Abstract
In the POLLUX study, daratumumab plus lenalidomide/dexamethasone significantly reduced risk of progression/death versus lenalidomide/dexamethasone alone in relapsed/refractory multiple myeloma. We provide one additional year of follow up and include the effect on minimal residual disease and in clinically relevant subgroups. After 25.4 months of follow up, daratumumab plus lenalidomide/dexamethasone prolonged progression-free survival versus lenalidomide/dexamethasone alone (median not reached vs. 17.5 months; hazard ratio, 0.41; 95% confidence interval, 0.31-0.53; P<0.0001). The overall response rate was 92.9% versus 76.4%, and 51.2% versus 21.0% achieved a complete response or better, respectively (both P<0.0001). At the 10−5 sensitivity threshold, 26.2% versus 6.4% were minimal residual disease–negative, respectively (P<0.0001). Post hoc analyses of clinically relevant patient subgroups demonstrated that progression-free survival was significantly prolonged for daratumumab plus lenalidomide/dexamethasone versus lenalidomide/dexamethasone regardless of number of prior lines of therapy. Patients previously treated with lenalidomide or thalidomide and those refractory to bortezomib received similar benefits (all P<0.01). Treatment benefit with daratumumab plus lenalidomide/dexamethasone was maintained in high-risk patients (median progression-free survival 22.6 vs. 10.2 months; hazard ratio, 0.53; 95% confidence interval, 0.25-1.13; P=0.0921) and patients with treatment-free intervals of >12 and ≤12 months and >6 and ≤6 months. No new safety signals were observed. In relapsed/refractory multiple myeloma patients, daratumumab plus lenalidomide/dexamethasone continued to improve progression-free survival and deepen responses versus lenalidomide/dexamethasone. Trial Registration: clinicaltrials.gov identifier: 02076009.
Introduction
Novel therapeutics approved during the last decade for relapsed or refractory multiple myeloma (RRMM), including proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs), are now standard of care treatments.1–3 However, patients eventually relapse and subpopulations such as those with high-risk cytogenetic abnormalities may not achieve the same benefits as standard-risk patients.4 Treatment choices must be based on patient factors (comorbidities, frailty, preferences), disease factors (burden, molecular risk), and treatment factors (prior therapies, refractoriness, and toxicity).
Daratumumab is a human monoclonal antibody targeting CD38, a cell surface receptor highly expressed on multiple myeloma (MM) cells,5,6 that exerts its effects via direct on-tumor and immunomodulatory mechanisms of action.7–11 Daratumumab is approved in many countries as monotherapy for patients with heavily treated RRMM.12,13 In combination with standard of care regimens, including bortezomib/dexamethasone (Vd; CASTOR study)14 and lenalidomide/dexamethasone (Rd; POLLUX study),15 daratumumab significantly prolonged progression-free survival (PFS) and deepened responses in patients with RRMM. This led to regulatory approval in many countries for patients with ≥1 prior line of therapy.16
In the prespecified interim analysis of POLLUX, after a median follow up of 13.5 months, daratumumab in combination with Rd (D-Rd) resulted in a 63% reduction in the risk of disease progression or death, significantly higher overall response rates (ORRs; 92.9% vs. 76.4%), and significantly higher minimal residual disease (MRD)-negativity rates at multiple sensitivity thresholds versus Rd.15
To identify patients who may benefit more from D-Rd treatment, we conducted subgroup analyses of POLLUX after a longer follow up of 25.4 months. The efficacy of D-Rd versus Rd was compared according to the number of prior lines of therapy received, prior IMiD exposure, bortezomib refractoriness, treatment-free interval after previous treatment line, and cytogenetic risk. We also assessed the ability of daratumumab to drive deep clinical responses beyond complete responses (CRs) through assessment of MRD.
Methods
Study Design
POLLUX is an ongoing randomized, open-label, multicenter, phase 3 study conducted in patients with RRMM (clinicaltrials.gov identifier: 02076009). An independent ethics committee or institutional review board at each site approved the trial. The study pro tocol was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The study design and primary results have been previously published.15 Briefly, eligible patients had progressive disease according to International Myeloma Working Group (IMWG) criteria18,19 during or after their last regimen and had received and responded to ≥1 line of prior therapy; lenalidomide-refractory patients were ineligible. Patients were randomized 1:1 to Rd (lenalidomide: 25 mg orally on Days 1-21 of each 28-day cycle; dexamethasone: 40 mg orally weekly) with or without daratumumab (16 mg/kg intravenously weekly for 8 weeks, every 2 weeks for 16 weeks, and then every 4 weeks) until progression.
