Abstract
Laccases are multi-copper oxidoreductases with broad biotechnological applications. Here, we report detailed biochemical characterization of purified recombinant laccases originating from Myceliophthora thermophila (MtL) and Trametes trogii (TtL). We identified optimal conditions for decolorization of commercial dyes and textile wastewater samples. We also tested the toxicity of decolorized wastewater samples using human peripheral blood mononuclear cells. MtL and TtL were expressed in Saccharomyces cerevisiae, and secreted enzymes were purified by consecutive hydrophobic and gel chromatography. The molecular masses of TtL (~ 65 kDa) and MtL (> 100 kDa) suggested glycosylation of the recombinant enzymes. Deglycosylation of MtL and TtL led to 25% and 10% decreases in activity, respectively. In a thermal stability assay, TtL retained 61% and MtL 86% of the initial activity at 40 °C. While TtL retained roughly 50% activity at 60 °C, MtL lost stability at temperatures higher than 40 °C. MtL and TtL preferred syringaldazine as a substrate, and the catalytic efficiencies for ABTS oxidation were 7.5 times lower than for syringaldazine oxidation. In the presence of the mediator HBT, purified TtL almost completely decolorized dyes within 30 min and substantially decolorized wastewater samples from a textile factory (up to 74%) within 20 h. However, products of TtL-catalyzed decolorization were more toxic than MtL-decolorized products, which were almost completely detoxified.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1525-3) contains supplementary material, which is available to authorized users.
Keywords: Laccases, Textile dyes, Wastewater, Decolorization, Toxicity, PBMCs
Introduction
Laccases (EC 1.10.3.2) belong to a group of oxidoreductases that accept a broad range of substrates, including phenols, aromatic and aliphatic amines, and organometallic compounds. Laccases, often referred to as “green catalysts,” reduce molecular oxygen to water during monoelectronic oxidation of substrates (Riva 2006). The enzymes have great potential for biotechnological applications in the pulp and paper industry (Widsten and Kandelbauer 2008), the food industry (Osma et al. 2010), nanobiotechnology, biosensor research (Arca-Ramos et al. 2016), bioremediation processes (Kadri et al. 2017), and the textile industry (Chatha et al. 2017). Moreover, in the presence of mediators, laccases can oxidize substrates with a high redox potential (Khlifi et al. 2010) and catalyze conversion of non-phenolic substrates (Mate and Alcalde 2015). This environmentally friendly oxidation has contributed to the increasing use of laccases in the synthesis of organic compounds (Cannatelli and Ragauskas 2017).
Use of laccases in the textile industry is relatively well-established. These enzymes can replace hydrogen peroxide in biobleaching processes to remove natural pigments from cellulosic materials prior to dyeing them or treating them with sodium hypochlorite, as in “denim washing.” Laccases also can modify protein fibers to improve their water or shrink resistance (Pezzella et al. 2015). However, laccases are most frequently used for decolorization and detoxification of dyes and wastewaters (WWs) from textile factories (Forgacs et al. 2004; Singh et al. 2015). The dye chemical structure, specifically the number and locations of functional groups, influences the laccase decolorization efficiency. Dyes containing electron-donating substituents (–CH3, –NH2, –OH) are degraded by laccases more efficiently than dyes with electron-withdrawing substituents (–Cl, –NO2, –SO3H, –COOH). Laccases with high redox potential, originating mainly from ligninolytic fungi, are generally preferred for dye decolorization (Legerská et al. 2016). Some dyes, such as anthraquinone, can serve as laccase catalysis mediators for azo dye degradation (Senthivelan et al. 2016; Zeng et al. 2012).
WW from textile factories typically has a high pH and temperature and often contains other chemicals including salts, heavy metals, detergents, and surfactants (Ali et al. 2009). Decolorization procedures must be optimized to account for these factors, and additional procedures, such as membrane filtration, adsorption, coagulation, Fenton’s oxidation, and photo-catalytic oxidation, may need to be employed (Singh and Arora 2011). Hybrid processes combining two or more treatment methods represent an efficient and low-cost alternative. Chemical methods for pre-treatment and biological methods for post-treatment (e.g., ozonation or irradiation for pre-treatment followed by microbial degradation) or vice versa have been studied for these purposes (Holkar et al. 2016). Treatment with cross-linked aggregates of laccases combined with different filtration techniques to eliminate pollutants is another example of a hybrid process (Grandclément et al. 2017). Biological methods based on laccases or a laccase/mediator system have been studied extensively and are often used for WW treatment, as well as bioremediation processes in general (Archna and Kiran 2012; Barrios-Estrada et al. 2018; Naghdi et al. 2018; Sharma et al. 2018). The stability of laccases used in decolorization processes can be enhanced by immobilizing the enzymes [for review, see Bilal et al. (2017)] or the microorganisms producing recombinant laccases (Herkommerova et al. 2018). Engineering appropriate expression vectors (Antosova and Sychrova 2016; Ranieri et al. 2009), altering the laccase structure (Luo et al. 2018; Mate and Alcalde 2015; Wang et al. 2017), and optimizing the host cultivation conditions also contribute to laccase decolorization efficiency (Antosova and Sychrova 2016; Patel and Gupte 2016; Zhu et al. 2016).
The toxicity of decolorized products is another important parameter to consider in WW treatment. In general, lower toxicity is achieved in the presence of natural mediators (e.g., acetosyringone, syringaldehyde, vanillin) (Khlifi et al. 2010) or by application of synthetic mediators (e.g., 2,6-dimethoxyphenol or 1-hydroxybenzotriazole). The latter often yields better decolorization but can have both positive and negative effects on the final sample toxicity. Tests based on inhibition of the growth of different microorganisms or seed germination (phytotoxicity test) (Benzina et al. 2013) can be used to determine toxicity. Both increases in toxicity of WW treated with laccases (Benzina et al. 2013; Dellai et al. 2013) and detoxification of WW by laccases (Guan et al. 2015; Khlifi et al. 2010) have been reported. Optimizing the WW decolorization conditions based on the properties of the laccases used and incorporating product toxicity analysis are, therefore, important considerations for effective biotechnological application of these enzymes.
Here, we biochemically characterized two purified laccases originating from Myceliophthora thermophila (MtL) and Trametes trogii (TtL). Our analysis was aimed at their optimal application in decolorization of synthetic dyes and WWs from textile factories. In parallel, we performed toxicity analysis of decolorized synthetic dyes and WW samples following laccase treatment using a luminescent cell viability assay. To the best of our knowledge, this is the first report describing optimization of decolorization processes by recombinant MtL, as well as the first to assess toxicity of decolorized products using human cells. Our findings suggest that some decolorized WWs from textile factories can pose a health risk for humans, highlighting the importance of product toxicity testing following laccase treatment.
Materials and methods
Chemicals
Synthetic dyes (Bromophenol Blue, Coomassie Brilliant Blue, Remazol Brilliant Blue R), substrates (Syringaldazine, ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), Metol), mediator (HBT: 1-hydroxybenzotriazole) and N-glycosidase F enzyme for deglycosylation of purified laccases were purchased from Sigma-Aldrich (St. Louis, MO, USA). The synthetic dye Saturn Blue was purchased from Synthesia (Semetín, Czech Republic). Samples of textile WW were obtained from CNM textil a.s. (Baška, Czech Republic). Other chemicals used for buffer preparation were purchased from PENTA s.r.o. (Prague, Czech Republic).
Microorganisms and cultivation conditions
The yeast strain Saccharomyces cerevisiae BW31a transformed with expression plasmid pVT-100U-MtL [T2 mutant of MtL; MtL gene was mutated by directed evolution to enhance its expression in S. cerevisiae, Bulter et al. (2003)] or pVT-100U-TtL (LCC1 isoform of TtL; TtL gene was synthetized with optimized codons for S. cerevisiae) was used for recombinant production of secreted laccases MtL and TtL from Myceliophthora thermophila and Trametes trogii, respectively (Antosova et al. 2018). The yeast cells were cultivated in 500 mL (in a 2 L shake flask) of minimal YNB medium (6.7 g/L) without amino acids (ForMedium™) supplemented with 2% (w/v) glucose, 0.8% (w/v) alanine, 0.6 mM CuSO4 and BSM-Ura (Brent supplement mixture drop-out uracil, ForMedium™) at the recommended concentration according to the provider’s manual, at 30 °C for 3 days.
