Abstract
In standard-risk acute promyelocytic leukemia, recent results have shown that all-trans retinoic acid plus arsenic trioxide combinations are at least as effective as classical all-trans retinoic acid plus anthracycline-based chemotherapy while being less myelosuppressive. However, the role of frontline arsenic trioxide is less clear in higher-risk acute promyelocytic leukemia, and access to arsenic remains limited for front-line treatment of standard-risk acute promyelocytic leukemia in many countries. In this randomized trial, we compared arsenic, all-trans retinoic acid and the “classical” cytarabine for consolidation treatment (after all-trans retinoic acid and chemotherapy induction treatment) in standard-risk acute promyelocytic leukemia, and evaluated the addition of arsenic during consolidation in higher-risk disease. Patients with newly diagnosed acute promyelocytic leukemia with a white blood cell count <10x109/L, after an induction treatment consisting of all-trans retinoic acid plus idarubicin and cytarabine, received consolidation chemotherapy with idarubicin and cytarabine, arsenic or all-trans retinoic acid. Patients with a white blood cell count >10x109/L received consolidation chemotherapy with or without arsenic. Overall, 795 patients with acute promyelocytic leukemia were enrolled in this trial. Among those with standard-risk acute promyelocytic leukemia (n=581), the 5-year event-free survival rates from randomization were 88.7%, 95.7% and 85.4% in the cytarabine, arsenic and all-trans retinoic acid consolidation groups, respectively (P=0.0067), and the 5-year cumulative incidences of relapse were was 5.5%, 0% and 8.2%. (P=0.001). Among those with higher-risk acute promyelocytic leukemia (n=214), the 5-year event-free survival rates were 85.5% and 92.1% (P=0.38) in the chemotherapy and chemotherapy plus arsenic groups, respectively, and the corresponding 5-year cumulative incidences of relapse were 4.6% and 3.5% (P=0.99). Given the prolonged myelosuppression that occurred in the chemotherapy plus arsenic arm, a protocol amendment excluded cytarabine during consolidation cycles in the chemotherapy plus arsenic group, resulting in no increase in relapse. Our results therefore advocate systematic introduction of arsenic in the first-line treatment of acute promyelocytic leukemia, but probably not concomitantly with intensive chemotherapy, a situation in which we found myelosuppression to be significant. (ClinicalTrials.gov Identifier: NCT00378365)
Introduction
Acute promyelocytic leukemia (APL) is a specific subtype of acute myeloid leukemia (AML) characterized by its morphology, the presence of t(15;17), and marked sensitivity to the differentiating effect of all-trans retinoic acid (ATRA) and the pro-apoptotic effect of arsenic trioxide (ATO).1 The combination of ATRA and anthracycline-based chemotherapy has been the mainstay of the treatment of newly diagnosed APL over the last two decades.2–4 Published results have shown that cytarabine (cytosine arabinoside, AraC) could be omitted from chemotherapy in standard-risk APL [i.e., with a baseline white blood cell count (WBC) <10x109/L] but appeared to be useful in high-risk APL (with a WBC >10x109/L), possibly at high doses, to reduce the incidence of relapse.5 A beneficial role for prolonged maintenance treatment with continuous low-dose chemotherapy (6-mercaptopurine and methotrexate) and intermittent ATRA was also suggested, especially in high-risk APL, following in particular randomized results from our group,5,6 and from a recent meta-analysis7 of several trials. With regards to anthracyclines, at least one study suggested that idarubicin gave better results than daunorubicin,8 while non-randomized studies suggested a potential benefit of adding ATRA during consolidation cycles, at least if AraC was omitted.2,4
Recently, however, ATO has been demonstrated to have pronounced efficacy in newly diagnosed APL. In particular, it was shown in two large randomized trials that the combination of ATO and ATRA without chemotherapy was at least equal and, with longer term follow-up, even superior to ATRA plus chemotherapy combinations in standard-risk APL.9,10,11 In high-risk APL, ATO plus ATRA combinations, with very limited added chemotherapy, also appear very promising,10,12 and are currently being compared with the conventional ATRA chemotherapy approach in randomized trials.
When the APL 2006 trial was launched, ATO was mainly considered as an adjunct to ATRA chemotherapy combinations in the first-line treatment of APL, aimed at reducing the relapse rate (especially in high-risk APL) and/or diminishing the amount of chemotherapy administered (especially in standard-risk APL).
