Abstract
BAY 1143572 is a highly selective inhibitor of cyclin-dependent kinase 9/positive transcription elongation factor b. It has entered phase I clinical studies. Here, we have assessed the utility of BAY 1143572 for treating natural killer (NK) cell leukemias/lymphomas that have a poor prognosis, namely extranodal NK/T-cell lymphoma, nasal type and aggressive NK-cell leukemia, in a preclinical mouse model in vivo as well as in tissue culture models in vitro. Seven NK-cell leukemia/lymphoma lines and primary aggressive NK-cell leukemia cells from two individual patients were treated with BAY 1143572 in vitro. Primary tumor cells from an aggressive NK-cell leukemia patient were used to establish a xenogeneic murine model for testing BAY 1143572 therapy. Cyclin-dependent kinase 9 inhibition by BAY 1143572 resulted in prevention of phosphorylation at the serine 2 site of the C-terminal domain of RNA polymerase II. This resulted in lower c-Myc and Mcl-1 levels in the cell lines, causing growth inhibition and apoptosis. In aggressive NK-cell leukemia primary tumor cells, exposure to BAY 1143572 in vitro resulted in decreased Mcl-1 protein levels resulting from inhibition of RNA polymerase II C-terminal domain phosphorylation at the serine 2 site. Orally administering BAY 1143572 once per day to aggressive NK-cell leukemia-bearing mice resulted in lower tumor cell infiltration into the bone marrow, liver, and spleen, with less export to the periphery relative to control mice. The treated mice also had a survival advantage over the untreated controls. The specific small molecule targeting agent BAY1143572 has potential for treating NK-cell leukemia/lymphoma.
Introduction
Extranodal natural killer (NK)/T-cell lymphoma (ENKTL), nasal type and aggressive NK-cell leukemia (ANKL), are both representative NK-cell leukemia/lymphoma in which Epstein-Barr virus (EBV) is considered to play a critical role.1,2 ANKL is a systemic neoplastic proliferation of NK cells that has an aggressive clinical course, and a seriously poor prognosis, with a median survival of < 2 months.2–5 There can be overlap with ENKTL, nasal type, showing systemic organ involvement; thus, it is unclear whether ANKL is the leukemic counterpart of ENKTL, nasal type.1,2 A regimen not containing anthracyclines, SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) has brought some improvement in the treatment of these systemic NK-cell leukemia/lymphoma.6,7 However, the prognosis of these neoplasms is still unsatisfactory,8,9 and the development of novel therapeutic agents remains an urgent issue. Nevertheless, to the best of our knowledge, until now there have been very few preclinical studies on the development of novel antitumor agents targeting NK-cell leukemia/lymphoma.
We have been focusing on cyclin-dependent kinase 9 (CDK9) as a potential molecular target for NK-cell leukemia/lymphoma. CDK9 is a serine (Ser)/threonine kinase, and constitutes a subunit of the positive transcription elongation factor b (P-TEFb) complex. This plays a vital role in regulating gene transcription elongation via phosphorylation of the C-terminal domain (CTD) of RNA polymerase II (RNAPII).10–12 Accumulating reports indicate that CDK9 kinase activity is crucial during the evolution and/or maintenance of many types of human malignancy.10–17 CDK9 is also known to have an important role for Epstein–Barr Nuclear Antigen 2 (EBNA2)-dependent transcriptional activation and immortalization of EBV-infected cells.18–20 Taken together, these findings suggest that CDK9 could represent a new molecular target for treating systemic NK-cell neoplasms, such as ENKTL, nasal type with systemic organ involvement, as well as ANKL. Here, we begin to test this hypothesis by investigating the therapeutic potential of BAY 1143572 (Bayer AG Pharmaceuticals Division, Berlin, Germany), which is a new, highly selective inhibitor of CDK9/P-TEFb.21
Methods
NK-cell leukemia/lymphoma lines
SNT-8, SNK-1, SNT-16, NK-92 and KAI-3 are EBV-positive, but MTA and KHYG-1 are EBV-negative lines.22–26 NK-92 was purchased from ATCC (Manassas, VA), and MTA, KAI-3 and KHYG-1 were purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan).
Primary tumor cells from patients with ANKL and cells from control subjects
Primary tumor cells were isolated using anti-human CD56 microbeads (Miltenyi Biotec, Bergisch Gladbach) from peripheral blood mononuclear cells (PBMC) of two patients (patient A and B, Online Supplementary Figure S1). Five healthy volunteers participated as control subjects, and their CD56-positive cells were isolated from their PBMC in the same manner. All donors provided written informed consent before blood sampling according to the Declaration of Helsinki, and the present study was approved by the institutional ethics committees of Nagoya City University Graduate School of Medical Sciences.
Cell proliferation and apoptosis assays
Cell proliferation and apoptosis were assessed as previously described.17,27
Western blotting
Antibodies to RNAPII (N-20), c-Myc, Mcl-1, actin, phosphorylated RNAPII (phospho-RNAPII) (Ser2 of the CTD) and phospho-RNAPII (Ser5 of the CTD) were as previously described.17,28
Histological analysis
Hematoxylin and eosin (HE) staining was performed on formalin-fixed, paraffin-embedded sections, as previously described.17,29
Animals
All in vivo experiments of NOD/Shi-scid, IL-2Rγnull (NOG) mice were performed as previously described.17
Establishment of the primary ANKL cell-bearing mouse model
Patient A’s PBMC, consisting of almost 90% CD56-positive atypical lymphoid tumor cells, were injected intraperitoneally (i.p.) into naïve NOG mice (1 × 107/mouse). Three to 4 weeks after i.p. injection, the NOG mice became weaker and exhibited clinical features of cachexia. The tumor cells were recovered and i.p. inoculated into other naïve NOG mice, and after three to four weeks, they displayed features almost identical to those of the donor mice. This procedure of transfer from mouse to mouse was repeated successfully until at least the fifth passage.