Purification of laccases
Secreted laccases were isolated from cell-free supernatants obtained from S. cerevisiae cultivation. The yeast cells were harvested by centrifugation (5000×g for 15 min), and the supernatants were filtered over Durapore Membrane Filters, PVDF, pore size 0.45 µm, diameter 47 mm (Sigma-Aldrich, St. Louis, MO, USA). Sodium dihydrogen phosphate and ammonium sulfate were added to the filtrates to final concentrations of 50 mM and 1.5 M, respectively, and the pH was adjusted to 7. These samples were applied onto a Phenyl Sepharose 6 Fast Flow column (HiTrap™ Phenyl FF, GE Healthcare, Piscataway, NJ, USA) equilibrated with 50 mM phosphate buffer (pH 7) supplemented with 1.5 M ammonium sulfate. Fractions containing active laccases were eluted with 50 mM phosphate buffer (pH 7) and concentrated with Amicon Ultra filters (30 K, Millipore, Tullagreen, Carrigtwohill, Co. Cork, Ireland) to a volume of 5 mL. The concentrated samples were loaded onto a Superdex™ 75 10/300 GL column (GE Healthcare, Piscataway, NJ, USA) equilibrated with 50 mM phosphate buffer (pH 7). Eluted fractions containing active laccases were pooled. The efficiency of each purification step was analyzed by laccase activity measurement and SDS-PAGE. Purified laccases were identified by N-terminal sequencing and peptide mass fingerprinting. For the further experiments, purified laccases were dialyzed to remove salts (D-TUBE DIALYZER MEGA, 20 mL, MWCO 6–8 kDa, Merck, Darmstadt, Germany) overnight at 4 °C against distilled water.
Laccase activity assay
During the purification process, laccase activity was measured spectrophotometrically using ABTS as a substrate. Each 200 µL reaction mixture contained 20 µL of eluted protein fractions, 1 µL of 100 mM ABTS (in 100 mM citrate buffer, pH 4.5) and 179 µL of 100 mM citrate buffer, pH 4.5. Reactions were incubated at 25 °C in microtiter plates, and oxidation of ABTS was followed by a linear increase in absorbance at 418 nm (εABTS = 36,000 M−1cm−1). One unit of laccase activity was defined as the amount of enzyme oxidizing 1 µmol of the substrate per minute. Laccase activity was expressed as units per milliliter (U/mL).
ABTS was also used as a substrate for determination of the pH and temperature dependence of laccase activity. The activity of purified laccases in various buffers with pH values ranging from 2 to 8.5 was measured in a mixture containing 194 µL of an appropriate 100 mM buffer, 5 µL of enzyme (initial concentration was 0.05 mg/mL) and 1 µL of 100 mM ABTS. For thermostability measurements, 5 µL of enzyme (initial concentration was 0.05 mg/mL) was incubated in 194 µL of 100 mM citrate buffer, pH 4.5, at various temperatures in the 25–70 °C range for 1 h. Then, 1 µL of 100 mM ABTS was added to the reaction to determine the residual laccase activity. The activity of laccases measured at 25 °C after 1 h was set at 100%.
All experiments were performed in triplicates, and data are shown as averages ± standard deviation.
Substrate specificity measurement
Substrate specificity assays involved one non-phenolic ABTS substrate and two phenolic substrates: metol and syringaldazine. Each reaction contained 5 µL of ~ 0.01 mg/mL purified enzyme and 195 µL of substrate in an appropriate buffer. Reactions were incubated at 25 °C in microtiter plates. Substrate oxidation was measured spectrophotometrically as an absorbance change. The conditions for each substrate were as follows: 0.01–50 mM ABTS in 100 mM citrate buffer (pH 4.5), absorbance measurement at 418 nm (ɛABTS = 36,000 M−1 cm−1); 10–250 mM metol in 100 mM acetate buffer (pH 4.5), 540 nm (ɛmetol = 2,000 M−1 cm−1); 0.0001–0.05 mM syringaldazine in 100 mM phosphate buffer (pH 6), 530 nm (ɛsyringaldazine = 65,000 M−1 cm−1). The Michaelis constant (Km) and the maximal reaction velocity (Vmax) were calculated according to the Michaelis–Menten equation: V = Vmax × [S]/Km + [S], where V is reaction rate and S is substrate concentration. Non-linear regressions were performed with SigmaPlot software, version 11.0 (Systat Software Inc., USA). All experiments were performed in triplicate, and data are shown as averages ± standard deviation.
Deglycosylation assay
The NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/) was used to predict N-glycosylation sites in MtL and TtL sequence. Deglycosylation reactions, in a total volume of 265 µL containing 10 µg purified laccase, 5 µL N-glycosidase F, 10 µL of 0.5 M sodium citrate buffer (pH 5.5) and an appropriate amount of sterile distilled water, were incubated overnight at 37 °C. To compare the glycosylated and deglycosylated forms of laccases, SDS-PAGE (protein bands visualized by Coomassie-based staining solution; Instant Blue™, Expedeon, Cambridge, UK) and native-PAGE (protein bands visualized by 100 mM ABTS in citrate buffer, pH 4.5) analysis were carried out. Laccase activity was assessed before and after treatment with N-glycosidase F. The activity measurements were performed in triplicate, and data are shown as averages ± standard deviation.
Decolorization studies
The purified laccases were used to decolorize two triphenylmethane dyes, Bromophenol Blue (BB, λmax = 595 nm) and Coomassie Brilliant Blue (CBB, λmax = 612 nm); one azo dye, Saturn Blue (SB, λmax = 595 nm); and one antraquinonic dye, Remazol Brilliant Blue R (RBB, λmax = 595 nm). Stock solutions of dyes (1 mg/mL) were prepared in sterile water, pH was adjusted to 4.5 with NaOH, and the solutions were filtered (Millex-GP syringe filter unit, disposable, 0.22 µm pore size, 33 mm diameter, Millipore, Tullagreen, Carrigtwohill, Co. Cork, Ireland). A 10 mg/mL stock solution of the 1-hydroxybenzotriazole (HBT) mediator was prepared in DMF. In the initial assay, the reaction was carried out in a microtiter plate in a total volume of 200 µL in sterile distilled water with final concentrations of 50 mg/L dye and 0.05 U/mL laccase. The mixture was incubated for 20 h at 30 °C, and decolorization was monitored spectrophotometrically as the relative decrease in absorbance at the appropriate wavelength after 20 h. During optimization of decolorization conditions, different laccase concentrations, pH values, and temperatures, as well as the presence or absence of HBT mediator, were tested. In these experiments, the decrease in absorbance was monitored continuously for 30 min (for TtL) and also after 60, 90, and 120 min (for MtL).
Samples of textile factory WW (WW 1–5) contained dyes and many other chemicals from textile production (CNM textil a.s., Baška, Czech Republic). WW no. 4 (black with pH 11.2) and WW no. 5 (violet with pH 9.1) were filtered (Millex-GP syringe filter unit, disposable, 0.22 µm pore size, 33 mm diameter, Millipore, Tullagreen, Carrigtwohill, Co. Cork, Ireland) to remove solid particles prior to decolorization tests. Similar to our decolorization experiments with dyes, we optimized conditions for WW decolorization (laccase concentration, pH, temperature, presence of a mediator). During these assays, the decrease in absorbance was measured after 20 h.
Decolorization was calculated as follows: decolorization (%) = [(A0 − At)/A0] × 100, where A0 is the initial absorbance of the dye and At is the absorbance of the dye after time. All experiments were performed in triplicate, and data are shown as averages ± standard deviation. Dyes with heat-inactivated laccases were used as negative controls only in the experiments under initial conditions (not under optimized conditions for dye and WW decolorization). Every experiment included blanks containing all components of the reaction mixture except dyes and WWs.
