106–10. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Sequeira V, Wijnker PJ, Nijenkamp LL, Kuster DW, Najafi A, Witjas-Paalberends ER, Regan JA, Boontje N, Ten Cate FJ, Germans T, Carrier L, Sadayappan S, van Slegtenhorst MA, Zaremba R, Foster DB, Murphy AM, Poggesi C, Dos Remedios C, Stienen GJ, Ho CY, Michels M, van der Velden J. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ Res. 2013;112:1491–505. doi: 10.1161/CIRCRESAHA.111.300436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YH, Zhang Y, Ding Y, Ross CA, Li H, Olson TM, Xu X. Cardiac transcriptome and dilated cardiomyopathy genes in zebrafish. Circ Cardiovasc Genet. 2015;8:261–9. doi: 10.1161/CIRCGENETICS.114.000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleman C, Holtzman NG. Analysis of postembryonic heart development and maturation in the zebrafish, Danio rerio. Dev Dyn. 2012;241:1993–2004. doi: 10.1002/dvdy.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Dyer E, Stuckey D, Leung MC, Memo M, Mansfield C, Ferenczi M, Liu K, Redwood C, Nowak K, Harding S, Clarke K, Wells D, Marston S. Investigation of a transgenic mouse model of familial dilated cardiomyopathy. J Mol Cell Cardiol. 2010;49:380–9. doi: 10.1016/j.yjmcc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Song W, Vikhorev PG, Kashyap MN, Rowlands C, Ferenczi MA, Woledge RC, MacLeod K, Marston S, Curtin NA. Mechanical and energetic properties of papillary muscle from ACTC E99K transgenic mouse models of hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2013;304:H1513–24. doi: 10.1152/ajpheart.00951.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Hoage T, Bai P, Ding Y, Chen Z, Zhang R, Huang W, Jahangir A, Paw B, Li YG, Xu X. Cardiac hypertrophy involves both myocyte hypertrophy and hyperplasia in anemic zebrafish. PLoS One. 2009;4:e6596. doi: 10.1371/journal.pone.0006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Fang Y, Xu X, Lu G, Chen Z. Evidence of an Association between Age-Related Functional Modifications and Pathophysiological Changes in Zebrafish Heart. Gerontology. 2015;61:435–47. doi: 10.1159/000369094. [DOI] [PubMed] [Google Scholar]
- Talavera J, Giraldo A, Fernandez-Del-Palacio MJ, Garcia-Nicolas O, Seva J, Brooks G, Moraleda JM. An Upgrade on the Rabbit Model of Anthracycline-Induced Cardiomyopathy: Shorter Protocol, Reduced Mortality, and Higher Incidence of Overt Dilated Cardiomyopathy. Biomed Res Int. 2015;2015:465342. doi: 10.1155/2015/465342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter MS, Ryba DM, Li BH, Wolska BM, Solaro RJ. Omecamtiv Mecarbil, a Cardiac Myosin Activator, Increases Ca2+ Sensitivity in Myofilaments With a Dilated Cardiomyopathy Mutant Tropomyosin E54K. J Cardiovasc Pharmacol. 2015;66:347–53. doi: 10.1097/FJC.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikhorev PG, Smoktunowicz N, Munster AB, Copeland O, Kostin S, Montgiraud C, Messer AE, Toliat MR, Li A, Dos Remedios CG, Lal S, Blair CA, Campbell KS, Guglin M, Knoll R, Marston SB. Abnormal contractility in human heart myofibrils from patients with dilated cardiomyopathy due to mutations in TTN and contractile protein genes. Sci Rep. 2017;7:14829. doi: 10.1038/s41598-017-13675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikhorev PG, Song W, Wilkinson R, Copeland O, Messer AE, Ferenczi MA, Marston SB. The dilated cardiomyopathy-causing mutation ACTC E361G in cardiac muscle myofibrils specifically abolishes modulation of Ca(2+) regulation by phosphorylation of troponin I. Biophys J. 2014;107:2369–80. doi: 10.1016/j.bpj.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cao J, Dickson AL, Poss KD. Epicardial regeneration is guided by cardiac outflow tract and Hedgehog signalling. Nature. 