Primary ANKL cell-bearing mice treated with BAY 1143572
Leukemic cells from ANKL patient A, which could be serially transplanted into NOG mice, were i.p. injected into 10 naïve NOG mice (1×107/mouse). The animals were randomly divided into two groups seven days after ANKL cell inoculation, and were treated with oral application of 12.5 mg/kg BAY 1143572 or vehicle, for 15 days (7–21 days after tumor inoculations). Therapeutic efficacy was then evaluated 22 days after tumor inoculation. In another experiment, ANKL cells from the mice suspended were also inoculated i.p. into another 12 naive NOG mice (0.8×107/mouse). These animals were randomly divided into two groups and were treated by oral application of 12.5 mg/kg BAY1143572 or vehicle for 15 days (7–21 days after tumor inoculation). The therapeutic efficacy of BAY 1143572 was evaluated by survival times.
Flow cytometry analysis of cells inoculated into mice
The following mAbs were used for flow cytometry: BD MultitestTM CD3/CD16+CD56/CD45/CD19 (No. 342416, BD Biosciences), and stained cells were analyzed as previously described.17
Statistical analysis
All statistical analyses were performed using SPSS Statistics 17.0 software (SPSS Inc., Chicago, IL), as previously described.17
Results
In vitro inhibitory effect of BAY 1143572 on the proliferation of NK-cell leukemia/lymphoma lines
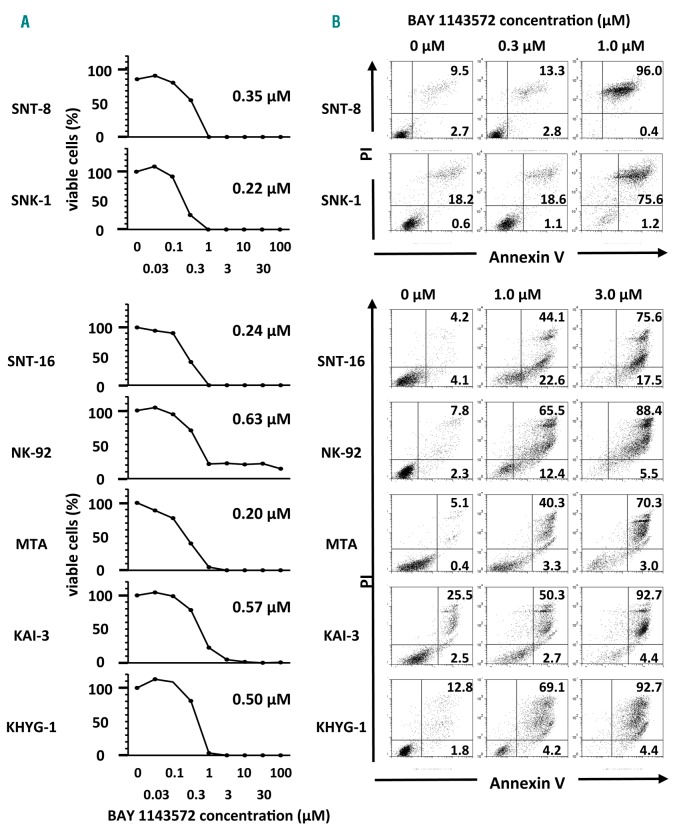
BAY 1143572 was found to inhibit NK-cell leukemia/lymphoma cell line proliferation in a dose-dependent manner (Figure 1A). IC50 values for BAY1143572 after 72 hours of incubation for SNT-8, SNK-1, SNT-16, NK-92, MTA, KAI-3, and KHYG-1 were 0.35, 0.22, 0.24, 0.63, 0.20, 0.57, and 0.50 μM, respectively.
Figure 1.
BAY 1143572 inhibits proliferation and induces apoptosis in natural killer (NK) cell leukemia/lymphoma lines. (A) Viability of NK-cell leukemia/lymphoma lines on exposure to different concentrations of BAY 1143572 for 72 hours. The IC50 value is shown for each line. IC50 was defined as the concentration of an inhibitor that reduced cell survival to 50% of the untreated control value, with the highest viability (no inhibitor) defined as 100%, and the lowest viability defined as 0%. Each graph shows one representative result of three independent experiments. (B) NK-cell leukemia/lymphoma lines were treated with different concentrations of BAY 1143572 for 72 hours followed by assessing apoptosis via Annexin V and propidium iodide (PI; nuclear) staining. BAY 1143572 concentrations are indicated above the panels, and the percentage of cells in each quadrant is given. Each graph shows one representative result of three independent experiments.
BAY 1143572 induces apoptosis in NK-cell leukemia/lymphoma lines
BAY 1143572 was found to induce apoptosis of the tested NK-cell leukemia/lymphoma lines in a dose-dependent manner. Nearly half of the MTA and KAI-3 cells, 70% of SNT-16 and KHYG-1 cells, 80% of SNK-1 and NK-92 cells, and 100% of SNT-8 cells underwent apoptosis on treatment with 1.0 μM BAY 1143572 for 72 hours (Figure 1B).
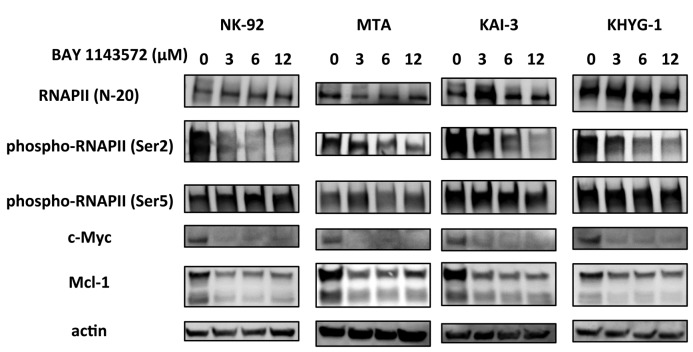
Effect of BAY 1143572 on CDK9 activity in NK-cell leukemia/lymphoma lines
BAY 1143572 had very little effect on the total amount of RNAPII protein in any of the cell lines tested but inhibited phosphorylation of RNAPII CTD at the Ser2 site in a dose-dependent manner. In contrast, phosphorylation of RNAPII at the Ser5 site was not affected by this agent in any of the lines. BAY 1143572 treatment did decrease c-Myc and Mcl-1 protein levels in a dose-dependent manner in all of the lines (Figure 2).
Figure 2.
