Abstract
Mitochondrial disorders are genetic diseases that result in a deficiency of energy metabolism (ATP production). A “mitochondrial crisis” can occur in the setting of infection, dehydration, or physiologic stress. The hallmark of a mitochondrial crisis is failure of multiple individual organ systems. The mortality of mitochondrial crisis is high and therapy is supportive but involves a specific strategy of hydration with dextrose-containing IV fluids, avoidance of many medications known to worsen mitochondrial function, and limitations of oxygenation as this can promote free radical production. We report a case of a patient with known mitochondrial disease that presented with a mitochondrial crisis with prominent and life-threatening cardiac manifestations including long QT, ventricular arrhythmias, and acute left ventricular systolic dysfunction in addition to rhabdomyolysis, lactic acidosis, and an acute kidney injury. This patient was managed successfully with a specifically tailored supportive strategy, a high-dose metabolic cocktail, permissive hypoxia, and low-protein diet. At 10 weeks post discharge all electrocardiographic abnormalities resolved and ventricular recovery has been observed. Given the increased survival of this population of patients into adulthood it is important that these adjunctive therapeutic strategies require consideration by clinicians treating this group of patients.
Keywords: Mitochondrial crisis, Cardiomyopathy, Arrhythmia, Clinical management
Introduction
Mitochondrial disorders are increasingly encountered by adult and pediatric clinicians. Adults with mitochondrial disorders usually fall into well characterized “mitochondrial syndromes” that are often the result of a known mitochondrial DNA mutation. Children with suspected mitochondrial syndromes present a greater diagnostic challenge as they commonly have nuclear DNA mutations and the classic signs of a mitochondrial syndrome may be missing and genetic confirmation is commonly elusive [1]. Reports suggest that the prevalence of these disorders may exceed 1 in 5000 births making these disorders more common than other inherited myopathies including Duchenne or myotonic myopathies [2]. Common to all mitochondrial disorders are deficiencies of energy metabolism and ATP production that affect cells in many organ systems. A mitochondrial crisis is defined by the failure of multiple organ systems, commonly precipitated by a minor infection, exposure to medications known to interfere with respiratory chain function, or other physiologic stressors. A crisis can occur over hours to weeks and is associated with a high mortality. Unfortunately, therapeutic strategies to manage cardiac mitochondrial crisis have not been reported.
Case report
A 24-year-old female initially presented at the age of 5 months with ketotic hypoglycemia, increased liver enzymes, and lactic acidosis. She was subsequently recognized to have sensorineural hearing loss, developmental delay, and short stature and was followed in the Center for Mitochondrial Disease. Genetic testing did not identify a known mutation. Four years prior to the current admission the patient was admitted for rhabdomyolysis and myocarditis which was treated with intravenous immunoglobulin, and a seizure disorder which was treated with levetiracetam. Several months prior to the current illness the patient was weaned off her seizure medication by her neurologist, as she had remained seizure-free for many years. The patient was well and functioning at her baseline attending a daycare program.
Six days prior to admission the patient was noted by the daycare worker to be lethargic. She was taken to a community emergency department where she was found to have an elevated white blood cell (WBC) count to 22,000/mm3, deranged liver function tests, and highly elevated creatinine kinase (CK) levels >10,000 IU/ml consistent with rhabdomyolysis. She was admitted to the community hospital where she received IV ceftriaxone and hydration. She was discharged when her CK levels fell below 5000 IU/ml. The following day, the patient suffered a series of tonic-clonic movements consistent with seizures and her parents brought her to the emergency department of the tertiary care hospital where she has admitted by the neurology department with a diagnosis of breakthrough seizures. A baseline electrocardiogram (ECG) was performed and showed new T-wave changes and ventricular bigeminy. The patient was moved to a pediatric cardiac telemetry unit where she was noted to have further seizure activity.
