Abstract
Daratumumab, a CD38 human monoclonal antibody, demonstrated significant clinical activity in combination with bortezomib and dexamethasone versus bortezomib and dexamethasone alone in the primary analysis of CASTOR, a phase 3 study in relapsed and/or refractory multiple myeloma. A post hoc analysis based on treatment history and longer follow up is presented. After 19.4 (range: 0–27.7) months of median follow up, daratumumab plus bortezomib and dexamethasone prolonged progression-free survival (median: 16.7 versus 7.1 months; hazard ratio, 0.31; 95% confidence interval, 0.24-0.39; P<0.0001) and improved the overall response rate (83.8% versus 63.2%; P<0.0001) compared with bortezomib and dexamethasone alone. The progression-free survival benefit of daratumumab plus bortezomib and dexamethasone was most apparent in patients with 1 prior line of therapy (median: not reached versus 7.9 months; hazard ratio, 0.19; 95% confidence interval, 0.12-0.29; P<0.0001). Daratumumab plus bortezomib and dexamethasone was also superior to bortezomib and dexamethasone alone in subgroups based on prior treatment exposure (bortezomib, thalidomide, or lenalidomide), lenalidomide-refractory status, time since last therapy (≤12, >12, ≤6, or >6 months), or cytogenetic risk. Minimal residual disease–negative rates were >2.5-fold higher with daratumumab across subgroups. The safety profile of daratumumab plus bortezomib and dexamethasone remained consistent with longer follow up. Daratumumab plus bortezomib and dexamethasone demonstrated significant clinical activity across clinically relevant subgroups and provided the greatest benefit to patients treated at first relapse. Trial registration: clinicaltrials.gov identifier: 02136134.
Introduction
As multiple myeloma (MM) progresses, a reduction in the duration and depth of response is observed with each treatment relapse, as a result of diminished sensitivity of heavily treated patients to subsequent therapies.1
Proteasome inhibitors (PIs) are widely used due to their clinical effectiveness, manageable safety profile, and combinability with other therapies.2 However, in several studies of novel PI-based regimens in relapsed and/or refractory MM (RRMM), deep clinical responses were uncommon.3–6 PI-based regimens that generate deeper responses in RRMM are an unmet need.
Daratumumab, a human IgGκ monoclonal antibody targeting CD38, has a direct on-tumor and immunomodulatory mechanism of action.7–12 In combination with standard of care regimens, (bortezomib and dexamethasone [Vd; CASTOR] or lenalidomide and dexamethasone [Rd; POLLUX]), daratumumab induced rapid, deep, and durable responses, reducing the risk of disease progression or death by >60%, versus Vd or Rd in relapsed patients.13,14 Based on the superior progression-free survival (PFS) benefit, daratumumab-Vd (D-Vd) and daratumumab-Rd (D-Rd) were approved in the United States and Europe for MM patients who have received ≥1 prior therapy.15,16 In addition, daratumumab plus pomalidomide and dexamethasone was approved in the United States for MM patients after 2 prior therapies including lenalidomide and a PI.15 More recently, daratumumab in combination with bortezomib, melphalan, and prednisone was approved in the United States for patients with newly diagnosed MM who are ineligible for autologous stem cell transplantation.15
At the time of the event-driven, pre-specified primary analysis (median follow up: 7.4 months) of the CASTOR study, PFS was significantly prolonged with D-Vd versus Vd (median: not reached versus 7.2 months; hazard ratio [HR], 0.39; 95% confidence interval [CI], 0.28-0.53; P<0.0001).13 This updated analysis provides an additional 12 months of follow up for efficacy and safety compared with the primary analysis, including updated PFS in the intent-to-treat population, and presents an exploratory post hoc analysis of CASTOR to identify patient subgroups that may benefit most from D-Vd.
Methods
Study Design
CASTOR (clinicaltrials.gov identifier: 02136134) is an ongoing multi-center, open-label, randomized, active-controlled, phase 3 study of D-Vd versus Vd in patients with RRMM who received ≥1 prior line of therapy. The study design and primary results were previously published.13 Briefly, patients were randomized 1:1 to D-Vd or Vd. Randomization was balanced and stratified by International Staging System (I, II, or III) at screening (central laboratory results), number of prior lines of therapy (1 versus 2 or 3 versus >3), and prior bortezomib exposure (no versus yes). The study protocol was approved by an independent ethics committee or institutional review board at each study center, and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent.
Patients
Eligible patients had ≥1 prior line of therapy, achieved at least a partial response to ≥1 prior MM treatment, and had progressive disease per International Myeloma Working Group (IMWG) criteria17,18 on or after their last regimen. Patients refractory to bortezomib or another PI (ixazomib or carfilzomib following a protocol amendment) were ineligible.
Procedures
Patients received 8 cycles of bortezomib (1.3 mg/m2 subcutaneously on Days 1, 4, 8, 11) and dexamethasone (20 mg orally on Days 1, 2, 4, 5, 8, 9, 11, 12) with or without daratumumab (16 mg/kg intravenously once weekly in Cycles 1-3, Day 1 of Cycles 4-8, then every 4 weeks until disease progression, unacceptable toxicity, or withdrawal of consent). Cycle durations were 21 days for Cycles 1 to 8 and 28 days for Cycle 9 onwards. A protocol amendment after the primary analysis allowed patients who progressed on Vd to receive daratumumab monotherapy.
