Abstract
Actin cytoskeleton dynamics depend on a tight regulation of actin filament formation from an intracellular pool of monomers, followed by their linkage to each other or to cell membranes, followed by their depolymerization into a fresh pool of actin monomers. The ubiquitous requirement for continuous actin remodeling that is necessary for many cellular functions is orchestrated in large part by actin binding proteins whose affinity for actin is altered by inositol phospholipids, most prominently PI(4,5)P2 (phosphatidylinositol 4,5bisphosphate). The kinetics of PI(4,5)P2 synthesis and hydrolysis, its lateral distribution within the lipid bilayer, and coincident detection of PI(4,5)P2 and another signal, all play a role in determining when and where a particular PI(4,5)P2-regulated proteins is inactivated or activated to exert its effect on the actin cytoskeleton. This review summarizes a range of models that have been developed to explain how PI(4,5)P2 might function in the complex chemical and structural environment of the cell based on a combination of experiment and computational studies.
Keywords: PI(4,5)P2; actin binding proteins; actin assembly
Background and introduction.
It has been 35 years since the landmark report by Lassing and Lindberg that phosphatidylinositol 4,5 bisphosphate [PI(4,5)P2], but not other lipids nor IP3 the isolated headgroup of PI(4,5)P2, was able to remove actin monomers bound to profilin and promote their assembly to actin filaments (F-actin) [1, 2]. Since then, hundreds of different proteins, many of them actin regulators, have been shown to bind PI(4,5)P2 [3–5] with similar affinity and specificity as profilin, placing the primacy of profilin-actin binding in doubt as the major PI(4,5)P2-mediated control of actin assembly (Table 1). Even the mechanism by which profilin exerts its control over actin is no longer generally considered to be the same as studied by Lassing and Lindberg. When first discovered, profilin was reported to be a monomer-sequestering agent that kept actin assembly suppressed, so release of it by PI(4,5)P2 would lead to rapid actin assembly. Currently profilin is more often considered to be a shuttle that carries actin to the barbed end of growing filaments, where, release by PI(4,5)P2 might play an ancillary role, but is not required for the shuttling activity. Nonetheless, the cellular effect of manipulating PI(4,5)P2 levels in cells is relatively clear: increasing its production leads to a large increase in cellular Factin [6, 7] and decreasing PI(4,5)P2 levels or sequestering it by overexpression of PI(4,5)P2 scavengers leads to decreased actin assembly and detachment of the membrane from the interior cytoskeleton [8].
Table 1.
Actin binding proteins regulated by PI(4,5)P2.
| Protein | PI(4,5)P2 binding domain | PI(4,5)P2 effect | References |
|---|---|---|---|
| Alpha-actinin | TAPYRNVNIQNFHLSWK[73] | stimulates gelation of F-actin and decreases its proteolysis | [42, 73–75] |
| Gelsolin | two or more distinct 10–20 amino acid sequences including QRLFQKRGG | inhibits severing and capping of actin filaments to prevent disassembly | [27–29, 48, 76, 77] |
| ezrin | within N-terminal (1–309) part, amino acids 12–115 and 233–310. | targeting to membrane and activation of actin binding | [78–82] |
| Profilin | Short sequence centered on Arg88 | release profilin from G-actin would lead to rapid actin assembly | [1, 2, 83] |
| Cofilin | WAPECAPLKSKM | inhibits severing activity | [21]. |
| Formins | basic domain of mDia | promotes actin filament nucleation and elongation | [31, 32, 84] |
| Talin | cationic site in FERM domain | localization to the plasma membrane followed by its activation. | [43, 85] |
| Vinculin | multiple polycationic sites that can bind different acidic lipids | links actin to transmembrane adhesion complexes. | [42] |
| Filamin | FTRWCNEHLKCVSKRIANLQTDL | inhibits actin gelation activity | [86, 87] |
| WASP | polybasic domain | releases N-WASP from an auto-inhibited state to promote actin assembly by activating the nucleating Arp2/3 complex; | [30] |
PI(4,5)P2 constitutes only on the order of 1% of the total lipid in the plasma membrane, but it is an important regulator of cytoskeletal and membrane dynamics [9–16]. Relevance of PI(4,5)P2 to cytoskeletal assembly was first suggested by biochemical studies of its interaction with actin binding proteins [17]. Subsequently, manipulation of enzymes involved in PI(4,5)P2 production showed that increasing cellular PI(4,5)P2 levels massively increased actin assembly [6] and stress fiber formation [7], whereas increasing PI(4,5)P2 degradation destabilized actin assembly [18, 19].
Pathophysiological consequence of altered PIP2-cytoskeleton interactions.
Expression of the phospholipase C beta PLCB3 hypomorphic variant in subjects with syndromic autosomal recessive spondylometaphyseal dysplasia leads to elevated levels of PI(4,5)P2 in patient fibroblasts, causing disorganization of the F-actin cytoskeleton [18]. On the other hand, local decrease of host plasma membrane PI(4,5)P2 acompanied with dissociation of ezrin and breakdown of the host cell actin cytoskeleton represents an egress strategy of Plasmodium parasites infecting hepatocytes [20]. The enzymes PTPRN2 and PLCbeta1 decrease plasma membrane PI(4,5)P2 concentration in metastatic breast cancer cells, leading to release of the PI(4,5)P2-binding protein cofilin from its inactive membrane-associated state into the cytoplasm where it accelerates actin turnover dynamics, thereby enhancing cellular migration and metastatic capacity [21]. Polyunsaturated fatty acids (n-3 PUFA) decrease the amount of PI(4,5)P2, in CD4(+) T cells and deplete the plasma membrane non-raft PI(4,5)P2 pool, leading to suppressed actin remodeling upon activation of the cells [22].
In vitro evidence of control of actin assembly by PIP2.
Introduction of lipid vesicles containing PI(4,5)P2 or PI(3,4,5)P3 into a Xenopus egg extract is sufficient to cause actin assembly at the vesicle that drives its motility through the extract (Figure 1), whereas vesicles with PI had no effect [23]. Such studies showed a requirement for activators of the ARP2/3 complex including Cdc42 [23] and have identified many proteins involved in actin assembly that are affected by PI(4,5)P2. In vitro studies have not yet led to a clear understanding of how cellular PI(4,5)P2 distribution is controlled in the plasma membrane, or how the proteins it potentially regulates compete for this scarce phospholipid.
Figure 1.
Pyrene-actin fluorescence after adding vesicles containing PPIs to Xenopus egg extract, and labeled actin comet tail at base of PPI-containing vesicle. From [1].