Toxicity study
Human peripheral blood mononuclear cells (PBMCs) were separated from the peripheral blood of healthy donors by gradient centrifugation on Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO, USA) at room temperature (RT). Briefly, PBS-diluted blood (1:1) was layered onto the Ficoll Paque medium without disturbing the layer and centrifuged at 400×g for 35 min at RT without brake. Cells at the Ficoll-plasma interface were collected and washed with PBS containing 2 mM EDTA. The remaining red blood cells were lysed with ammonium chloride-based red blood cell (RBC) lysis buffer.
The CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, Madison, WI, USA) was used to determine the number of viable cells according to the manufacturer’s instructions. This luminescent method is based on quantitation of ATP present in the cell lysate, which corresponds to the number of metabolically active cells. The concentration of isolated PBMCs was adjusted to 2.2 × 106 cells/mL in RPMI 1640 medium (Biowest, Nuaillé, France) supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS; Biosera, Nuaillé, France) and glutamine (2 mM). A 90 µL aliquot of the cell suspension (~ 200,000 cells) was seeded into each well of a white 96-well flat-bottomed plate (Thermo Fisher Scientific, Waltham, MA, USA) and incubated at 37 °C in a 5% CO2 atmosphere with 95% humidity. After overnight culture, 10 µL of sample (WWs, dyes or purified laccases) were added to wells, and cells were incubated for an additional 72 h. Each sample was tested in quadruplicate wells. After incubation, an equal volume of CellTiter-Glo™ reagent (100 µL) was added to each well to lyse the cells and generate a luminescent signal. The plate was shaken at 500 RPM for 10 min at RT, the signal was allowed to stabilize for 15 min at RT, and luminescence was measured in a Tecan Genios microplate reader (Tecan, Austria GmbH, Grödig, Austria) with an integration time of 1 s per well. Cell viability was calculated by normalizing the sample luminescence to that of a control containing 10 µL MilliQ water.
Results and discussion
Production and purification of recombinant laccases
First, we tested different cultivation conditions to optimize production of MtL in S. cerevisiae. We detected MtL activity in crude S. cerevisiae supernatants in the presence of different concentrations of Cu2+ under various cultivation temperatures (Online Resource, Fig. S1a, b). We achieved the highest MtL production in YNB medium containing 0.8% (w/v) alanine and 0.6 mM CuSO4 with cultivation at 30 °C for 3 days. These cultivation conditions were used for production of both laccases.
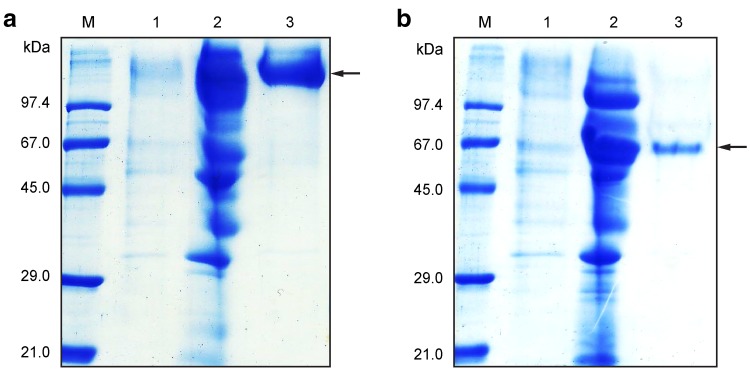
MtL and TtL were purified from the cultivation media by consecutive hydrophobic and gel chromatography (Fig. 1), and the purity of the isolated proteins was determined by mass spectrometry analysis and N-terminal sequencing. Protein yields from each purification step are summarized in Table 1. The molecular weights of native MtL and TtL are 85 kDa (Hollmann et al. 2008) and ~ 60 kDa (Ai et al. 2015; Zouari-Mechichi et al. 2006), respectively. However, the molecular weights of recombinant laccases may be higher due to hyperglycosylation in different microorganisms (Colao et al. 2006; Chen et al. 2012). MtL and TtL purified from S. cerevisiae displayed molecular weights > 100 kDa and ~ 65 kDa, respectively, confirming hyperglycosylation of MtL and indicating a low degree of glycosylation of TtL.
Fig. 1.
Purification of a MtL and b TtL. Lane M, protein marker; lane 1, crude culture filtrate; lane 2, laccase purified by hydrophobic chromatography (HiTrap™ Phenyl FF) and concentrated for the next purification step; lane 3, laccase purified by gel chromatography (Superdex™ 75 10/300 GL). Protein fractions were separated on 12% polyacrylamide gels and stained using Instant Blue™ solution
Table 1.
Summary of purification steps and yields of recombinant MtL and TtL
| Laccase | Purification step | Volume (mL) | Activity (U/mL) | Protein (mg/mL) | Specific activity (U/mg) | Activity yield (%) | Purification (fold) |
|---|---|---|---|---|---|---|---|
| MtL | Culture filtrate | 1900 | 0.03 | 0.01 | 1.69 | 100 | 1 |
| HiTrap™ Phenyl FF | 25 | 1.88 | 0.17 | 10.81 | 99 | 6 | |
| Superdex™ 75 10/300 GL | 5 | 4.50 | 0.23 | 19.68 | 47 | 12 | |
| TtL | Culture filtrate | 1900 | 0.003 | 0.016 | 0.207 | 100 | 1 |
| HiTrap™ Phenyl FF | 25 | 0.241 | 0.084 | 2.857 | 95 | 14 | |
| Superdex™ 75 10/300 GL | 2 | 0.34 | 0.11 | 3.13 | 11 | 15 |
We measured the activities of isolated protein fractions spectrophotometrically using ABTS as a substrate. The specific activity of MtL was six times higher than that of TtL (see Table 1). This can be attributed to our use of the T2 mutant of MtL, designed by Bulter et al. (2003), which allows production of a laccase with higher-specific activity, but can lead to lower protein stability.
Syringaldazine is the preferred substrate for MtL and TtL
The kinetic parameters (Km, kcat and Vmax) for laccase-mediated oxidation of two phenolic substrates (syringaldazine and metol) and one non-phenolic substrate (ABTS) (for chemical formulas, see Online Resource, Table S1) were estimated from Michaelis–Menten nonlinear regression curves using SigmaPlot software (Table 2). Both laccases exhibited the highest catalytic efficiency (kcat/Km) for oxidation of syringaldazine and the lowest for metol, which was a relatively poor substrate. In contrast to previous studies (Ai et al. 2015; Colao et al. 2006; Zouari-Mechichi et al. 2006) indicating that ABTS is a preferred substrate, we found that the catalytic efficiencies of both laccases were roughly six to seven times lower for ABTS oxidation than syringaldazine oxidation. This may be caused by different reaction conditions and a lower degree of purity of the laccase preparations used in previous studies. Our work with highly purified laccases strongly suggests that syringaldazine is the preferred substrate for MtL and TtL.
Table 2.