Assessments and Endpoints
The primary endpoint was PFS; secondary endpoints included time to disease progression, overall response rate (ORR), minimal residual disease (MRD), and safety. This exploratory, post hoc, secondary analysis examined subpopulations according to prior lines of therapy (1, 2-3, >3, or 1-3), prior treatment exposure (bortezomib, thalidomide, or lenalidomide), refractoriness to lenalidomide at the last prior line of therapy, time since last therapy (≤12, >12, ≤6, or >6 months), and cytogenetic risk assessed centrally by next-generation sequencing.19 Site investigators determined numbers of prior lines of therapy using IMWG guidelines.18 Time since last therapy was the duration between the end date of the last line of prior therapy and the randomization date. PFS, ORR, and MRD-negativity at 10−5 and 10−6 sensitivity thresholds were assessed for each subgroup. PFS based on MRD (10−5), and cytogenetic risk status was also examined. Health-related quality of life (HRQoL) was assessed by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 EORTC QLQ-C30 and the EuroQol 5 Dimensions Questionnaire (EQ-5D-5L) tools.
The Online Supplementary Appendix provides full details of statistical analyses and MRD, cytogenetic, and HRQoL assessments.
Results
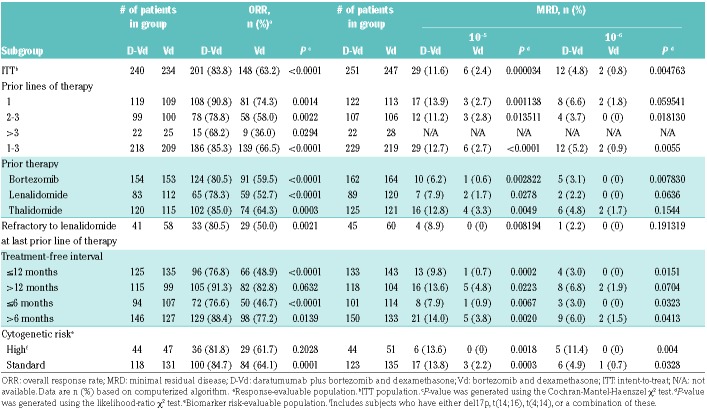
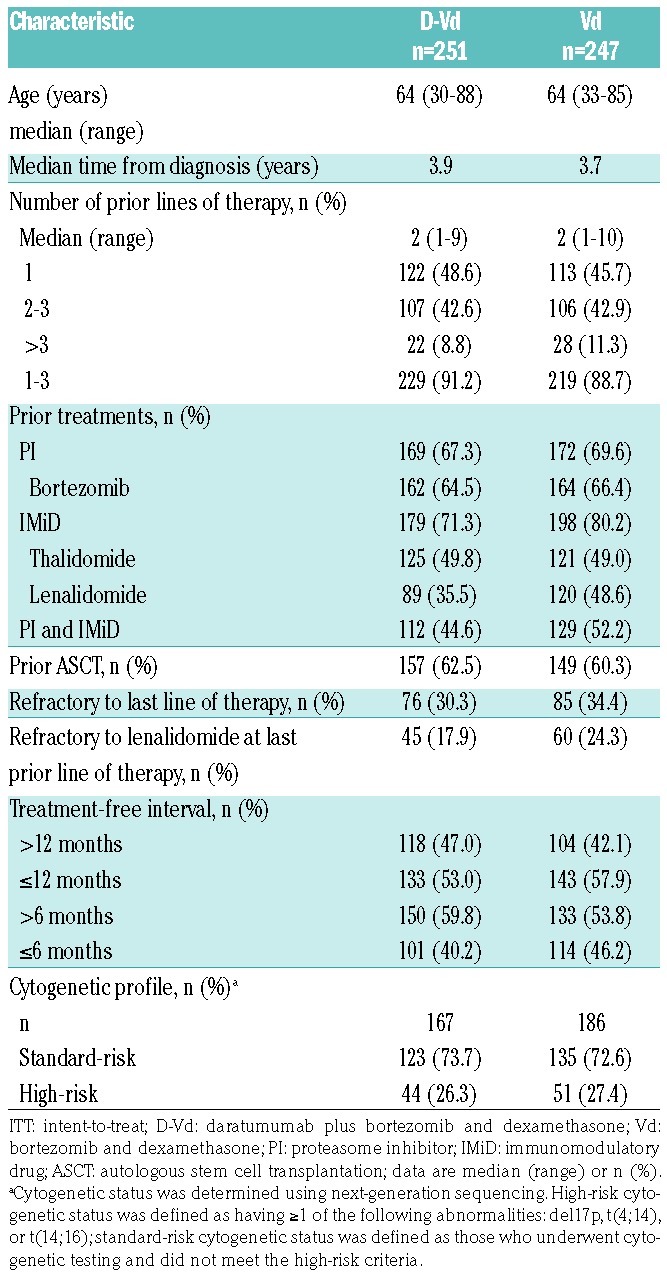
Of 498 patients, 251 and 247 were randomized to D-Vd and Vd, respectively (Online Supplementary Figure S1). Patient demographics and baseline clinical characteristics were previously published and are well-balanced between groups.13 Relevant clinical characteristics, including treatment history and cytogenetic-risk status, were balanced between groups and are summarized in Table 1 and Online Supplementary Table S1. Briefly, patients in CASTOR received a median of 2 prior lines of therapy. Overall, 47.2% received 1 prior line of therapy, 28.9% received 2 prior lines, 13.9% received 3 prior lines, and 10.0% received >3 prior lines of therapy. A total of 21.1% of patients were refractory to lenalidomide at their last line of therapy.
