Sialylation is known to regulate platelet clearance.1 Sialic acids are terminal sugar components of the oligosaccharide chains of glycoproteins and glycolipids. During platelet ageing, surface modifications such as the loss of sialic acid (i.e., desialylation) expose β-galactose residues. These senescence antigens facilitate platelet clearance, influence the platelet life span, and stimulate thrombopoietin (TPO) production.2 Greater exposure of non-sialylated glycan chains may be associated with accelerated platelet clearance. Sialyltransferases constitute a family of glycosyltransferases that transfer sialic acid from the donor substrate (cytidine-5’-monophosphate (CMP)-sialic acid) to acceptor oligosaccharide substrates.3 Mice in which the St3gal4 sialyltransferase gene has been knocked out are thrombocytopenic; the absence of sialic acid increases β-galactose exposure and thus results in the rapid clearance of platelets from the circulation.4 In sialic acid metabolism, a specific transporter [solute carrier family 35 member A1 (SLC35A1)] transfer CMP-sialic acid to the medial- and trans-Golgi apparatus, where it is used as a substrate for the sialylation of proteins by various sialyltransferases (Figure 1).
Figure 1.
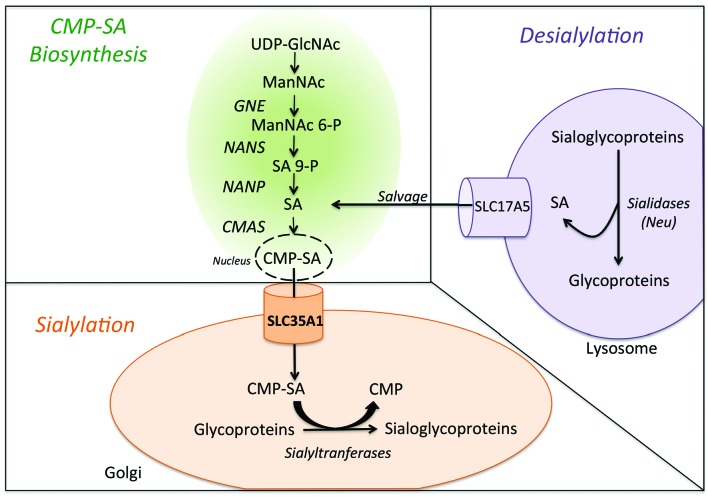
A simplified diagram of sialic acid metabolism in human. Biosynthesis of sialic acid (SA) mostly takes place in the cytoplasm, although the CMP–sialic acid synthase (CMAS) reaction occurs in the nucleus. The synthesis of ManNAc (N-acetylmannosamine) is carried out in two steps by UDP-GLcNAc–2 epimerase (GNE). NeuNAc synthase (NANS) generates phosphorylated forms of sialic acid, which must be dephosphorylated by a specific phosphatase (Neu5Ac-9-P-phosphatase, NANP) to yield free sialic acid in the cytoplasm. The free sialic acid can then reenter the biosynthesis pathway (indicated as “salvage” in the diagram). The CMP-SA (produced in the nucleus) is transferred by SLC35A1 into the Golgi apparatus, where it is used as a substrate for sialylation by sialyltransferases.
Herein, we report on a congenital deficiency in SLC35A1 in two siblings born to consanguineous parents and who displayed moderate macrothrombocytopenia. The sister (II:1, born in 2001) and brother (II:2, born in 2005) shared similar clinical presentations from birth, including delayed psychomotor development, epilepsy, ataxia, microcephaly, choreiform movements, and macrothrombocytopenia. This presentation was highly suggestive of an inherited disease. The hemorrhagic symptoms consisted of easy bruising for both siblings, and menorrhagia (leading to iron deficiency) for II:1. Persistent thrombocytopenia was observed in both siblings, with a mean ± standard error of the mean (SEM) platelet count of 95 ±10 G/L (n=31 analyses between 2002 and 2017) for II:1 and 60 ± 6 G/L (n=21 analysis between 2010 and 2015) for II:2. Comparative with normal values (6.5 to 12 fL), the platelet volume was high (mean ± SEM: 15.7 ± 3.9 fL (n=31) for II:1, and 19.1 and 20.4 fL for II:2). A peripheral blood smear review revealed the presence of giant platelets and macroplatelets (Figure 2A). Fluorescence analysis of the immature platelet fraction (on a Sysmex XE 5000 hematology analyzer) indicated high levels of reticulated platelets: 54% and 66% (n=2) for II:1, and 46% and 60% (n=2) for II:2; the normal range is <5%). Bone marrow aspirate smears of II:1 revealed an elevated megakaryocyte count (>2 megakaryocytes per low-power field), with a predominance of immature forms (characterized by small size, hypolobulation, and a basophilic cytoplasm, as observed in peripheral thrombocytopenia; Figure 2B). The elevated proportion of immature platelets and the bone marrow analysis results were suggestive of a compensatory mechanism for peripheral thrombocytopenia.