Kinetic parameters of recombinant MtL and TtL
| Substrate | MtL | TtL | ||||||
|---|---|---|---|---|---|---|---|---|
| K m (µM) | V max (µM/sec) | k cat (s−1) | k cat/Km | K m (µM) | V max (µM/s) | k cat (s−1) | k cat/Km | |
| ABTS | 20.9 ± 5 | 0.0019 | 13.2 ± 0.5 | 0.6 | 38 ± 9.6 | 0.0009 | 8.5 ± 0.2 | 0.2 |
| Metol | 21 800 ± 6 600 | 0.00016 | 19.8 ± 0.5 | 0.0009 | 57 600 ± 23 700 | 0.000005 | 0.1 ± 0.003 | 0.000002 |
| Syringaldazine | 17 ± 5 | 0.00063 | 63.7 ± 0.8 | 3.7 | 0.074 ± 0.014 | 0.00018 | 0.11 ± 0.003 | 1.5 |
Parameters were calculated according to the Michaelis–Menten equation
Glycosylation is important for laccase activity
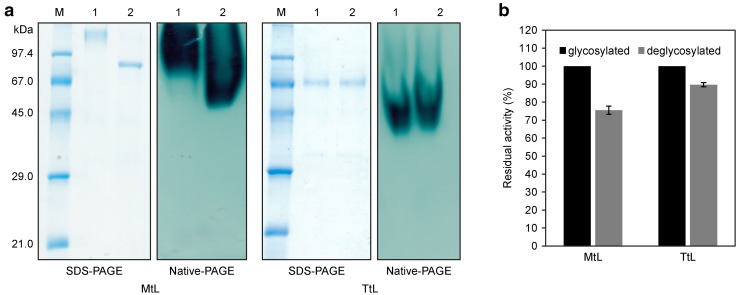
We predicted six N-glycosylation sites in the MtL sequence (Asn 92, 135, 248, 263, 291 and 336) using the NetNGlyc 1.0 Server. In the TtL sequence, we found only two Asn residues, at positions 72 and 454, that may be glycosylated. To assess the effect of glycosylation on laccase activity, we treated the purified enzymes with N-glycosidase F and analyzed the glycosylated and deglycosylated forms by SDS-PAGE and native-PAGE stained with ABTS (Fig. 2a). Deglycosylation of MtL resulted in a decrease in molecular weight of ~ 25 kDa (confirmed by peptide mass fingerprinting; data not shown), which is in agreement with results obtained by Bulter et al. (2003) and Piscitelli et al. (2005) for MtL produced in S. cerevisiae. Our prediction of a lower glycosylation level for TtL was confirmed; no significant differences in the molecular weights of the glycosylated and deglycosylated forms were detected by SDS–PAGE. However, the activity of deglycosylated TtL was 10% lower than that of glycosylated TtL (Fig. 2b). The activity of deglycosylated MtL decreased by 25% (Fig. 2b). Similarly, other researchers have found that deglycosylation of laccase from the white-rot fungi Pycnoporus sanguineus led to the an 18% reduction in its oxidation activity (Vite-Vallejo et al. 2009), while laccases from Lentinus sp. lost up to 50% of activity after mutation of predicted glycosylation sites (Maestre-Reyna et al. 2015). Our results, as well as previous findings, clearly show that laccase glycosylation contributes to efficient oxidation.
Fig. 2.
a Analysis of glycosylated and deglycosylated forms of purified MtL and TtL. Right side—SDS-PAGE, 12% polyacrylamide gels stained by Coomassie-based staining solution; left side—native-PAGE, 12% polyacrylamide gels stained with 100 mM ABTS (in 100 mM citrate buffer, pH 4.5). b Detection of enzymatic activity of glycosylated and deglycosylated purified MtL and TtL using 100 mM ABTS (in 100 mM citrate buffer, pH 4.5) as a substrate. The experiments were performed in triplicate, and data are shown as averages ± standard deviation
pH, salt, and temperature influence laccase activity
The efficiency of laccase-mediated WW decolorization can be influenced by several factors, including the high pH of WW and the presence of different cations, salts, and other chemicals.
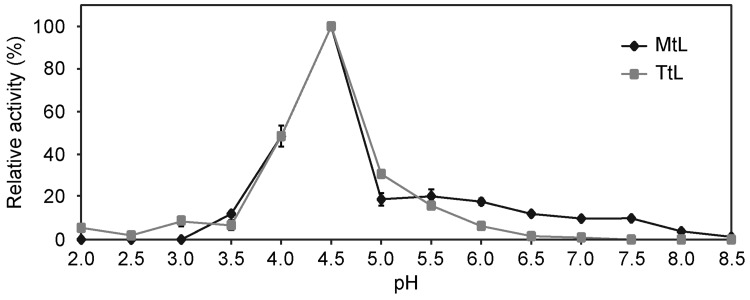
To identify the pH optima of the enzymes, we tested their activity at different pH values using ABTS as a substrate (Fig. 3). Both laccases exhibited highest activity at pH 4.5, and activity decreased at lower and higher pH values. MtL became completely inactive at pH > 8, and TtL at pH > 6.5. The loss of activity at higher pH was likely caused by hydroxide anions binding to the catalytic center (especially to the T2/T3 copper center) and decreasing the oxygen reduction potential (Xu 1997). The absence or very low activity of MtL at pH < 4 is a result of MtL gene mutation by directed evolution. The T2 mutant of MtL has only 50% of the wild-type activity at pH 3 and no activity at pH 2 (Bulter et al. 2003).
Fig. 3.
pH–activity profiles of purified MtL (filled diamond) and TtL (filled square). Laccase activities were measured in 100 mM buffers (sodium acetate buffer for pH 2, 3, 4; sodium citrate buffer for pH 2.5, 3.5, 4.5, 5.5; potassium phosphate buffer for pH 5, 6, 7; sodium phosphate buffer for pH 6.5 and Tris buffer for pH 7.5, 8, 8.5) at 25 °C using ABTS as a substrate (in 100 mM citrate buffer, pH 4.5). The experiments were performed in triplicate, and data are shown as averages ± standard deviation
We next determined the effect of 80 mM NaCl, the most common salt present in textile WWs, on laccase activities in buffers with various pH values (Online Resource, Fig. S2a, b). At pH 4.5, the activity of MtL and TtL decreased by 37% and 45%, respectively, in the presence of NaCl compared to buffers without NaCl. Our results are in agreement with those of Zilly et al. (2011) and Champagne et al. (2013), who determined the effect of NaCl on decolorization of dyes. Chloride ions bind to the catalytic center of laccases (especially to the T2 site) and block electron transfer from the T1 site to the T2/T3 cluster site, similar to the effect of hydroxide anions (Kepp 2015; Raseda et al. 2014).
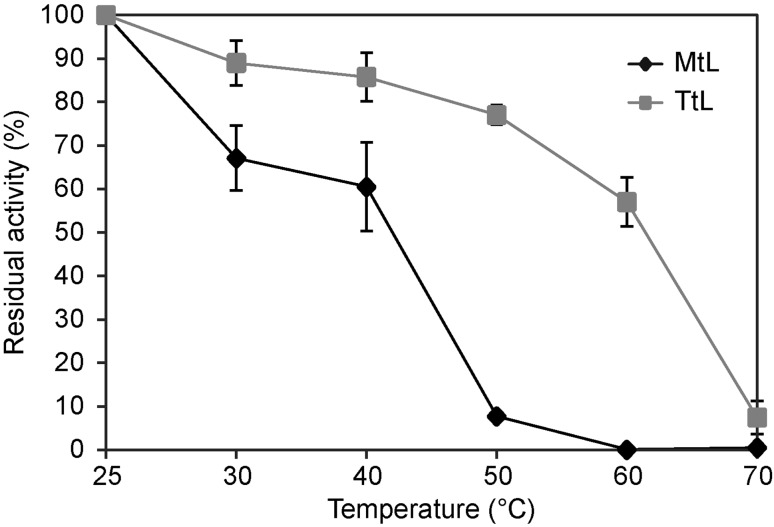
Dye decolorization by laccases is more effective at higher temperatures (Nyanhongo et al. 2002), and we, therefore, assessed the thermal stability of purified laccases (Fig. 4). At 40 °C, MtL retained 61% and TtL 86% of the initial activity determined at 25 °C. At 50 °C, the activity of TtL decreased by only 23%, whereas MtL lost 92% of its initial activity. TtL retained more than 50% activity even at 60 °C. Daâssi et al. (2013) reported an optimal temperature of 50 °C for TtL-catalyzed decolorization of azo dye Acid Orange 51, indicating the broader potential of TtL for dye decolorization.
Fig. 4.