Endpoints and Assessments
The primary efficacy endpoint was PFS, and secondary efficacy endpoints included ORR, rates of very good partial response (VGPR) or better and CR or better, MRD, time to response, and overall survival.
This exploratory, post hoc, secondary analysis examined patient populations according to prior lines of therapy received (1, 2-3, 1-3), prior treatment exposure (bortezomib, lenalidomide, and thalidomide), refractoriness to bortezomib, time since last therapy (>12 months, ≤12 months, >6 months, and ≤6 months prior to randomization), and cytogenetic risk. Numbers of prior lines of therapy were determined by investigators according to the IMWG consensus guidelines.19 Treatment-free interval was defined as the duration between the end date of the last line of prior therapy and randomization. Cytogenetic abnormalities were determined with CD138+ selected cells at the screening visit prior to randomization by centralized next-generation sequencing. The presence of 1 or more of t(4;14), t(14;16), or del17p (utilizing >50% deletion cutoff) defined a patient as high risk. Standard-risk cytogenetic status was assigned to those not meeting the high-risk criteria. Due to limitations with sample quality or quantity, and because cytogenetic evaluation was mandated mid-study, cytogenetic risk was not evaluated in all patients. PFS, ORR, and MRD negativity at sensitivity thresholds of 10−5 and 10−6 were assessed for each subgroup. PFS based on MRD and cytogenetic risk status was also examined. Health-related quality of life (HRQoL) was assessed using the EuroQol 5-Dimension Questionnaire (EQ-5D-5L) and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 (EORTC QLQ-C30).
Minimal Residual Disease
MRD was assessed (blinded to treatment group) at the time of suspected CR (i.e., CR/stringent CR or VGPR with suspected interference20,21), and at 3 and 6 months after suspected CR for patients who maintained this response. The MRD-negativity rate per treatment arm was determined as the proportion of patients with negative MRD at any time point after the first dose and compared using the likelihood-ratio test.
Additional details on assessments for safety, MRD, cytogenetic risk, and HRQoL and statistical analyses are provided in the Appendix.
Results
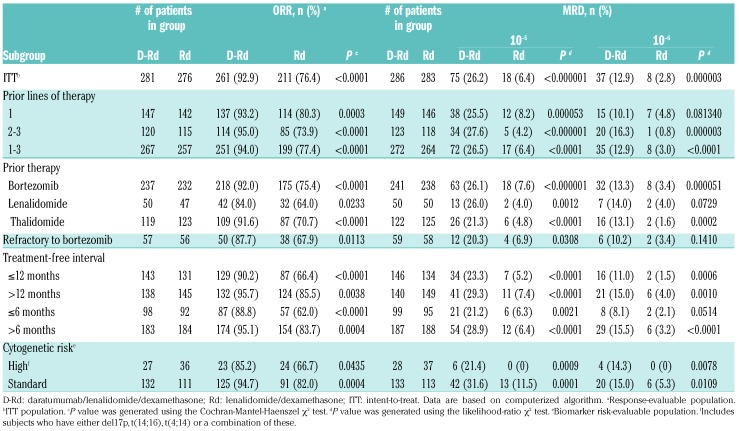
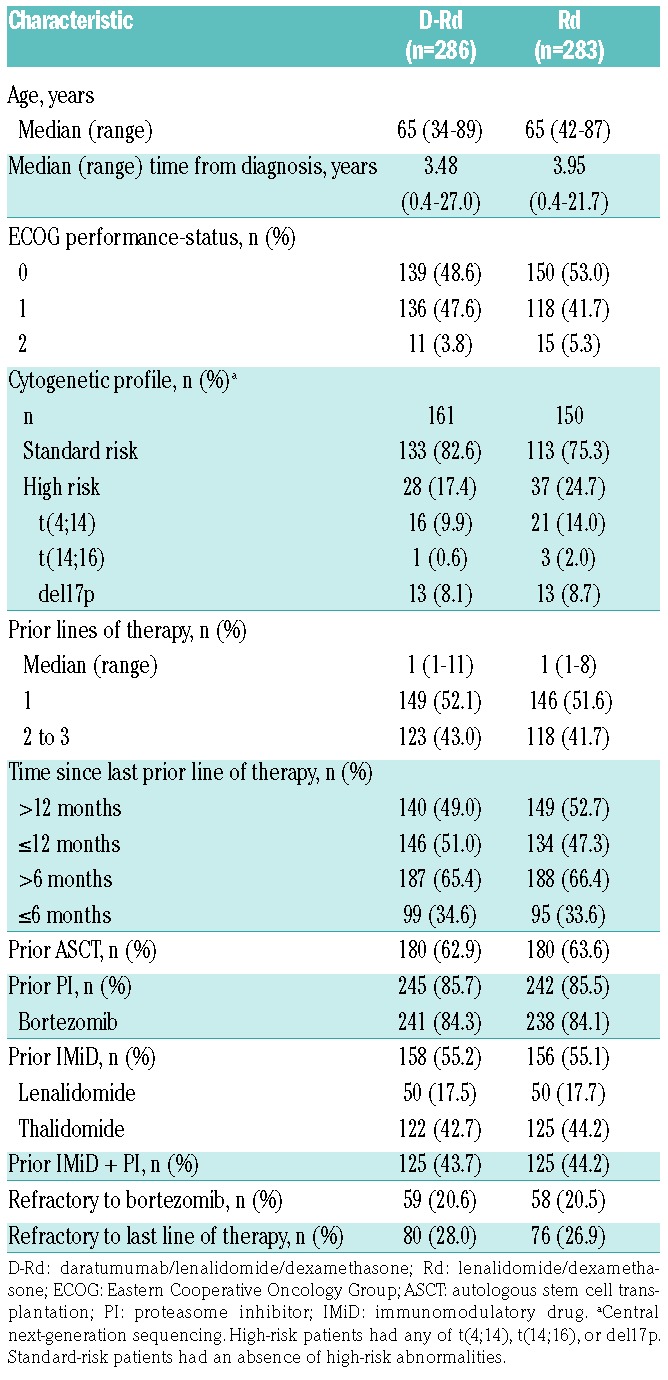
Of 569 enrolled patients in POLLUX, 286 were assigned to D-Rd and 283 to Rd (Appendix Figure 1). Baseline patient demographics and prior treatment history were generally balanced and have been previously reported.15 Additional baseline clinical and cytogenetic characteristics are summarized in Table 1. The median (range) duration of study treatment was 24.5 (0-32.7) months in the D-Rd group and 16.0 (0.20-32.2) months in the Rd group.
Table 1.
Baseline demographic and clinical characteristics.