Table 1.
Baseline demographics and clinical characteristics of the ITT population.
Among patients treated with D-Vd, the median duration of treatment was 13.4 months (range: 0-26.7) versus 5.2 months (range: 0.2-8.0) with Vd. Following a protocol amendment after the primary analysis, patients who progressed on Vd had the option to receive daratumumab monotherapy.13 At a median follow up of 19.4 months, all patients in both groups had discontinued or completed Vd treatment per protocol; in the D-Vd group, 41% of patients remained on daratumumab monotherapy. A total of 64 patients in the Vd group opted to receive daratumumab monotherapy following disease progression.
The clinical cut-off date was January 11, 2017. At a median duration of follow up of 19.4 months (range: 0-27.7) months, D-Vd significantly prolonged PFS versus Vd (median: 16.7 versus 7.1 months; HR, 0.31; 95% CI, 0.24-0.39; P<0.0001 [Figure 1A]), with 18-month PFS rates of 48.0% and 7.9%, respectively. Among response-evaluable patients (D-Vd, n=240; Vd, n=234), ORR was significantly improved with D-Vd versus Vd (83.8% versus 63.2%; P<0.0001 [Table 2]), including higher rates of stringent complete response (CR) (8.8% versus 2.6%), CR or better (28.8% versus 9.8%; P<0.0001), and very good partial response or better (62.1% versus 29.1%; P<0.0001 [Online Supplementary Table S2]).
Figure 1.
PFS (A) in the ITT population and (B) in patients who received 1 prior line of therapy or (C) 2 to 3 prior lines of therapy. Kaplan-Meier estimates of PFS. in (A) the the ITT population and in patients who received (B) 1 prior line of therapy or (C) 2 to 3 prior lines of therapy. D-Vd: daratumumab plus bortezomib and dexamethasone; Vd: bortezomib and dexamethasone; HR: hazard ratio; CI: confidence interval.
Table 2.
ORR and MRD based on prior treatment history.
MRD was evaluated for the ITT population at pre-specified time points using a stringent, unbiased approach with IMWG criteria of a minimum sensitivity threshold of 10−5 for next-generation sequencing evaluation.20 At this threshold, 11.6% of D-Vd–treated patients were MRD-negative versus 2.4% of Vd-treated patients (P=0.000034 [Table 2]). Consistent findings were observed at a higher sensitivity threshold of 10−6 (D-Vd: 4.8%; Vd: 0.8%; P=0.004763). Overall survival (OS) remained immature at the time of this analysis, and survival follow up will continue until 320 deaths are reported, per protocol.
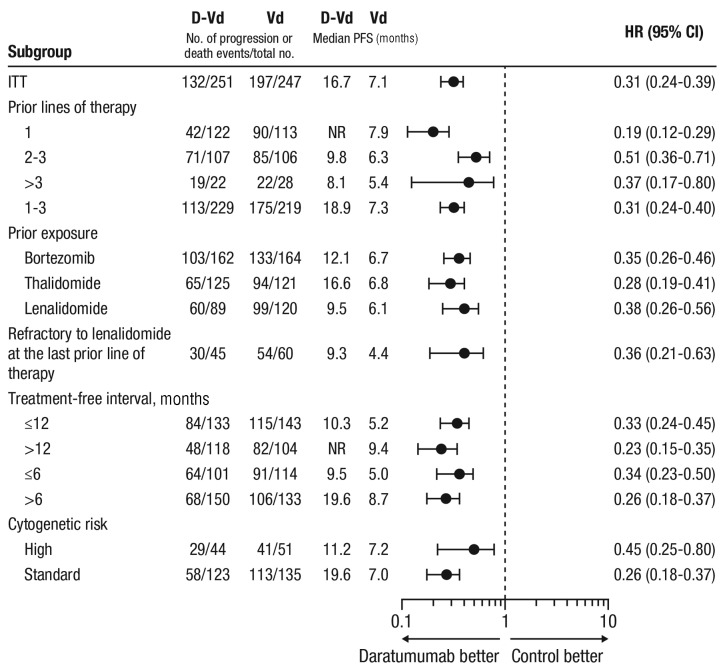
Subgroup analyses showed the clinical benefit of daratumumab by prolonging PFS and improving ORR and MRD negativity across all clinical populations (Table 2 and Figure 2). Patients who received D-Vd at first relapse (D-Vd, n=122; Vd, n=113) achieved the greatest benefit [Table 2 and Figure 2]. In this population, PFS was significantly prolonged with D-Vd versus Vd (median: not reached versus 7.9 months; HR, 0.19; 95% CI, 0.12-0.29; P<0.0001 [Figure 1B]), an 81% reduction in the risk of disease progression or death with 18-month PFS of 68.0% versus 11.5%, respectively. Among patients with 2 to 3 prior lines of therapy (D-Vd, n=107; Vd, n=106), PFS was also significantly prolonged with D-Vd versus Vd (median: 9.8 versus 6.3 months; HR, 0.51; 95% CI, 0.36-0.71; P<0.0001), with 18-month PFS of 31.2% versus 5.5%, respectively (Figure 1C). Likewise, in patients with 1 to 3 prior lines of therapy (D-Vd, n=229; Vd, n=219), D-Vd significantly prolonged PFS versus Vd (median: 18.9 versus 7.3 months; HR, 0.31; 95% CI, 0.24-0.40; P<0.0001), with 18-month PFS rates of 51.2% versus 8.7%, respectively (Online Supplementary Figure S2).