Some cytoskeletal proteins bind PI(4,5)P2 by canonical PPI-binding motifs such as the FERM domain [24] but very few contain PH domains, the most prominent PI(4,5)P2-binding motif [25]. Most often, actin regulatory proteins bind PI(4,5)P2 using less structured motifs that contain clusters of basic and hydrophobic amino acids [26] and are likely to respond to changes in the local charge density and access to disordered acyl chains in the bilayers. Such proteins include the Wiskott Aldrich Syndrome protein (WASP) superfamily that promotes actin assembly by activating the nucleating Arp2/3 complex, gelsolin family proteins, which sever and cap actin filaments to promote dynamic actin reorganization, and formin family proteins that promote actin filament nucleation and elongation. Gelsolin lacks traditional PI(4,5)P2 binding motifs and binds both the charged inositol headgroup and the hydrophobic acyl chains of PI(4,5)P2 [27–29]. N-WASP can bind several PI(4,5)P2 molecules via its polybasic domain and responds to small changes in PI(4,5)P2 surface density [30]. Formins also appear to interact with PI(4,5)P2 and other acidic phospholipids [31, 32] and PI(3,5)P2 was found to be essential for targeting of formin to the cell cortex [33].
A challenge in understanding how PPIs function in vivo is the knowing the specificities and contexts in which the large number of phosphoinositide (usually PI(4,5)P2) binding proteins that have been well-characterized biochemically as high affinity ligands for these lipids operate to regulate signaling. Measurement of PI(4,5)P2 diffusion shows that most of the plasma membrane PI(4,5)P2 pool is bound or sequestered to some extent [34].
Specificity in PIP2-protein regulation.
Several different mechanisms have been proposed to explain how different PI(4,5)P2-binding proteins are differentiated at the membrane interface. Here we briefly summarize five of these ideas, based, respectively on coincident detection of PI(4,5)P2 plus another membrane ligand; recruitment to the membrane by association with a lipid kinase; domain formation in membranes containing PI(4,5)P2; kinetics of the protein-PI(4,5)P2 binding; and curvature of the membrane containing PI(4,5)P2. A final section summarizes recent results showing that many of the proteins that are regulated by PI(4,5)P2 are also regulated by forces, suggesting that domain exposure or occlusion that occurs after binding PI(4,5)P2 can also occur when biologically relevant forces are applied to the PI(4,5)P2-sensitive protein.
Coincident binding.
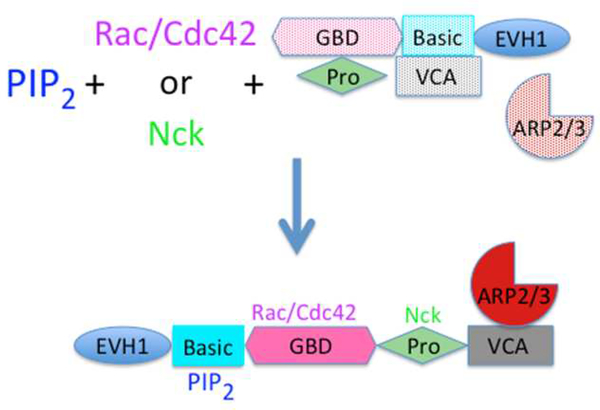
Among the best-studied examples of actin regulatory protein activation by multiple ligands is the release of Wiskott Aldrich syndrome protein (WASP) and its homolog N-WASP from an auto-inhibited state after binding PI(4,5)P2. Figure 2 shows that in its inactive state WASP is a cytosolic protein in which multiple domains interact with each other to prevent the binding of WASP to the ARP2/3 complex, which would activate its actin nucleation activity. Mutation of a PI(4,5)P2 binding site in N-WASP reduced its actin nucleating activity in cells [1], and addition of PI(4,5)P2 to an inactive complex of the N- and C- terminal domains of N-WASP dissociated them and restored the ARP2/3 activating function of the C- terminal VCA domain [35]. The combination of PI(4,5)P2 and the GTPase Cdc42 produced greater nucleating activity and many subsequent studies have shown that binding of Cdc42 to a domain of NWASP distinct from its PI(4,5)P2-binding site can itself partly enhance the nucleating activity of N-WASP/ARP2/3 but the combination of Cdc42 and PI(4,5)P2 is stronger than the effect of either ligand alone. More recent studies show that Nck and other Src-homology domain containing proteins can also act in concert with PI(4,5)P2 by binding to the proline-rich domain of N-WASP or WASP distinct from the site to which Cdc42 binds [35–38].
Figure 2.
Coincident activation of WASP/N-WASP by PI(4,5)P2 and either small GTPases or Nck. ARP2/3 activators related to Wiskott-Aldrich syndrome protein (WASP) are multiple domain proteins that in solution are auto-inhibited due to multiple inter-domain interactions that prevent activation of Arp2/3. The small GTPases Rac1 or Cdc42 activate the nucleation activity of Apr2/3 in the presence of specific WASP family members by binding to their GBD domains. Alternatively, some WASP variants can be activated by the multiple Src homology domains in the protein NCK binding the proline-rich domain. The extent of Arp2/3 activation and the resulting rate of actin polymerization are strongly augmented by the coincident binding of PI(4,5)P2 along with either of the protein activators.
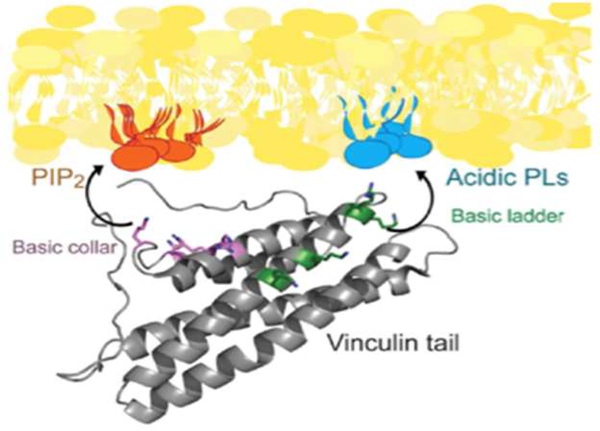
Coincidence detection can also involve PI(4,5)P2 and another lipid. Multiple PI(4,5)P2-regulated cytoskeletal proteins have more than one, usually polycationic, site that can bind multiple acidic lipids. Figure 3 shows, for example, the structure of the membrane-binding domain of vinculin, a protein that links actin to transmembrane adhesion complexes. A combination of numerous structure data and computational modeling revealed that residues in one folded domain of vinculin interact specifically with PI(4,5)P2, while residues in a different domain simultaneously bind other acidic lipids, PI(4,5)P2 binding appears to be required for vinculin activation and dimerization, but not necessarily for binding to focal adhesions once activated [39–42].