At the clinical cut-off on March 7, 2017, the median (range) duration of follow up was 25.4 (0-32.7) months. Consistent with the primary analysis, D-Rd improved PFS compared with Rd (median not reached [NR] vs. 17.5 months; hazard ratio [HR], 0.41; 95% confidence interval [CI], 0.31-0.53; P<0.0001), with 24-month PFS rates of 68.0% versus 40.9%, respectively (Figure 1A). In the response-evaluable population, D-Rd (n=281) compared with Rd alone (n=276) significantly improved the ORR (92.9% vs. 76.4%, respectively; P<0.0001 [Table 2]), and rates of ≥VGPR and ≥CR (Appendix Table 1). Stringent CRs were achieved by 26.0% and 8.7% of patients receiving D-Rd and Rd, respectively (Appendix Table 1). Overall survival data remain immature, and the final analysis is planned after 330 events are observed.
Figure 1.
(A) PFS in the ITT population and (B) a forest plot summary of PFS HRs in subgroups by prior lines, prior therapies, treatment-free intervals. Kaplan- Meier analysis of PFS among patients in the ITT population. aTreatment-free interval was defined as the duration between the end date of the last line of prior therapy and randomization. bHigh-risk patients had any of t(4;14), t(14;16), or del17p as assessed by next generation sequencing. cStandard-risk patients had an absence of highrisk abnormalities. PFS: progression- free survival; ITT: intenttotreat; HR: hazard ratio; D-Rd: daratumumab/lenalidomide/dex amethasone; Rd: lenalidomide/dexamethasone; CI: confidence interval; NR: not reached; TFI: treatment-free interval; std: standard.
Table 2.
ORR and MRD disease based on prior treatment history.
D-Rd significantly improved PFS in RRMM patients who received several prior lines of therapy, a population for whom more effective therapies are needed. In patients who received 1 prior line of therapy, D-Rd significantly prolonged PFS compared with Rd (D-Rd, n=149; Rd, n=146; median NR vs. 19.6 months; HR, 0.39; 95% CI, 0.26-0.58, P<0.0001 [Figure 1B, Appendix Figure 2A]), with 24-month PFS rates of 70.3% and 45.0%, respectively. A similar benefit was observed in patients who received 2 to 3 prior lines of therapy (Figure 1B, Appendix Figure 2B). ORR was also significantly improved in patients treated with 1 prior line of therapy (D-Rd, n=147; Rd, n=142; 93.2% vs. 80.3%; P=0.0003 [Table 2]) and 2 to 3 prior lines of therapy (D-Rd, n=120; Rd, n=115; 95.0% vs. 73.9%; P<0.0001 [Table 2]). It is considered preferable for MM patients who relapse to switch drug classes for subsequent therapy,22 and, in bortezomib-refractory patients, D-Rd significantly prolonged PFS compared with Rd (D-Rd, n=59; Rd, n=58; median 26.1 vs. 11.3 months; HR, 0.46; 95% CI, 0.26-0.80; P=0.0051 [Figure 1B]), with 24-month PFS rates of 60.0% and 29.7%, respectively, and improved ORR (D-Rd, n=57; Rd, n=56; 87.7% vs. 67.9%; P=0.0113 [Table 2]).