BAY 1143572 affects CDK9 activity in NK-cell leukemia/lymphoma lines. NK-92, MTA, KAI-3, and KHYG-1 cells were treated with the indicated concentrations of BAY 1143572 for 5 hours, followed by Western blotting probing RNA polymerase II (RNAPII), phospho-RNAPII (the serine-2 residue of the C-terminal domain [CTD] [Ser2]), phospho-RNAPII (Ser5), c-Myc, and Mcl-1. Actin was the loading control.
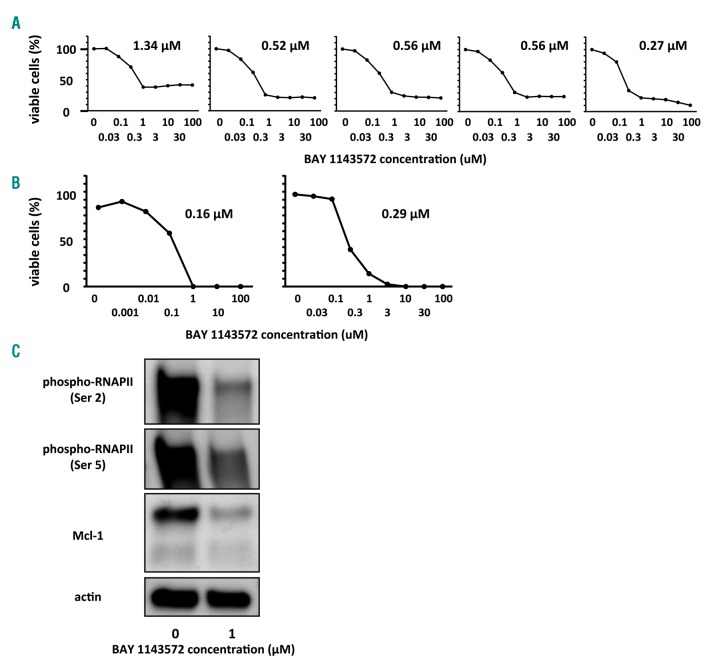
BAY 1143572 inhibits growth of primary ANKL cells isolated from patients
IC50 values for BAY 1143572 after 24 hours of incubation with CD56-positive cells isolated from five healthy volunteers were 0.65, 0.56, and 0.27–1.34 μM, (mean, median, and range, respectively). Cell viability curves for BAY 1143572 showed an initial decrease at concentrations up to around 1.0 uM, almost reaching a plateau at around 100.0 uM. Thus, some of the CD56-positive cells isolated from healthy volunteers remained viable even after exposure to 100.0 uM BAY 1143572 for 24 hours (Figure 3A). On the other hand, IC50 values for BAY 1143572 after 24 hours of incubation with CD56-positive tumor cells obtained from two different patients with ANKL were 0.16 uM and 0.29 uM (Figure 3B). In contrast to the CD56-positive cells from healthy volunteers, most of the tumor cells were dead after exposure to 3.0 uM BAY 1143572 for 24 hours. Bay 1143572 inhibited phosphorylation of RNAPII CTD at the Ser2 site and reduced the expression of Mcl-1 proteins in the primary tumor cells (Figure 3C).
Figure 3.
BAY 1143572 inhibits proliferation and affects CDK9 activity in primary ANKL cells. (A) Viability of human CD56-positive cells in control donor PBMC (n=5) assessed in the presence of recombinant human IL-2 (rIL-2, Miltenyi Biotec) at a final concentration of 100 IU/mL together with different concentrations of BAY 1143572 for 24 hours. The IC50 value is indicated in each graph. (B) Viability of primary aggressive NK-cell leukemia (ANKL) cells from two separate patients (patient A, left panel, and patient B, right panel) assessed in the presence of rIL-2 at 100 IU/mL together with different concentrations of BAY 1143572 for 24 hours. The IC50 value is indicated in each graph. IC50 was defined as the concentration of an inhibitor that reduced cell survival to 50% of the untreated control value, with the highest viability (no inhibitor) defined as 100%, and the lowest viability defined as 0%. (C) Primary ANKL cells from patient A were treated with the indicated concentrations of BAY 1143572 for 12 hours in the presence of 100 IU/mL rIL-2, followed by Western blotting probed with antibodies to phospho-RNAPII (Ser2), phospho-RNAPII (Ser5), and Mcl-1. Actin was the loading control.
Phosphorylation status of RNAPII at the Ser2 site in tumor lesions of ENKTL, nasal type
RNAPII in ENKTL, nasal type, tumor cells were highly phosphorylated at Ser2 sites. This was also the case in non-tumorous lymphocytes from reactive lymph nodes. Indeed, the degree of phosphorylation of RNAPII at the Ser2 site was identical in both the tumor cells and non-tumor lymphocytes. Three representative cases from each group are shown in Online Supplementary Figure S2.