Figure 3.
Docking of vinculin to the plasma membrane through interactions with a lipid domain enriched in PI(4,5)P2 and a separate region with other acidic lipids, usually phosphatidylserine. From [3].
Recruitment to the membrane by association with a lipid kinase.
An interesting variant of coincident activation that is proposed is that PI(4,5)P2 participates in the localization of the actin-membrane linker talin to the plasma membrane followed by its activation. Talin, like N-WASP is a multi-domain protein that is held in an inactive cytosolic state by inhibitory interactions among its various domains. This auto-inhibition can be relieved by PI(4,5)P2 [43] to activate binding of talin to both the F-actin and the transmembrane adhesion protein integrin. Talin also binds phosphatidylinositol 4-phosphate 5-kinase gamma (PIPKIγ2) once that enzyme is phosphorylated by AKT [44]. PIPKIγ2 binds to the plasma membrane where it produces PI(4,5)P2 from its precursor PIP, and the newly synthesized PI(4,5)P2 then binds to adjacent talin (Figure 4).
Figure 4.
The lipid kinase PIPKIγi2, is activated by phosphorylation to bind talin in the cytosol and then it is recruited to the plasma membrane where it phosphorylates PI(4)P to PI(4,5)2. Talin is then activated by newly synthesized PI(4,5)P2 which relieves the intramolecular inhibition of talin, to enable both its actin binding and binding to the cytoplasmic tail of β-integrins. From [6].
Domain formation in membranes containing PI(4,5)P2.
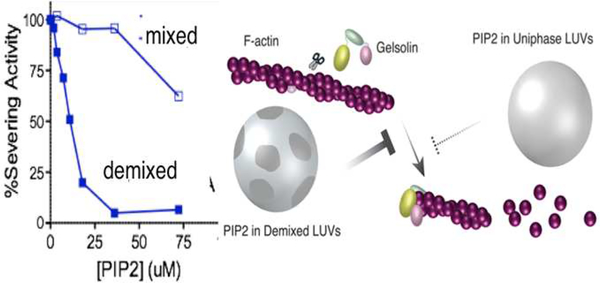
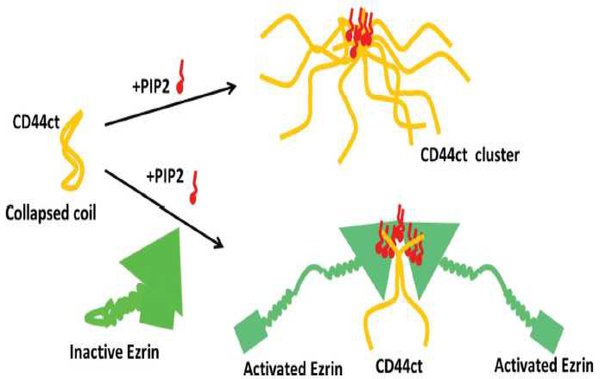
A major unresolved question is how PI(4,5)P2 distributes laterally within the plasma membrane and whether all PI(4,5)P2 molecules are equally effective at binding their targets. From the first studies of profilin and gelsolin, it was clear that these proteins bound more tightly to lipid micelles and bilayer vesicles that contained high mole fractions of PI(4,5)P2, suggesting that local clustering of PI(4,5)P2 that formed domains enriched in local charge density were important for the protein-lipid interaction [45]. The finding that IP3, the headgroup of PI(4,5)P2 neither bound tightly nor displaced PI(4,5)P2 from these proteins also suggested that unlike binding of pleckstrin homology domains or other PI(4,5)P2 binding motifs, the interaction of several cytoskeletal proteins involved more than just the headgroup of a single PI(4,5)P2. Three different mechanisms have been shown to lead to regulate the local concentration of PI(4,5)P2 on one leaflet of the bilayer. These include sequestration of PI(4,5)P2 in the liquid disordered phase after cholesterol-dependent lipid demixing, formation of nanoscale domains highly enriched in PI(4,5)P2 driven by Ca2+[46, 47], and aggregation due to binding of peripheral membrane proteins with large polycationic domains capable of binding multiple acidic lipids. Local demixing of PI(4,5)P2 can lead to selective activation of some proteins even without a net increase in PI(4,5)P2 levels, as shown by the effects of domain formation on the inhibition of gelsolin by PI(4,5)P2. When PI(4,5)P2 is distributed evenly in mixed lipid vesicles at mole fractions below 10% the vesicles have very weak ability to inactivate the F-actin severing activity of gelsolin. However, when the lipids demix into liquid ordered and disordered domains, the inhibitory activity of PI(4,5)P2 can increase by an order of magnitude (Figure 5). The demixing can occur either by small changes in the lipid composition of the bilayer, for example by increasing cholesterol, or by changing temperature to bring vesicles with identical lipid compositions across the demixing phase transition [48]. A second example of PI(4,5)P2 clustering leading to enhanced activity of PI(4,5)P2 involve the local concentration of PI(4,5)P2 by the cytoplasmic tail of the transmembrane protein receptor for hyaluronic acid CD44 (Figure 6). A combination of spectroscopic and biochemical studies shows that upon binding PI(4,5)P2, the cytoplasmic tail of CD44 aggregates and brings together multiple PI(4,5)P2s. As a result, the cytoskeletal protein ezrin, which is activated by PI(4,5)P2, binds to the CD44/PI(4,5)P2 aggregates and thereby links the actin cytoskeleton to HAenriched extracellular matrices through CD44 receptors. An interesting conclusion of this study is that ezrin and other ezrin related PI(4,5)P2-binding proteins bearing a FERM domain need not bind CD44 directly to link actin the transmembrane protein. Instead the cluster of PI(4,5)P2 acts as the bridge between these two proteins [49].
Figure 5.
Gelsolin’s severing activity is inhibited better when PI(4,5)P2 partitions into liquid disordered phase.
Figure 6.
A schematic diagram showing how binding of CD44 to PI(4,5)P2 leads to cluster formation and subsequent recruitment and activation of ezrin at the membrane. From [2].