Figure 2.
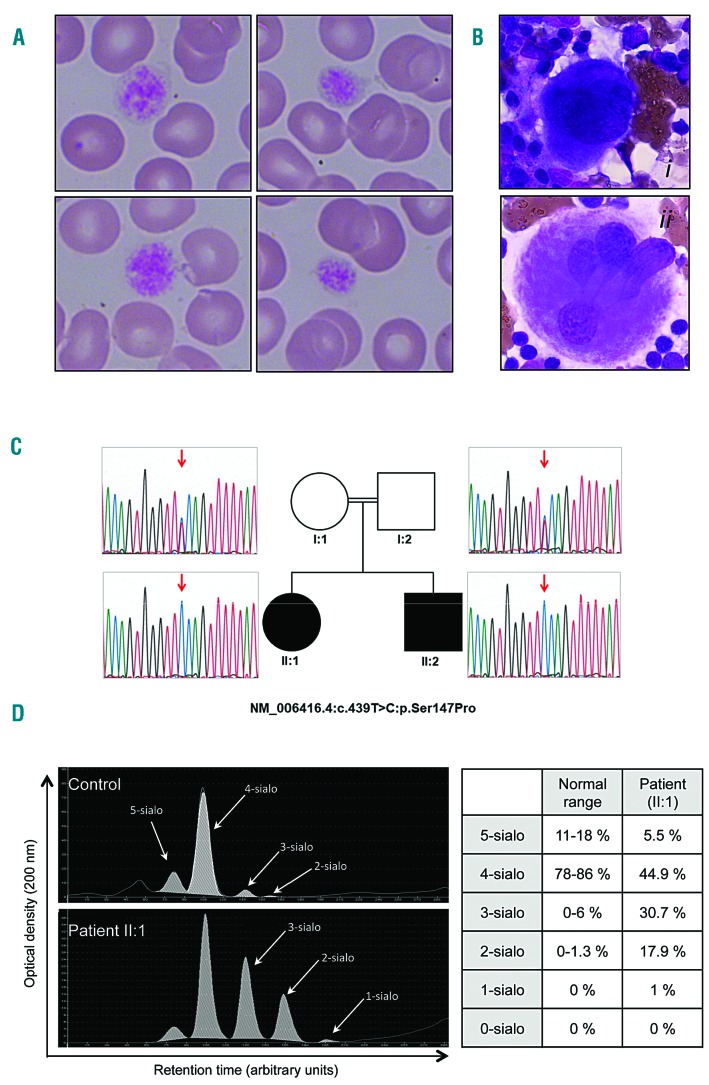
Blood and bone marrow smears, characterization of the SLC35A1 mutation in the family, and the capillary electrophoresis transferrin profile. Blood (A) and bone marrow (B) smears of patient II:1 were stained with May-Grünwald Giemsa reagent. The blood smear showed the presence of large platelets (relative to the size of red blood cells). The MKs from patient II:1’s bone marrow were immature (ii), as characterized by small size, hypolobulation and a basophilic cytoplasm, and (ii) granular MKs. C) The family’s pedigree, and Sanger sequencing results. Arrows indicate the position of the mutated nucleotide. Genetic mapping of disease loci in the family was carried out using an Affymetrix GeneChip Human Mapping 250K NspI SNP microarray. Multipoint linkage analysis of single-nucleotide polymorphism (SNP) data was performed using Alohomora and Merlin software. We performed homozygosity mapping of SNP data, and found 18 candidate loci of at least 1 Mb in size with a maximum logarithm of odds score (Zmax) of 1.8. Whole exome sequencing (WES) was performed in DNA from the index case (II:1), using the Exome Capture Agilent SureSelect XT V5 kit for library preparation and exome enrichment. Sequencing was performed on a Genome Analyzer IIx Illumina instrument in paired-end mode with a read length of 2x100bp. The median WES coverage was 60. Reads were aligned with the human reference genome sequence (UCSC hg19, NCBI build 37.3) using the BWA software package. Variants were selected using SAMtools, and then annotated using Annovar software. Variants in coding regions (including non-synonymous and nonsense mutations), intron-exon junctions or short coding insertions or deletions were selected when the minor allele frequency was less than 0.0030. The homozygous c.439T>C mutation in SLC35A1 was confirmed by Sanger sequencing, using primers flanking the mutations (SLC960-F: 5’-GCCCGGCCATTATCAAATA-3’, SLC960-R: 5’-AAATCAAAGAAATGTAGTCATGCTG-3’) in both affected individuals. Both affected individuals were homozygous for the mutation (II:1 and II:2, c.439T>C: p.Ser147Pro). The nucleotide and amino acid changes are indicated with respect to the reference sequences (Genbank: NM_006416.4 and NP_006407.1, respectively). Open symbols: unaffected; filled symbols: affected. D) The capillary electrophoresis transferrin profile for a control (top panel) and for patient II:1 (bottom panel) was performed as previously described.15 x axis: the migration time in arbitrary units. y axis: the optical density in arbitrary units. 5-sialo, 4-sialo, 3-sialo, 2-sialo, and 0-sialo: penta-, tetra-, tri-, di- and asialotransferrin, respectively. The isoform distributions for the control and for patient II:1 are indicated in the table.