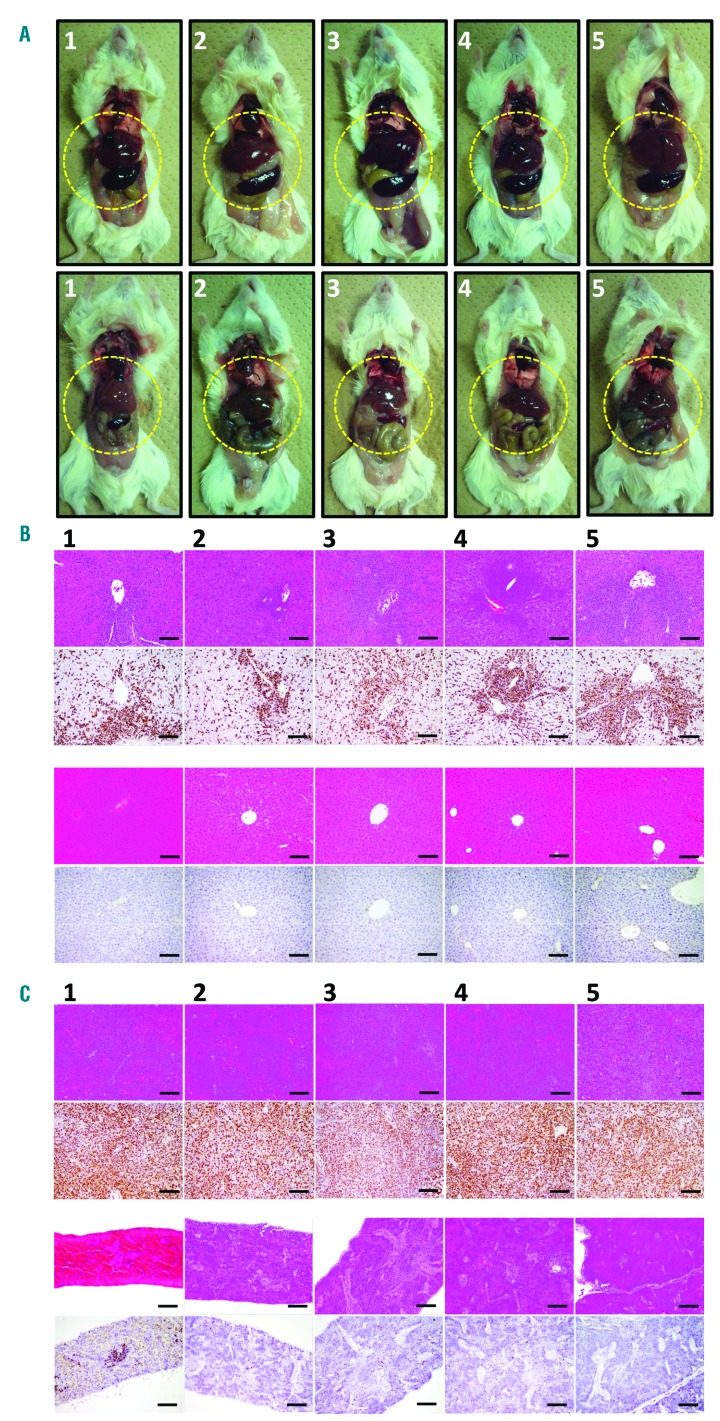
Macroscopic and microscopic findings in primary ANKL cell-bearing mice with or without BAY 1143572 therapy
The appearance of mice treated with vehicle only and those treated with BAY 1143572, 22 days after primary ANKL cell inoculation, is shown in Figure 4A, upper and lower panels, respectively. Hepatosplenomegaly was observed in all of the untreated mice, but not in those treated with BAY 1143572 (liver and spleen are demarcated by narrow yellow lines). Immunohistological analyses revealed that the livers of the control mice were infiltrated by atypical lymphoid cells, and the normal architecture was destroyed (Figure 4B, top panels). ISH showed that these atypical cells were positive for EBER (Figure 4B, second panels from the top). On the other hand, the liver architecture of the treated mice was almost intact (Figure 4B, third panels from the top), and EBER-positive cells were rare (Figure 4B, bottom panels). The same immunohistological analyses also revealed that the spleens of the untreated mice were infiltrated by atypical lymphoid cells, and the normal architecture was completely destroyed (Figure 4C, top panels). ISH also showed that these atypical cells were positive for EBER (Figure 4C, second panels from the top). On the other hand, the splenic architecture of the treated mice, like the liver, was essentially intact (Figure 4C, third panels from the top), and EBER-positive cells were rarely observed (Figure 4C, bottom panels).
Figure 4.
Macroscopic and microscopic findings in primary ANKL cell-bearing mice treated or not treated with BAY 1143572. (A) Macroscopic appearance of control mice treated with vehicle (control; top panels) or BAY 1143572 (bottom panels). Liver and spleen are demarcated by narrow yellow lines. (B) Photomicrographs of control mouse liver (hematoxylin and eosin [HE] staining) (top panels), and with in situ hybridization (ISH) using an Epstein–Barr virus (EBV)-encoded RNA (EBER) probe (panels second from top). HE-stained liver (third panels from the top), together with ISH using an EBER Probe (bottom panels) from BAY 1143572-treated mice. Scale bars represent 100 μm. (C) HE staining of the spleen of control mice (top panels), together with ISH using an EBER Probe (Leica Microsystems Newcastle Ltd.) (second panels from the top). HE staining of the spleen (third panels from the top), and ISH using an EBER Probe (bottom panels) from mice treated with BAY 1143572. Scale bars represent 100 μm.
BAY 1143572 treatment reduces primary ANKL cells in the blood of mice
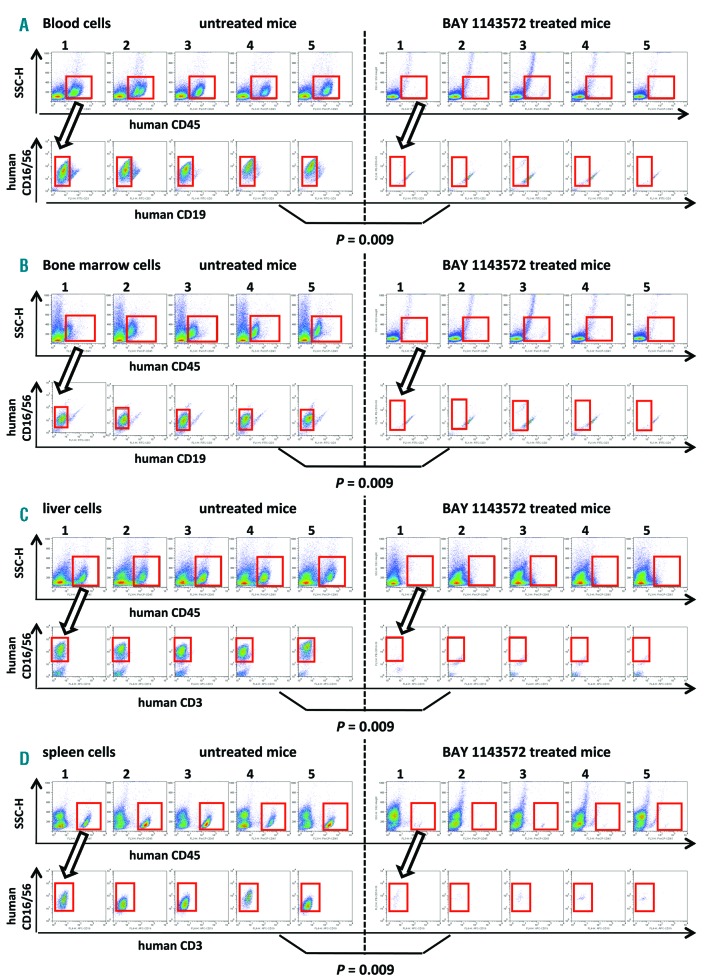
Twenty-two days after inoculation of primary ANKL cells, the percentage of human tumor cells (identified as CD45- and CD16/56-positive but CD19-negative) in the whole blood of control NOG mouse No.1 was 17.1% (i.e., 17.3% [CD45-positive lymphocyte population] × 98.7% [CD16/56-positive, but CD19-negative cells] = 17.1%) (Figure 5A, the two upper left panels). In control NOG mice Nos. 2, 3, 4, and 5, and in BAY1143572-treated NOG mice Nos. 1, 2, 3, 4, and 5, the percentages of ANKL cells in whole blood, calculated in the same manner, were 30.9, 45.0, 11.1, and 38.4%; and 0.6, 0.1, 0.4, 0.4, and 0.4%, respectively. Thus, BAY 1143572 treatment resulted in significantly decreased percentages of ANKL cells in the blood of these xenogeneic primary tumor-bearing mice (P=0.009; Figure 5A).