Based on the results of the APL 2006 trial, reported here, we evaluated the role of ATO in the treatment of standard-and high-risk APL, in addition to the “classical” ATRA plus chemotherapy backbone regimens.
Methods
Patients
Between 2006 and 2013, patients from French, Belgian and Swiss centers with documented (by cytogenetics and or molecular biology), newly diagnosed APL who were aged 70 years or less were eligible for inclusion in the APL 2006 trial, after giring informed consent. The trial was approved by local ethical committees (ClinicalTrials.gov Identifier: NCT00378365). Eligibility criteria in this trial were a morphological diagnosis of APL based on French-American-British criteria and no contraindication to intensive chemotherapy. No minimal performance status was required and patients with therapy-related APL could be enrolled.
Induction treatment consisted of ATRA 45 mg/m2/day until complete remission with idarubicin 12 mg/m2/day for 3 days and AraC 200 mg/m2/day for 7 days starting on day 3.
Patients with a baseline WBC <10x109/L who achieved a complete remission were randomized for consolidation between three groups given treatment containing AraC, ATO or ATRA. The AraC group (standard group) received a first consolidation course with idarubicin 12 mg/m2/day for 3 days and AraC 200 mg/m2/day for 7 days, a second consolidation course with idarubicin 9 mg/m2/day for 3 days and AraC 1 g/m2/12 h for 4 days, and maintenance therapy for 2 years with intermittent ATRA 15 days/3 months and continuous treatment with 6 mercaptopurine (90 mg/m2/day orally) and methotrexate (15 mg/m2/week orally).
The ATO and ATRA groups received the same treatment as the AraC group, but AraC was replaced by, respectively, ATO 0.15 mg/kg/day on days 1 to 25 and ATRA 45 mg/m2/day on days 1 to 15 for both consolidation courses. The rationale for the ATRA consolidation treatment was based on results of a Spanish PETHEMA group trial, suggesting that AraC could be omitted from chemotherapy consolidation cycles in standard-risk APL, and that there could be a benefit from adding ATRA to consolidation cycles. The use of prolonged maintenance treatment was based on our previous results in a randomized phase III trial supporting the interest of this approach in reducing relapses after a conventional ATRA chemotherapy regimen.
Patients with a baseline WBC >10x109/L were randomized to consolidation with either chemotherapy or chemotherapy combined with ATO. The chemotherapy group received a first consolidation course with idarubicin 12 mg/m2/day for 3 days and AraC 200 mg/m2/day for 7 days, a second consolidation course with idarubicin 9 mg/m2/day for 3 days and AraC 1 g/m2/12 h for 4 days, and 2-year maintenance therapy with intermittent ATRA and continuous 6-mercaptopurine plus methotrexate. The chemotherapy plus ATO group received the same treatment except that ATO 0.15 mg/kg/day was added from day 1 to day 25 during both consolidation courses. After a first interim analysis in September 2010 on data from 81 patients, AraC was deleted from consolidation cycles of the chemotherapy plus ATO group.
Treatment of coagulopathy during the induction phase was based on platelet support to maintain the platelet count at a level greater than 50x109/L until the disappearance of the coagulopathy. The use of heparin, tranexamic acid, fresh-frozen plasma, and fibrinogen transfusions was optional, according to each center’s policy.
Prophylaxis and treatment of ATRA syndrome consisted of dexamethasone 10 mg/12 h given intravenously for at least 3 days if the WBC was above 10x109/L (before or during treatment with ATRA) or at the earliest sign of the ATRA syndrome (dyspnea, lung infiltrates, pleural effusion, unexplained renal failure). In the absence of rapid improvement of symptoms (within 24 h), ATRA was transiently stopped until clinical control was obtained.
Statistical methods
The primary endpoint was event-free survival from the time of achieving complete remission. Relapse, survival, side effects of the treatment and duration of hospitalization were secondary endpoints.
Analyses were performed on a modified intent-to-treat principle, excluding only diagnostic errors and withdrawals of consent. Censored endpoints were estimated by the nonparametric Kaplan-Meier method13 and then compared between randomized groups by the log-rank test. In estimating relapses, we took into account competing risks, i.e., deaths in first complete remission, using cumulative incidence curves and then compared results using the Gray test, whereas a cause-specific Cox model was used to estimate cause-specific hazard ratios.14 The type I error was fixed at the 5% level. All tests were two-tailed. Statistical analyses were performed using SAS 9.1 (SAS Inc, Cary, NC, USA) and R software packages.