Figure 2.
PFS based on prior treatment history and cytogenetic risk (ITT population). Subgroup analysis of PFS based on prior lines of therapy, prior treatment exposure, refractoriness to lenalidomide at the last prior line of therapy, treatment-free interval, and cytogenetic risk. Patients with high-risk cytogenetics had any of t(4;14), t(14;16), or del17p cytogenetic abnormalities as determined by central next-generation sequencing. Standard-risk patients had an absence of high-risk abnormalities. PFS: progression-free survival; ITT: intent-to-treat; D-Vd: daratumumab plus bortezomib and dexamethasone; Vd: bortezomib and dexamethasone; CI: confidence interval; NR: not reached.
The PFS benefit of daratumumab was maintained in patients who received prior bortezomib (D-Vd, n=162; Vd, n=164; median: 12.1 versus 6.7 months; HR, 0.35; 95% CI, 0.26-0.46; P<0.0001 [Online Supplementary Figure S3]), with 18-month PFS rates of 37.9% and 1.8%, respectively. In this subgroup, D-Vd improved ORR (80.5% versus 59.5%) and increased MRD-negative rates (6.2% versus 0.6%) versus Vd (Table 2). Importantly, the PFS benefit of daratumumab was maintained in patients who received prior bortezomib in their only line of therapy (D-Vd, n=62; Vd, n=57; median: 19.6 versus 8.0 months; HR, 0.20; 95% CI, 0.12-0.35; P<0.0001 [Online Supplementary Figure S4]), with 18-month PFS rates of 58.1% and 2.1%, respectively.
Patients refractory to lenalidomide at their last prior line of therapy (D-Vd, n=45; Vd, n=60) also achieved a significant PFS benefit with D-Vd versus Vd (median: 9.3 versus 4.4 months; HR, 0.36; 95% CI, 0.21-0.63; P=0.0002 [Figure 2]), with 18-month PFS rates of 33.5% versus 2.0%, respectively. In this subgroup, D-Vd improved ORR (80.5% versus 50.0%) and increased MRD negativity (8.9% versus 0%) versus Vd [Table 2].
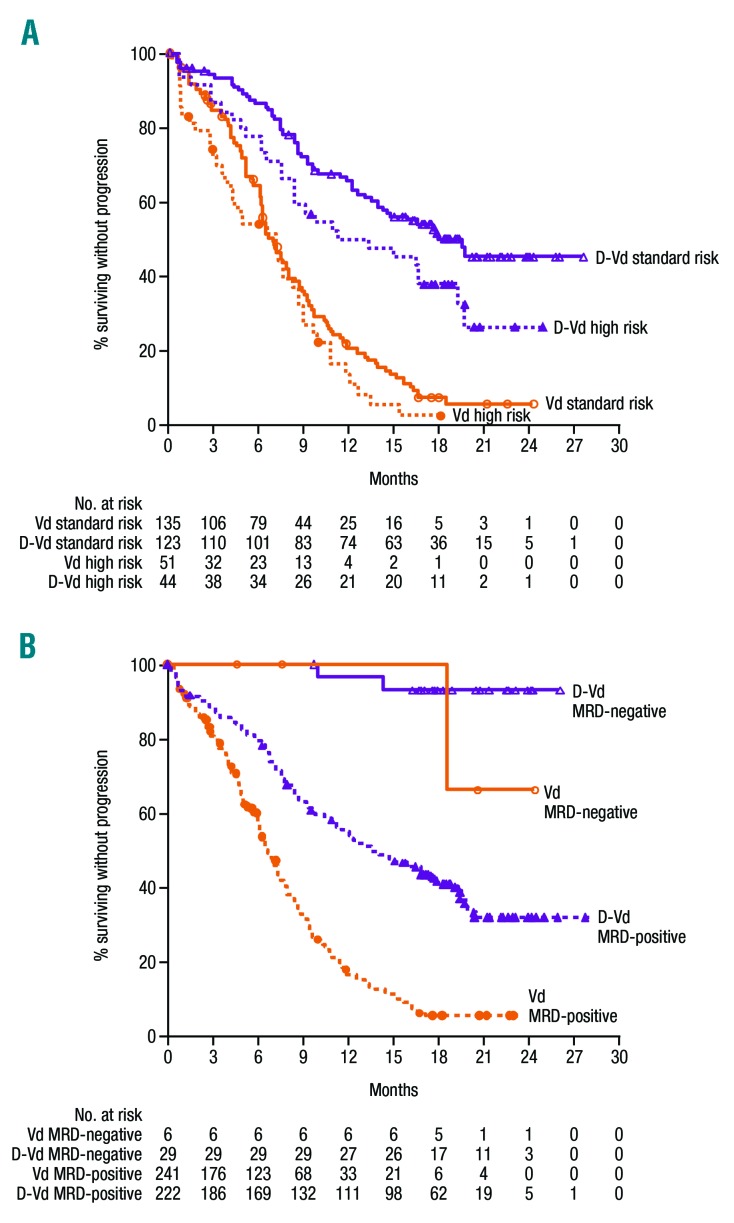