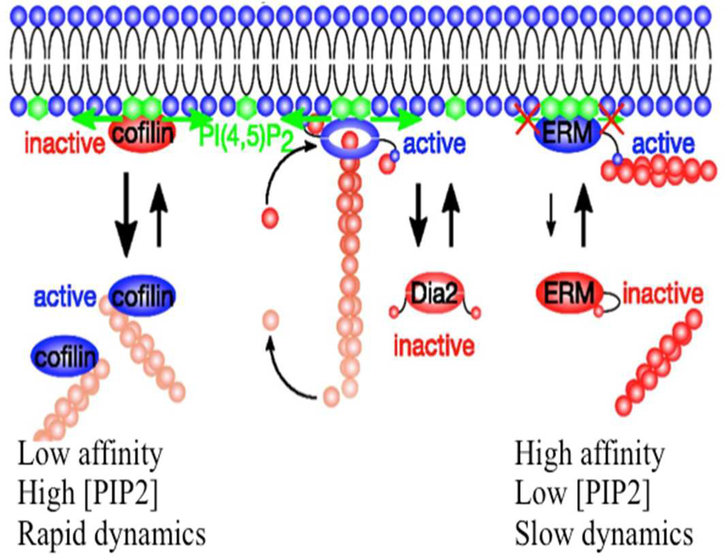
Kinetics of the protein-PI(4,5)P2 binding.
A recent analysis of on and off rates of different PI(4,5)P2 regulated proteins showed large differences in both the kinetics and affinity of proteins for PIP in membranes as well as different effects of the proteins on PI(4,5)P2 lateral mobility in the membrane [50]. The actin filament severing protein cofilin, for example, which is inhibited by a PI(4,5)P2 binds rapidly and with relatively low affinity to a small number of PI(4,5)P2 molecules and does not restrict the lateral diffusion of the phospholipids in the plasma membrane bilayer (Figure 7). In contrast, ERM proteins, which are activated by PI(4,5)P2 to link the actin network to the plasma membrane might bind fewer lipids but prevent their lateral diffusion, consistent with the mechanisms shown in Figures 3 and 4. The formin-class actin nucleator mDia appears to be intermediate, with fast on rate and moderately higher affinity but with sufficient dynamics to allow PI(4,5)P2 diffusion and release of formin from the membrane. Such kinetics differences suggest that the type of PI(4,5)P2 binding protein recruited to the membrane is influenced by the lateral diffusion of the lipid in the membrane, and that each protein might create an environment that is either conducive or inhibitory to binding of other proteins, depending on their binding kinetics and requirements to bind multiple PI(4,5)P2s or other acidic lipids.
Figure 7.
A range of kinetic and equilibrium constant characterize the binding of different cytoskeletal proteins to PIP2, resulting in different dependencies on lipid clustering and different effects of lipid lateral mobility. From [5].
Curvature of the membrane containing PI(4,5)P2.
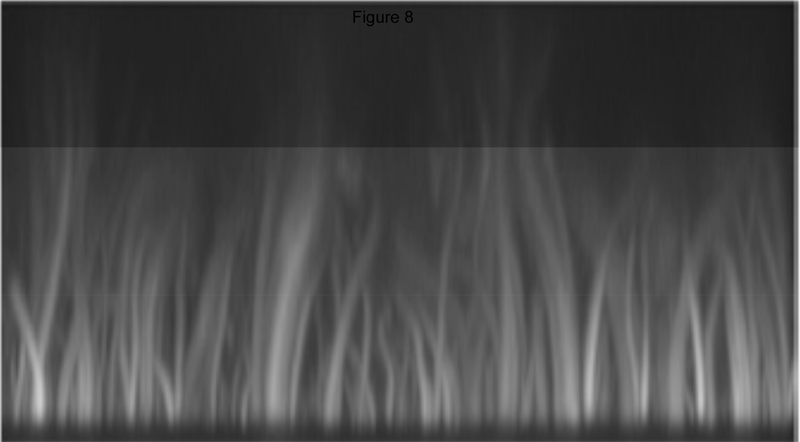
An intriguing idea based on a series of reports is that the type of actin nucleators recruited to PIP-enriched areas of the membrane depends on membrane curvatures. Flat membranes, such as glass-supported lipid bilayers containing PI(4,5)P2 recruit primarily formins and VASP to initiate formation of straight and bundles actin filaments similar to those in filopodia as shown in Figure 8 [51]. In contrast, chemically similar vesicles or supported bilayers attached to glass beads with micron or submicron diameters recruit primarily N-WASP, Cdc42, and other factors involved in making branched actin networks [51–53] as seen in the example in Figure 1. An interesting feature in the nucleation from curved surfaces in Xenopus extracts is that if PI(4,5)P2 is the only inositol lipid in the membrane, then activation of Cdc42 by transducer of Cdc42 activation-1 (toca-1), for example, is essential for N-WASp/Arp2.3-mediated nucleation, but if PI(3,4,5)P3 is also present in the membrane, then Cdc42 activation is no longer required [52]. Effects of PI(3,4,5)P3 on sorting nexin 9 can also promote its stimulation of actin assembly by activation of N-Wasp/Arp2.3 [52–54]. The requirement for high curvature suggests that the conformation of PI(4,5)P2 or another phosphoinositide, which generally have larger headgroups than other bilayer lipids [55, 56], might be more accessible to the proteins they regulate, as seen in the large effect of submicron membrane curvature on the enzymatic acidity of PI3-kinase [57]. Alternatively high curvature might alter the ionization state of PI(4,5)P2, as predicted from the effects of lateral packing density on the net charge of PI(4,5)P2 and other inositol lipids [58].
Figure 8.
Predominantly long straight F-actin nucleated by formins in a Xenopus extract at a flat membrane containing PIP2. From [4].
Computational Investigations of Membrane Recognition of Actin Binding and Associating Proteins
As discussed above, many globular domains of peripheral proteins bind to the specific lipid headgroups presented on cellular membranes, and this binding is often tightly regulated. Examples include pleckstrin homology (PH) and C2 domains, which are among the largest domain families. Structural structures, binding studies and analyses of subcellular localization have provided insight the features they recognize and the molecular mechanisms they utilize for binding. Structural insights are available for C1, C2, PH, PX, and FYVE domains, which specifically recognize single tightly regulated membrane components such as diacylglycerol or phosphoinositides [59, 60]. A more complete description of the molecular interactions between the protein and the membrane in this context is emerging from studies reporting molecular dynamics simulations. Computational approaches to investigate the molecular interactions and dynamics of a lipid recognition modules of the PH domain on biological membranes have recently been reported. Using multiscale approaches using combinations of atomistic and coarse-grained models yielded results comparable to those of actual experiments; the studies revealed specific modes of interactions of PH domains with membranes, and the observation of PIP clusters around the proteins [61]. Principles underlying regulation of the actin cytoskeleton by phosphoinositides were addressed in a recent report using a combination of biochemical assays, biophysical approaches, and molecular dynamics simulations to uncover the molecular principles by which actin binding proteins interact with phosphoinositide-rich membranes. The study concluded that despite using different domains for lipid binding, these proteins associate with membranes through similar multivalent electrostatic interactions, without specific binding pockets or penetration into the lipid bilayer. But strikingly, the results suggested that these proteins display enormous differences in the dynamics of membrane interactions and in the ranges of phosphoinositide densities that they sense, which implies that control of lipid composition and heterogeneity through signaling and biophysical mechanisms will have a big impact on actin assembly. The study concluded that profilin and cofilin display transient, low-affinity interactions with phosphoinositide-rich membranes, whereas F-actin assembly factors mDia2 and N-WASP reside on phosphoinositide-rich membranes for longer time periods and complement the experimental view on which Figure 7 is based [50].