Here we present the results based on all patients included in the trial and data collected before June, 2017.
Results
Eight-hundred and seven patients were included in the trial. The diagnosis of APL could be confirmed in 795 of the patients who had t(15;17) and/or a PML-RAR rearrangement. The remaining 12 patients were excluded as diagnostic errors. The further analyses only dealt with the 795 patients with a confirmed diagnosis of APL who gave their consent to participation in the study and comprised 581 patients with standard-risk APL and 214 with high-risk APL.
Standard-risk acute promyelocytic leukemia
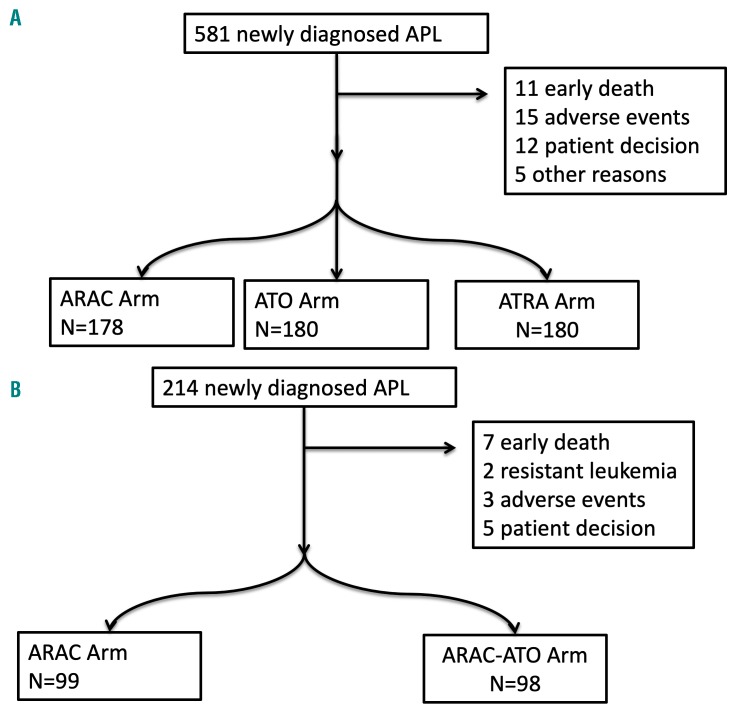
Of the 581 patients with standard-risk APL; 570 (98.1%) achieved a complete remission; the others died early. Forty-three patients were not randomized for consolidation treatment, including the 11 patients who did not achieve a complete remission, 15 due to adverse events, 12 due to the patients’ decision and five for other reasons (Figure 1).
Figure 1.
Consort diagrams. (A) Patients with a white blood cell count <109/L; (B) Patients with a white blood cell count >109/L. APL: acute promyelocytic leukemia; ARAC: cytarabine; ATO: arsenic trioxide; ATRA: all-trans retinoic acid.
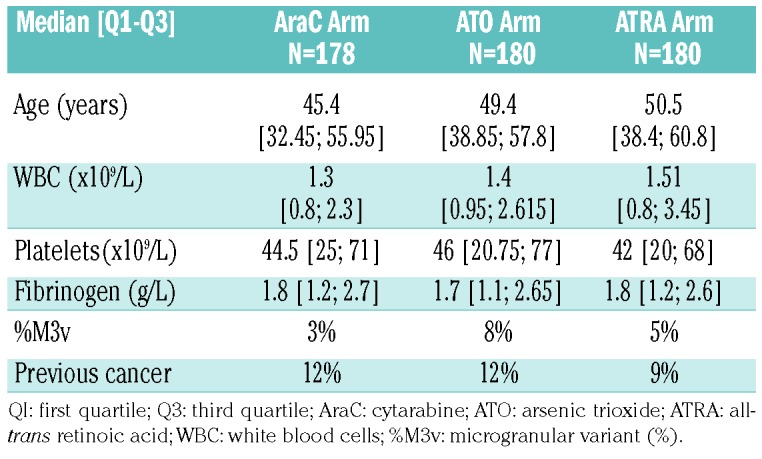
Five-hundred and thirty-eight patients were randomized to different consolidation treatment (178, 180 and 180 in the AraC, ATO and ATRA arms, respectively). Pre-treatment characteristics were well-balanced between the three consolidation groups (Table 1).
