Abstract
A 52-year-old woman presenting with shortness of breath and having no related past medical history was diagnosed with takotsubo cardiomyopathy. However, she revealed respiratory failure atypical with takotsubo cardiomyopathy.
We diagnosed myasthenia gravis with myasthenic crisis by acetylcholine receptor-binding antibody titer with mediastinal tumor.
Physical or emotional stress is well known to trigger the onset of takotsubo cardiomyopathy. Similarly, myasthenia crisis is also triggered by stress. Here, we report a case of simultaneous occurrence of takotsubo cardiomyopathy and myasthenia crisis.
Keywords: Takotsubo cardiomyopathy, Myasthenia gravis
Introduction
Takotsubo cardiomyopathy (TC) is a type of non-ischemic cardiomyopathy, in which there is a sudden temporary weakening of the myocardium, and it is known to be triggered by physical or emotional stress 1, 2, 3, 4, 5, 6. Electrocardiography findings feature ST-segment elevation and/or T wave inversion, suggestive of acute myocardial infarction, while echocardiography usually shows transient hypokinesis, akinesis, or dyskinesis of the left mid-ventricular segments, with or without apical segment involvement 2, 6. Typically left ventricular wall motion returns to normal within 1–4 weeks 3, 4, 5.
On the other hand, myasthenic crisis of myasthenia gravis (MG) is considered to be a consequence of various factors such as infection, surgery, pregnancy, childbirth, tapering of immunosuppressive medications, certain antibiotics, and cardiac drugs. Similar to TC, one of the etiologies of myasthenic crisis is emotional and physical stress [4].
Here, we report a rare case of a woman without any previous related medical history, who was simultaneously diagnosed with TC and MG.
Case report
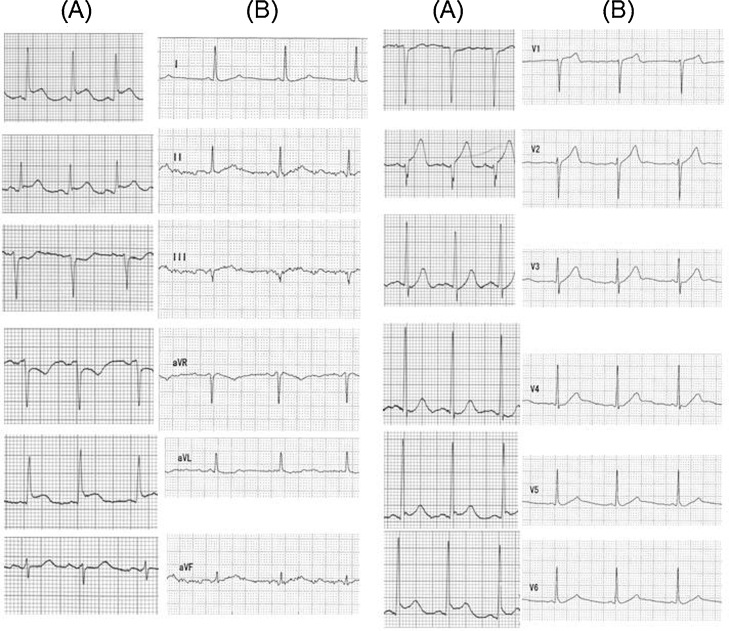
A 52-year-old woman presented to our emergency department with breathlessness, which had begun shortly after her dinner that evening. Two weeks prior to admission, she had visited another medical center, complaining of general fatigue for 3 months, where she was diagnosed with hypoferric anemia and hyperthyroidism. She had been smoking for 27 years and had a familial history of coronary artery disease. On admission, her heart rate was 120 beats/min with regularity, blood pressure was 182/134 mmHg and percutaneous oxygen saturation was 99% with a mask of 6 L/min of oxygen. Her heart sounds revealed a pansystolic murmur at apex with Levine III/IV. Jugular venous distention and pretibial edema were not observed. Coarse crackles were heard in her lower lungs, and blood gas analysis showed hypoventilation with respiratory acidosis (pH = 7.267; pCO2 = 58.3; HCO3− = 26.0). Chest radiography revealed an enlargement of the mediastinal shadow, but it was highly unlikely that she had aortic dissection because her d-dimer level was not elevated. At presentation, her electrocardiogram showed sinus rhythm with ST elevations in almost all leads (Fig. 1A), and the echocardiogram showed apical akinesia and excessive contraction at the proximal segments with 45% left ventricular ejection fraction (LVEF) measured by the modified Simpson method.
