More than half of all Swedish adult patients with acute myeloid leukemia (AML) are 70 years or older at diagnosis.1,2 The incidence of AML currently peaks at age 80–84, and declines among even older adults, in accordance with the general cancer incidence by age.3–5 AML biology according to age has again come into focus due to the concept of age-related clonal hematopoiesis.6 We have previously shown that most patients up to 80 years tol erate and benefit from intensive chemotherapy,2 and the outcome has improved over the last twenty years,7 whereas patients older than 80 have a very poor outcome with no improvement over time. However, now new therapies with much less toxicity are available8,9 which could potentially be tolerated by the oldest patients, who therefore need to be better characterized.
We previously reported the karyotypic profile in older patients.10 Herein we report the clinical and diagnostic features according to age with a specific focus on the very old (Table 1) and a comparison to younger patients. Data from patients diagnosed between 1997 and 2016 was extracted in March 2017 from the Swedish AML registry.1,2 Laboratory data, body mass index (BMI) and information on hypomethylating therapy were available from 2007. Data on geriatric assessment11 and detailed comorbidity12 were not available.
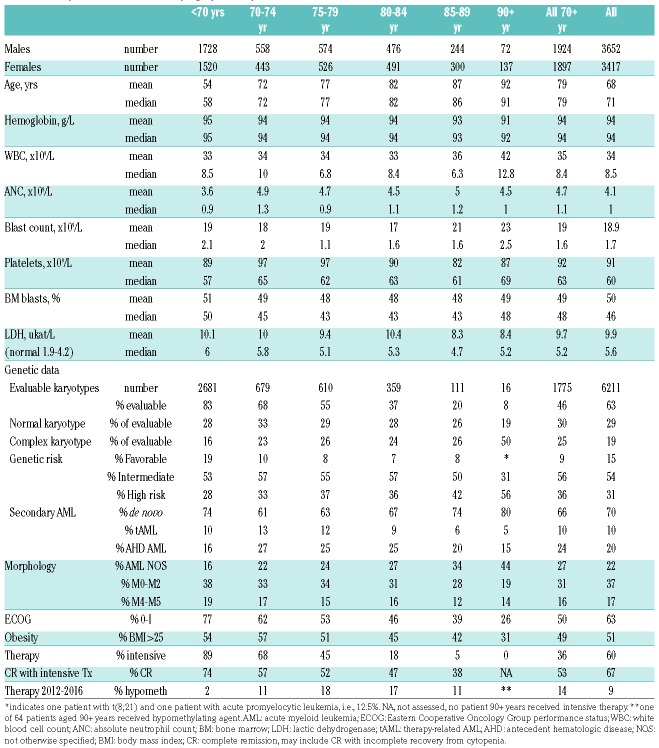
Table 1.
AML patient characteristics by age (n=7069).
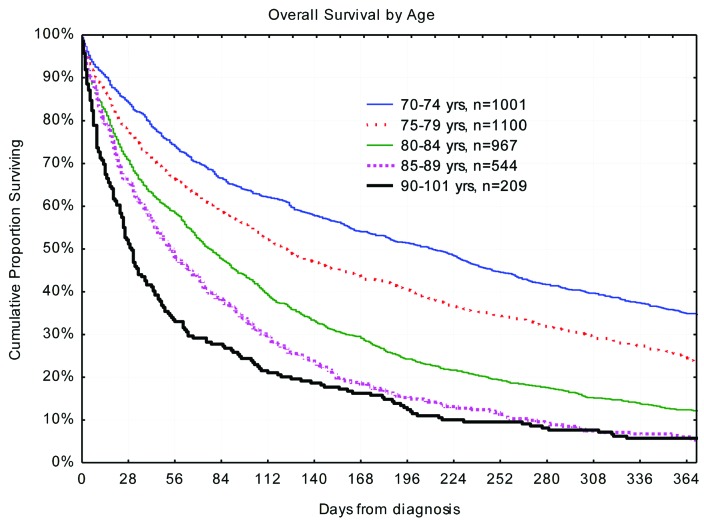
There are obvious clinical differences by age. The male to female patient number ratio is lower in older patients, despite the higher male incidence up to age 95 years,1 due to the corresponding sex ratio in the population. Older patients undergo less diagnostic procedures, such as morphological subclassification and genetic evaluation, due to the lack of impact on clinical management in the past. Nevertheless, AML subclassification according to The French-American-British/The World Health Organization (FAB/WHO) was performed in a higher proportion of Swedish patients over 90 years than in all ages according to The Surveillance, Epidemiology, and End Results (SEER) program.13 Older patients have poorer Eastern Cooperative Oncology Group (ECOG) performance status at diagnosis, and fewer receive intensive therapy, of whom a minority achieve complete remission (CR)2 (Table 1). Older patients are, according to Swedish guidelines, less likely to receive salvage therapy if they do not respond to the first induction attempt. Thus, survival is increasingly poor with advancing age (Figure 1).