Table 1.
Baseline characteristics of the patients aged <70 years with standard-risk acute promyelocytic leukemia.
Overall, 8, 0, and 14 patients relapsed (P=0.001) and 11, 5, and 11 patients (P=0.28) died in complete remission in the AraC, ATO and ATRA consolidation groups, respectively. Causes of death in complete remission were sepsis (n=2), hemorrhage (n=16), AML/myelodysplastic syndrome (MDS) (n=2), other (n=7). Overall, five patients developed AML/MDS including two, one and two patients treated in the AraC, ATO and ATRA arms, respectively.
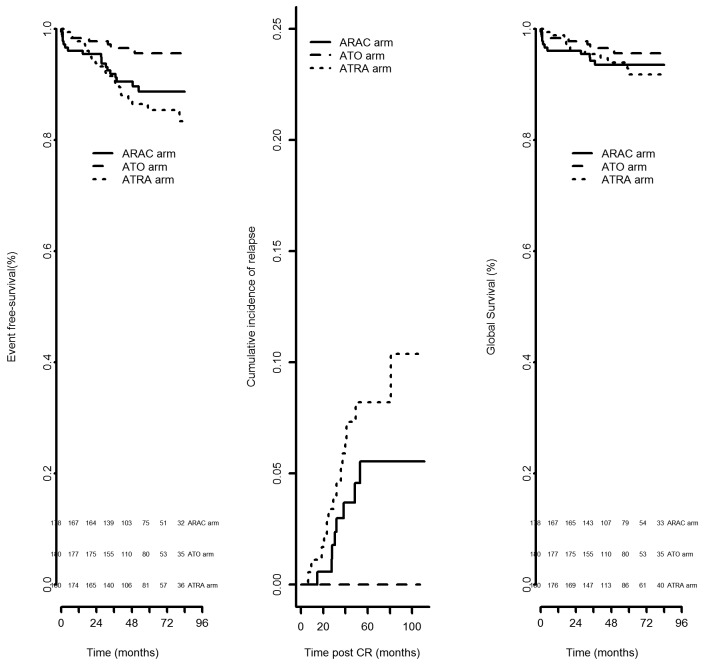
Five-year event-free survival rates from randomization were 88.7%, 95.7% and 85.4% in the AraC, ATO and ATRA consolidation groups, respectively (P=0.0067). The 5-year cumulative incidences of relapse were 5.5%, 0% and 8.2% (P=0.001) and the 5-year overall survival rates were 93.6%, 95.7% and 91.9% (P=0.349) in the AraC, ATO and ATRA consolidation groups, respectively (Figure 2).
Figure 2.
Event-free survival, cumulative incidence of relapse and overall survival in patients with standard-risk acute promyelocytic leukemia. ARAC: cytarabine; ATO: arsenic trioxide; ATRA: all-trans retinoic acid; CR: complete remission.
The median times to an absolute neutrophil count >1x109/L after the first consolidation course were 23.5, 22.8 and 18 days in the AraC, ATO and ATRA groups, respectively (P<0.0001). Similarly, the times to an absolute neutrophil count >1x109/L after the second consolidation course were 23.3, 18.2 and 13.8 days (P<0.0001). The median durations of hospitalization after the first and the second consolidation courses were 31.5, 32.2, and 19.5 days (P<0.0001) and 28.2, 29.9, and 16.5 days in the AraC, ATO and ATRA group, respectively (P<0.0001).
High-risk acute promyelocytic leukemia
Of the 214 patients with high-risk APL, 205 (95.7%) achieved a complete remission, seven (3.2%) died early (1 from bleeding, 3 from thrombosis, 1 from sepsis and 2 from other causes) and two (0.9%) had resistant leukemia. Seventeen patients were not randomized to consolidation treatment, including the nine patients who did not achieve a complete remission, three due to adverse events and five consequent to the patients’ decision.