Figure 5.
Significant therapeutic effect of BAY 1143572 in ANKL cell-bearing NOG mice. (A) Flow cytometry of mouse blood cells. Human CD45-positive cells among the mouse cells are indicated in each panel by a red square (upper panels) and are plotted to show CD16/56 and CD19 expression; ANKL cells are CD16/56-positive and CD19-negative as indicated by a red square (lower panels). The percentages of ANKL cells in whole blood of control and BAY 1142572-treated NOG mice are 28.5%, 30.9% (mean, median), and 0.4%, 0.4%, respectively. (B) Flow cytometry of mouse bone marrow cells. Human CD45-expressing cells are again indicated in each panel by red squares (upper panels) and plotted to show CD16/56 and CD19 expression; ANKL cells are CD16/56-positive and CD19-negative as indicated by red squares (lower panels). The percentages of ANKL cells in the bone marrow of control and BAY 1142572-treated NOG mice are 20.8%, 18.3% (mean, median), and 0.2%, 0.2%, respectively. (C) Flow cytometry of mouse liver cell suspensions. Human CD45-expressing cells are indicated in each panel by red squares (upper panels) and are stained for CD16/56 and CD3 expression; ANKL cells are CD16/56-positive and CD3-negative (red squares, lower panels). The percentages of ANKL cells in the livers of control and BAY 1142572-treated NOG mice are 25.4%, 27.6% (mean, median), and 0.7%, 0.5%, respectively. (D) Flow cytometry of mouse spleen cell suspensions. As above, the human CD45-expressing cells are indicated in each panel by red squares (upper panels) and ANKL cells are CD16/56-positive and CD3-negative (red squares, lower panels). The percentages of ANKL cells in the spleens of control and BAY 1142572-treated NOG mice are 18.1%, 19.3% (mean, median), and 1.0%, 0.9%, respectively. The significance of the difference (P value) in the percentages of ANKL cells between two groups is shown in each panel (A) (B) (C), and (D).
BAY 1143572 treatment reduces primary ANKL cells in the bone marrow
Twenty-two days after inoculation, the percentage of ANKL tumor cells in the bone marrow of control NOG mouse No.1 was 18.3% (i.e., 26.5% [CD45-positive lymphocyte population] × 69.2% [CD16/56-positive, CD19-negative] = 18.3%) (Figure 5A, the two upper left panels). In control NOG mice Nos. 2, 3, 4, and 5, and in BAY 1143572-treated NOG mice Nos. 1, 2, 3, 4, and 5, the percentages of ANKL cells in bone marrow, calculated in the same manner, were 17.0, 26.0, 26.0, and 16.8%; and 0.2, 0.3, 0.3, 0.2, and 0.2%, respectively. Thus, BAY1143572 treatment resulted in lowered percentages of ANKL cells infiltrating into the bone marrow (P=0.009; Figure 5B).
BAY 1143572 treatment reduces primary ANKL cells in the liver
The percentage of leukemia cells infiltrating into the liver of control NOG mouse No.1 was 32.4% (i.e., 36.9% [CD45-positive lymphocyte population] × 87.8% [CD16/56-positive, CD3-negative] = 32.4%) (Figure 5C, the two upper left panels), 22 days after tumor inoculation. In control NOG mice Nos. 2, 3, 4, and 5, and in BAY 1143572 treated NOG mice Nos. 1, 2, 3, 4, and 5, these figures were 27.6, 29.7, 15.8, and 21.4%; and 0.3, 0.6, 1.8, 0.5, and 0.1%, respectively. Thus, the percentage of primary ANKL cells present in the liver was also decreased by BAY1143572 treatment in these animals (P=0.009; Figure 5C).
BAY 1143572 treatment reduces primary ANKL cells in the spleen
Using the same experimental protocol as described above, 22 days after inoculation of primary ANKL tumor cells, the percentage of leukemia cells in the spleen of control NOG mouse No.1 was 11.7% (Figure 5D, the upper left two panels) whereas in control NOG mice Nos. 2, 3, 4, and 5, and in BAY1143572-treated NOG mice Nos.1, 2, 3, 4, and 5, these percentages were 17.6, 21.0, 21.1, and 19.3%; and 0.3, 0.5, 1.0, 0.9, and 2.5%, respectively. Thus, BAY1143572 treatment also decreases primary ANKL cell infiltration into the spleens of these animals (P=0.009; Figure 5D).