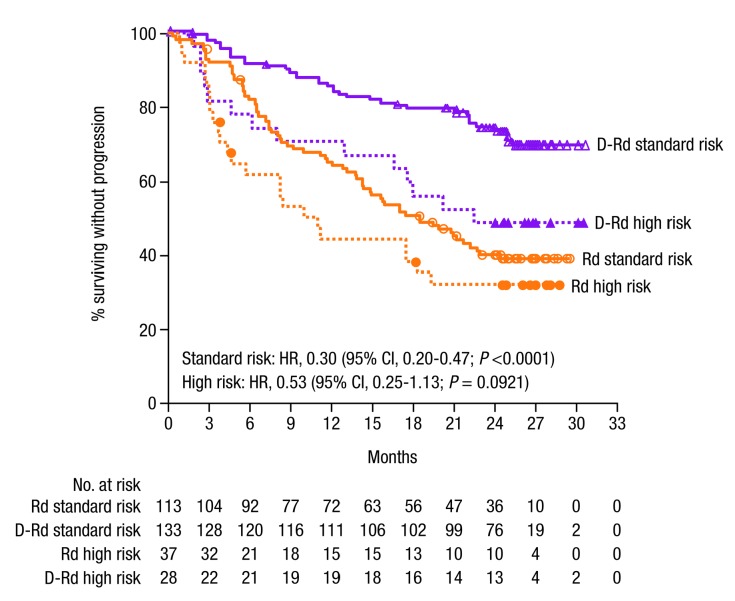
Patients with high-risk cytogenetic status have had historically poor outcomes, but, regardless of cytogenetic risk status, D-Rd improved outcomes compared with Rd (Figures 1B and 2, Table 2). PFS was longer for D-Rd-treated (n=28) versus Rd-treated patients (n=37) with high cytogenetic risk (median 22.6 vs. 10.2 months; HR, 0.53; 95% CI, 0.25-1.13; P=0.0921; 24-month PFS rate, 48.1% vs. 31.7%), and significantly longer for patients with standard-risk disease (D-Rd, n=133; Rd, n=113; median NR vs. 18.5 months; HR, 0.30; 95% CI, 0.20-0.47; P<0.0001; 24-month PFS rate, 74.3% vs. 40.0% [Figures 1B and 2]). With D-Rd versus Rd, significantly higher ORRs were observed for both high-risk (D-Rd, n=27; Rd, n=36; 85.2% vs. 66.7%; P=0.0435) and standard-risk patients (D-Rd, n=132; Rd, n = 111; 94.7% vs. 82.0%; P=0.0004 [Table 2]). Further subgroup analyses determined that the clinical benefit of daratumumab was maintained in patients regardless of prior lines of therapy received (1-3), prior treatment exposure (thalidomide or lenalidomide), or time since last therapy (≤12, >12, ≤6, or >6 months [Figure 1B, Table 2, Appendix Figure 2C]).
Figure 2.
PFS by cytogenetic risk status. Cytogenetic risk was assessed via nextgeneration sequencing. High-risk patients had any of t(4;14), t(14;16), or del17p. Standard-risk patients had an absence of high-risk abnormalities. PFS: progression- free survival; D-Rd: daratumumab/lenalidomide/dexamethasone; Rd: lenalidomide/dexamethasone; HR: hazard ratio; CI: confidence interval
Although D-Rd was associated with a significant treatment benefit in subpopulations that received their last line of prior therapy either ≤12 or >12 months before randomization into the study (Figure 1B, Table 2), the proportion of patients who remained progression-free and alive at 24 months was smaller among patients who relapsed earlier in both the D-Rd (63.6% vs. 72.4%) and Rd (29.2% vs. 51.4%) treatment groups. However, the ORR and PFS in patients with a shorter treatment-free interval was more severely impacted in patients treated with Rd compared with D-Rd (Figure 1B, Table 2).
