Summary
5-Fluorouracil (5-FU) is a key component in the treatment of many gastrointestinal tract adenocarcinomas. Despite its proven therapeutic efficacy, this chemotherapeutic agent also possesses several associated cardiac toxicities, including coronary vasospasm and coronary thrombosis. We report a case of acute coronary syndrome with thrombotic subtotal coronary artery occlusion in the setting of home 5-FU infusion highlighting the importance of careful pre-administration cardiac evaluation and close monitoring during drug administration.
Keywords: 5-Fluorouracil, Acute coronary syndrome, Cardiotoxicity
Introduction
Acute coronary syndrome (ACS) precipitated by the administration of 5-fluorouracil (5-FU) is a rare but well-established event 1, 2 and only one of the several side effects of this chemotherapeutic agent. Other cardiotoxicities include cardiomyopathy, vasospastic angina, and malignant arrhythmias 1, 2, 3, 4, 5. Following an initial cardiac complication, it is essential to weigh the risks of repeat drug administration against the potential for cure of the malignancy given the effectiveness of this chemotherapeutic regimen. This case report and review of the literature examines the cardiac complications of 5-FU and their effect on the therapeutic decision-making process.
Case report
A 55-year-old male with a history of hypertension, hyperlipidemia, and recent subtotal gastrectomy for stage IA gastric cancer developed chest tightness within an hour of the initial administration of FOLFOX (5-FU, oxaliplatin, and folinic acid). These symptoms lasted for only 5 min and resolved spontaneously following termination of the infusion. The infusion was subsequently restarted without any recurrent chest pain. The patient was sent home with an infusion pump to receive a continuous infusion of 5-FU for an additional 24 h. Six hours later he developed recurrent severe chest pressure with radiation to both arms accompanied by nausea and diaphoresis. On presentation to the emergency department an electrocardiogram (ECG) demonstrated anterolateral ST-segment depressions and T wave inversions. His infusion was stopped and he was treated with sublingual nitroglycerine with complete symptom resolution. His initial troponin-I was elevated at 0.19 ng/mL, later peaking at 28 ng/mL.
He was admitted to the coronary care unit and a left heart catheterization was performed demonstrating mildly depressed global left ventricular systolic function, nonobstructive disease in the left anterior descending and right coronary arteries and a thrombotic subtotal occlusion of the left circumflex coronary artery (Fig. 1A). He underwent successful mechanical thrombectomy and placement of a bare metal stent (Fig. 1B) without complication. During post-procedure observation he had no symptom recurrence and was discharged on hospital day 4 on a beta-blocker, angiotensin-converting enzyme inhibitor, aspirin, statin, and clopidogrel.
Figure 1.
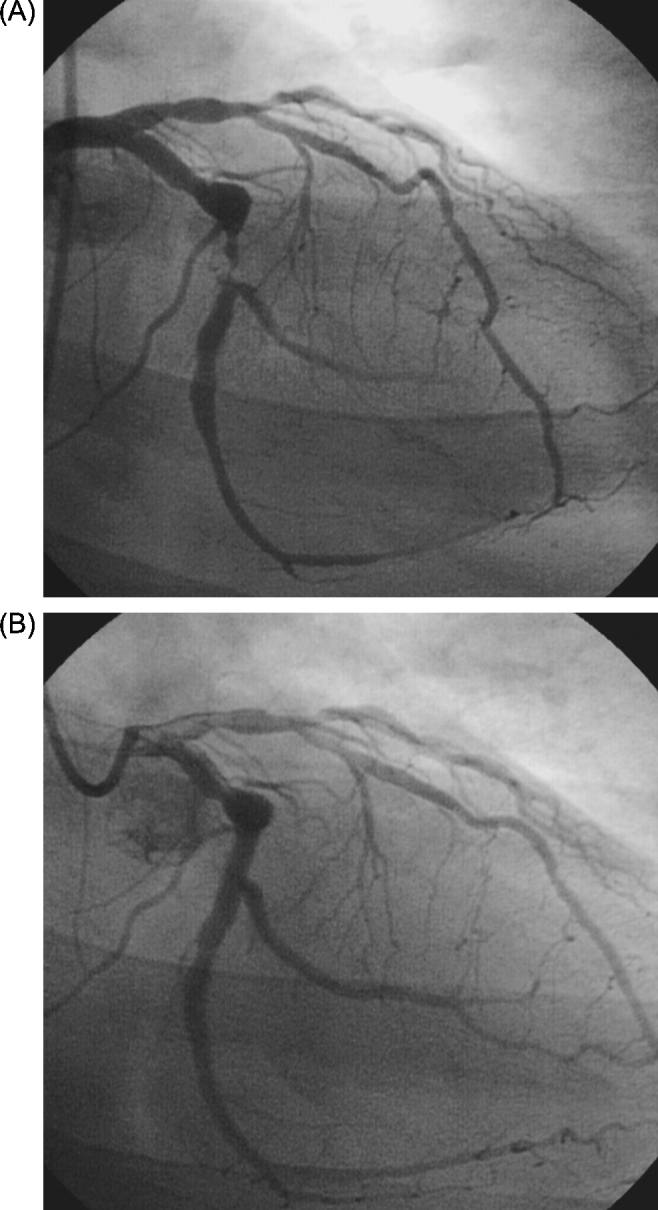
(A) Angiogram demonstrating thrombotic subtotal occlusion of the left circumflex coronary artery on right anterior oblique caudal projection. (B) Angiogram in the same projection following percutaneous coronary intervention with a bare metal stent.