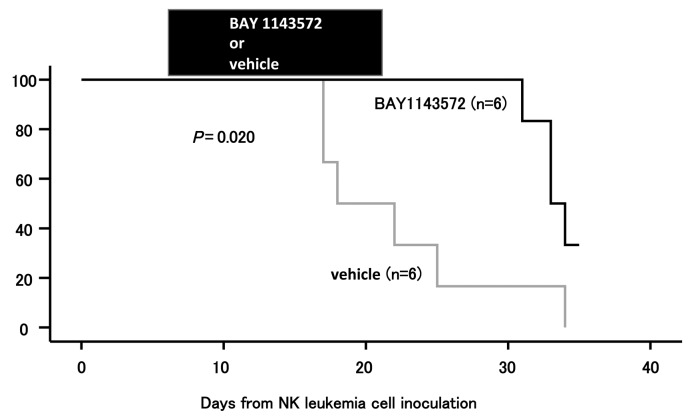
BAY1143572 prolongs the survival of mice inoculated with primary ANKL cells
Two of the 6 BAY 1143572-treated mice survived up to 34 days after inoculation of the primary ANKL tumor cells, whereas none of the vehicle-treated control mice were alive at that time (P=0.020; Figure 6). No toxicity attributable to BAY 1143572 was observed over this time period in any of the mice. Thus, we conclude that BAY 1143572-treatment of mice inoculated with primary ANKL cells significantly extended their survival relative to the untreated controls.
Figure 6.
Kaplan-Meier survival curves of ANKL cell-bearing NOG mice treated with BAY 1143572 or vehicle. Mice were treated orally with 12.5 mg/kg BAY 1143572 or vehicle (n=6 for both) once daily for 15 days (7-21 days after ANKL cell inoculation). The significance of the difference in survival is shown on the graph.
Discussion
NK-cell leukemia/lymphoma have a very poor prognosis necessitating improved treatment regimens for these patients.1–9 Here, we begin to assess whether a selective CDK9/P-TEFb inhibitor, BAY 1143572, may have clinical utility in this regard. We found that BAY 1143572 possessed notable antitumor activity, not only against established NK cell leukemia/lymphoma lines, but also against primary ANKL cells in vitro and in primary ANKL cell-bearing mice in vivo. Hence, CDK9 activity is crucial for NK-cell leukemia/lymphoma pathogenesis, implying that transcriptional machinery could represent an appropriate molecular target for developing new treatments for this disease.
First, we aimed to investigate the mechanism of action of BAY 1143572 using NK-cell leukemia/lymphoma lines in vitro and determined that it specifically inhibited the phosphorylation of RNAPII CTD at the Ser2 but not the Ser5 site. This resulted in transcriptional repression of RNAPII and thus decreased levels of the downstream proteins c-Myc and Mcl-1. This in turn causes inhibition of growth and induction of apoptosis in NK-cell leukemia/lymphoma lines whether they originate from NK cells (SNK-1, NK-92, KAI-3, or KHY-G) or T cells (SNT-8, SNT-16, or MTA). Whether they were EBV-positive (SNT-8, SNK-1, SNT-16, NK-92, or KAI-3) or -negative (MTA or KHY-G) was also found not to affect the anticancer activity of BAY 1143572. Very similar results were obtained using primary ANKL cells freshly isolated from a patient, rather than established cell lines. BAY 1143572 markedly reduced phosphorylation of RNAPII CTD at the Ser2 site, but only weakly at the Ser5 site, which results in downregulation of the downstream protein, Mcl-1.
Importantly, our study showed that NK-cell leukemia/lymphoma are more sensitive to the CDK9/P-TEFb inhibitor than their normal cell counterparts. Exposure to ≥1.0 μM of BAY 1143572 for 24 hours killed almost all primary ANKL cells from two different patients, but also a fraction of CD56-positive cells from healthy volunteers. Nonetheless, after 24 hours’ exposure to 10.0 μM BAY 1143572, 20-40% of CD56-positive cells from controls were still viable. This clearly documents that BAY 1143572 is far less toxic to CD56-positive cells from healthy donors than to CD56-positive primary ANKL tumor cells. It remains unclear why NK-cell leukemia/lymphoma cells are more sensitive to the CDK9/P-TEFb inhibitor. One possibility is that CDK9 kinase and RNAPII are more highly activated in malignant than in normal cells. Supporting this, another group has reported higher CDK9 expression accompanied by greater phosphorylation of RNAPII CTD in tumor cells than in normal cells.30 Additionally, a different group reported that CDK9 expression appeared to be related to particular stages of lymphoid differentiation/activation.31 However, we did not observe any significant differences between tumor cells and non-tumor lymphocytes in the phosphorylation level of RNAPII at the Ser2 site in the present study. A possible explanation for this is that the malignant cells are more “dependent” on the transcriptional machinery involving RNAPII to sustain the expression of critical oncogenes supporting their survival and proliferation, such as Mcl-1 and c-Myc.32,33 In this context, inhibition of the transcriptional machinery would result in an acute and concurrent downregulation of these oncogenes, thereby leading to cell death. In fact, inhibition of CDK7 or BET bromodomain 4, which also block the activity of RNAPII in a similar manner to CDK9/PTEF-b inhibition, has been reported to result in potent anti-cancer activity in several malignancies.34–38 Importantly, many types of cancer and leukemia cells are more sensitive to these inhibitors than non-transformed cells.39,40 These studies suggest that expression of oncogenes may need to be maintained at a high level, and thus that cancer cells are likely to be more sensitive to RNAPII inhibition. Collectively, these earlier investigations and the present studies suggest a great advantage for the clinical use of CDK9 inhibitors. Future investigation is needed to analyze the status of regulatory elements after BAY 1143572 treatment.