The role of curvature originating from lipid composition and heterogeneity was recently addressed through simulations of a complex asymmetric plasma membrane model, which contained seven different lipids species including the glycolipid GM3 in the outer leaflet and PIP2 in the inner leaflet. In these simulations the most striking feature was the formation of nano-clusters of GM3 within the outer leaflet. Moreover, the dynamics revealed correlations between curvature of the bilayer surface and clustering of lipid molecules [62]. Protein-induced curvature on membranes have also been investigated in several computational studies as reviewed in [63]. In curvature remodeling proteins (CRPs), while electrostatic interactions coupled with perturbation of bilayer structure has been shown to induce curvature, other mechanisms such as molecular crowding [2] as well as manipulation of membrane undulations can also lead to stable induction of membrane curvature [64]. Multiscale methodologies to model CRPs at the molecular as well as at the mesoscopic and cellular scales including a free energy perspective of membrane remodeling through the organization and assembly of CRPs is described in [2]. Since several CRPs can simultaneously function as localizers of actin binding proteins and sensors of membrane curvature and tension [65], collectively these theoretical studies compliment the experimental literature to rationalize the effects of mechanics and curvature on protein-membrane recognition.
PIP2 and mechanical stress.
Several cytoskeletal regulators shown to be activated by the altered tension that arises when cells are grown on stiff substrates, or that respond to cortical tension, are also activated by PPIs in the absence of force. These proteins include talin [66], alpha-actinin [42], and vinculin [42, 67] which localize to focal adhesions and ezrin, a protein immediately downstream of CD44 [68]. A variety of cellular functions such as motility, cell division, and endocytosis are controlled by plasma membrane tension, which is dominated by the attachment of the actin cortex to the inner leaflet of the plasma membrane. In MDCK II cells a substantial stiffening of cells and an increase in membrane tension was observed upon microinjection of PI(4,5)P2 micelles, but weakening of linkage between plasma membrane and actin-cortex was observed by ezrin siRNA and administration of neomycin [69]. The actin nucleators N-WASP [70] and formins [32, 71] also respond to mechanical stress that leads to increased actin assembly.
All of these proteins are autoinhibited by interactions between distinctly folded domains in their tertiary or quaternary structure. A reasonable hypothesis is that autoinhibition can be relieved either by competitive binding or allosteric effects caused by PI(4,5)P2 that release the inhibitory interactions, or by forces applied to distal ends of the proteins that would also destabilize the inhibitory interactions. The distinct effects of force and PI(4,5)P2 could act in combination to promote PI(4,5)P2 binding at sites of mechanical stress, or alternatively reduce the stress required for activation if PI(4,5)P2 is also bound. The ability of PI(4,5)P2 or other inositol lipids to mimic or reproduce the actin-polymerization and focal adhesion growth at sites of mechanical stress is consistent with the finding that disruption of PI(3,4,5)P3 synthesis leads to rapid loss of actin bundles and focal adhesions in cells grown on soft gels containing both the CD44 ligand hyaluronan, and an integrin ligand, but has little effect on cells bound to rigid substrates where mechanical stresses alone might be sufficient to maintain actin and focal adhesion assembly [72]. Whether this partnership of mechanical stress and phosphoinositide signaling is a significant biological regulator of the actin cytoskeleton remains to be seen.
Highlights:
PI(4,5)P2 synthesis and its lateral distribution within the lipid bilayer play a role in determining when and where a particular PI(4,5)P2-regulated protein is inactivated or activated to exert its effect on the actin cytoskeleton
Acknowledgments
Funding
This work is supported by Grants: GM083272 (to P.A.J) from NIH, R01 GM111942 from the General Medicine Institute of the NIH (to P.A.J.) and National Science Center, Poland, UMO-2015/17/B/NZ6/03473 (to RB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- [1].Higgs HN, Pollard TD, Activation by Cdc42 and PIP(2) of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex, The Journal of cell biology, 150 (2000) 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lassing I, Lindberg U, Specificity of the interaction between phosphatidylinositol 4,5-bisphosphate and the profilin:actin complex, Journal of cellular biochemistry, 37 (1988) 255–267. [DOI] [PubMed] [Google Scholar]
- [3].Catimel B, Schieber C, Condron M, Patsiouras H, Connolly L, Catimel J, Nice EC, Burgess AW, Holmes AB, The PI(3,5)P2 and PI(4,5)P2 Interactomes, J Proteome Res, (2008). [DOI] [PubMed] [Google Scholar]
- [4].Tsujita K, Itoh T, Phosphoinositides in the regulation of actin cortex and cell migration, Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids, 1851 (2015) 824–831. [DOI] [PubMed] [Google Scholar]
- [5].Tsujita K, Itoh T, Kondo A, Oyama M, Kozuka-Hata H, Irino Y, Hasegawa J, Takenawa T, Proteome of acidic phospholipid-binding proteins: spatial and temporal regulation of Coronin 1A by phosphoinositides, The Journal of biological chemistry, 285 (2010) 6781–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shibasaki Y, Ishihara H, Kizuki N, Asano T, Oka Y, Yazaki Y, Massive Actin Polymerization Induced by Phosphatidylinositol-4-phosphate 5-Kinase in Vivo, J. Biol. Chem, 272 (1997) 7578–7581. [DOI] [PubMed] [Google Scholar]
- [7].Yamamoto M, Hilgemann DH, Feng S, Bito H, Ishihara H, Shibasaki Y, Yin HL, Phosphatidylinositol 4,5-bisphosphate induces actin stress-fiber formation and inhibits membrane ruffling in CV1 cells, The Journal of cell biology, 152 (2001) 867876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bittner MA, Holz RW, Phosphatidylinositol-4,5-bisphosphate: actin dynamics and the regulation of ATP-dependent and -independent secretion, Mol Pharmacol, 67 (2005) 1089–1098. [DOI] [PubMed] [Google Scholar]