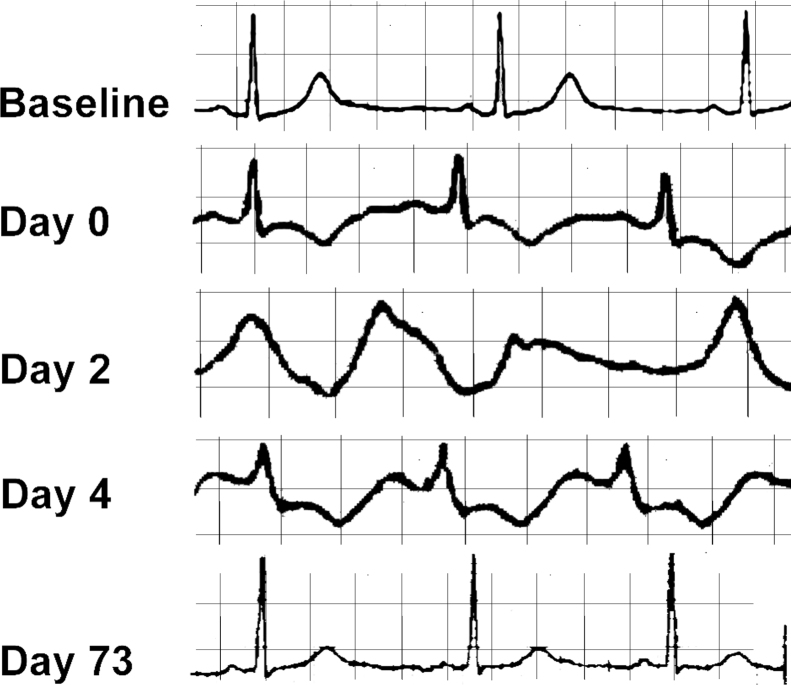
On physical examination, the patient was sleepy and lethargic. She was afebrile. She had small stature, microcephaly with low-set ears, and a left eye ptosis. Her cardiac exam revealed tachycardia and she had scant crackles at the bases of her lungs. She was warm and well perfused. Initial laboratory investigations revealed a WBC count of 22,000/mm3, elevated liver enzymes, and total CK levels were 5489 IU/ml. MB fraction was 97.9 IU/ml, troponin-T levels were 0.989 ng/ml, and there was lactic acidosis (lactic acid 2.4 IU/ml). An echocardiogram revealed depressed left ventricular systolic function (Table 1). Later that day she developed progressive respiratory distress and bradycardia, which culminated in a pulseless electrical activity arrest. The patient was resuscitated with cardiopulmonary resuscitation and intubated. She was commenced on an epinephrine drip and had further episodes of unstable ventricular tachycardia and ventricular fibrillation. She was transferred to the adult congestive heart failure service where repeat echocardiogram now revealed progressively declining ventricular function. The patient was commenced on IV amiodarone but the QT interval was observed to be dramatically increasing (Fig. 1) and the patient had further episodes of polymorphic ventricular tachycardia. The patient was switched to an isoproterenol infusion. The patient became febrile and was treated with broad-spectrum antibiotics and suffered an acute kidney injury with her creatinine increasing from a baseline of 0.5 to 2.3 mg/dl. On day 3 of admission the patient remained critically ill and unstable. Withdrawal of care was discussed with the family given the worsening clinical picture. On day 4, after discussion with the mitochondrial disease center we elected to commence the patient on a “mitochondrial cocktail” for adjunctive treatment of mitochondrial crisis. This consisted of riboflavin 200 mg q 12 hourly, ubiquinone 1.4 g bid, arginine 20 g IV q 6 h for 3 days, then 15 g IV daily as continuous infusion, levocarnitine 1.8 g IV q 8 h, folic acid 1 mg IV bid, and thiamine 100 mg IV daily. In addition to the mitochondrial cocktail we also treated the patient with dextrose containing IV fluids, fed the patient enterally with low-protein feeds, allowed a permissive hypoxia, and strictly avoided all medications known to interfere with respiratory chain function. By hospital day 4 the patient's condition was stabilizing, her ECG was normalizing, and her ejection fraction improved (Fig. 2). The patient was extubated after 12 days and she was discharged home on hospital day 24 with a percutaneous endoscopic gastrostomy tube for supplemental feedings and for continuance of an outpatient version of the mitochondrial cocktail that requires frequent dosing. She had home physical therapy for her severe de-conditioning but has since returned to her baseline functional status.