In the present study, we have established a mouse xenogeneic model for pre-clinical testing of a CDK9/P-TEFb inhibitor. Severe immune deficiencies in NOG mice permit engraftment of human immune cells, where they may retain very similar functions in an in vivo environment that can be manipulated experimentally.41,42 In these mice, primary EBV-positive, CD16/CD56-positive, CD3− and CD19-negative cells from ANKL patients were found to robustly infiltrate several organs such as the spleen, liver and bone marrow. These features are very similar to those seen in the ANKL patients who donated the cells. To the best of our knowledge, this is the first report of primary ANKL cell-bearing mice in which it could be shown that ANKL cells are maintained by serial transplantations. This is a useful model because these tumor cells cannot be cultured as cell lines in vitro, implying that the in vivo microenvironment is an absolute requirement for tumor cell survival. Hence, our present in vivo ANKL model represents the in vivo human ANKL environment better than other models using established tumor cell lines. Thus, this in vivo xenogeneic primary tumor model offers a useful methodology for probing ANKL pathogenesis and provides a more relevant tool for testing novel antitumor agents. Here, we report that BAY 1143572 possesses strong antitumor activity, as demonstrated in vivo by reduced ANKL cell infiltration into blood, bone marrow, liver and spleen in this model. Moreover, this model also allowed us to demonstrate that BAY 1143572 monotherapy can prolong survival of ANKL-bearing hosts. It should also be noted that the in vivo antitumor activity of BAY 1143572 in the primary tumor cell-bearing NOG mice was actually mediated by the on-target effect of BAY 1143572; namely, the inhibition of CDK9 and subsequent inhibition of phosphorylation at the Ser2 site of the RNAPII CTD was clearly demonstrated in our previous study on ATL.17 It is also important to note that no BAY 1143572 toxicity was observed in any of the mice studied here. Taken together, these findings in the primary ANKL mice strongly suggest that targeting CDK9 in human ANKL patients could be a promising therapeutic approach.
To the best of our knowledge, this is the first report evaluating the efficacy of CDK9-targeted therapy in NK-cell leukemia/lymphoma, including ANKL. It must be noted that several other anti-cancer agents targeting CDK9 have already been tested in the clinic, but with little benefit and they were accompanied by numerous adverse events.43–47 The latter may have been due to their insufficient selectivity for CDK9.48 In contrast, BAY 1143572 exhibits marked selectivity for CDK9/PTEFb,21 and possesses strong antitumor effects against NK-cell leukemia/lymphoma including ANKL, not only in vitro, but also in animal models in vivo. Because, in clinical practice, NK-cell leukemia/lymphoma is highly aggressive and refractory, combination strategies such as CDK9 inhibition together with PD-1 blockade should be worth considering.49,50
In conclusion, we propose that BAY 1143572 holds great promise as a potential agent to treat NK-cell leukemia/lymphoma, including ANKL patients. The mechanism of action is via CDK9/P-TEFb inhibition. Clinical evaluation of CDK9/P-TEFb selective inhibitors in patients with NK cell leukemia/lymphoma is therefore warranted.
Supplementary Material
Acknowledgments
We thank Ms Chiori Fukuyama for excellent technical assistance and Ms Naomi Ochiai for excellent secretarial assistance. We also thank Stuart Ince (Bayer US, LLC) for the kind contribution of obtaining BAY 1143572.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/12/2059
Funding
This work was supported by research funding from Bayer AG to Nagoya City University Graduate School of Medical Sciences. This work was also supported by grants-in-aid for scientific research (B) (No. 16H04713 to Takashi Ishida), grants-in-aid from the National Cancer Center Research and Development Fund (Nos. 29-A-3 to Takashi Ishida, and Shinsuke Iida), and grants-in-aid from the Japan Agency for Medical Research and Development (Nos. 17ck0106287h0001 to Takashi Ishida, 16cm0106301h0001 to Takashi Ishida, and 15ck0106132h0002 to Takashi Ishida, Ryuzu Ueda, and Shinsuke Iida).
References
- 1.Chan JKC, Quintanilla-Martinez L, Ferry JA. Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO classification of tumours of haematopoietic and lymphoid tissues (Revised 4th Edition, pp 368–371). Lyon, France, International Agency for Research on Cancer (IARC), 2017. [Google Scholar]
- 2.Chan JKC, Jaffe ES, Ko YH. Aggressive NK-cell leukaemia. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO classification of tumours of haematopoietic and lymphoid tissues (Revised 4th Edition, pp 353–354). Lyon, France, International Agency for Research on Cancer (IARC), 2017. [Google Scholar]
- 3.Ishida F, Ko YH, Kim WS, et al. Aggressive natural killer cell leukemia: therapeutic potential of L-asparaginase and allogeneic hematopoietic stem cell transplantation. Cancer Sci. 2012;103(6):1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song SY, Kim WS, Ko YH, Kim K, Lee MH, Park K. Aggressive natural killer cell leukemia: clinical features and treatment outcome. Haematologica. 2002;87(12):1343–1345. [PubMed] [Google Scholar]
- 5.Suzuki R, Suzumiya J, Nakamura S, et al. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia. 2004;18(4):763–770. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29(33):4410–4416. [DOI] [PubMed] [Google Scholar]
- 7.Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120(15):2973–2980. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17(3):389–400. [DOI] [PubMed] [Google Scholar]
- 9.Jung KS, Cho SH, Kim SJ, Ko YH, Kang ES, Kim WS. L-asparaginase-based regimens followed by allogeneic hematopoietic stem cell transplantation improve outcomes in aggressive natural killer cell leukemia. J Hematol Oncol. 2016;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bywater MJ, Pearson RB, McArthur GA, Hannan RD. Dysregulation of the basal RNA polymerase transcription apparatus in cancer. Nat Rev Cancer. 2013;13(5):299–314. [DOI] [PubMed] [Google Scholar]
- 11.Morales F, Giordano A. Overview of CDK9 as a target in cancer research. Cell Cycle. 2016;15(4):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson CM, Jiang B, Erb MA, et al. Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nat Chem Biol. 2018;14(2):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CH, Lujambio A, Zuber J, et al. CDK9-mediated transcription elongation is required for MYC addiction in hepatocellular carcinoma. Genes Dev. 2014;28(16):1800–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory GP, Hogg SJ, Kats LM, et al. CDK9 inhibition by dinaciclib potently suppresses Mcl-1 to induce durable apoptotic responses in aggressive MYC-driven B-cell lymphoma in vivo. Leukemia. 2015;29(6):1437–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker A, Gregory GP, Verbrugge I, et al. The CDK9 inhibitor dinaciclib exerts potent apoptotic and antitumor effects in preclinical models of MLL-rearranged acute myeloid leukemia. Cancer Res. 2016;76(5):1158–1169. [DOI] [PubMed] [Google Scholar]