- [9].Yin HL, Janmey PA, Phosphoinositide regulation of the actin cytoskeleton, Annu Rev Physiol, 65 (2003) 761–789. [DOI] [PubMed] [Google Scholar]
- [10].Mao YS, Yin HL, Regulation of the actin cytoskeleton by phosphatidylinositol 4-phosphate 5 kinases, Pflugers. Arch, 455 (2007) 5–18. [DOI] [PubMed] [Google Scholar]
- [11].van den Bout I, Divecha N, PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions, Journal of cell science, 122 (2009) 3837–3850. [DOI] [PubMed] [Google Scholar]
- [12].Kwiatkowska K, One lipid, multiple functions: how various pools of PI(4,5)P(2) are created in the plasma membrane, Cell Mol Life Sci, 67 (2010) 3927–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Funakoshi Y, Hasegawa H, Kanaho Y, Regulation of PIP5K activity by Arf6 and its physiological significance, J Cell Physiol, (2010). [DOI] [PubMed] [Google Scholar]
- [14].Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y, SchneidmanDuhovny D, Wolfson HJ, Backer JM, Williams RL, Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit, Science, 317 (2007) 239–242. [DOI] [PubMed] [Google Scholar]
- [15].Bunney TD, Katan M, Phosphoinositide signalling in cancer: beyond PI3K and PTEN, Nat Rev Cancer, 10 (2010) 342–352. [DOI] [PubMed] [Google Scholar]
- [16].Sun Y, Turbin DA, Ling K, Thapa N, Leung S, Huntsman DG, Anderson RA, Type I gamma phosphatidylinositol phosphate kinase modulates invasion and proliferation and its expression correlates with poor prognosis in breast cancer, Breast Cancer Res, 12 (2010) R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Khuong TM, Habets RL, Slabbaert JR, Verstreken P, WASP is activated by phosphatidylinositol-4,5-bisphosphate to restrict synapse growth in a pathway parallel to bone morphogenetic protein signaling, Proceedings of the National Academy of Sciences of the United States of America, 107 (2010) 17379–17384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sakisaka T, Itoh T, Miura K, Takenawa T, Phosphatidylinositol 4,5bisphosphate phosphatase regulates the rearrangement of actin filaments, Mol Cell Biol, 17 (1997) 3841–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ben-Salem S, Robbins SM, Lm Sobreira N, Lyon A, Al-Shamsi AM, Islam BK, Akawi NA, John A, Thachillath P, Al Hamed S, Valle D, Ali BR, Al-Gazali L, Defect in phosphoinositide signalling through a homozygous variant in PLCB3 causes a new form of spondylometaphyseal dysplasia with corneal dystrophy, J Med Genet, 55 (2018) 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Burda PC, Caldelari R, Heussler VT, Manipulation of the Host Cell Membrane during Plasmodium Liver Stage Egress, MBio, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sengelaub CA, Navrazhina K, Ross JB, Halberg N, Tavazoie SF, PTPRN2 and PLCbeta1 promote metastatic breast cancer cell migration through PI(4,5)P2dependent actin remodeling, The EMBO journal, 35 (2016) 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hou TY, Barhoumi R, Fan YY, Rivera GM, Hannoush RN, McMurray DN, Chapkin RS, n-3 polyunsaturated fatty acids suppress CD4(+) T cell proliferation by altering phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] organization, Biochimica et biophysica acta, 1858 (2016) 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ma L, Cantley LC, Janmey PA, Kirschner MW, Corequirement of specific phosphoinositides and small GTP-binding protein Cdc42 in inducing actin assembly in Xenopus egg extracts, The Journal of cell biology, 140 (1998) 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Elliott PR, Goult BT, Kopp PM, Bate N, Grossmann JG, Roberts GC, Critchley DR, Barsukov IL, The Structure of the talin head reveals a novel extended conformation of the FERM domain, Structure, 18 (2010) 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kapus A, Janmey P, Plasma Membrane-Cortical Cytoskeleton Interactions: A Cell Biology Approach with Biophysical Considerations, Compr Physiol, 3 (2013) 1231–1281. [DOI] [PubMed] [Google Scholar]
- [26].Janmey PA, Bucki R, Yin HL, Phosphoinositides and actin cytoskeletal rearrangement, in: Bradshaw T, Dennis E (Eds.) The Handbook of Cell signaling, elsevier; 2009. [Google Scholar]
- [27].Janmey PA, Stossel TP, Modulation of Gelsolin Function by Phosphatidylinositol 4,5-Bisphosphate, Nature, 325 (1987) 362–364. [DOI] [PubMed] [Google Scholar]
- [28].Lassing I, Lindberg U, Specific interaction between phosphatidylinositol 4,5bisphosphate and profilactin, Nature, 314 (1985) 472–474. [DOI] [PubMed] [Google Scholar]
- [29].Gorbatyuk VY, Nosworthy NJ, Robson SA, Bains NPS, Maciejewski MW, dos Remedios CG, King GF, Mapping the Phosphoinositide-Binding Site on Chick Cofilin Explains How PIP2 Regulates the Cofilin-Actin Interaction, Molecular cell, 24 (2006) 511–522. [DOI] [PubMed] [Google Scholar]
- [30].Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ, Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway, The Journal of biological chemistry, 276 (2001) 26448–26452. [DOI] [PubMed] [Google Scholar]
- [31].Gorelik R, Yang C, Kameswaran V, Dominguez R, Svitkina T, Mechanisms of plasma membrane targeting of formin mDia2 through its amino terminal domains, Molecular biology of the cell, 22 (2011) 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ramalingam N, Zhao H, Breitsprecher D, Lappalainen P, Faix J, Schleicher M, Phospholipids regulate localization and activity of mDia1 formin, European journal of cell biology, 89 (2010) 723–732. [DOI] [PubMed] [Google Scholar]
- [33].van Gisbergen PA, Li M, Wu SZ, Bezanilla M, Class II formin targeting to the cell cortex by binding PI(3,5)P(2) is essential for polarized growth, The Journal of cell biology, 198 (2012) 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Golebiewska U, Nyako M, Woturski W, Zaitseva I, McLaughlin S, Diffusion coefficient of fluorescent phosphatidylinositol 4,5-bisphosphate in the plasma membrane of cells, Molecular biology of the cell, 19 (2008) 1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rohatgi R, Ho HY, Kirschner MW, Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate, The Journal of cell biology, 150 (2000) 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL, Phosphatidylinositol 4,5bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3, Current biology : CB, 10 (2000) 311–320. [DOI] [PubMed] [Google Scholar]