Thermostability of purified MtL (filled diamond) and TtL (filled square). The activities were measured in 100 mM citrate buffer, pH 4.5, at various temperatures between 25 and 70 °C for 1 h using ABTS (in 100 mM citrate buffer, pH 4.5) as a substrate. The experiments were performed in triplicate, and data are shown as averages ± standard deviation
MtL and TtL serve as non-toxic, effective catalysts for decolorization of synthetic textile dyes
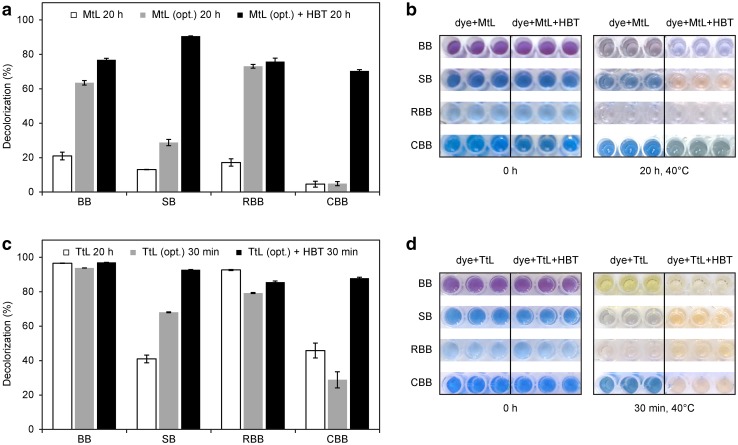
We assessed the abilities of laccases to decolorize two triphenylmethane dyes, Bromophenol Blue (BB) and Coomassie Brilliant Blue (CBB); one azo dye, Saturn Blue (SB); and one antraquinonic dye, Remazol Brilliant Blue R (RBB) (for chemical formulas, see Online Resource, Table S1). Under the initial conditions (50 mg/L dye, 0.05 U/mL purified laccase, pH 7, 30 °C, 20 h), purified MtL-decolorized all the dyes very poorly compared with the negative control (heat-inactivated laccase). In contrast, purified TtL decolorized the dyes with the following efficiencies: BB − 99%, RBB − 95%, CBB − 47%, and SB − 42%. We next measured decolorization under optimized conditions (0.2 U/mL purified laccase, citrate buffer, pH 4.5, 40 °C) with or without HBT mediator with 30 min reaction times for TtL and 20 h for MtL (Fig. S3 and S4 in Online Resource). Compared with the initial conditions, these conditions significantly improved the ability of MtL to decolorize BB and RBB (by 56% and 58%, respectively) and moderately increased its activity toward SB and CBB (see Fig. 5). Addition of HBT improved the MtL-catalyzed decolorization of SB and CBB by 78% and 65%, respectively. Decolorization of all dyes by TtL was more effective than the MtL-catalyzed reactions under the initial conditions and was significantly faster under optimized conditions. Under optimized conditions, we observed some degree of decolorization of all dyes within 30 min. Use of optimized conditions and addition of HBT increased decolorization of SB to nearly 100% and RBB to 90%. A high degree of CBB decolorization by TtL occurred only in the presence of HBT. Several previous studies have described decolorization of synthetic dyes by TtL (Colao et al. 2006; Grassi et al. 2011; Ranieri et al. 2009; Zouari-Mechichi et al. 2006), but the efficiency of decolorization differs among these studies due to different experimental conditions (e.g., concentrations of enzymes, dyes and mediators; temperature; pH; time of incubation). To the best of our knowledge, this is the first report describing recombinant MtL as an efficient tool for decolorization of synthetic dyes.
Fig. 5.
a Decolorization of synthetic dyes by purified MtL under initial and optimized (opt.) conditions after 20 h. The experiments were performed in triplicate, and data are shown as averages ± standard deviation. b Photographic record of dyes before and after treatment with purified MtL (under optimized conditions). c Decolorization of synthetic dyes by purified TtL under initial and optimized (opt.) conditions after 20 h and 30 min, respectively. The experiments were performed in triplicate, and data are shown as averages ± standard deviation. d Photographic record of dyes before and after treatment with purified TtL under optimized conditions
In general, many synthetic dyes, particularly azo dyes, are potentially toxic or mutagenic and negatively influence the environment and human health (Carneiro et al. 2010; Mahmood et al. 2016; Robinson et al. 2001; Sen et al. 2016). Dye toxicity is usually tested in bioassays based on inhibition of microorganism or plant root growth or germination of plant seeds (Abadulla et al. 2000; Chhabra et al. 2015; Yang et al. 2015). Only a few studies have tested dye toxicity using human cells (Vanhulle et al. 2008). Here, we used human PBMCs to determine the toxicity of dye decolorization products. First, we tested the toxicity of the purified laccases. Both laccases were non-toxic (data not shown), and the results are in agreement with data obtained for MtL by Brinch and Pedersen (2002). The dyes used in this study did not show any toxicity either before or after decolorization by laccases, even when HBT was present in the reaction (Online Resource, Fig. S5a, b). Nevertheless, the ability of laccases to reduce the toxicity of several toxic dyes has been described by Rezaei et al. (2015), Daâssi et al. (2013) and Shanmugam et al. (2017).
Efficiencies of WW decolorization and detoxification differ among laccases and depend on wastewater properties
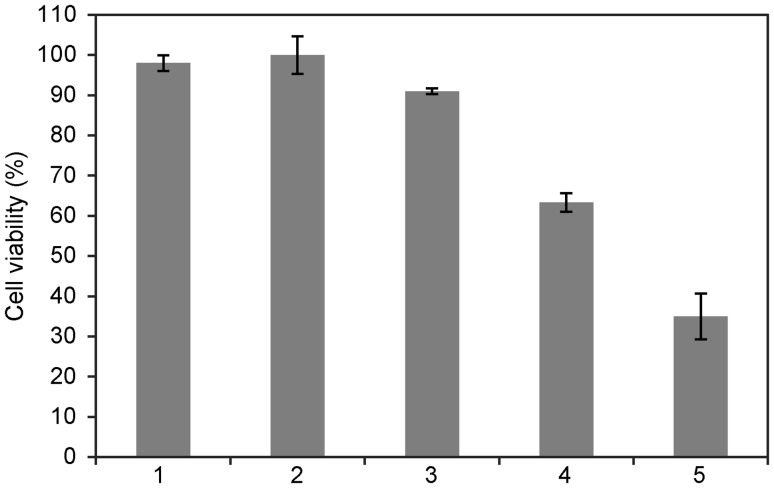
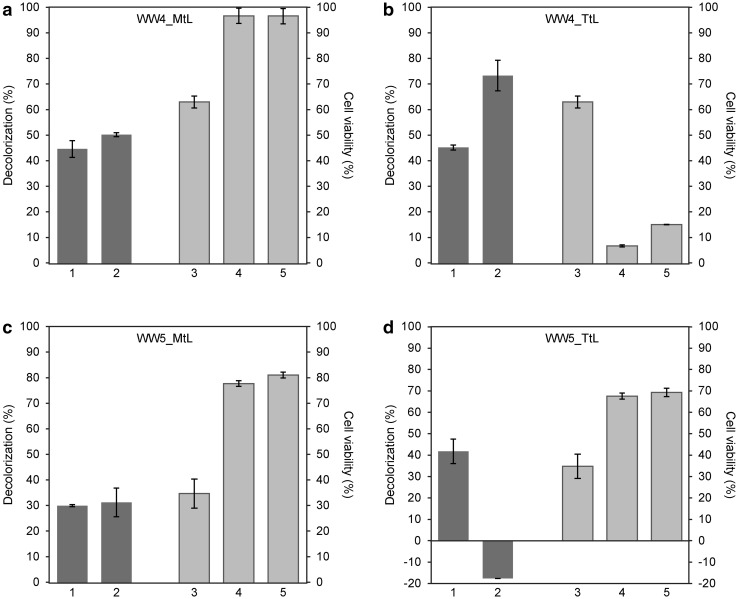
Five samples of WW collected at different times from a textile factory in the Czech Republic were initially tested for toxicity using human PBMCs. Information about the WW content was not available, as the textile factory runs a combination of different manufacturing processes and the content of individual dyes varies over time. Of the five WW samples analyzed, two (WW4 and WW5) were toxic (see Fig. 6). PBMC viabilities in the presence of WW4 and WW5 were 63% and 35%, respectively. These two samples were used for further decolorization and detoxification analysis. For WW decolorization, we applied the optimal conditions used in our prior experiments with dyes. The WW pH was adjusted to 4.5 with HCl, and samples were incubated at 40 °C for 20 h in the presence or absence of purified laccases. No changes in absorbance were detected when WWs were incubated without laccases, indicating that the selected conditions did not affect WW properties and that WW decolorization can be attributed to laccase activity. Purified MtL and TtL decolorized WW4 with the same efficiency (45%), and addition of HBT increased the extent of decolorization to 50% and 74%, respectively (Fig. 7a, b). Similar results were obtained for WW5. MtL and TtL decolorized WW5 with 30% and 42% efficiency, respectively (Fig. 7 c, d). However, addition of HBT influenced the reactions catalyzed by MtL and TtL very differently. While decolorization of WW5 by MtL slightly increased in the presence of the mediator, a more colored product was formed during the TtL-catalyzed reaction (Fig. 7d). Fragments of azo bond cleavage in the presence of HBT, which enables laccases to oxidize non-phenolic substrates or substrates with a high redox potential (Moilanen et al. 2014), can undergo autooxidation under aerobic conditions and form colored products (Tauber et al. 2008). Our results suggest that decolorization efficiency can be affected not only by the type of laccase, but also by the type of mediator, pH value, reaction temperature, and presence of other inhibitors in the reaction. The decolorization of industrial WWs by different laccase systems has been studied by Antosova et al. (2018); Bello-Gil et al. (2018) and Sondhi et al. (2018); these studies have reported decolorization with up to 90% efficiency.