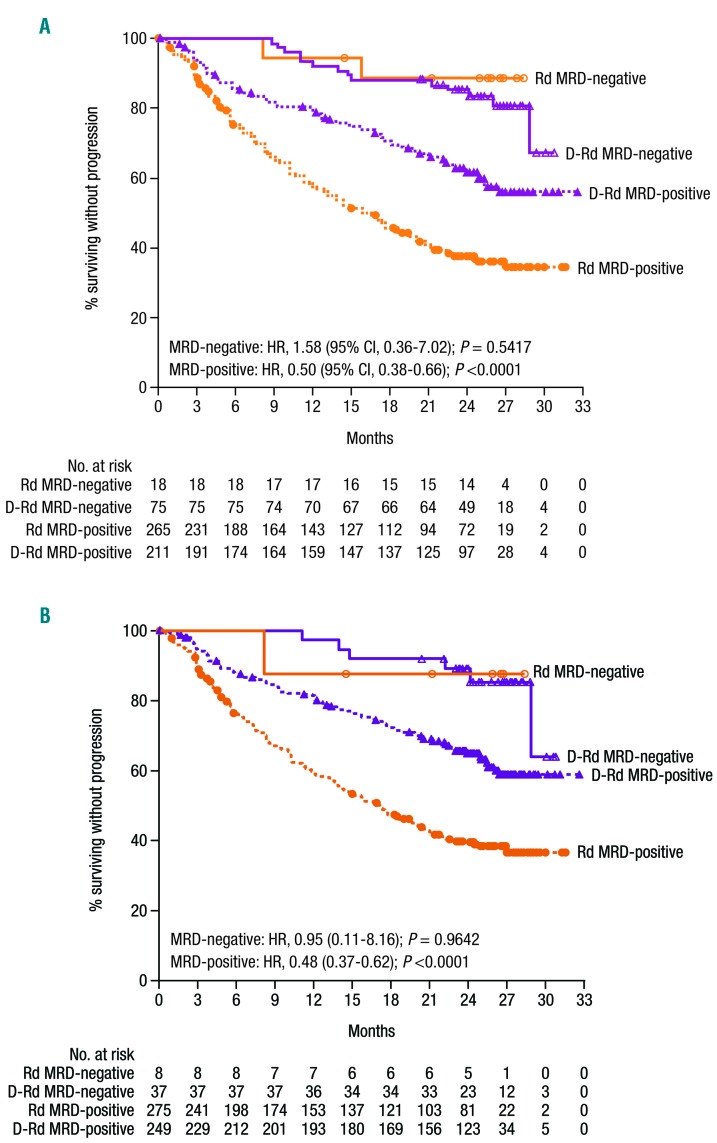
MRD status was evaluated to determine the ability of daratumumab to generate deeper responses in patients achieving conventional CR. The IMWG established MRD-negative criteria requiring evaluation by next-generation sequencing to be conducted at a minimum sensitivity threshold of 10−5.23 At this threshold, of the ITT population, 26.2% of patients treated with D-Rd achieved MRD negativity compared with 6.4% of patients who received Rd (P<0.000001 [Table 2]). Consistent findings were also observed for D-Rd versus Rd at a sensitivity of 10−6 (12.9% vs. 2.8%; P=0.000003). D-Rd generated deeper responses and higher MRD-negativity rates than Rd in all subgroups evaluated (Table 2), including patients who received 1 prior line of therapy (D-Rd, n=149; Rd, n=146; 10−5: 25.5% vs. 8.2%; P=0.000053; 10−6: 10.1% vs. 4.8%; P=0.08134) and 2 to 3 prior lines of therapy (D-Rd, n=123; Rd, n=118; 10−5: 27.6% vs. 4.2%; P<0.000001; 10−6: 16.3% vs. 0.8%; P=0.000003), patients who were bortezomib refractory (D-Rd, n=59; Rd, n=58; 10−5: 20.3% vs. 6.9%; P=0.0308; 10−6: 10.2% vs. 3.4%; P=0.1410), and patients who had high-cytogenetic risk at a sensitivity threshold of 10−5 (D-Rd, n=28; Rd, n=37; 21.4% vs. 0.0%, P=0.0009) and 10−6 (D-Rd, n=28; Rd, n=37; 14.3% vs. 0.0%, P=0.0078 [Table 2]). PFS was prolonged in patients who achieved MRD negativity compared with MRD-positive patients in both treatment arms (Figure 3A-B). D-Rd also significantly prolonged PFS compared with Rd in patients with MRD-positive status at the 10−5 and 10−6 sensitivity thresholds (P<0.001 [Figure 3A-B]). At the time of the analysis, the Table 2. ORR and MRD disease based on prior treatment history. majority of patients maintained MRD-negative status; patients will continue to be assessed annually.
Figure 3.