- [37].Benesch S, Lommel S, Steffen A, Stradal TE, Scaplehorn N, Way M, Wehland J, Rottner K, Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2, The Journal of biological chemistry, 277 (2002) 37771–37776. [DOI] [PubMed] [Google Scholar]
- [38].Miki H, Miura K, Takenawa T, N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases, The EMBO journal, 15 (1996) 5326–5335. [PMC free article] [PubMed] [Google Scholar]
- [39].Dwivedi M, Winter R, Binding of Vinculin to Lipid Membranes in Its Inhibited and Activated States, Biophysical journal, 111 (2016) 1444–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chinthalapudi K, Patil DN, Rangarajan ES, Rader C, Izard T, Lipid-directed vinculin dimerization, Biochemistry, 54 (2015) 2758–2768. [DOI] [PubMed] [Google Scholar]
- [41].Chinthalapudi K, Rangarajan ES, Patil DN, George EM, Brown DT, Izard T, Lipid binding promotes oligomerization and focal adhesion activity of vinculin, The Journal of cell biology, 207 (2014) 643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fukami K, Endo T, Imamura M, Takenawa T, alpha-Actinin and vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase, The Journal of biological chemistry, 269 (1994) 1518–1522. [PubMed] [Google Scholar]
- [43].Ye X, McLean MA, Sligar SG, Conformational equilibrium of talin is regulated by anionic lipids, Biochimica et biophysica acta, 1858 (2016) 1833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Le OTT, Cho OY, Tran H, Kim JA, Chang S, Jou I, Lee SY, Phosphorylation of phosphatidylinositol 4-phosphate 5-kinase gamma by Akt regulates its interaction with talin and focal adhesion dynamics, Biochimica Et Biophysica Acta-Molecular Cell Research, 1853 (2015) 2432–2443. [DOI] [PubMed] [Google Scholar]
- [45].Hubner S, Couvillon AD, Kas JA, Bankaitis VA, Vegners R, Carpenter CL, Janmey PA, Enhancement of phosphoinositide 3-kinase (PI 3-kinase) activity by membrane curvature and inositol-phospholipid-binding peptides, European journal of biochemistry, 258 (1998) 846–853. [DOI] [PubMed] [Google Scholar]
- [46].Wang YH, Slochower DR, Janmey PA, Counterion-mediated cluster formation by polyphosphoinositides, Chemistry and physics of lipids, 182 (2014) 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang YH, Collins A, Guo L, Smith-Dupont KB, Gai F, Svitkina T, Janmey PA, Divalent cation-induced cluster formation by polyphosphoinositides in model membranes, Journal of the American Chemical Society, 134 (2012) 3387–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Naughton FB, Kalli AC, Sansom MSP, Modes of Interaction of Pleckstrin Homology Domains with Membranes: Toward a Computational Biochemistry of Membrane Recognition, Journal of molecular biology, 430 (2018) 372–388. [DOI] [PubMed] [Google Scholar]
- [49].Chen X, Khajeh JA, Ju JH, Gupta YK, Stanley CB, Do C, Heller WT, Aggarwal AK, Callaway DJ, Bu Z, Phosphatidylinositol 4,5-bisphosphate clusters the cell adhesion molecule CD44 and assembles a specific CD44-Ezrin heterocomplex, as revealed by small angle neutron scattering, The Journal of biological chemistry, 290 (2015) 6639–6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Senju Y, Kalimeri M, Koskela EV, Somerharju P, Zhao HX, Vattulainen I, Lappalainen P, Mechanistic principles underlying regulation of the actin cytoskeleton by phosphoinositides, Proceedings of the National Academy of Sciences of the United States of America, 114 (2017) E8977–E8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Walrant A, Saxton DS, Correia GP, Gallop JL, Triggering actin polymerization in Xenopus egg extracts from phosphoinositide-containing lipid bilayers, in: Ross J, Marshall WF (Eds.) Building a Cell from Its Component Parts 2015, pp. 125–147. [DOI] [PubMed] [Google Scholar]
- [52].Gallop JL, Walrant A, Cantley LC, Kirschner MW, Phosphoinositides and membrane curvature switch the mode of actin polymerization via selective recruitment of toca-1 and Snx9, Proceedings of the National Academy of Sciences of the United States of America, 110 (2013) 7193–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Daste F, Walrant A, Holst MR, Gadsby JR, Mason J, Lee JE, Brook D, Mettlen M, Larsson E, Lee SF, Lundmark R, Gallop JL, Control of actin polymerization via the coincidence of phosphoinositides and high membrane curvature, Journal of Cell Biology, 216 (2017) 3745–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bucki R, Bachelot-Loza C, Zachowski A, Giraud F, Sulpice JC, Calcium induces phospholipid redistribution and microvesicle release in human erythrocyte membranes by independent pathways, Biochemistry, 37 (1998) 15383–15391. [DOI] [PubMed] [Google Scholar]
- [55].Levental I, Janmey PA, Cebers A, Electrostatic contribution to the surface pressure of charged monolayers containing polyphosphoinositides, Biophysical journal, 95 (2008) 1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Levental I, Cebers A, Janmey PA, Combined electrostatics and hydrogen bonding determine intermolecular interactions between polyphosphoinositides, Journal of the American Chemical Society, 130 (2008) 9025–9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mondal S, Chandra A, Venkatramani R, Datta A, Optically sensing phospholipid induced coil-helix transitions in the phosphoinositide-binding motif of gelsolin, Faraday discussions, (2018). [DOI] [PubMed] [Google Scholar]
- [58].Kooijman EE, King KE, Gangoda M, Gericke A, Ionization properties of phosphatidylinositol polyphosphates in mixed model membranes, Biochemistry, 48 (2009) 9360–9371. [DOI] [PubMed] [Google Scholar]
- [59].Moravcevic K, Oxley CL, Lemmon MA, Conditional peripheral membrane proteins: facing up to limited specificity, Structure, 20 (2012) 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bucki R, Giraud F, Sulpice JC, Phosphatidylinositol 4,5-bisphosphate domain inducers promote phospholipid transverse redistribution in biological membranes, Biochemistry, 39 (2000) 5838–5844. [DOI] [PubMed] [Google Scholar]