One hundred and ninety-seven patients were randomized to consolidation therapy, 99 in the chemotherapy group and 98 in the chemotherapy plus ATO groups. Pretreatment characteristics were well balanced between the two groups (Table 2). With a median follow-up of 52 months, eight patients (4 in the chemotherapy group versus 4 in the chemotherapy plus ATO group) had relapsed leading to 5-year cumulative incidence rates of 4.6% [95% confidence interval (95% CI: 1.5; 10.6) and 3.5% (95% CI: 0.9; 9.2), P=0.99] and 13 patients had died in complete remission including nine in the chemotherapy arm and four in the chemotherapy plus arm (P=0.98). One patient, randomized to the chemotherapy plus ATO arm, developed AML/MDS. The 5-year overall survival rates were 90% and 93% in the chemotherapy and chemotherapy plus ATO groups, respectively (P=0.62), while the corresponding 5-year event-free rates were 85.5% and 92.1% (P=0.38) (Figure 3).
Table 2.
Baseline characteristics of the patients aged less than 70 years with high-risk acute promyelocytic leukemia.
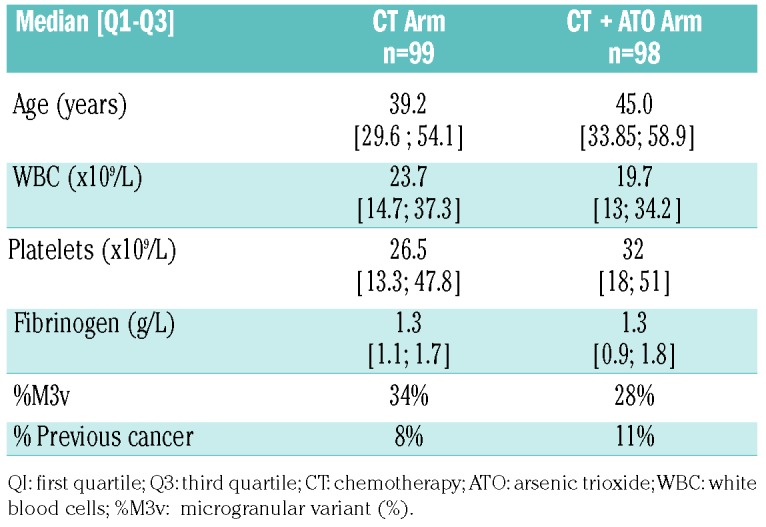
Figure 3.
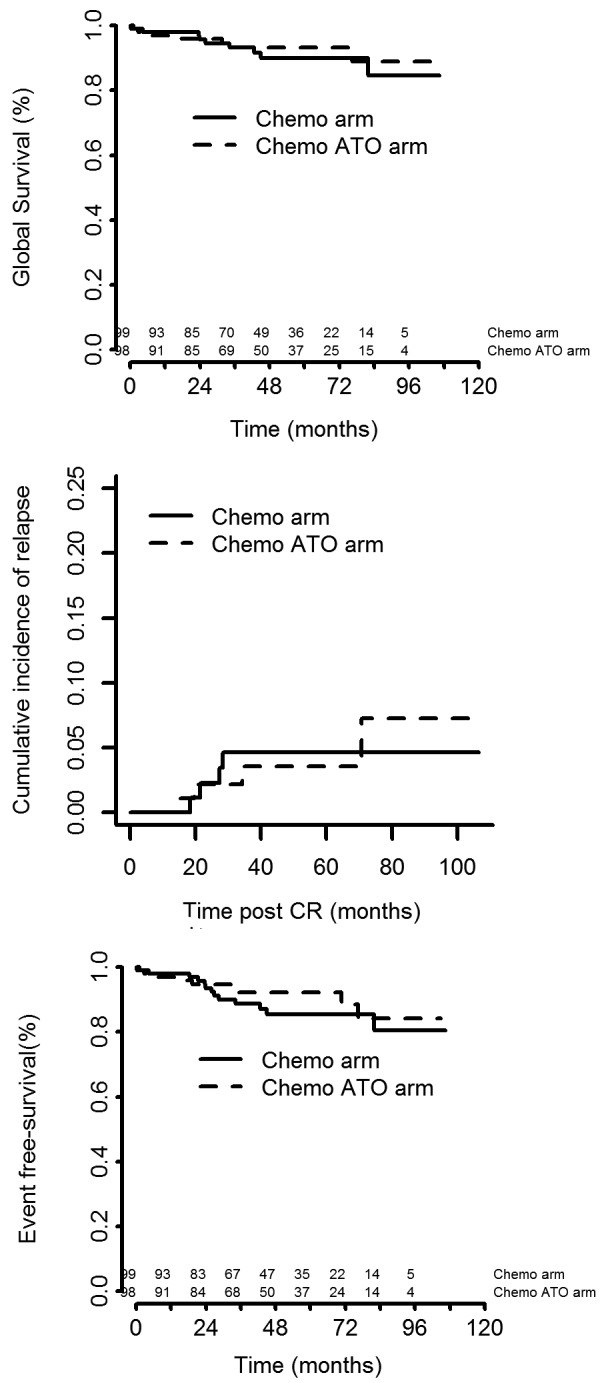
Overall survival, cumulative incidence of relapse and event-free survival in patients with high-risk acute promyelocytic leukemia. Chemo: chemotherapy; ATO: arsenic trioxide; CR: complete remission.