Fig. 1.
12-Lead electrocardiograms at admission (A) and 4 months from onset (B). 12-Lead electrocardiogram at admission demonstrated ST elevation in leads I, II, aVL, V2, V5, V6, and reciprocating ST depression in leads III, aVR, and V1 (A). At 4 months from onset, the electrocardiogram showed a completely normal pattern (B).
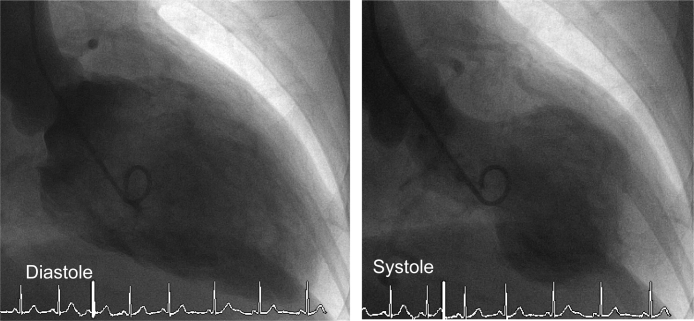
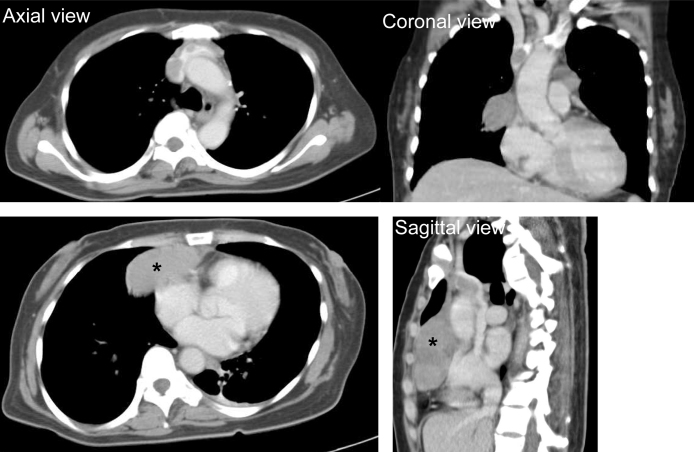
Based on these findings, acute coronary syndrome was suspected, and she underwent emergency coronary angiography; however, cardiac catheterization revealed no significant coronary artery disease. The left ventriculogram showed apical ballooning with hyperdynamic proximal segments and sparing of the apex (Fig. 2), consistent with the features of TC, and her laboratory workup showed an elevated catecholamine titer. She had an enzymatic curve with maximumcreatinine kinase (CK) and CK-MB levels of 266 IU/L and 40 IU/L respectively (Table 1). She underwent computed tomography of the chest, which revealed an anterior mediastinal mass invading the vessel (Fig. 3). On the second night, she experienced respiratory failure and significant muscle weakness. Thereafter, she developed muscle weakness, hypoventilation and respiratory failure, requiring continuous positive airway pressure.
Fig. 2.
Left ventriculogram demonstrated left ventricular apical ballooning on end systolic frame.
Table 1.
Blood sample findings.
| Leukocytes | 9900/μL | AST | 26 IU/L |
| Erythrocytes | 421 × 104/μL | ALT | 16 IU/L |
| Hemoglobin | 7.4 g/dL | LDH | 219 IU/L |
| Hematocrit | 26.8% | γ-GTP | 16 IU/L |
| MCV | 63.7 fl | Total protein | 7.9 g/dL |
| MCH | 17.6 pg | Creatinine | 0.41 mg/dL |
| MCHC | 27.6% | BUN | 18.2 mg/dL |
| Platelets | 54.6 × 104/μL | Uric acid | 4.6 mg/dL |
| Sodium ion | 138 mequiv/L | ||
| Reticulocytes | 7092/μL | Potassium ion | 3.9 mequiv/L |
| Chloride ion | 100 mequiv/L | ||
| PT | 14.4 s | Glucose | 229 mg/dl |
| APTT | 25.0 s | CK | 76 IU/L |
| d-Dimer | 0.8 g/mL | CK-MB | 17 IU/L |
| Fibrinogen | 344 mg/dL | CRP | 0.09 mg/dL |
| NT-pro BNP | 219.9 pg/mL | ||
| Adrenaline | 79 pg/mL | AchR antibodies | 48 nmol/L |
| Noradrenaline | 1277 pg/mL | ||
| Dopamine | 41 pg/mL |
MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PT, prothrombin time; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; γ-GTP, γ-glutamyltranspeptidase; BUN, blood urea nitrogen; CK, creatine kinase; CRP, C-reactive protein; NT-pro BNP, N-terminal pro-B-type natriuretic peptide; AchR, acetylcholine receptor.