- [61].Yamamoto E, Computational and theoretical approaches for studies of a lipid recognition protein on biological membranes, Biophysics and physicobiology, 14 (2017) 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Koldsø H, Shorthouse D, Hélie J, Sansom MSP, Lipid Clustering Correlates with Membrane Curvature as Revealed by Molecular Simulations of Complex Lipid Bilayers, PLoS Computational Biology, 10 (2014) e1003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ramakrishnan N, Bradley RP, Tourdot RW, Radhakrishnan R, Biophysics of membrane curvature remodeling at molecular and mesoscopic length scales,, J. Phys. B: Condensed Matter, (2018) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bradley RP, Radhakrishnan R, Curvature-undulation coupling as a basis for curvature sensing and generation in bilayer membranes, Proceedings of the National Academy of Sciences of the United States of America, 113 (2016) E51175124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Diz-Munoz A, Fletcher DA, Weiner OD, Use the force: membrane tension as an organizer of cell shape and motility, Trends in cell biology, 23 (2013) 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sen S, Ng WP, Kumar S, Contributions of talin-1 to glioma cell-matrix tensional homeostasis, Journal of the Royal Society, Interface, 9 (2012) 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rubashkin MG, Cassereau L, Bainer R, DuFort CC, Yui Y, Ou G, Paszek MJ, Davidson MW, Chen YY, Weaver VM, Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3,4,5)triphosphate, Cancer research, 74 (2014) 4597–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Herrig A, Janke M, Austermann J, Gerke V, Janshoff A, Steinem C, Cooperative adsorption of ezrin on PIP2-containing membranes, Biochemistry, 45 (2006) 13025–13034. [DOI] [PubMed] [Google Scholar]
- [69].Rouven Bruckner B, Pietuch A, Nehls S, Rother J, Janshoff A, Ezrin is a Major Regulator of Membrane Tension in Epithelial Cells, Scientific reports, 5 (2015) 14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kawamura K, Takano K, Suetsugu S, Kurisu S, Yamazaki D, Miki H, Takenawa T, Endo T, N-WASP and WAVE2 acting downstream of phosphatidylinositol 3-kinase are required for myogenic cell migration induced by hepatocyte growth factor, The Journal of biological chemistry, 279 (2004) 54862–54871. [DOI] [PubMed] [Google Scholar]
- [71].Kozlov MM, Bershadsky AD, Processive capping by formin suggests a forcedriven mechanism of actin polymerization, The Journal of cell biology, 167 (2004) 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pogoda K, Bucki R, Byfield FJ, Cruz K, Lee T, Marcinkiewicz C, Janmey PA, Soft Substrates Containing Hyaluronan Mimic the Effects of Increased Stiffness on Morphology, Motility, and Proliferation of Glioma Cells, Biomacromolecules, 18 (2017) 3040–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fukami K, Sawada N, Endo T, Takenawa T, Identification of a phosphatidylinositol 4,5-bisphosphate-binding site in chicken skeletal muscle alpha-actinin, Journal of Biological Chemistry, 271 (1996) 2646–2650. [DOI] [PubMed] [Google Scholar]
- [74].Fukami K, Furuhashi K, Inagaki M, Endo T, Hatano S, Takenawa T, Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function, Nature, 359 (1992) 150–152. [DOI] [PubMed] [Google Scholar]
- [75].Corgan AM, Singleton C, Santoso CB, Greenwood JA, Phosphoinositides differentially regulate alpha-actinin flexibility and function, Biochem J, 378 (2004) 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Janmey PA, Lamb J, Allen PG, Matsudaira PT, Phosphoinositide-Binding Peptides Derived from the Sequences of Gelsolin and Villin, Journal of Biological Chemistry, 267 (1992) 11818–11823. [PubMed] [Google Scholar]
- [77].Cunningham CC, Vegner R, Bucki R, Funaki M, Korde N, Hartwig JH, Stossel TP, Janmey PA, Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly, Journal of Biological Chemistry, 276 (2001) 43390–43399. [DOI] [PubMed] [Google Scholar]
- [78].Niggli V, Andreoli C, Roy C, Mangeat P, Identification of a phosphatidylinositol-4,5-bisphosphate-binding domain in the N-terminal region of ezrin, FEBS Lett, 376 (1995) 172–176. [DOI] [PubMed] [Google Scholar]
- [79].Al-Momany A, Li L, Alexander RT, Ballermann BJ, Clustered PI(4,5)P(2) accumulation and ezrin phosphorylation in response to CLIC5A, Journal of cell science, 127 (2014) 5164–5178. [DOI] [PubMed] [Google Scholar]
- [80].Shabardina V, Kramer C, Gerdes B, Braunger J, Cordes A, Schafer J, Mey I, Grill D, Gerke V, Steinem C, Mode of Ezrin-Membrane Interaction as a Function of PIP2 Binding and Pseudophosphorylation, Biophysical journal, 110 (2016) 27102719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sun F, Schroer CFE, Xu L, Yin H, Marrink SJ, Luo SZ, Molecular Dynamics of the Association of L-Selectin and FERM Regulated by PIP2, Biophysical journal, 114 (2018) 1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bosk S, Braunger JA, Gerke V, Steinem C, Activation of F-Actin Binding Capacity of Ezrin: Synergism of PIP2 Interaction and Phosphorylation, Biophysical journal, 100 (2011) 1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sohn RH, Chen J, Koblan KS, Bray PF, Goldschmidtclermont PJ, Localization of a Binding-Site for Phosphatidylinositol 4,5-Bisphosphate on Human Profilin, Journal of Biological Chemistry, 270 (1995) 21114–21120. [DOI] [PubMed] [Google Scholar]
- [84].Ramalingam N, Zhao HX, Breitsprecher D, Pekka L, Faix J, Schleicher M, Phospholipids regulate localization and activity of mDia1 formin, European journal of cell biology, 89 (2010) 723–732. [DOI] [PubMed] [Google Scholar]
- [85].Song XQ, Yang J, Hirbawi J, Ye S, Perera HD, Goksoy E, Dwivedi P, Plow EF, Zhang RG, Qin J, A novel membrane-dependent on/off switch mechanism of talin FERM domain at sites of cell adhesion, Cell Res, 22 (2012) 1533–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Furuhashi K, Inagaki M, Hatano S, Fukami K, Takenawa T, Inositol phospholipid-induced suppression of F-actin-gelating activity of smooth muscle filamin, Biochemical and biophysical research communications, 184 (1992) 12611265. [DOI] [PubMed] [Google Scholar]
- [87].Kallikourdis M, Trovato AE, Roselli G, Muscolini M, Porciello N, Tuosto L, Viola A, Phosphatidylinositol 4-Phosphate 5-Kinase beta Controls Recruitment of Lipid Rafts into the Immunological Synapse, J Immunol, 196 (2016) 1955–1963. [DOI] [PubMed] [Google Scholar]