Fig. 6.
Cell viability in the presence of different wastewaters (1–5). Cell viability tests were carried out on PBMCs using the CellTiter-Glo® Luminescent Cell Viability Assay, which determines the number of viable cells based on the cellular ATP level. The experiments were performed in triplicate, and data are shown as averages ± standard deviation
Fig. 7.
Decolorization and detoxification of WW4 and WW5 by purified MtL and TtL (0.2 U/mL) at pH 4.5. Decolorization of WWs was tested after 20 h of incubation at 40 °C (dark gray columns). WW toxicity was assessed on PBMCs using the CellTiter-Glo® Luminescent Cell Viability Assay (for more details, see Methods) (light gray columns). The experiments were performed in triplicate, and data are shown as averages ± standard deviation. Columns: (1) decolorization of WWs by laccases; (2) decolorization of WWs by laccases in the presence of 1 mM HBT mediator; (3) cell viability in the presence of untreated WWs; (4) cell viability in the presence of WWs treated with laccases; (5) cell viability in the presence of WWs treated with laccases and 1 mM HBT
We next assessed the toxicity of decolorized WWs. The change in pH and higher temperature had no effect on WW toxicity (data not shown). Treatment of WW4 with MtL with or without HBT, which decolorized the samples to 50%, nearly eliminated their toxicity (cell viability within treated WW4 was 97%) (Fig. 7a, light gray columns). In contrast, the WW4 decolorization by TtL to 55 and 26% (in the presence of mediator) led to more toxic products than prior to laccase treatment (cell viability was only 7% in the absence of HBT, and 15% in its presence) (Fig. 7b). We observed different results for WW5 treated with MtL and TtL. While detoxification and decolorization of WW5 by MtL was comparable to that obtained for WW4 with exception of higher product toxicity (cell viability in the presence and in the HBT absence was only 78 and 81%, respectively) (Fig. 7c), the decolorization of WW5 by TtL in the presence of HBT resulted in a more colored product, but cell viability was 70% (Fig. 7d). In summary, our results show that the efficiency of WW detoxification differs among laccases and is dependent on the chemical composition of WWs. Previous work has demonstrated WW decolorization and detoxification by laccases from Pseudomonas peli (Dellai et al. 2013) and Trametes hirsuta (Abadulla et al. 2000). Application of a natural laccase-mediator such as acetosyringone is a less effective decolorizing agent but usually generates non-toxic products. In contrast, addition of HBT contributes to a higher rate of WW decolorization but typically does not significantly decrease the toxicity of treated WW (Khlifi et al. 2010).
Conclusion
Our experiments with MtL and TtL purified to high homogeneity provided accurate data on multiple parameters important for optimization of decolorization processes, including the thermal stabilities of these enzymes, their activity dependence on salt concentration and pH, and their substrate specificities. Determining the precise concentrations of purified laccases enabled us to directly compare the decolorization and detoxification efficiencies of laccases from different microorganisms. Our results suggest the great importance of toxicity testing during laccase-mediated decolorization of WWs. The favorable properties of laccases continue to be improved by genetic engineering and other methods, and use of these enzymes—in combination with other physicochemical methods—has great potential application in WW treatment processes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Grant NPU LO 1302 from the Ministry of Education, Youth and Sports of the Czech Republic. The authors thank Zdeněk Voburka for N-terminal protein sequencing, Martin Hubálek for performing the peptide mass fingerprinting analysis, Miroslav Hájek for the toxicity study, Petr Beier for a consultation in dye chemistry, Zuzana Antošová for a consultation of decolorization experiments and Radek Stloukal (Lentikat’s a.s., Straž pod Ralskem, Czech Republic) for providing samples of textile industry wastewater originating from CNM textile a.s. (Baška, Czech Republic.)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Gubitz GM. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl Environ Microbiol. 2000;66:3357–3362. doi: 10.1128/AEM.66.8.3357-3362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai MQ, Wang FF, Huang F. Purification and characterization of a thermostable laccase from Trametes trogii and its ability in modification of kraft lignin. J Microbiol Biotechnol. 2015;25:1361–1370. doi: 10.4014/jmb.1502.02022. [DOI] [PubMed] [Google Scholar]
- Ali N, Hameed A, Ahmed S. Physicochemical characterization and Bioremediation perspective of textile effluent, dyes and metals by indigenous Bacteria. J Hazard Mater. 2009;164:322–328. doi: 10.1016/j.jhazmat.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Antosova Z, Sychrova H. Yeast hosts for the production of recombinant laccases: a review. Mol Biotechnol. 2016;58:93–116. doi: 10.1007/s12033-015-9910-1. [DOI] [PubMed] [Google Scholar]
- Antosova Z, Herkommerova K, Pichova I, Sychrova H. Efficient secretion of three fungal laccases from Saccharomyces cerevisiae and their potential for decolorization of textile industry effluent—a comparative study. Biotechnol Prog. 2018;34:69–80. doi: 10.1002/btpr.2559. [DOI] [PubMed] [Google Scholar]
- Arca-Ramos A, et al. Assessing the use of nanoimmobilized laccases to remove micropollutants from wastewater. Environ Sci Pollut Res. 2016;23:3217–3228. doi: 10.1007/s11356-015-5564-6. [DOI] [PubMed] [Google Scholar]
- Archna LKN, Kiran S. Biological methods of dye removal from textile effluents—a review. J Biochem Tech. 2012;3:177–180. [Google Scholar]
- Barrios-Estrada C, de Jesús Rostro-Alanis M, Muñoz-Gutiérrez BD, Iqbal HMN, Kannan S, Parra-Saldívar R. Emergent contaminants: Endocrine disruptors and their laccase-assisted degradation—a review. Sci Total Environ. 2018;612:1516–1531. doi: 10.1016/j.scitotenv.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Bello-Gil D, et al. An enzymatic system for decolorization of wastewater dyes using immobilized CueO laccase-like multicopper oxidase on poly-3-hydroxybutyrate. Microbial Biotechnol. 2018 doi: 10.1111/1751-7915.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzina O, et al. Decolorization and detoxification of two textile industry effluents by the laccase/1-hydroxybenzotriazole system. Environ Sci Pollut Res. 2013;20:5177–5187. doi: 10.1007/s11356-013-1491-6. [DOI] [PubMed] [Google Scholar]
- Bilal M, Asgher M, Parra-Saldivar R, Hu HB, Wang W, Zhang XH, Iqbal HMN. Immobilized ligninolytic enzymes: An innovative and environmental responsive technology to tackle dye-based industrial pollutants—a review. Sci Total Environ. 2017;576:646–659. doi: 10.1016/j.scitotenv.2016.10.137. [DOI] [PubMed] [Google Scholar]