Discussion
The chemotherapeutic agent 5-FU is a fluoropyrimidine antimetabolite agent key to several chemotherapy regimens, particularly in the treatment of gastrointestinal tract adenocarcinomas. There is evidence that 5-FU therapy combined with surgical resection offers an impressive absolute risk reduction of 5-year mortality of 5.7% [6]. Unfortunately 5-FU also has poorly defined cardiotoxic effects. Various studies demonstrate direct endothelial cardiotoxicity, arterial vasospasm, cardiomyopathy with significant left ventricular systolic dysfunction, sinoatrial and atrioventricular node dysfunction, and ventricular arrythmias 5, 7, 8.
The precise etiology and pathophysiology of 5-FU cardiotoxicity is still unknown and based primarily on limited animal studies, case reports, and small clinical studies. Clinically significant coronary vasospasm causing myocardial ischemia is the most commonly suspected mechanism, occurring in 1.2–18% of cases, even in the absence of coronary artery disease 1, 3, 7, 9, 10. Both animal and human studies demonstrate a dose-dependent vasospasm that ceases with cessation of drug administration 2, 7. Experimental evidence also supports a direct toxic effect of 5-FU on the coronary endothelium [11] and a hypercoagulable state which together precipitate acute thrombotic events 10, 12.
A study by Rezkalla et al. [4] demonstrated ischemic and arrhythmic ECG changes, including ST-segment deviation and QT-prolongation, in nearly 68% of patients receiving infusions of 5-FU. And while overall cardiac toxicity with 5-FU administration ranges from 1 to 3%, it approaches 5% in patients with underlying heart disease 2, 4. One retrospective examination of 377 case reports revealed acute myocardial infarction in 22% of patients during 5-FU administration [8]. Another prospective study of 26 patients suggested infarction by ECG and symptom criteria in 35% of patients receiving 5-FU [1].
It is believed that the newer prolonged infusion schedules of 5-FU can lead to even more vasospastic and thrombotic complications [9]. Additionally, the median time for symptom initiation is 12 h following initiation of the infusion [3], although in animal studies, the median time was reported to be as long as 48 h [13] and have a variable point of symptom onset. Our patient, although early in presentation, is still within the range of 5-FU-induced thrombosis. Although our patient experienced chest pain during his initial observed 5-FU infusion, as there was no recurrence with later resumption of the infusion, he was sent home with a continuous infusion pump. In retrospect, it may have been preferable to conduct a more prolonged observation with ECG and hemodynamics during reinitiation of his 5-FU infusion prior to home administration. Additionally, several studies have demonstrated that having a lower dose of 5-FU is preferable if absolutely necessary [14], which is likely why this patient went home on a lower dose infusion pump and did well post infusion.
This case highlights the importance of the pre-chemotherapy history and physical examination. Patients should have a careful evaluation for cardiovascular risk factors as well as subclinical coronary artery disease or cardiomyopathies which may be exacerbated by 5-FU administration. Future administration of 5-FU in our patient is controversial. However, there is no absolute evidence that repeat 5-FU administration is contraindicated, although it is typical practice to at least lower the infusion dose or add antianginals such as nitrates or calcium-channel blockers [14]. And in this case, the culprit coronary lesion was treated successfully with percutaneous coronary intervention and angiography demonstrated minimal residual coronary artery disease in the other territories. This patient did run a risk of having potential in-stent thrombosis, along with de novo lesions (particularly in the left anterior descending artery region) with further 5-FU; ultimately, this patient was treated with aggressive risk factor modification as well as aspirin and statin for their antithrombotic and plaque stabilization properties in order to continue his chemotherapy regimen. He continued 5-FU therapy under close monitoring in an effort to achieve complete cure of his malignancy and experienced no further treatment complications.