Fig. 3.
Thorax contrast enhanced computed tomography (CT) scan. CT scan of the thorax revealed the presence of a round mass at the level of the anterior mediastinum (*) and it invaded the superior vena cava.
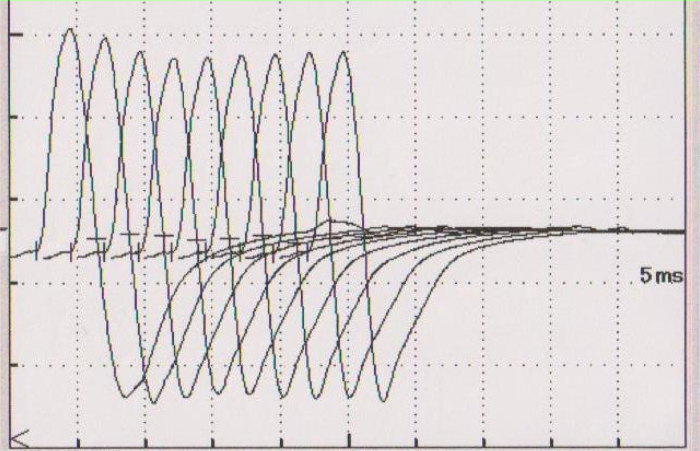
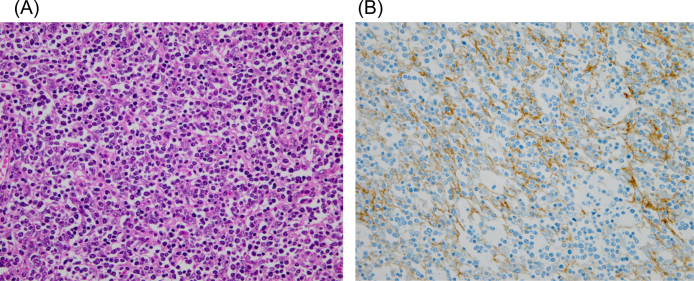
As she complained about the weakness of her eyelid muscles from the first visit to the emergency department, we suspected MG with thymoma and bilateral ptosis, and thus injected edrophonium chloride. We obtained a positive result, and her muscle weakness improved significantly. Repetitive nerve stimulation at a frequency of 3 Hz showed a 12% decremental response of the median nerve compound muscle action potential amplitude in the electromyogram (Fig. 4), supporting a post-synaptic neuromuscular junction dysfunction. Her laboratory workup showed an elevated acetylcholine receptor-binding antibody titer of 48 nmol/L (normal <0.30 nmol/L). Based on these findings, she was diagnosed with myasthenic crisis of MG. We immediately started administering pyridostigmine (180 mg daily) and methylprednisolone (1000 mg daily). On the fourth day, she was transferred to another better equipped healthcare facility where she was kept on mechanical ventilation due to worsening respiratory dysfunction and the need for long-term endotracheal intubation. Her treatment also included plasmapheresis, steroid pulse therapy, and immunosuppressants. About 2 months after admission, her LVEF recovered to 57%, as measured by cardiac ultrasonography. Four months after admission, her anti-acetylcholine receptor antibody titers had decreased to 17 nmol/L and she was weaned off the ventilator. Her electrocardiogram also normalized 4 months after onset (Fig. 1B). She underwent anterior mediastinal tumor and intravenous mass resection 5 months after onset and the isolated tissue was pathologically confirmed to be a type B1 thymoma (Fig. 5). At this time, her anti-acetylcholine receptor antibody titers had decreased further to 7.4 nmol/L, and she could finally be discharged from the hospital, 151 days after symptom onset, while receiving radiation therapy.
Fig. 4.