- Brinch DS, Pedersen PB. Toxicological studies on laccase from Myceliophthora thermophila expressed in Aspergillus oryzae. Regul Toxicol Pharmacol. 2002;35:296–307. doi: 10.1006/rtph.2002.1538. [DOI] [PubMed] [Google Scholar]
- Bulter T, Alcalde M, Sieber V, Meinhold P, Schlachtbauer C, Arnold FH. Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution. Appl Environ Microbiol. 2003;69:987–995. doi: 10.1128/AEM.69.2.987-995.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannatelli MD, Ragauskas AJ. Two decades of laccases: advancing sustainability in the chemical industry. Chem Rec. 2017;17:122–140. doi: 10.1002/tcr.201600033. [DOI] [PubMed] [Google Scholar]
- Carneiro PA, Umbuzeiro GA, Oliveira DP, Zanoni MVB. Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. J Hazard Mater. 2010;174:694–699. doi: 10.1016/j.jhazmat.2009.09.106. [DOI] [PubMed] [Google Scholar]
- Champagne PP, Nesheim ME, Ramsay JA. A mechanism for NaCl inhibition of Reactive Blue 19 decolorization and ABTS oxidation by laccase. Appl Microbiol Biotechnol. 2013;97:6263–6269. doi: 10.1007/s00253-012-4525-y. [DOI] [PubMed] [Google Scholar]
- Chatha SAS, Asgher M, Iqbal HMN. Enzyme-based solutions for textile processing and dye contaminant biodegradation—a review. Environ Sci Pollut Res. 2017;24:14005–14018. doi: 10.1007/s11356-017-8998-1. [DOI] [PubMed] [Google Scholar]
- Chen SC, et al. Biochemical characterization of a novel laccase from the basidiomycete fungus Cerrena sp. WR1. Protein Eng Des Sel. 2012;25:761–769. doi: 10.1093/protein/gzs082. [DOI] [PubMed] [Google Scholar]
- Chhabra M, Mishra S, Sreekrishnan TR. Immobilized laccase mediated dye decolorization and transformation pathway of azo dye acid red 27. J Environ Health Sci Eng. 2015;13:38. doi: 10.1186/s40201-015-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colao MC, Lupino S, Garzillo AM, Buonocore V, Ruzzi M. Heterologous expression of lcc 1 gene from Trametes trogii in Pichia pastoris and characterization of the recombinant enzyme. Microb Cell Fact. 2006;5:31. doi: 10.1186/1475-2859-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daâssi D, Zouari-Mechichi H, Frikha F, Martinez MJ, Nasri M, Mechichi T. Decolorization of the azo dye Acid Orange 51 by laccase produced in solid culture of a newly isolated Trametes trogii strain. 3 Biotech. 2013;3:115–125. doi: 10.1007/s13205-012-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellai A, Dridi D, Lemorvan V, Robert J, Cherif A, Mosrati R, Mansour HB. Decolorization does not always mean detoxification: case study of a newly isolated Pseudomonas peli for decolorization of textile wastewater. Environ Sci Pollut Res. 2013;20:5790–5796. doi: 10.1007/s11356-013-1603-3. [DOI] [PubMed] [Google Scholar]
- Forgacs E, Cserháti T, Oros G. Removal of synthetic dyes from wastewaters: a review. Environ Int. 2004;30:953–971. doi: 10.1016/j.envint.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Grandclément C, et al. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: a review. Water Res. 2017;111:297–317. doi: 10.1016/j.watres.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Grassi E, Scodeller P, Filiel N, Carballo R, Levin L. Potential of Trametes trogii culture fluids and its purified laccase for the decolorization of different types of recalcitrant dyes without the addition of redox mediators. Int Biodeterior Biodegrad. 2011;65:635–643. doi: 10.1016/j.ibiod.2011.03.007. [DOI] [Google Scholar]
- Guan ZB, Shui Y, Song CM, Zhang N, Cai YJ, Liao XR. Efficient secretory production of CotA-laccase and its application in the decolorization and detoxification of industrial textile wastewater. Environ Sci Pollut Res. 2015;22:9515–9523. doi: 10.1007/s11356-015-4426-6. [DOI] [PubMed] [Google Scholar]
- Herkommerova K, Zemancikova J, Sychrova H, Antosova Z. Immobilization in polyvinyl alcohol hydrogel enhances yeast storage stability and reusability of recombinant laccase-producing S. cerevisiae. Biotechnol Lett. 2018;40:405–411. doi: 10.1007/s10529-017-2485-0. [DOI] [PubMed] [Google Scholar]
- Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB. A critical review on textile wastewater treatments: possible approaches. J Environ Manage. 2016;182:351–366. doi: 10.1016/j.jenvman.2016.07.090. [DOI] [PubMed] [Google Scholar]
- Hollmann F, Gumulya Y, Tölle C, Liese A, Thum O. Evaluation of the laccase from Myceliophthora thermophila as industrial biocatalyst for polymerization reactions. Macromolecules. 2008;41:8520–8524. doi: 10.1021/ma801763t. [DOI] [Google Scholar]
- Kadri T, Rouissi T, Brar SK, Cledon M, Sarma S, Verma M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J Environ Sci. 2017;51:52–74. doi: 10.1016/j.jes.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Kepp KP. Halide binding and inhibition of laccase copper clusters: The role of reorganization energy. Inorg Chem. 2015;54:476–483. doi: 10.1021/ic5021466. [DOI] [PubMed] [Google Scholar]
- Khlifi R, Belbahri L, Woodward S, Ellouz M, Dhouib A, Sayadi S, Mechichi T. Decolourization and detoxification of textile industry wastewater by the laccase-mediator system. J Hazard Mater. 2010;175:802–808. doi: 10.1016/j.jhazmat.2009.10.079. [DOI] [PubMed] [Google Scholar]
- Legerská B, Chmelová D, Ondrejovič M. Degradation of synthetic dyes by laccases—a mini-review. Nova Biotechnol Chim. 2016;15:90–106. doi: 10.1515/nbec-2016-0010. [DOI] [Google Scholar]
- Luo Q, Chen Y, Xia J, Wang K-Q, Cai Y-J, Liao X-R, Guan Z-B. Functional expression enhancement of Bacillus pumilus CotA-laccase mutant WLF through site-directed mutagenesis. Enzyme Microbial Technol. 2018;109:11–19. doi: 10.1016/j.enzmictec.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Maestre-Reyna M, et al. Structural and functional roles of glycosylation in fungal laccase from Lentinus sp. PLoS One. 2015;10:e0120601. doi: 10.1371/journal.pone.0120601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S, Khalid A, Arshad M, Mahmood T, Crowley DE. Detoxification of azo dyes by bacterial oxidoreductase enzymes. Crit Rev Biotechnol. 2016;36:639–651. doi: 10.3109/07388551.2015.1004518. [DOI] [PubMed] [Google Scholar]
- Mate DM, Alcalde M. Laccase engineering: From rational design to directed evolution. Biotechnol Adv. 2015;33:25–40. doi: 10.1016/j.biotechadv.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Moilanen U, Kellock M, Várnai A, Andberg M, Viikari L. Mechanisms of laccase-mediator treatments improving the enzymatic hydrolysis of pre-treated spruce. Biotechnol Biofuels. 2014;7:177. doi: 10.1186/s13068-014-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghdi M, Taheran M, Brar SK, Kermanshahi-pour A, Verma M, Surampalli RY. Removal of pharmaceutical compounds in water and wastewater using fungal oxidoreductase enzymes. Environ Pollut. 2018;234:190–213. doi: 10.1016/j.envpol.2017.11.060. [DOI] [PubMed] [Google Scholar]