Excluding AraC (after the protocol amendment) from the consolidation cycles in the chemotherapy plus ATO group did not increase the 5-year cumulative incidence of relapse (4.6% in the chemotherapy arm, 5.3% in the chemotherapy plus ATO with AraC arm and 2.7% in the chemotherapy plus ATO without AraC arm, P=0.61). On the other hand, excluding AraC from consolidation cycles in the chemotherapy plus ATO arm significanty reduced myelosuppression: the median times to an absolute neutrophil count >1x109/L after the second consolidation course were 22, 25 and 18 days in, respectively, the chemotherapy arm, the chemotherapy plus ATO with AraC arm, and the chemotherapy plus ATO without AraC arm (P<0.001), while the median times to a platelet count >50x109/L were 24, 26 and 18 days (P<0.001). Similarly, the median durations of hospitalization after the first and the second consolidation courses were 29 days, 34 days, and 33 days (P<0.0001) and 28 days, 32 days and 31 days (P=0.0005), respectively.
Discussion
The main results of this study are that, in standard-risk APL, addition of ATO to a “classical” ATRA chemotherapy regimen further reduces the incidence of relapse and that, in high-risk APL, AraC (including high-dose AraC) can be replaced by ATO without increasing the relapse risk and with more limited myelosuppression, thus potentially reducing the risk of death in complete remission.
A first finding was the very high complete remission rate obtained in the APL 2006 trial, both in standard-risk and high-risk APL (98.1% and 95.7%, respectively), even though patients could be included up to the age of 70. Recent reports have suggested that, even in the ATRA era, early death rates could be as high as 15% to 20% in “real-life” APL patients.15–19 On the other hand, we previously published that, during the 2006 to 2011 period, 75% of the patients in the 17 French largest centers participating in the APL 2006 trial could be included in the trial, while 25% could not, mainly based on age, major comorbidities or direct admission to an intensive care unit.15 The overall complete remission rate was 91.4% and the overall rate of early death was 8.6%. All studies suggest that, if APL is suspected and before the diagnosis is confirmed, the immediate institution of ATRA treatment can reduce the risk of early death. Intensive platelet support during induction treatment can probably also contribute to reducing the risk of early death, particularly in patients with high-risk APL.
In the APL 2006 trial, in standard-risk APL patients aged less than 70 years of age, our aim was to show that by substituting ATO or ATRA for AraC during consolidation cycles, we would not increase the relapse rate, but would reduce myelosuppression, thereby potentially reducing the incidence of deaths in complete remission, which was 5% in our previous experience with AraC-containing consolidation cycles (at a conventional dose for the first consolidation cycle, and intermediate dose for the second). The ATRA and chemotherapy regimen chosen appeared to be an “optimal” regimen, using in particular high cumulative doses of anthracyclines, idarubicin rather than daunorubicin (as the latter may lead to more relapses8), AraC during consolidation and prolonged maintenance treatment with 6-mercaptopurine, methotrexate and intermittent ATRA, which may also contribute to reducing the relapse rate.7 This reference treatment proved effective, as the incidence of relapse after 5 years was only 5.5%.
Substituting ATRA for AraC did not significantly increase the relapse rate (8.2% at 5 years, compared to 5.5% in the AraC group) , but the replacement significantly reduced the time to recovery from neutropenia after the first and second consolidation cycles, and the duration of hospitalization during those two consolidation cycles. It did not reduce the incidence of deaths in complete remission, but among the six, six and five deaths in complete remission occurring in the three consolidation groups, only two in each arm were due to myelosuppression (the remaining being due to inter-current disease or secondary AML/MDS).