Electromyogram with repetitive nerve stimulation at a frequency of 3 Hz showed 12% decremental response of the median nerve compound muscle action potential amplitude.
Fig. 5.
Microscopic appearance of the thymoma [hematoxylin and eosin stain, 40× (A), cytokeratin stain, 40× (B)]. Microscopic appearance of the thymoma, which was a type B1 thymoma, showing ovoid spindle shaped cells with mature lymphocytes.
Discussion
We report a case of TC leading to the diagnosis of MG. Although cases in which the onset of TC and myasthenic crisis, during the course of MG, have been reported 7, 8, 9, we are not aware of any previous reports describing the diagnosis of MG following the onset of myasthenic crisis during an already existing TC as this case.
This case revealed typical TC findings such as ST segment elevation, increase in troponins and CK-MB level, apical ballooning with pressure gradient on left ventriculography, and no stenosis in coronary arteries.
However, atypical symptoms of TC were observed such as muscle weakness leading to hypercapnic respiratory failure. Then, we confirmed the diagnosis of MG by the presence of circulating acetylcholine receptor antibodies, a sensitive measurement in 88–93% of generalized MG cases, especially those presenting with thymoma [10] as well as after the improvement of extremity muscle strength following the injection of 10 mg edrophonium intravenously and repetitive nerve stimulation.
This case revealed simultaneous onset of TC and myasthenic crisis. Is there any relation between the two diseases? The occurrence of TC has been shown to be associated with physical and emotional stress 1, 2, 3, 4, 5, 6. Although the relationship between myasthenic crisis and plasma norepinephrine levels has not been demonstrated, the same condition may have triggered TC and MG crisis simultaneously. Considering the same trigger between TC and MG crisis, we should add MG crisis into differential diagnosis when patients have respiratory failure following TC.
Conclusion
Here, we report a case of TC that led to the diagnosis of MG, highlighting the possibility of MG crisis having a risk of developing TC.
References
- 1.Tsuchihashi K., Ueshima K., Uchida T., Oh-mura N., Kimura K., Owa M., Yoshiyama M., Miyazaki S., Haze K., Ogawa H., Honda T., Hase M., Kai R., Morii I. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heartsyndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 2.Abe Y., Kondo M., Matsuoka R., Araki M., Dohyama K., Tanio H. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol. 2003;41:737–742. doi: 10.1016/s0735-1097(02)02925-x. [DOI] [PubMed] [Google Scholar]
- 3.Sharkey S.W., Lesser J.R., Zenovich A.G., Maron M.S., Lindberg J., Longe T.F., Maron B.J. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 4.Wittstein I.S., Thiemann D.R., Lima J.A., Baughman K.L., Schulman S.P., Gerstenblith G., Wu K.C., Rade J.J., Bivalacqua T.J., Champion H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 5.Desmet W.J., Adriaenssens B.F., Dens J.A. Apical ballooning of the left ventricle: first series in white patients. Heart. 2003;89:1027–1031. doi: 10.1136/heart.89.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bybee K.A., Prasad A., Barsness G.W., Lerman A., Jaffe A.S., Murphy J.G., Wright R.S., Rihal C.S. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94:343–346. doi: 10.1016/j.amjcard.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Beydoun S.R., Wang J., Levine R.L., Farvid A. Emotional stress as a trigger of myasthenic crisis and concomitant takotsubo cardiomyopathy: a case report. J Med Case Rep. 2010;4:393. doi: 10.1186/1752-1947-4-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai M., Ukigai H., Miyata H.A. case of transient left ventricular ballooning (“Takotsubo”-shaped cardiomyopathy) developed during plasmapheresis for treatment of myasthenic crisis. Rinsho Shinkeigaku. 2004;44:207–210. [PubMed] [Google Scholar]
- 9.Sousa J.M., Knobel M., Buchelle G., Sousa J.A., Fisher C.H., Born D., Akamine N., Knobel E. Transient ventricular dysfunction (Takotsubo cardiomyopathy) Arq Bras Cardiol. 2005;84:340–342. doi: 10.1590/s0066-782x2005000400013. [DOI] [PubMed] [Google Scholar]
- 10.Lennon V.A. Serologic profile of myasthenia gravis and distinction from the Lambert-Eaton myasthenic syndrome. Neurology. 1997;48(Suppl. 5):S23–S27. [Google Scholar]