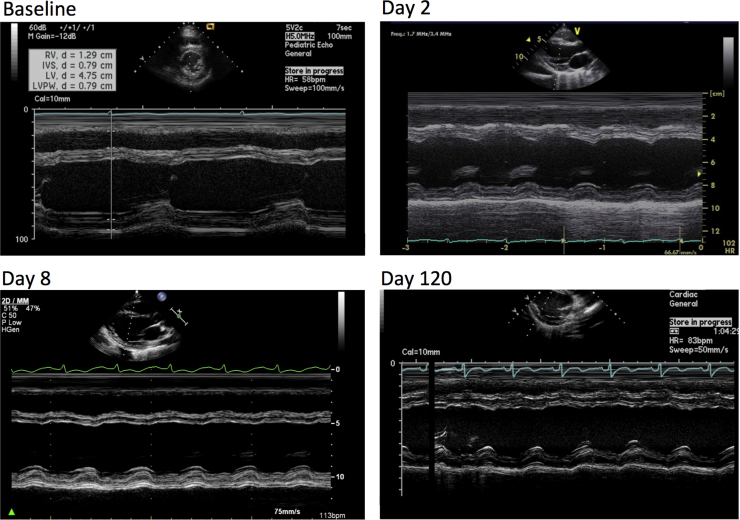
Table 1.
Progression of echocardiographic changes at baseline, during the mitochondrial crisis, and in follow up.
| DOS | Baseline | Day 2 | Day 8 | Day 120 |
|---|---|---|---|---|
| EDD (cm) | 4.7 | 5.1 | 5.1 | 3.8 |
| EDS (cm) | 3.1 | 4.0 | 4.2 | 2.9 |
| EF (%) | 64 | 40 | 28 | 40 |
| RV function | Normal | Normal | Normal | Normal |
| MR | Trace | Mild | Mild | Trace |
| TR | Trace | Mild | Trace | Mild |
| RVSP (mm Hg) | Not done | 35 | Not measurable | 28 |
EDD, end diastolic diameter; EDS, end systolic diameter; EF, ejection fraction; RV, right ventricular; MR, mitral regurgitation; TR, tricuspid regurgitation; RVSP, right ventricular systolic pressure.
Fig. 1.
Electrocardiographic changes at baseline, during the mitochondrial crisis, and in follow up after resolution of the crisis state.
Fig. 2.
M-mode images from echocardiography performed at various time points during the course of the illness. Baseline study was performed two years before the patient developed the mitochondrial crisis (no parasternal short-axis view was available for comparison). The Day 2 and Day 8 echocardiograms show progressive deterioration of left ventricular systolic function. The final study, performed 3 months into the patient's clinical recovery, demonstrates improvement back to baseline.
Discussion
Mitochondrial disorders predominantly affect tissues dependent on aerobic metabolism including brain, retina, nerve, and muscle tissue. Cardiac involvement is ubiquitous in mitochondrial disorders with multi-systemic involvement. Cardiomyopathy associated with mitochondrial disorders, termed as ‘mitochondrial cardiomyopathy’ is characterized by abnormal structure and/or function of the heart muscle secondary to genetic defects involving the mitochondrial respiratory chain, in the absence of other known structural heart disease. Cardiac abnormalities such as left ventricular non-compaction (LVNC) have been previously reported in patients with Barth syndrome [3], a mitochondrial disorder characterized by X-linked cardiomyopathy, mitochondrial myopathy, and cyclic neutropenia. Moreover, stressors like fever, acute illness, heat, or medication can precipitate an acute mitochondrial cardiac crisis, which is characterized by multi-organ failure and elevated lactate levels due to ATP depletion. Cardiac complications during an acute mitochondrial crisis can result in acute heart failure leading to cardiogenic shock, ventricular arrhythmias, murmurs, and sudden cardiac death. Successful recovery is dependent on identification and correction of precipitating factors. Work up should include complete blood count, liver function tests, renal function tests, lactate, arterial blood gas, IgG, amylase, lipase levels, ECG, and echocardiogram. Since most patients have baseline acidosis correction of severe acidosis should be gradual. If infection is suspected, antibiotics should be initiated. Partial pressure of O2 should be maintained between 50 and 60 mm Hg as excessive oxygenation may worsen a crisis by increasing free radical production. The pharmacologic strategy includes administration of a “mitochondrial cocktail,” which consists of respiratory chain co-factors including coenzyme Q10 (CoQ10), thiamine, riboflavin, anti-oxidants such as vitamin E, agents to correct secondary biochemical deficits including l-carnitine, creatine, and folate and vasodilators such as l-arginine. There is some controversy regarding this approach as a systematic review failed to provide clear evidence supporting the use of CoQ10, creatine, dichloroacetate, and dimethylglycine in mitochondrial disorders 4, 5, 6. However, a partial improvement in clinical features using anti-oxidants including coenzyme Q10, idebenone (a coenzyme Q10 analog), vitamin E, and dihydrolipoate has been reported 4, 5, 6. Patients with mitochondrial cytopathies taking creatine monohydrate show increased strength of high-intensity anaerobic and aerobic type activities [7]. l-Arginine infusion improves clinical symptoms in patients