Figure 1.
Overall survival in days by age at diagnosis.
On the other hand, there were no major clinically distinct differences by age in blood counts at presentation. The total white blood cell (WBC) counts and blood absolute blast counts were slightly higher in patients over 85 years (P=0.03 and P=0.0001, respectively), but lactic dehydrogenase (LDH) was less elevated in older subjects (P=0.02 by non-parametric statistics). Obesity is a risk factor for AML,14 but fewer older patients were obese (BMI>25, P<0.0001). Underweight (BMI<18.5) was present in 2.5% of both patients younger than 70 years and those over 70 years, but possibly somewhat more common in patients of 90 years or over.
The pattern of genetic changes is hard to interpret due to low coverage among the oldest patients. It is likely that adverse findings, such as complex and monosomic karyotypes, deletions of 5q and 7q, and TP53-mutations are more common in this group.10 However, we also found acute promyelocytic leukemia and core-binding factor leukemia with t(8;21) in patients over 90 years. Secondary AML was most common in those aged between 70 and 80,2,15 and, perhaps surprisingly, less common among the very old (P<0.0001), despite the fact that the age-related incidence of myelodysplastic syndromes (MDS) is more skewed towards the elderly than in AML.
Twenty-six patients aged 85 or over (89 being the oldest) received intensive therapy, and half of them survived for more than six months; the longest survival time was three years. Nineteen 85-year-old or older patients received primary hypomethylating agents, and 11/19 (58%) survived for more than six months (median 205 days), with the longest survival time being 46 months. The oldest recipient of AML-specific treatment was a 91-year-old male with AML M2, performance status 0, bone marrow blasts of 26% and a WBC count of 1.0×109/L who received azacytidine, and survived for 640 days.
Twelve patients of 90 years or older survived for one year or more; eight of them had ECOG performance status 0-I, and their median marrow blast count and WBC count were below average at 26% and 1.7×109/L, respectively, but their median hemoglobin and platelet counts and morphologic subtype were similar to that of others [hemoglobin 86 g/L, platelets 45×109/L, M1 (n=1), M2 (n=1), M5 (n=2), AML with multilineage dysplasia (n=2), and without further specification (n=6)]. One patient had a normal karyotype, whereas the others did not have their karyotypes evaluated.
The interpretation of biological differences and similarities between very old and younger patients is hampered by the lack of diagnostic procedures in the very old, and even the primary diagnosis may be missing in critically ill older people. Older patients with modest MDS-related cytopenia may go undiagnosed until they develop AML, and older patients with end-stage MDS may not have their AML transformation diagnosed and reported.
In summary, AML is a very heterogeneous disease, but in this unique population-based analysis of very old patients there seems to be only modest differences in the clinical subsets of AML between younger and older patients, with the proviso that molecular data are as yet not available. Thus, therapeutic improvements which are being developed for patients aged from 70 to 80 may also benefit even older patients, which should be studied specifically.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Contributor Information
Collaborators: for the Swedish AML Registry
References
- 1.Juliusson G, Abrahamsson J, Lazarevic V, et al. Prevalence and characteristics of survivors from acute myeloid leukemia in Sweden. Leukemia. 2017;31(3):728–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179–4187. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Research UK [Updated June 15, 2018; cited October 23, 2018]. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/age#heading-Zero.
- 4.Pavlidis N, Stanta G, Audisio RA. Cancer prevalence and mortality in centenarians: a systematic review. Crit Rev Oncol Hematol. 2012;83(1):145–152. [DOI] [PubMed] [Google Scholar]
- 5.Nolen SC, Evans MA, Fischer A, et al. Cancer-Incidence, prevalence and mortality in the oldest-old. A comprehensive review. Mech Ageing Dev. 2017;164:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juliusson G, Lazarevic V, Lehmann S, et al. Improved survival of patients with acute myeloid leukemia following implementation of Swedish National Guidelines: Results from the AML Registry 1997–2013. Blood. 2014;124(21):2269. [Google Scholar]
- 8.Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. 2016;127(1):42–52. [DOI] [PubMed] [Google Scholar]
- 9.Stein EM, Tallman MS. Emerging therapeutic drugs for AML. Blood. 2016;127(1):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarevic V, Hörstedt AS, Johansson B, et al. Incidence and prognostic significance of karyotypic subgroups in older patients with acute myeloid leukemia: the Swedish population-based experience. Blood Cancer J. 2014;4:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood. 2018;131(5):515–524. [DOI] [PubMed] [Google Scholar]
- 12.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://seer.cancer.gov/
- 14.Poynter JN, Richardson M, Blair CK, et al. Obesity over the life course and risk of acute myeloid leukemia and myelodysplastic syndromes. Cancer Epidemiol. 2016;40:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulegårdh E, Nilsson C, Lazarevic V, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol. 2015;90(3):208–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.