- Nyanhongo GS, Gomes J, Guebitz G, Zvauya R, Read J, Steiner W. Decolorization of textile dyes by laccases from a newly isolated strain of Trametes modesta. Water Res. 2002;36:1449–1456. doi: 10.1016/S0043-1354(01)00365-7. [DOI] [PubMed] [Google Scholar]
- Osma JF, Toca-Herrera JL, Rodríguez-Couto S. Uses of Laccases in the food industry. Enzyme Res. 2010 doi: 10.4061/2010/918761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H, Gupte A. Optimization of different culture conditions for enhanced laccase production and its purification from Tricholoma giganteum AGHP. Bioresour Bioprocess. 2016;3:11. doi: 10.1186/s40643-016-0088-6. [DOI] [Google Scholar]
- Pezzella C, Guarino L, Piscitelli A. How to enjoy laccases. Cell Mol Life Sci. 2015;72:923–940. doi: 10.1007/s00018-014-1823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli A, Giardina P, Mazzoni C, Sannia G. Recombinant expression of Pleurotus ostreatus laccases in Kluyveromyces lactis and Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2005;69:428–439. doi: 10.1007/s00253-005-0004-z. [DOI] [PubMed] [Google Scholar]
- Ranieri D, Colao MC, Ruzzi M, Romagnoli G, Bianchi MM. Optimization of recombinant fungal laccase production with strains of the yeast Kluyveromyces lactis from the pyruvate decarboxylase promoter. FEMS Yeast Res. 2009;9:892–902. doi: 10.1111/j.1567-1364.2009.00532.x. [DOI] [PubMed] [Google Scholar]
- Raseda N, Hong S, Kwon OY, Ryu K. Kinetic evidence for the interactive inhibition of laccase from Trametes versicolor by pH and chloride. J Microbiol Biotechnol. 2014;24:1673–1678. doi: 10.4014/jmb.1408.08012. [DOI] [PubMed] [Google Scholar]
- Rezaei S, Tahmasbi H, Mogharabi M, Ameri A, Forootanfar H, Khoshayand MR, Faramarzi MA. Laccase-catalyzed decolorization and detoxification of Acid Blue 92: statistical optimization, microtoxicity, kinetics, and energetics. J Environ Health Sci Eng. 2015;13:31. doi: 10.1186/s40201-015-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S. Laccases: blue enzymes for green chemistry. Trends Biotechnol. 2006;24:219–226. doi: 10.1016/j.tibtech.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Robinson T, McMullan G, Marchant R, Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol. 2001;77:247–255. doi: 10.1016/S0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Sen SK, Raut S, Bandyopadhyay P, Raut S. Fungal decolouration and degradation of azo dyes: a review. Fungal Biol Rev. 2016;30:112–133. doi: 10.1016/j.fbr.2016.06.003. [DOI] [Google Scholar]
- Senthivelan T, Kanagaraj J, Panda RC. Recent trends in fungal laccase for various industrial applications: an eco-friendly approach—a review. Biotechnol Bioprocess Eng. 2016;21:19–38. doi: 10.1007/s12257-015-0278-7. [DOI] [Google Scholar]
- Shanmugam S, Ulaganathan P, Swaminathan K, Sadhasivam S, Wu Y-R. Enhanced biodegradation and detoxification of malachite green by Trichoderma asperellum laccase: degradation pathway and product analysis. Int Biodeterior Biodegrad. 2017;125:258–268. doi: 10.1016/j.ibiod.2017.08.001. [DOI] [Google Scholar]
- Sharma B, Dangi AK, Shukla P. Contemporary enzyme based technologies for bioremediation: a review. J Environ Manage. 2018;210:10–22. doi: 10.1016/j.jenvman.2017.12.075. [DOI] [PubMed] [Google Scholar]
- Singh K, Arora S. Removal of synthetic textile dyes from wastewaters: a critical review on present treatment technologies. Crit Rev Environ Sci Technol. 2011;41:807–878. doi: 10.1080/10643380903218376. [DOI] [Google Scholar]
- Singh RL, Singh PK, Singh RP. Enzymatic decolorization and degradation of azo dyes—a review. Int Biodeterior Biodegrad. 2015;104:21–31. doi: 10.1016/j.ibiod.2015.04.027. [DOI] [Google Scholar]
- Sondhi S, Kaur R, Kaur S, Kaur PS. Immobilization of laccase-ABTS system for the development of a continuous flow packed bed bioreactor for decolorization of textile effluent. Int J Biol Macromol. 2018;117:1093–1100. doi: 10.1016/j.ijbiomac.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Tauber MM, Gubitz GM, Rehorek A. Degradation of azo dyes by oxidative processes—laccase and ultrasound treatment. Bioresour Technol. 2008;99:4213–4220. doi: 10.1016/j.biortech.2007.08.085. [DOI] [PubMed] [Google Scholar]
- Vanhulle S, et al. Cytotoxicity and genotoxicity evolution during decolorization of dyes by White Rot Fungi. World J Microbiol Biotechnol. 2008;24:337–344. doi: 10.1007/s11274-007-9475-7. [DOI] [Google Scholar]
- Vite-Vallejo O, Palomares LA, Dantán-González E, Ayala-Castro HG, Martínez-Anaya C, Valderrama B, Folch-Mallol J. The role of N-glycosylation on the enzymatic activity of a Pycnoporus sanguineus laccase. Enzyme Microb Technol. 2009;45:233–239. doi: 10.1016/j.enzmictec.2009.05.007. [DOI] [Google Scholar]
- Wang J, Lu L, Feng F. Improving the Indigo Carmine decolorization ability of a Bacillus amyloliquefaciens laccase by site-directed mutagenesis. Catalysts. 2017;7:275. doi: 10.3390/catal7090275. [DOI] [Google Scholar]
- Widsten P, Kandelbauer A. Laccase applications in the forest products industry: a review. Enzyme Microb Technol. 2008;42:293–307. doi: 10.1016/j.enzmictec.2007.12.003. [DOI] [Google Scholar]
- Xu F. Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J Biol Chem. 1997;272:924–928. doi: 10.1074/jbc.272.2.924. [DOI] [PubMed] [Google Scholar]
- Yang J, Yang XD, Lin YH, Ng TB, Lin J, Ye XY. Laccase-catalyzed decolorization of Malachite Green: Performance optimization and degradation mechanism. PLoS One. 2015;10:14. doi: 10.1371/journal.pone.0127714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Cai Y, Liao X, Zeng X, Luo S, Zhang D. Anthraquinone dye assisted the decolorization of azo dyes by a novel Trametes trogii laccase. Process Biochem. 2012;47:160–163. doi: 10.1016/j.procbio.2011.10.019. [DOI] [Google Scholar]
- Zhu C, Bao G, Huang S. Optimization of laccase production in the white-rot fungus Pleurotus ostreatus (ACCC 52857) induced through yeast extract and copper. Biotechnol Biotechnol Equip. 2016;30:270–276. doi: 10.1080/13102818.2015.1135081. [DOI] [Google Scholar]
- Zilly A, da Silva Coelho-Moreira J, Bracht A, Marques de Souza CG, Carvajal AE, Koehnlein EA, Peralta RM. Influence of NaCl and Na2SO4 on the kinetics and dye decolorization ability of crude laccase from Ganoderma lucidum. Int Biodeterior Biodegrad. 2011;65:340–344. doi: 10.1016/j.ibiod.2010.12.007. [DOI] [Google Scholar]
- Zouari-Mechichi H, Mechichi T, Dhouib A, Sayadi S, Martínez AT, Martínez MJ. Laccase purification and characterization from Trametes trogii isolated in Tunisia: decolorization of textile dyes by the purified enzyme. Enzyme Microb Technol. 2006;39:141–148. doi: 10.1016/j.enzmictec.2005.11.027. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.