By combining whole-exome sequencing with the genetic mapping of disease loci (assuming autosomal recessive inheritance), we found that patient II:1 was homozygous for a missense mutation in the SLC35A1 gene (NM_006416.4: c.439T>C: p.Ser147Pro, Figure 2C). The presence of this variant in both siblings was confirmed by Sanger sequencing (Figure 2C). The variant was absent from the dbSNP150, 1000Genomes or ExAC Browser databases, and was predicted to be pathogenic (Polyphen-2 score: 1).5 Both parents were heterozygous for the mutation (Figure 2C). The SLC35A1 protein comprises 10 transmembrane domains (TMDs), and a topol ogy analysis indicated that the p.Ser147Pro mutation was located in TMD-5. Given that the insertion of a Pro residue into a TMD is predicted to have a profound impact on a protein’s activity, the mutation p.Ser147Pro is expected to be deleterious. The cytoplasmic loop between TMDs 4 and 5 is important for SLC35A1’s transporter activity,6 and so a mutation in TMD-5 might change the protein’s activity via a conformational change in this loop and/or by destabilizing the transmembrane helix. Capillary electrophoresis of patient II:1’s serum transferrin (Figure 2D) showed elevated levels of hyposialylated glycoforms (3-sialo to 1-sialo), which is highly suggestive of a congenital disorder of glycosylation and thus the impaired maturation of protein-linked N-glycans in the Golgi apparatus. SLC35A1 deficiency has been referred to as congenital disorder of N-linked glycosylation IIf (CDG-IIf), with the new nomenclature SLC35A1-CDG.7
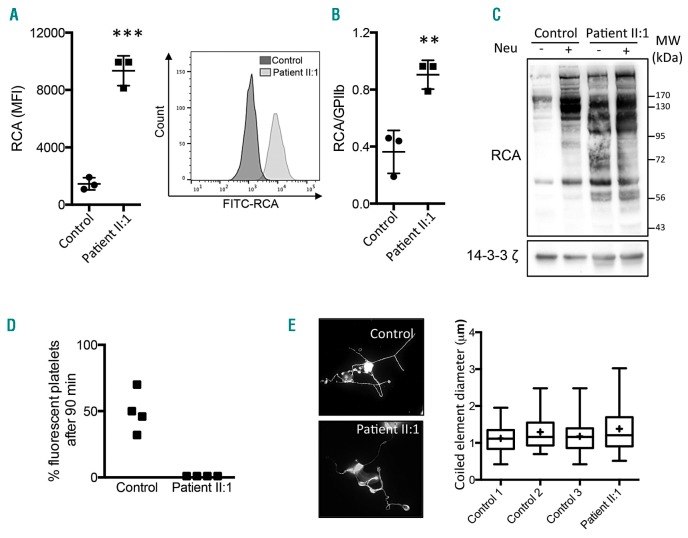
Given the recent discovery of sialylation’s importance in senescent platelet clearance, a congenital deficiency in the CMP-sialic acid transporter might account for the thrombocytopenia identified in the two probands. Since SLC35A1 is expressed in megakaryocytes and platelets (data not shown), we analyzed platelets from patient II:1. We performed a flow cytometry assay for platelet β-galactose exposure by using the lectin Ricinus communis agglutinin (RCA), which is specific for the β-galactose exposed in the absence of sialic acid. The significant elevation (P<0.001) in platelet surface RCA labeling for II:1 was highly suggestive of a sialylation defect (Figure 3A). To take platelet size into account, we also measured the ratio of the mean fluorescence intensity (MFI) of RCA to the MFI of GPIIb. A significant elevation (P<0.01) in II:1 (relative to a control) was still observed (Figure 3B) - indicating the presence of a qualitative defect in platelet sialylation. This defect could not be attributed to anti-GPIb antibodies [which have been reported elsewhere to induce platelet desialylation in immune thrombocytopenia (ITP)]8 because none were present in II:1’s plasma, according to a conventional assay. Moreover, we confirmed the absence of a plasma effect on platelet sialylation; when normal platelets were incubated with either normal plasma or patient II:1’s plasma, no difference in the mean ± SEM RCA binding was observed (21552 ± 1003 and 23080 ± 1930 arbitrary units (AU), respectively; n=3). In contrast, the incubation of normal platelets with control plasma in the presence of botrocetin (a positive control) induced greater RCA binding (38845 ± 880 AU, n=3; P<0.001), as expected.9 Next, we investigated the sialylation defect by Western blotting II:1’s platelet lysate. The RCA intensity was four-fold higher in II:1’s platelets than in a control sample (Figure 3C), and several bands were only present in patient II:1 (at around 100 kDa, 70-72 kDa, and 56-58 kDa). Interestingly, treatment with neuraminidase increased the RCA intensity in control experiments but not in experiments with patient II:1’s platelets – indicating that the latter are already markedly hyposialylated. Taken as a whole, our results suggest that SLC35A1 has a major role in platelet sialylation.