PFS by MRD at sensitivity threshold of (A) 10−5 and (B) 10−6. Kaplan-Meier estimates of PFS among patients in the intent-to-treat population. MRD-negative status was evaluated at sensitivity thresholds of 10−5 and 10−6 using bone marrow aspirate samples that were prepared using Ficoll and analyzed by the clonoSEQTM assay. PFS: progression-free survival; MRD: minimal residual disease; D-Rd: daratumumab/lenalidomide/dexamethasone; Rd: lenalidomide/dexamethasone.
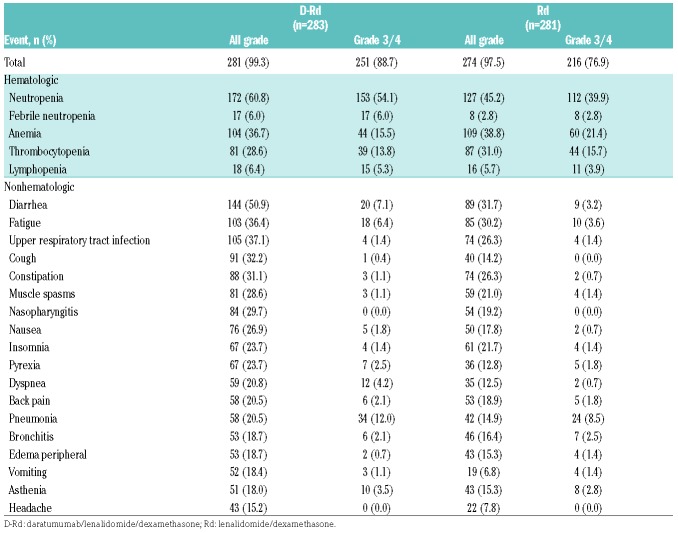
The safety profile remained unchanged from the primary analysis, with no new safety signals reported in either treatment group with longer follow up (Table 3). The most common treatment-emergent adverse events (≥15%) of any grade included neutropenia, anemia, thrombocytopenia, diarrhea, fatigue, upper respiratory tract infection, cough, constipation, muscle spasms, nasopharyngitis, and nausea (Table 3). The most common grade 3/4 treatment-emergent adverse events (≥5%) included neutropenia, febrile neutropenia, anemia, thrombocytopenia, lymphopenia, diarrhea, fatigue, and pneumonia (Table 3). The percentage of patients with adverse events leading to treatment discontinuation was similar between groups (12.0% for D-Rd, 12.8% for Rd). The most common adverse events (≥1%) leading to treatment discontinuation in D-Rd compared with Rd included pneumonia (1.4% vs. 0.7%), pulmonary embolism (0% vs. 1.1%), general physical health deterioration (1.1% vs. 0%), and renal failure (0.4% vs. 1.1%), respectively.
Table 3.
Most common all grade (≥15%) and grade 3/4 (≥5%) treatment-emergent adverse events in the safety population
The incidence of second primary malignancies between the D-Rd (n=283) and Rd (n=281) groups was the same (5.7% vs. 5.7%), consistent with previous observations that had no notable differences.15 The proportion of patients who received transfusions while on study drug was also similar between the D-Rd and Rd groups (24.4% vs. 25.3%).
There was no decline in HRQoL measures with the addition of daratumumab to Rd. Statistically significant differences in the change from baseline were observed in favor of D-Rd at Weeks 48 and 56 with the Utility Score and at Weeks 40, 48, and 56 with the Visual Analog Scale Score of the EQ-5D-5L questionnaire. With the EORTC QLQ-C30 Global Health Status Score, statistically significant differences in the change from baseline were observed in favor of D-Rd at Weeks 40, 48, 52, 68, 84, and 116. However, these improvements did not last beyond 3 consecutive assessments in either questionnaire. No significant differences for median time to improvement (6.6 months vs. 6.5 months; HR, 1.03; 95% CI, 0.81-1.30; P=0.820) were reported for EORTC QLQ-C30 Global Health Status Scores in the D-Rd and Rd groups. Similarly, no significant differences in median time to improvement were observed between treatment groups for either the EQ-5D-5L Utility Score (6.6 months vs. 10.2 months; HR, 1.23; 95% CI, 0.97-1.57; P=0.089) or Visual Analog Scale Score (6.9 months vs. 9.3 months; HR, 1.14; 95% CI, 0.89-1.45; P=0.283).