However, the main result in this standard-risk APL group was that no relapses were seen in the ATO arm, and that this relapse rate was significantly lower than in the AraC and ATRA consolidation arms. These results suggest that adding ATO to an already highly effective ATRA chemotherapy regimen may further improve the regimen’s anti-leukemic effect, and that ATO may not be dispensable in the treatment of standard-risk APL. On the other hand, substituting ATO for AraC did not reduce the duration of neutropenia after consolidation cycles, and neutropenia was longer with ATO and idarubicin than with ATRA and idarubicin consolidation cycles. This finding suggests that ATO, a non-myelosuppressive drug when used alone or combined with ATRA, may worsen myelosuppression when used concomitantly with chemotherapy. The duration of hospitalization was, however, shorter after ATO and idarubicin than after AraC and idarubicin consolidation cycles. The incidence of deaths in complete remission was not reduced in the ATO group, but only two deaths in complete remission were attributable to myelosuppression in the three consolidation arms. Finally, the incidence of secondary AML/MDS was similar in the three treatment arms, and similar to that reported in APL patients treated with ATRA chemotherapy regimens, i.e., between 1% and 2%.20–22 By contrast, in the follow up of the two main clinical trials that used ATRA-ATO regimens without chemotherapy in newly diagnosed APL, no case of secondary AML/MDS has been reported so far (Lo Coco and Russell, personal communications).
Thus, in standard-risk APL, and in spite of very high complete remission and very low relapse rates obtained with ATRA chemotherapy combinations, our results confirm that the rates can be further improved by using ATO during the consolidation regimen. ATO in this situation did indeed reduce the relapse risk in standard-risk APL, confirming results of two recent, large studies.10,11 Long-term results of one of them, the Italian German study, show in particular that an ATRA-ATO regimen is not just equivalent but superior to ATRA chemotherapy regimens in terms of relapse rate and overall survival. Thus, ATRA-ATO (chemotherapy free) regimens are becoming reference treatments for standard-risk APL.
With regards to high-risk APL, only limited studies of ATO-ATRA regimens without chemotherapy have been published, and in those studies patients often also received myelosuppressive drugs, mainly gentuzumab.10,12 In the British study, this approach was found to give results equivalent to those of an ATRA chemotherapy regimen, but the overall number of patients included in the randomized study was only 56.10 A US intergroup study showed that addition of ATO to a classical ATRA chemotherapy regimen significantly reduced the relapse rate. The ATRA chemotherapy regimen was, however based on daunorubicin instead of idarubicin (with a total scheduled dose of 500 mg/m2), which may have contributed to higher relapse rates.
In the present study, among the patients with high-risk APL there was a very high complete remission rate (97.4%) and, contrary to the US intergroup study, a very low relapse rate (2.5%) was seen in the chemotherapy consolidation arm (without ATO), confirming our previous results.23
The fact that substituting ATO for AraC was not associated with an increased incidence of relapse (5.3% versus 2.7%), but with a reduced incidence of deaths in complete remission (from 7.8% to 0%) was, therefore, an important finding. This substitution also lead to less myelosuppression and less hospitalization for consolidation cycles.
By contrast, the chemotherapy plus ATO consolidation therapy, combining AraC and ATO, used during the first part of the trial, did not further reduce the relapse rate (which was, it should be noted, already very low in the conventional AraC arm) but was associated with increased myelosuppression and a 5% rate of deaths in complete remission. This finding supports the fact that ATO worsens myelosuppression when used concomitantly with chemotherapy, as in the standard-risk group.
Our results therefore support the addition of ATO during consolidation cycles, in high-risk APL, at least in order to reduce the amount of chemotherapy administered and, therefore, the rate of deaths in complete remission (as in our study) but also the relapse rate (according to other studies, including the US intergroup study). While ATO-ATRA regimens without chemotherapy can now probably be substituted for ATRA chemotherapy regimens in standard-risk APL, ongoing clinical trials will show to what extent chemotherapy can also be reduced or even avoided in high-risk APL.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/12/2033
Funding
This study was supported by the programme Hospitalier de Recherche Clinique and the Association pour la Recherche sur le Cancer (ARC).