- 16.Tong Z, Chatterjee D, Deng D, et al. Antitumor effects of cyclin dependent kinase 9 inhibition in esophageal adenocarcinoma. Oncotarget. 2017;8(17):28696–28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narita T, Ishida T, Ito A, et al. Cyclin-dependent kinase 9 is a novel specific molecular target in adult T-cell leukemia/lymphoma. Blood. 2017;130(9):1114–1124. [DOI] [PubMed] [Google Scholar]
- 18.Bark-Jones SJ, Webb HM, West MJ. EBV EBNA 2 stimulates CDK9-dependent transcription and RNA polymerase II phosphorylation on serine 5. Oncogene. 2006;25(12):1775–1785. [DOI] [PubMed] [Google Scholar]
- 19.Palermo RD, Webb HM, West MJ. RNA polymerase II stalling promotes nucleosome occlusion and pTEFb recruitment to drive immortalization by Epstein-Barr virus. PLoS Pathog. 2011;7(10):e1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaborowska J, Isa NF, Murphy S. P-TEFb goes viral. Inside Cell. 2016;1(2):106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucking U, Scholz A, Lienau P, et al. Identification of atuveciclib (BAY 1143572), the first highly selective, clinical PTEFb/CDK9 inhibitor for the treatment of cancer. ChemMedChem. 2017;12(21):1776–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Nagata H, Ikeuchi T, et al. Common cytological and cytogenetic features of Epstein-Barr virus (EBV)-positive natural killer (NK) cells and cell lines derived from patients with nasal T/NK-cell lymphomas, chronic active EBV infection and hydroa vacciniforme-like eruptions. Br J Haematol. 2003;121(5):805–814. [DOI] [PubMed] [Google Scholar]
- 23.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8(4):652–8. [PubMed] [Google Scholar]
- 24.Emi N, Abe A, Kasai M, et al. CD4- and CD56-positive T-cell line, MTA, established from natural killer-like T-cell leukemia/lymphoma. Int J Hematol. 1999;69(3):180–185. [PubMed] [Google Scholar]
- 25.Tsuge I, Morishima T, Morita M, Kimura H, Kuzushima K, Matsuoka H. Characterization of Epstein-Barr virus (EBV)-infected natural killer (NK) cell proliferation in patients with severe mosquito allergy; establishment of an IL-2-dependent NK-like cell line. Clin Exp Immunol. 1999;115(3):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagita M, Huang CL, Umehara H, et al. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia. 2000;14(5):922–930. [DOI] [PubMed] [Google Scholar]
- 27.Ishida T, Iida S, Akatsuka Y, et al. The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-Cell leukemia/lymphoma. Clin Cancer Res. 2004;10(22):7529–7539. [DOI] [PubMed] [Google Scholar]
- 28.Ri M, Iida S, Ishida T, et al. Bortezomib-induced apoptosis in mature T-cell lymphoma cells partially depends on upregulation of Noxa and functional repression of Mcl-1. Cancer Sci. 2009;100(2):341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishida T, Utsunomiya A, Iida S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9(10):3625–3634. [PubMed] [Google Scholar]
- 30.Zhang Y, Zhou L, Leng Y, Dai Y, Orlowski RZ, Grant S. Positive transcription elongation factor b (P-TEFb) is a therapeutic target in human multiple myeloma. Oncotarget. 2017;8(35):59476–59491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellan C, De Falco G, Lazzi S, et al. CDK9/CYCLIN T1 expression during normal lymphoid differentiation and malignant transformation. J Pathol. 2004;203(4):946–952. [DOI] [PubMed] [Google Scholar]
- 32.Wong RWJ, Ishida T, Sanda T. Targeting general transcriptional machinery as a therapeutic strategy for adult T-cell leukemia molecules. 2018;23(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hnisz D, Abraham BJ, Lee TI, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155(4):934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roderick JE, Tesell J, Shultz LD, et al. c-Myc inhibition prevents leukemia initiation in mice and impairs the growth of relapsed and induction failure pediatric T-ALL cells. Blood. 2014;123(7):1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong RWJ, Ngoc PCT, Leong WZ, et al. Enhancer profiling identifies critical cancer genes and characterizes cell identity in adult T-cell leukemia. Blood. 2017;130(21):2326–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hnisz D, Shrinivas K, Young RA, Chakraborty AK, Sharp PA. A phase separation model for transcriptional control. Cell. 2017;169(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwiatkowski N, Zhang T, Rahl PB, et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature. 2014;511(7511):616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsby E, Pratt G, Shao H, et al. A novel Cdk9 inhibitor preferentially targets tumor cells and synergizes with fludarabine. Oncotarget. 2014;5(2):375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori F, Ishida T, Ito A, et al. Potent antitumor effects of bevacizumab in a microenvironment-dependent human lymphoma mouse model. Blood Cancer J. 2012;2(4):e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito A, Ishida T, Utsunomiya A, et al. Defucosylated anti-CCR4 monoclonal antibody exerts potent ADCC against primary ATLL cells mediated by autologous human immune cells in NOD/Shi-scid, IL-2R gamma(null) mice in vivo. J Immunol. 2009;183(7):4782–4791. [DOI] [PubMed] [Google Scholar]
- 43.Kar JE, Garrett-Mayer E, Estey EH, et al. Randomized phase II study of two schedules of flavopiridol given as timed sequential therapy with cytosine arabinoside and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Haematologica. 2012;97(11):1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemunaitis JJ, Small KA, Kirschmeier P, et al. A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J Transl Med. 2013;11:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Tourneau C, Faivre S, Laurence V, et al. Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur J Cancer 2010;46(18):3243–3250. [DOI] [PubMed] [Google Scholar]
- 46.Tong WG, Chen R, Plunkett W, et al. Phase I and pharmacologic study of SNS-032, a potent and selective Cdk2, 7, and 9 inhibitor, in patients with advanced chronic lymphocytic leukemia and multiple myeloma. J Clin Oncol. 2010;28(18):3015–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Biessen DA, Burger H, de Bruijn P, et al. Phase I study of RGB-286638, a novel, multitargeted cyclin-dependent kinase inhibitor in patients with solid tumors. Clin Cancer Res. 2014;20(18):4776–4783. [DOI] [PubMed] [Google Scholar]
- 48.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14(2):130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwong YL, Chan TSYD, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437–2442. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Cheng Y, Zhang M, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.