In a pre-specified subgroup analysis of cytogenetic risk, D-Vd prolonged PFS and improved ORR versus Vd (Table 2, Figures 2 and 3A). PFS was prolonged with D-Vd versus Vd in both high-risk (median: 11.2 versus 7.2 months; HR, 0.45; 95% CI, 0.25-0.80; P=0.0053; D-Vd, n=44; Vd, n=51) and standard-risk disease (median: 19.6 versus 7.0 months; HR: 0.26; 95% CI, 0.18-0.37; P<0.0001; D-Vd, n=123; Vd, n=135 [Figures 2 and 3A]). ORRs were higher with D-Vd for both high-risk (D-Vd, n=44; Vd, n=47; 81.8% versus 61.7%; P=0.2028) and standard-risk (D-Vd, n=118; Vd, n=131; 84.7% versus 64.1%; P=0.0001) subgroups (Table 2). Higher D-Vd response rates aligned with MRD negativity. In the D-Vd group, 13.8% (17/123) of evaluable, standard-risk patients reached MRD negativity at 10−5 sensitivity versus 2.2% (3/135) in the Vd group (P=0.0003 [Table 2]). No high-risk Vd group patients (n=51) achieved MRD negativity at 10−5, unlike 13.6% (6/44) of high-risk D-Vd group patients (P=0.0018). The PFS benefit of D-Vd versus Vd was also maintained irrespective of the time since last therapy (≤12, >12, ≤6, or >6 months [Figure 2]).
Figure 3.
PFS survival based on (A) cytogenetic risk and (B) MRD status. (A) Kaplan-Meier estimates of PFS among patients evaluated for cytogenetic risk. High-risk patients had any of t(4;14), t(14;16), or del17p cytogenetic abnormalities as determined by central next-generation sequencing. Standard-risk patients had an absence of high-risk abnormalities. (B) Kaplan-Meier estimates of PFS among patients in the ITT population population. MRD-negative status was evaluated at a sensitivity threshold of 10−5 using bone marrow aspirate samples that were prepared using Ficoll and analyzed by the clonoSEQ® assay. MRD: minimal residual disease; D-Vd: daratumumab plus bortezomib and dexamethasone; Vd: bortezomib and dexamethasone.
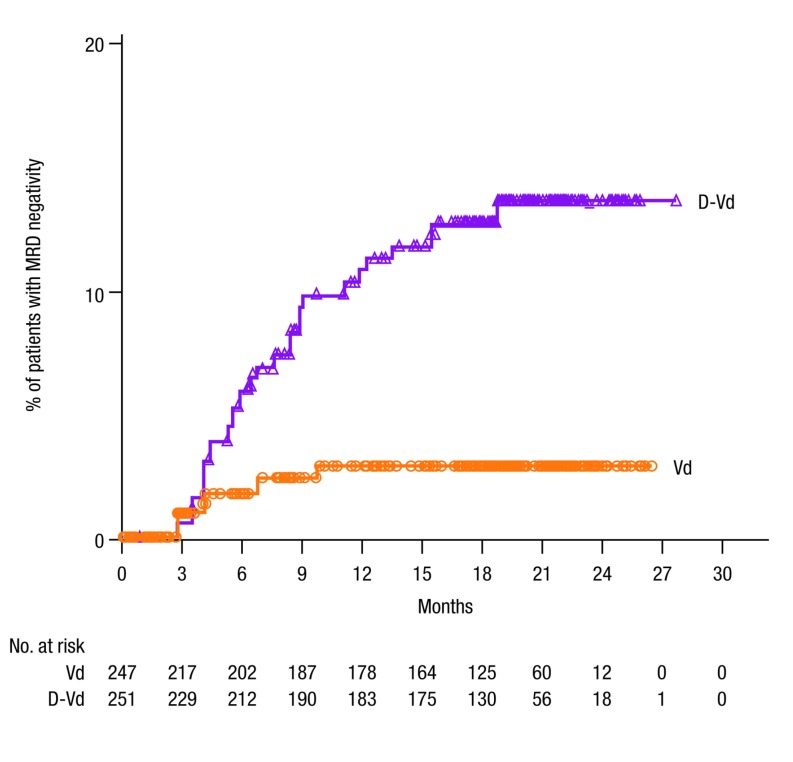
Regardless of treatment group, PFS was prolonged in patients who achieved MRD-negative status (median: not reached in either group [Figure 3B]). Conversely, among patients with MRD-positive status (10−5), D-Vd significantly prolonged PFS versus Vd (median: not reached versus 16.2 months; HR, 0.19; 95% CI, 0.05-0.73; P=0.0080 [Figure 3B]). The rate of MRD-negativity (10–5) continued to increase over time for patients in the overall study population who received D-Vd versus Vd (Figure 4).