2015;522:226–230. doi: 10.1038/nature14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Panakova D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin YF, Sabeh MK, Werdich AA, Yelon D, Macrae CA, Poss KD. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development. 2011;138:3421–30. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LW, Huttner IG, Santiago CF, Kesteven SH, Yu ZY, Feneley MP, Fatkin D. Standardized echocardiographic assessment of cardiac function in normal adult zebrafish and heart disease models. Dis Model Mech. 2017;10:63–76. doi: 10.1242/dmm.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pinto JR, Solis RS, Dweck D, Liang J, Diaz-Perez Z, Ge Y, Walker JW, Potter JD. Generation and functional characterization of knock-in mice harboring the cardiac troponin I-R21C mutation associated with hypertrophic cardiomyopathy. J Biol Chem. 2012;287:2156–67. doi: 10.1074/jbc.M111.294306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CM, Karam CN, Wolska BM, Kobayashi T, de Tombe PP, Arteaga GM, Bos JM, Ackerman MJ, Solaro RJ. Green Tea Catechin Normalizes the Enhanced Ca2+ Sensitivity of Myofilaments Regulated by a Hypertrophic Cardiomyopathy-Associated Mutation in Human Cardiac Troponin I (K206I) Circ Cardiovasc Genet. 2015;8:765–73. doi: 10.1161/CIRCGENETICS.115.001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder T, Ryba DM, Wieczorek DF, Wolska BM, Solaro RJ. N-acetylcysteine reverses diastolic dysfunction and hypertrophy in familial hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;309:H1720–30. doi: 10.1152/ajpheart.00339.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R, Song W, Smoktunowicz N, Marston S. A dilated cardiomyopathy mutation blunts adrenergic response and induces contractile dysfunction under chronic angiotensin II stress. Am J Physiol Heart Circ Physiol. 2015;309:H1936–46. doi: 10.1152/ajpheart.00327.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Labeit S, Lewinter MM, Granzier H. Titin: an endosarcomeric protein that modulates myocardial stiffness in DCM. J Card Fail. 2002;8:S276–86. doi: 10.1054/jcaf.2002.129278. [DOI] [PubMed] [Google Scholar]
- Xu X, Meiler SE, Zhong TP, Mohideen M, Crossley DA, Burggren WW, Fishman MC. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat Genet. 2002;30:205–9. doi: 10.1038/ng816. [DOI] [PubMed] [Google Scholar]
- Yang J, Shah S, Olson TM, Xu X. Modeling GATAD1-Associated Dilated Cardiomyopathy in Adult Zebrafish. J Cardiovasc Dev Dis. 2016:3. doi: 10.3390/jcdd3010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Li R, Parks E, Takabe W, Hsiai TK. Electrocardiogram signals to assess zebrafish heart regeneration: implication of long QT intervals. Ann Biomed Eng. 2010;38:2346–57. doi: 10.1007/s10439-010-9993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CC, Muthu P, Kazmierczak K, Liang J, Huang W, Irving TC, Kanashiro-Takeuchi RM, Hare JM, Szczesna-Cordary D. Constitutive phosphorylation of cardiac myosin regulatory light chain prevents development of hypertrophic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2015;112:E4138–46. doi: 10.1073/pnas.1505819112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto F, Lu QW, Morimoto S, Tanaka H, Kono N, Nagata K, Ojima T, Takahashi-Yanaga F, Miwa Y, Sasaguri T, Nishita K, Tanokura M, Ohtsuki I. Drastic Ca2+ sensitization of myofilament associated with a small structural change in troponin I in inherited restrictive cardiomyopathy. Biochem Biophys Res Commun. 2005;338:1519–26. doi: 10.1016/j.bbrc.2005.10.116. [DOI] [PubMed] [Google Scholar]
- Zhang R, Yang J, Zhu J, Xu X. Depletion of zebrafish Tcap leads to muscular dystrophy via disrupting sarcomere-membrane interaction, not sarcomere assembly. Hum Mol Genet. 2009;18:4130–40. doi: 10.1093/hmg/ddp362. [DOI] [PMC free article] [PubMed] [Google Scholar]