Figure 3.
Effect of SLC35A1 mutation on platelet sialylation, life span and proplatelet formation. A) Left panel: the mean fluorescence intensity (MFI) of Ricinus communis agglutinin I (RCA) for three healthy controls and patient II:1 in triplicate. Right panel: the histogram for FITC-RCA labeling on platelets from controls and II:1. Platelet surface β-galactose exposure was determined using flow cytometry with FITC-conjugated RCA (12.5 μg/mL, Vector Labs). Platelet-rich plasma (5 μL) was incubated with 12.5 μg/mL RCA for 30 min at room temperature in a final volume of 100 μL. The reaction was terminated by adding PBS (400 μL), and the samples were immediately analyzed in a flow cytometer (Beckman Coulter). For each sample, the MFI was determined for a total of 20,000 cells. GPIIb glycoprotein was also quantified by using the PLT GP/Receptors® assay (Biocytex). B) The mean ± SEM RCA/GPIIb ratio for three healthy controls and the patient II:1 in triplicate (**P<0.01, ***P<0.001 in Student’s t-test). C) A lectin blot of washed platelets. Washed platelets (3×108/mL) were completely desialylated (or not) by treatment with α2-3,6,8 neuraminidase from Clostridium perfringens (0.2 U/mL, 15 min, Sigma-Aldrich, indicated as Neu). After 15 min, washed platelets from the control and from II:1 were lysed in SDS denaturing buffer (50 mM Tris, 100 mM NaCl, 50 mM NaF, 5 mM EDTA, 40 mM β-glycerophosphate, 100 μM phenylarsine oxide, 1% SDS, 5 μg/mL leupeptin, 10 μg/mL aprotinin, pH 7.4). The proteins were subjected to SDS-PAGE, and were then transferred to nitrocellulose membranes. The latter were incubated with biotinylated RCA (20 μg/mL, Vector Labs). A loading control was monitored by the expression of 14-3-3ζ. Immunoreactive bands were visualized using Enhanced Chemiluminescence Detection Reagents (Pierce). Images of the chemiluminescent signal were captured using G:BOX Chemi XT16 Image Systems software and quantified using Gene Tools software (version 4.0.0.0, Syngene). MW: molecular weights of the protein ladder. D) Measurements of platelet life span in mice. Washed platelets were first stained with 5-chloromethylfluorescein diacetate (CMFDA, 10 mM), washed and then intravenously injected in male NOD SCID gamma mice as a single bolus (108 CMFDA-labeled platelets). Human platelets were monitored by flow cytometry of whole blood samples collected at 1 min and 90 min after injection. The proportion of fluorescent platelets at 90 min was calculated as a percentage of the fluorescent platelets at 1 min. Four mice were injected with 108 platelets from a control, and four other mice were injected with 108 platelets from patient II:1. In the scatter plot, each dot represents a mouse. E) Measurement of proplatelet formation. CD34+ cells were isolated from peripheral blood using an immunomagnetic technique (Miltenyi Biotec). Human TPO (50 nM) was added once to the culture medium on day 0, followed by 20 nM TPO on day 6. On day 12. The MKs were then observed and stained for α-tubulin (magnification: 600x, bottom panel). The coiled element diameters were then measured (right panel). The quantitative data are presented in box and whisker plots; the lower hinge represents the 25th percentile, and the upper hinge represents the 75th percentile. The line within the box marks the median, and the mean is represented by a cross (+). The maxima and minima are at the ends of the whiskers (n=65 MKs in control 1, 31 in control 2, 96 in control 3, and 53 for patient II:1).
Given that the sialic acid content and β-galactose exposure at the platelet surface are highly relevant for platelet clearance and platelet life span, we next measured the clearance of patient II:1’s hyposialylated platelets in mice. Platelet survival was evaluated in vivo after the injection of washed platelets into the circulation of non-obese diabetic/severe combined immunodeficiency (NOD SCID) gamma mice. As shown in Figure 3D, 46 ± 15% of the control platelets were detected in the circulation 90 minutes after platelet injection. In contrast, II:1’s platelets were virtually undetectable - strongly suggesting that the patient’s platelets had a very short life span. Given that the circulating platelet count results from the balance between platelet clearance and platelet production, we next looked at whether a mutation in SLC35A1 disturbed in vitro proplatelet formation in cultured human megakaryocytes (MKs). Peripheral blood CD34+ cells from the controls and patient II:1 were differentiated in vitro in the presence of TPO. We did not observe defective proplatelet formation in the patient’s MKs, which contained normal-sized coiled elements (i.e., future platelets) (Figure 3E). These results suggest that SLC35A1-dependent sialic acid transport in MKs is not involved in platelet formation. Macrothombocytopenia has already been reported in two patients with (respectively) a p.Gln101His mutation10 and a homozygous G>A substitution in the donor splice site of intron 6 in the SLC35A1 gene.11 Furthermore, mutations in the gene coding for glucosamine (UDP-N-acetyl)-2-epimerase/N-acetylmannosamine kinase (GNE) (Figure 1) – an enzyme involved in the CMP-SA pathway – has been linked to macrothombocytopenia.12,13 However, platelet sialylation and life span were not investigated, and a link between thrombocytopenia and defect in platelet sialylation was not highlighted. Herein, we demonstrated that a severe defect in the sialylation pathway resulted in (i) the hyposialylation of platelet glycoproteins, and (ii) a severely shortened platelet half-life. Furthermore, our results suggest that SLC35A1-regulated sialic acid transfer is not involved in proplatelet formation in MKs or that it is compensated for by another pathway. The presence of giant platelets in patient II:1 was probably not directly due to SLC35A1 dysfunction in MKs. The giant platelets might correspond to a compensatory mechanism in a context of thrombocytopenia, as suggested by elevated reticulated platelet levels and a high MK count in the bone marrow.