Figure 4.
Time to MRD negativity in the ITT population. MRD-negative status was evaluated over time at a sensitivity threshold of 10−5 using bone marrow aspirate samples that were prepared using Ficoll and analyzed by the clonoSEQ® assay. MRD: minimal residual disease; D-Vd: daratumumab plus bortezomib and dexamethasone; Vd: bortezomib and dexamethasone.
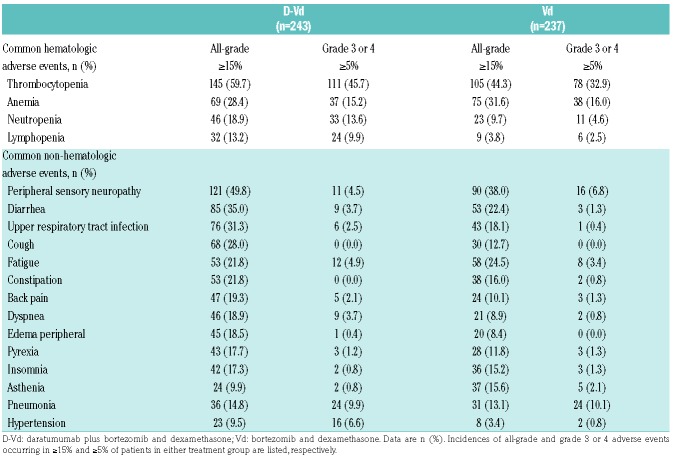
Within the safety population (D-Vd, n=243; Vd, n=237), longer follow up revealed a tolerability profile consistent with the primary analysis and no new emergent toxicities. Among the most common (≥15%) hematologic treatment-emergent adverse events (TEAEs) were thrombocytopenia and anemia. Among the most common (≥15%) non-hematologic TEAEs were peripheral sensory neuropathy, diarrhea, upper respiratory tract infection, and cough (Table 3).
Table 3.
Adverse events in the safety population.
The most common (≥5%) grade 3 or 4 hematologic TEAEs included thrombocytopenia, anemia, neutropenia, and lymphopenia (Table 3). The most common (≥5%) grade 3 or 4 non-hematologic TEAEs included pneumonia, hypertension, and peripheral sensory neuropathy. Discontinuations due to TEAEs remained low and balanced between groups (D-Vd: 9.5%; Vd: 9.3%). Transfusions were received by 26.3% versus 20.3% of patients (D-Vd versus Vd).
With longer follow up, second primary malignancies (SPMs) occurred in 10 (4.1%) patients who received D-Vd (4 new cases following the primary analysis13 included basal and squamous cell carcinoma, Bowen disease, and prostate cancer) versus 1 (0.4%) patient who received Vd (no new cases with longer follow up).
The EORTC QLQ-C30 and EQ-5D-5L tools showed that HRQoL was maintained during treatment for patients in both groups who remained on the study. Significant differences in the least squares mean changes from baseline were not observed between D-Vd and Vd at any time for the EORTC QLQ-C30 Global Health Status Scores or the EQ-5D-5L Utility Score. A significant difference was observed solely at Week 21 in favor of D-Vd for the Visual Analog Scale Score (P=0.0185). No significant differences in EORTC QLQ-C30 global health status were observed for median time to improvement (5.0 versus 5.1 months; HR, 0.99; 95% CI, 0.76-1.29; P=0.9163). Similarly, no significant differences in median time to improvement were observed for either the EQ-5D-5L Utility Score (7.7 versus 3.5 months; HR, 0.82; 95% CI, 0.62-1.08; P=0.1469) or the Visual Analog Scale Score (5.0 versus 5.0 months; HR, 1.03; 95% CI, 0.79-1.35; P=0.8072).
Discussion
These data confirm that D-Vd provides significant clinical benefit to patients with RRMM. D-Vd prolonged PFS, resulting in a 69% reduction in the risk of disease progression or death versus Vd. With an additional 12 months of follow up, responses to daratumumab deepened over time (≥CR: 28.8%) compared with the primary analysis (19.2%).13 Deeper responses to D-Vd were associated with significantly higher (>4 fold) MRD-negative rates at sensitivities of 10−5 and 10−6 versus Vd. We hypothesize that, as previous studies have demonstrated a correlation between MRD negativity and OS,21,22 this may translate into improved OS outcomes after longer follow up for patients treated with D-Vd. Analysis of OS is ongoing.