Discussion
These updated analyses with nearly one further year of follow up reinforce the initial findings of deep and durable responses achieved with D-Rd in RRMM patients.15 The benefit of D-Rd over Rd was consistently maintained across patients who received 1 or 2 to 3 prior lines of therapy, and the risk of progression or death was reduced by >60%. D-Rd was also superior to Rd regardless of prior treatment exposure (i.e., lenalidomide, thalidomide, bortezomibrefractory), time since last therapy, and cyto- genetic risk status. As one of the first studies to prospectively assess MRD in a phase 3 trial of RRMM, MRD-negativity rates were also significantly higher with D-Rd across all patient subgroups, including patients with high cytogenetic risk. These findings reinforce observations that highlight the depth, durability, and robustness of responses achieved with daratumumab-based regimens.24
These findings compare favorably with other studies of IMiD-containing regimens with subgroup analyses based on prior lines of therapy and/or prior treatment exposure (Appendix Table 2). In a large phase 3 study (ASPIRE) of carfilzomib-Rd (KRd) compared with Rd alone, a consistent PFS benefit was observed with KRd versus Rd among patients who received 1 and 2 to 3 prior lines of therapy.25 Prespecified PFS subgroup analyses showed a modest benefit with KRd over Rd for patients who previously received lenalidomide and patients nonresponsive to bortezomib in any previous regimen.26 The magnitude of benefit for KRd versus Rd was similar in patients with early disease relapse (≤12 months from starting the first prior regimen; ORR: 79% vs. 61%).27 In a phase 3 study of elotuzumab (ELOQUENT-2), the PFS benefit in combination with Rd was maintained among patients who received 2 to 3 prior lines of therapy or had prior exposure to bortezomib but not among patients who received 1 prior line of therapy or prior lenalidomide.28,29 Ixazomib in combination with lenalidomide and dexamethasone in the phase 3 TOURMALINE-MM1 study demonstrated a modest PFS benefit versus Rd in patients who received 1 or 2 prior lines, were previously treated with an IMiD or a PI, and were refractory to their last line of therapy; interestingly, patients who received 3 prior lines of therapy demonstrated an HR of 0.37.30
D-Rd improved responses, including MRD negativity in patients with high-risk cytogenetic status, suggesting that targeting CD38 in combination with Rd may improve outcomes for this challenging-to-treat population. Moreover, despite the small number of patients with high-risk disease, D-Rd demonstrated significantly higher MRD-negativity rates compared with standard-risk patients receiving Rd. While PFS was not statistically different between D-Rd versus Rd for patients with high-risk disease, a numerical improvement in PFS was observed in high-risk patients who received D-Rd versus Rd. As the magnitude of benefit for D-Rd was lower among high-risk patients, these findings suggest that while D-Rd is able to provide improvements in efficacy, it is not able to overcome the greater risk of progression and poor outcomes associated with high-risk disease. Although cross-study comparisons should be performed with caution, smaller differences in PFS and ORR between standard- and high-risk patients in the CASTOR study in patients treated with daratumumab and Vd were observed.31 Daratumumab-based combinations that include both a PI and an IMiD may further improve efficacy in patients with high-risk disease.
Prolonged treatment with D-Rd or Rd did not uncover new safety concerns, and the incidences of second primary malignancies were balanced between study arms. While certain adverse events, such as cough, dyspnea, and pneumonia occurred more frequently with daratumumab, the percentage of patients that discontinued treatment due to adverse events was similar between groups (12.0% for D-Rd, 12.8% for Rd). Additionally, patients in the D-Rd group were treated for longer, with a median duration of 24.5 months versus 16.0 months in the Rd group, which likely contributed to the increase in rates for certain adverse events. Finally, the addition of a third drug to the standard of care Rd regimen did not have a negative effect on HRQoL.
Several limitations of the study should be noted. First, although balanced between treatment arms, only a subset of patient samples was collected for central cytogenetic testing as outlined in the Methods. Second, not all eligible patients with suspected CR or CR had available MRD data, and these patients were therefore conservatively classified as MRD positive. Based on the experiences obtained from this phase 3 study, continuous improvements in MRD testing are being implemented across ongoing and future daratumumab studies in multiple myeloma. Finally, based on the limitations of post hoc analyses, the findings presented here require further confirmation in future studies prospectively assessing the efficacy of D-Rd in these clinically relevant patient populations.
In summary, these updated secondary subgroup analyses highlight the benefit of combining daratumumab with the standard of care regimen Rd in RRMM across examined subgroups. The addition of daratumumab to Rd drove deep responses, as shown by achievement of MRD negativity in many patients. These results suggest that D-Rd is a highly effective and well-tolerated regimen that may be recommended for RRMM after first relapse and beyond.