with acute stroke-like episodes of MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes) [8]. Careful attention is required to avoid medications that can interfere with respiratory chain function. These include valproic acid, statins, propofol, aminoglycosides, stretptomycin, tetracyclines, chloramphenicol, erythromycin, azithromycin, and metformin. l-Arginine mediates incorporation of ammonia into less toxic metabolites citrulline and arginosuccinate, which are easily secreted. A loading dose of 600 mg/kg of 10% l-arginine should be started through a central line as it may cause tissue necrosis and phlebitis if given peripherally, and maintained at 600 mg/kg/day. Side effects of intravenous l-arginine include hypotension, nausea, emesis, headache, and potentially life-threatening hyperkalemia. In patients with worsening renal function, severe lactic acidosis, hyperammonemia, and hyperkalemia, hemodialysis is recommended. Patients with fever or inability to drink or eat should be provided with dextrose containing IV fluids, preferably containing 10% dextrose at a maintenance dose regardless of the levels of blood glucose. Careful assessment of metabolic and volume status should be done at regular intervals. Serial echocardiography should be done at the time of discharge, at one month, and every three months to assess clinical recovery. In the absence of contraindications, β-blockers and angiotensin-converting enzyme inhibitors should be considered in all patients with cardiomyopathy.
In summary, successful management of mitochondrial crisis is based on prompt recognition of the problem, use of mitochondrial ‘rescue’ medications, supportive therapy aimed at treating precipitating factors including fever, infections, avoiding medications that interfere with respiratory chain function, adequate control of seizures, good respiratory support, correction of acid base and electrolyte disturbances, providing patient and family education and a multidisciplinary approach with dietary, physical, occupational, and speech therapy.
References
- 1.Koenig M.K. Presentation and diagnosis of mitochondrial disorders in children. Pediatric Neurol. 2008;38:305–313. doi: 10.1016/j.pediatrneurol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skladal D., Halliday J., Thornburn D.R. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain. 2003;126:1905–1912. doi: 10.1093/brain/awg170. [DOI] [PubMed] [Google Scholar]
- 3.Ichida F., Tsubata S., Bowles K.R., Haneda N., Uese K., Miyawaki T., Dreyer W.J., Messina J., Li H., Bowles N.E., Towbin J.A. Novel gene mutations in patients with left ventricular noncompaction or barth syndrome. Circulation. 2001;103:1256–1263. doi: 10.1161/01.cir.103.9.1256. [DOI] [PubMed] [Google Scholar]
- 4.Ikejiri Y., Mori E., Ishii K., Nishimoto K., Yasuda M., Sasaki M. Idebenone improves cerebral mitochondrial oxidative metabolism in a patient with MELAS. Neurology. 1996;47:583–585. doi: 10.1212/wnl.47.2.583. [DOI] [PubMed] [Google Scholar]
- 5.Bakker H.D., Scholte H.R., Jeneson J.A. Vitamin E in a mitochondrial myopathy with proliferating mitochondria. Lancet. 1993;342:175. doi: 10.1016/0140-6736(93)91379-z. [DOI] [PubMed] [Google Scholar]
- 6.Barbiroli B., Medori R., Tritschler H.J., Klopstock T., Seibel P., Reichmann H., Iotti S., Lodi R., Zaniol P. Lipoic (thioctic) acid increases brain energy availability and skeletal muscle performance as shown by in vivo 31P-MRS in a patient with mitochondrial cytopathy. J Neurol. 1995;242:472–477. doi: 10.1007/BF00873552. [DOI] [PubMed] [Google Scholar]
- 7.Tarnopolsky M.A., Roy B.D., MacDonald J.R. A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve. 1997;20:1502–1509. doi: 10.1002/(sici)1097-4598(199712)20:12<1502::aid-mus4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Koga Y., Akita Y., Nishioka J., Yatsuga S., Povalko N., Tanabe Y., Fujimoto S., Matsuishi T. l-Arginine improves the symptoms of stroke like episodes in MELAS. Neurology. 2005;64:710–712. doi: 10.1212/01.WNL.0000151976.60624.01. [DOI] [PubMed] [Google Scholar]