There were consistent clinical benefits with D-Vd versus Vd across subgroups based on prior lines of therapy, treatment exposure, or refractory status. These were also observed in patients regardless of time since last therapy or cytogenetic risk, as those patient subgroups were not evaluated in the primary analysis. Importantly, the benefit of D-Vd was maintained in patients who received prior bortezomib (including as their sole prior line of therapy) and those refractory to lenalidomide at their last prior line of therapy. Bortezomib- and lenalidomide-based combinations are common MM first-line and maintenance regimens. Thus D-Vd can be considered after bortezomib (if patients are not PI-refractory) or in lenalidomide-refractory patients, which is of particular importance considering the increased lenalidomide use as maintenance therapy in newly diagnosed MM regardless of transplant eligibility.23,24 D-Vd significantly prolonged PFS versus Vd across all lines of therapy with the greatest benefit achieved in patients who received 1 prior line in comparison to those who received 2 to 3 or >3 prior lines of therapy. Response rates, including the rates of MRD-negativity, were also highest in patients who received 1 prior line of therapy. As D-Vd showed the greatest benefit at first relapse, it may represent an optimal second-line treatment for patients after frontline lenalidomide or bortezomib.
The benefit of D-Vd was also maintained in patients regardless of cytogenetic risk, as D-Vd but not Vd induced MRD negativity in high-risk patients, suggesting that this combination may improve historically poor outcomes in this population.25–28
D-Vd–treated patients continued to receive daratumumab monotherapy after completing 8 cycles of Vd, reflected by the longer treatment duration (median: D-Vd, 13.4 months; Vd, 5.2 months). With longer follow up, the depth of response in the D-Vd arm, including CR rates and MRD negativity, continued to improve over time after patients entered the monotherapy phase, supporting the benefit of continued daratumumab treatment. Analyses are ongoing to quantify the therapeutic impact of maintenance therapy with single-agent daratumumab.
This was the first randomized, phase 3 clinical trial of RRMM with prospective MRD evaluation. MRD-negative status was associated with prolonged PFS in both treatment groups, but D-Vd increased MRD-negative rates at all sensitivity thresholds and evaluated subgroups. Additional longitudinal MRD evaluation in CASTOR is ongoing and the potential benefit of daratumumab-induced MRD negativity is being explored in studies of newly diagnosed MM (ALCYONE clinicaltrials.gov identifier 02195479; clinicaltrials.gov identifier 02252172; clinicaltrials.gov identifier 02541383; clinicaltrials.gov identifier 03301220. These studies aim to further validate MRD-negative status as a surrogate study endpoint.
Several new agents for RRMM have been approved based on robust clinical data, including carfilzomib29 and ixazomib30 (second-generation PIs), pomalidomide31,32 (a third-generation immunomodulatory drug), daratumumab13,14,33–35 and elotuzumab36 (monoclonal antibodies), and panobinostat4 (a histone deacetylase inhibitor). Approvals of many of these agents were based on superiority of PFS in phase 3 trials. These studies are beginning to report OS outcomes. In the ENDEAVOR study, carfilzomib and dexamethasone conferred an additional OS benefit of 7.6 months versus Vd.37 OS analysis in CASTOR is ongoing.
Clinical trials are not usually powered to determine optimal treatment sequencing or the most effective regimen for each disease subset.38 Although meta-analyses provide useful guides for selecting treatment options, physicians need to consider many different factors to optimize individual regimens, including numbers and types of prior regimens, duration of response to prior therapy, toxicities with prior therapies, disease aggressiveness, and performance status or frailty.38,39 Based on the current findings, and the findings of other studies,40 daratumumab combined with other anti-myeloma drugs such as bortezomib or lenalidomide, may provide significant benefit in patients with early relapsed MM regardless of prior treatment exposure. It remains to be seen whether this translates to prolonged survival.
The safety profile of D-Vd remained unchanged with approximately 1 year of additional follow up from the primary analysis,13 with no new unexpected TEAEs observed. Preliminary data indicated that adding a third agent to Vd did not worsen HRQoL, an evaluation that was not presented in the primary analysis. More SPMs were reported with D-Vd versus Vd (4.1% versus 0.4%); this rate is similar to the incidence of SPMs reported for patients in POLLUX (5.7% for both D-Rd and Rd; manuscript in preparation) and for RRMM patients in general (between 1%-6%).41 At clinical cut-off, all patients in the Vd group had discontinued or completed 8 treatment cycles, whereas 41% of patients receiving D-Vd remained on daratumumab treatment. Therefore, more frequent monitoring during active treatment may explain why a greater number of TEAEs (including grade 3 or 4 events) and SPMs were reported with D-Vd. After 8 cycles of D-Vd, patients were monitored every 4 weeks during daratumumab dosing, whereas patients who received Vd who did not receive daratumumab monotherapy were followed for survival via phone calls every 16 weeks following disease progression.
In conclusion, the original finding of significant benefit of D-Vd over Vd was confirmed regardless of treatment history or cytogenetic risk. Importantly, this clinical benefit was achieved without any emergent safety issues or decline in HRQoL. These results provide further support for the addition of daratumumab to a standard of care regimen in RRMM, particularly at first relapse. The CASTOR study is ongoing, and the feasibility of MRD negativity as a surrogate for OS in RRMM continues to be investigated. An analysis of OS will be conducted after 320 events are observed.