Supplementary Material
Acknowledgments
The authors wish to thank the patients participating in this study and their families, as well as the global network of investigators, research nurses, study coordinators, and operations staff (including Sonali Trivedi, Jaime Bald, Xiang Qin, and Christopher Velas). Medical writing and editorial support were provided by Jason Jung, PhD, and Sima Patel, PhD, of MedErgy, and were funded by Janssen Global Services, LLC.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/12/2088
Funding
This study was sponsored by Janssen Research & Development, LLC. The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu. JLK consulted for BMS, Janssen, Celgene, Karyopharm, and Pharmacyclics.
References
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv52–iv61. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): multiple myeloma. Version 4. 2018. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf
- 5.Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121(4):482–488. [DOI] [PubMed] [Google Scholar]
- 6.Santonocito AM, Consoli U, Bagnato S, et al. Flow cytometric detection of aneuploid CD38(++) plasmacells and CD19(+) B-lymphocytes in bone marrow, peripheral blood and PBSC harvest in multiple myeloma patients. Leuk Res. 2004;28(5):469–477. [DOI] [PubMed] [Google Scholar]
- 7.de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;1 86(3):1840–1848. [DOI] [PubMed] [Google Scholar]
- 8.Lammerts van Bueren J, Jakobs D, Kaldenhoven N, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124(21):3474. [Google Scholar]
- 9.Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol. 2016;197(3):807–813. [DOI] [PubMed] [Google Scholar]
- 11.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeage K, Lyseng-Williamson KA. Daratumumab in multiple myeloma: a guide to its use as monotherapy in the EU. Drugs Ther Perspect. 2016;32(11):463–469. [Google Scholar]
- 13.McKeage K. Daratumumab: first global approval. Drugs. 2016;76(2):275–281. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331. [DOI] [PubMed] [Google Scholar]
- 16.Blair HA. Daratumumab: A review in relapsed and/or refractory multiple myeloma. Drugs. 2017;77(18):2013–2024. [DOI] [PubMed] [Google Scholar]
- 17.DARZALEX® (daratumumab) injection, for intravenous use [package insert]. Horsham, PA: Janssen Biotech, Inc.; 2018. [Google Scholar]
- 18.Durie BGM, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. [DOI] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18): 4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCudden C, Axel AE, Slaets D, et al. Monitoring multiple myeloma patients treated with daratumumab: teasing out monoclonal antibody interference. Clin Chem Lab Med. 2016;54(6):1095–1104. [DOI] [PubMed] [Google Scholar]
- 21.Durie BG, Miguel JF, Blade J, Rajkumar SV. Clarification of the definition of complete response in multiple myeloma. Leukemia. 2015;29(12):2416–2417. [DOI] [PubMed] [Google Scholar]
- 22.Sonneveld P, Broijl A. Treatment of relapsed and refractory multiple myeloma. Haematologica. 2016;101(4):396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. [DOI] [PubMed] [Google Scholar]
- 24.Avet-Loiseau H, Casneuf T, Chiu C, et al. Evaluation of minimal residual disease (MRD) in relapsed/refractory multiple myeloma (RRMM) patients treated with daratumumab in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone. Blood. 2016;128(22):246. [Google Scholar]
- 25.Dimopoulos A, Stewart AK, Rajkumar SV, et al. Effect of carfilzomib, lenalidomide, and dexamethasone (KRd) vs lenalidomide and dexamethasone (Rd) in patients with relapsed multiple myeloma (RMM) by line of therapy: secondary analysis from an interim analysis of the phase III study ASPIRE (NCT01080391). J Clin Oncol. 2015;33(15_suppl):8525. [Google Scholar]
- 26.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig H, Dimopoulos MA, Masszi T, et al. Carfilzomib, lenalidomide, and dexamethasone (KRd) vs lenalidomide and dexamethasone (Rd) in patients with relapsed multiple myeloma (RMM) and early progression during prior therapy: Secondary analysis from the phase 3 study ASPIRE (NCT01080391). J Clin Oncol. 2016;34(15_suppl):8045. [Google Scholar]
- 28.Lonial S, Richardson PG, Mateos MV, et al. ELOQUENT-2 update: Phase III study of elotuzumab plus lenalidomide/dexamethasone (ELd) vs Ld in relapsed/refractory mul tiple myeloma (RRMM)-identifying responders by subset analysis. J Clin Oncol. 2016;34(15_suppl):8037. [Google Scholar]
- 29.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–631. [DOI] [PubMed] [Google Scholar]
- 30.Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–1634. [DOI] [PubMed] [Google Scholar]
- 31.Mateos MV, Estell J, Barreto W, et al. Efficacy of daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory myeloma based on prior lines of therapy: updated analysis of CASTOR. Presented at the 58th Annual Meeting & Exposition of the American Society of Hematology (ASH); December 3-6, 2016; San Diego, CA Abstract 1150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.