Conclusions
With the demonstrated efficacy of 5-FU based chemotherapeutic regimens more patients may expect to receive these agents in the future, oftentimes via home infusion. Thus it is important for clinicians to be aware of the various rare, but potentially serious, adverse cardiac effects. A high level of alertness is required when considering 5-FU based regimens. Cardiovascular risk factors and prior history of cardiac disease should be investigated and patients counseled appropriately. Ambulatory patients should be advised to seek medical attention without delay in the event of symptoms. Inpatients should be observed closely and 5-FU administration discontinued if cardiac symptoms develop. A rechallenge with 5-FU should be reserved for those patients in whom there is no reasonable alternative therapy and only under close monitoring. This case highlights the cardiotoxic effects of 5-FU and the cost-benefit analysis of continuing this cardiotoxic chemotherapeutic agent in the setting of intervened coronary artery disease.
References
- 1.Kosmas C., Kallistratos M.S., Kopterides P., Syrios J., Skopelitis H., Mylonakis N., Karabelis A., Tsavaris N. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. 2008;134:75–82. doi: 10.1007/s00432-007-0250-9. [DOI] [PubMed] [Google Scholar]
- 2.Labianca R., Beretta G., Clerici M., Frascini P., Luporini G. Cardiac toxicity of 5-flurouracil: a study on 1083 patients. Tumori. 1982;68:505–510. doi: 10.1177/030089168206800609. [DOI] [PubMed] [Google Scholar]
- 3.Becker K., Erckenbrecht J., Haussinger D., Frieling T. Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs. 1999;57:475–484. doi: 10.2165/00003495-199957040-00003. [DOI] [PubMed] [Google Scholar]
- 4.Rezkalla S., Kloner R.A., Ensley J., al-Sarraf M., Revels S., Olivenstein A., Bhasin S., Kerpel-Fronious S., Turi Z.G. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. J Clin Oncol. 1989;7:509–514. doi: 10.1200/JCO.1989.7.4.509. [DOI] [PubMed] [Google Scholar]
- 5.Stewart T., Pavlakis N., Ward M. Cardiotoxicity with 5-fluorouracil and capecitabine: more than just vasospastic angina. Intern Med J. 2010;40:303–307. doi: 10.1111/j.1445-5994.2009.02144.x. [DOI] [PubMed] [Google Scholar]
- 6.GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group. Paoletti X., Oba K., Burzykowski T., Michiels S., Ohashi Y., Pignon J.P., Rougier P., Sakamoto J., Sargent D., Sasako M., Van Cutsem E., Buyse M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 7.Mosseri M., Fingert H., Varticovski L., Chokshi S., Isner J.M. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res. 1993;53:3028–3033. [PubMed] [Google Scholar]
- 8.Saif M.W., Shah M.M., Shah A.R. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8:191–202. doi: 10.1517/14740330902733961. [DOI] [PubMed] [Google Scholar]
- 9.Gorgulu S., Celik S., Tezel T. A case of coronary spasm induced by 5-fluorouracil. Acta Cardiol. 2002;57:381–383. doi: 10.2143/AC.57.5.2005458. [DOI] [PubMed] [Google Scholar]
- 10.Kinhult S., Albertsson M., Eskilsson J., Cwikiel M. Antithrombotic treatment in protection against thrombogenic effects of 5-fluorouracil on vascular endothelium: a scanning microscopy evaluation. Scanning. 2001;23:1–8. doi: 10.1002/sca.4950230101. [DOI] [PubMed] [Google Scholar]
- 11.Cwikiel M., Eskilsson J., Wieslander J.B., Stjernquist U., Albertsson M. The appearance of endothelium in small arteries after treatment with 5-fluorouracil: an electron microscopic study of late effects in rabbits. Scanning Microsc. 1996;10:805–818. [PubMed] [Google Scholar]
- 12.Jensen S.A., Sorensen J.B. 5-Fluorouracil-based therapy induces endovascular injury having potential significance to development of clinically overt cardiotoxicity. Cancer Chemother Pharmacol. 2012;69:57–64. doi: 10.1007/s00280-011-1669-x. [DOI] [PubMed] [Google Scholar]
- 13.Cwikiel M., Eskilsson J., Albertsson M., Stavenow L. The influence of 5-fluorouracil and methotrexate on vascular endothelium. An experimental study using endothelial cells in the culture. Ann Oncol. 1996;7:731–737. doi: 10.1093/oxfordjournals.annonc.a010723. [DOI] [PubMed] [Google Scholar]
- 14.Lestuzzi C., Crivellari D., Rigo F., Viel E., Meneguzzo N. Capecitabine cardiac toxicity presenting as effort angina: a case report. J Cardiovasc Med (Hagerstown) 2010;11:700–703. doi: 10.2459/JCM.0b013e328332e873. [DOI] [PubMed] [Google Scholar]