Supplementary Material
Acknowledgments
Medical writing and editorial support were provided by Jason Jung, PhD and Kristin Runkle, PhD of MedErgy, and were funded by Janssen Global Services, LLC.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/12/2079
Funding
This study was sponsored by Janssen Research & Development, LLC. The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
References
- 1.Kurtin SE. Relapsed or relapsed/refractory multiple myeloma. J Adv Pract Oncol. 2013;4(Suppl 1):5–14. [Google Scholar]
- 2.Merin NM, Kelly KR. Clinical use of proteasome inhibitors in the treatment of multiple myeloma. Pharmaceuticals (Basel). 2015;8(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. [DOI] [PubMed] [Google Scholar]
- 4.San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–1206. [DOI] [PubMed] [Google Scholar]
- 5.San-Miguel JF, Hungria VTM, Yoon SS, et al. Final analysis of overall survival from the Phase 3 panorama 1 trial of panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2015;126(23):3026. [Google Scholar]
- 6.Jakubowiak A, Offidani M, Pegourie B, et al. Randomized phase 2 study of elotuzumab plus bortezomib/dexamethasone (Bd) versus Bd for relapsed/refractory multiple myeloma. Blood. 2016;127(33):2833–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848. [DOI] [PubMed] [Google Scholar]
- 8.Lammerts van Bueren J, Jakobs D, Kaldenhoven N, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124(21):3474. [Google Scholar]
- 9.Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol. 2016;197(3):807–813. [DOI] [PubMed] [Google Scholar]
- 11.van de Donk NWCJ, Janmaat ML, Mutis T, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270(1):95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. [DOI] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331. [DOI] [PubMed] [Google Scholar]
- 15.DARZALEX® (daratumumab) injection, for intravenous use [package insert]. Horsham, PA: Janssen Biotech, Inc.; 2018. [Google Scholar]
- 16.European Medicines Agency. DARZALEX summary of product characteristics, May 2016. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004077/WC500207296.pdf Last accessed July 2018.
- 17.Durie BGM, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9): 1467–1473. [DOI] [PubMed] [Google Scholar]
- 18.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18): 4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu C, Soong D, Spicka I, et al. Next generation sequencing (NGS) methodology for determining cytogenetic risk status in the daratumumab phase 3 CASTOR and POLLUX studies in relapsed or refractory multiple myeloma (RRMM). Presented at: the 22nd Congress of the European Hematology Association (EHA); June 22-25, 2017; Madrid, Spain Abstract S100. [Google Scholar]
- 20.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123(20):3073–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawstron AC, Gregory WM, de Tute RM, et al. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood. 2015;125(12):1932–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy PL, Palumbo A. Maintenance therapy for multiple myeloma. Hematol Oncol Clin North Am. 2014;28(5):839–859. [DOI] [PubMed] [Google Scholar]
- 24.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917. [DOI] [PubMed] [Google Scholar]
- 25.Dimopoulos MA, Weisel KC, Song KW, et al. Cytogenetics and long-term survival of patients with refractory or relapsed and refractory multiple myeloma treated with pomalidomide and low-dose dexamethasone. Haematologica. 2015;100(10):1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109(8):3489–3495. [DOI] [PubMed] [Google Scholar]
- 27.Avet-Loiseau H, Hulin C, Campion L, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the intergroupe francophone du myelome experience. J Clin Oncol. 2013;31(22):2806–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28(2): 269–277. [DOI] [PubMed] [Google Scholar]
- 29.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. [DOI] [PubMed] [Google Scholar]
- 30.Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–1634. [DOI] [PubMed] [Google Scholar]
- 31.Richardson PG, Siegel DS, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123(12):1826–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. [DOI] [PubMed] [Google Scholar]
- 33.Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207–1219. [DOI] [PubMed] [Google Scholar]
- 34.Lonial S, Weiss BM, Usmani S, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–1560. [DOI] [PubMed] [Google Scholar]
- 35.Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–631. [DOI] [PubMed] [Google Scholar]
- 37.Dimopoulos MA, Goldschmidt H, Niesvizky R, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327–1337. [DOI] [PubMed] [Google Scholar]
- 38.Boudreault JS, Touzeau C, Moreau P. Triplet combinations in relapsed/refractory myeloma: update on recent phase 3 trials. Expert Rev Hematol. 2017;10(3):207–215. [DOI] [PubMed] [Google Scholar]
- 39.Yee AJ, Raje NS. Sequencing of nontransplant treatments in multiple myeloma patients with active disease. Hematology Am Soc Hematol Educ Program. 2016;2016(1):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Beurden-Tan CH, Franken MG, Blommestein HM, Uyl-de Groot CA, Sonneveld P. Systematic literature review and network meta-analysis of treatment outcomes in relapsed and/or refractory multiple myeloma. J Clin Oncol. 2017;35:1313–1319. [DOI] [PubMed] [Google Scholar]
- 41.Areethamsirikul N, Reece DE. The risk of secondary primary malignancies after therapy for multiple myeloma. Leuk Lymphoma. 2015;56(11):3012–3021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.