A better understanding of the mechanism of thrombocytopenia will have consequences for patient care. Indeed, patients with unexplained macrothrombocytopenia are often misdiagnosed with ITP (an exclusion diagnosis). Even though patient II:1 may have experienced ITP in childhood, hyposialylation was responsible for her basal thrombocytopenia. This knowledge will influence the chosen treatment. Given that thrombocytopenia is associated with a shorter life span for hyposialylated platelets, one can legitimately expect the transfusion of normal platelets to be clinically effective. This was confirmed by Willig et al. report on platelet transfusion.14
Overall, our results demonstrated that sialic acid transport by SLC35A1 is required for platelet life span and sialic acid content in humans. A limitation in our study resides in the fact that due to lack of parental consent for additional blood sampling, megakaryocytes and in vivo platelet clearance experiments could only be performed once. The present case report establishes a link between progress in understanding platelet physiology on one hand and patient care on the other.
Supplementary Material
Acknowledgments
The authors wish to thank the patients who participated in this study.
Footnotes
Funding: this study was funded by grants from the INSERM, Force Hémato/Groupe GFHT (to AK), the Agence de Biomedecine (2014) (to JM), EURO-CDG2 and metabERN, and ANR-15RAR3-0004-06 within the framework of E-RARE-3 (to Pd L. and DB).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hoffmeister KM. The role of lectins and glycans in platelet clearance. J Thromb Haemost. 2011;9 Suppl 1:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grozovsky R, Begonja AJ, Liu K, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med. 2015;21(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulson JC, Colley KJ. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989;264(30):17615–17618. [PubMed] [Google Scholar]
- 4.Sorensen AL, Rumjantseva V, Nayeb-Hashemi S, et al. Role of sialic acid for platelet life span: exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood. 2009;114(8):1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan KF, Zhang P, Song Z. Identification of essential amino acid residues in the hydrophilic loop regions of the CMP-sialic acid transporter and UDP-galactose transporter. Glycobiology. 2010;20(6):689–701. [DOI] [PubMed] [Google Scholar]
- 7.Jaeken J, Hennet T, Matthijs G, Freeze HH. CDG nomenclature: time for a change! Biochim Biophys Acta. 2009;1792(9):825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun. 2015;6:7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng W, Xu Y, Chen W, et al. Platelet clearance via shear-induced unfolding of a membrane mechanoreceptor. Nat Commun. 2016;7:12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamed M, Ashikov A, Guillard M, et al. Intellectual disability and bleeding diathesis due to deficient CMP--sialic acid transport. Neurology. 2013;81(7):681–687. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Duncker I, Dupre T, Piller V, et al. Genetic complementation reveals a novel human congenital disorder of glycosylation of type II, due to inactivation of the Golgi CMP-sialic acid transporter. Blood. 2005;105(7):2671–2676. [DOI] [PubMed] [Google Scholar]
- 12.Futterer J, Dalby A, Lowe GC, et al. Mutation in GNE is associated with a severe form of congenital thrombocytopenia. Blood. 2018. June 25 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manchev VT, Hilpert M, Berrou E, et al. A new form of macrothrombocytopenia induced by a germ-line mutation in the PRKACG gene. Blood. 2014;124(16):2554–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willig TB, Breton-Gorius J, Elbim C, et al. Macrothrombocytopenia with abnormal demarcation membranes in megakaryocytes and neutropenia with a complete lack of sialyl-Lewis-X antigen in leukocytes--a new syndrome? Blood. 2001;97(3):826–828. [DOI] [PubMed] [Google Scholar]
- 15.Parente F, Ah Mew N, Jaeken J, Gilfix BM. A new capillary zone electrophoresis method for the screening of congenital disorders of glycosylation (CDG). Clin Chim Acta. 2010;411(1–2):64–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.