References
- 1.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–1891. [DOI] [PubMed] [Google Scholar]
- 2.Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA group. Blood. 2010;116(17):3171–3179. [DOI] [PubMed] [Google Scholar]
- 3.Adès L, Chevret S, Raffoux E, et al. Long-term follow-up of European APL 2000 trial, evaluating the role of cytarabine combined with ATRA and daunorubicin in the treatment of nonelderly APL patients. Am J Hematol. 2013;88(7):556–559. [DOI] [PubMed] [Google Scholar]
- 4.Sanz MA, Montesinos P, Rayón C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25): 5137–5146. [DOI] [PubMed] [Google Scholar]
- 5.Fenaux P, Chastang C, Chevret S, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL group. Blood. 1999;94(4): 1192–1200. [PubMed] [Google Scholar]
- 6.Adès L, Guerci A, Raffoux E, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL group experience. Blood. 2010;115(9):1690–1696. [DOI] [PubMed] [Google Scholar]
- 7.Muchtar E, Vidal L, Ram R, Gafter-Gvili A, Shpilberg O, Raanani P. The role of maintenance therapy in acute promyelocytic leukemia in the first complete remission. Cochrane Database Syst Rev. 2013;(3): CD009594. [DOI] [PubMed] [Google Scholar]
- 8.Adès L, Sanz MA, Chevret S, et al. Treatment of newly diagnosed acute promyelocytic leukemia (APL): a comparison of French-Belgian-Swiss and PETHEMA results. Blood. 2008;111(3):1078–1084. [DOI] [PubMed] [Google Scholar]
- 9.Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121. [DOI] [PubMed] [Google Scholar]
- 10.Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295–1305. [DOI] [PubMed] [Google Scholar]
- 11.Platzbecker U, Avvisati G, Cicconi L, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 Trial. J Clin Oncol. 2017;35(6):605–612. [DOI] [PubMed] [Google Scholar]
- 12.Abaza Y, Kantarjian H, Garcia-Manero G, et al. Long-term outcome of acute promyelo- cytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. 2017;129(10):1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53457–53481. [Google Scholar]
- 14.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446)496– 509. [Google Scholar]
- 15.Rahmé R, Thomas X, Recher C, et al. Early death in acute promyelocytic leukemia (APL) in French centers: a multicenter study in 399 patients. Leukemia. 2014;28(12): 2422–2424. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann S, Ravn A, Carlsson L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011;25(7): 1128–1134. [DOI] [PubMed] [Google Scholar]
- 17.McClellan JS, Kohrt HE, Coutre S, et al. Treatment advances have not improved the early death rate in acute promyelocytic leukemia. Haematologica. 2012;97(1):133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman JK, Rademaker A, Cull E, et al. Administration of ATRA to newly diagnosed patients with acute promyelocytic leukemia is delayed contributing to early hemorrhagic death. Leuk Res. 2013;37(9): 1004–1009. [DOI] [PubMed] [Google Scholar]
- 19.Paulson K, Serebrin A, Lambert P, et al. Acute promyelocytic leukaemia is characterized by stable incidence and improved survival that is restricted to patients managed in leukaemia referral centres: a pan-Canadian epidemiological study. Br J Haematol. 2014;166(5):660–666. [DOI] [PubMed] [Google Scholar]
- 20.Batzios C, Hayes LA, He SZ, et al. Secondary clonal cytogenetic abnormalities following successful treatment of acute promyelocytic leukemia. Am J Hematol. 2009;84(11):715–719. [DOI] [PubMed] [Google Scholar]
- 21.Lobe I, Rigal-Huguet F, Vekhoff A, et al. Myelodysplastic syndrome after acute promyelocytic leukemia: the European APL group experience. Leukemia. 2003;17(8): 1600–1604. [DOI] [PubMed] [Google Scholar]
- 22.Andersen MK, Pedersen-Bjergaard J. Therapy-related MDS and AML in acute promyelocytic leukemia. Blood. 2002;100(5):1928–1929. [DOI] [PubMed] [Google Scholar]
- 23.Kelaidi C, Chevret S, De Botton S, et al. Improved outcome of acute promyelocytic leukemia with high WBC counts over the last 15 years: the European APL Group experience. J Clin Oncol. 2009;27(16):2668–2676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.