Abstract
Mesenchymal stem cells (MSCs) are heterogeneous population of cells with great potential for regenerative medicine. MSCs are relatively easy to expand in a cell culture, however determination of their concentration in harvested tissue is more complex and is not implemented as routine procedure. To identify MSCs collected from bone marrow we have used two combinations of cell markers (CD45−/CD73+/CD90+/CD105+ and CD45−/CD271+) and fibroblast colony-forming unit (CFU-F) assay. Further, in donors of various ages, mesenchymal stem cell concentration was compared with the result of CFU-F assay and with hematopoietic stem cell concentration, determined by a standardized flow cytometric assay. A positive correlation of MSC populations to the CFU-F numbers is observed, the population of the CD45−/CD271+ cells correlates better with CFU-F numbers than the population of the CD45−/CD73+/CD90+/CD105+ cells. The relationship between the hematopoietic CD45dim/CD34+ cell concentration and mesenchymal CFU-Fs or CD45−/CD271+ cells shows a positive linear regression. An age-related quantitative reduction of hematopoietic CD45dim/CD34+, mesenchymal CD45−/CD73+/CD90+/CD105+ and CD45−/CD271+ stem cells, and CFU-F numbers were noted. Additionally, statistically significant higher CFU-F numbers were observed when bone marrow samples were harvested from three different sites from the anterior iliac crest instead of harvesting the same sample amount only from one site.
Keywords: Mesenchymal stem cells, CD271+ cells, Bone marrow, CFU-F assay, Hematopoietic stem cells
Introduction
Bone marrow is a source of multipotential stem cells, including hematopoietic stem cells (HSCs) which are extensively used for the treatment of various malignant and non-malignant diseases for more than half a century (Eaves 2015; Passweg et al. 2012; Kresnik et al. 2016). In the last 20 years, mesenchymal stem cells (MSCs) are another bone marrow cell type often applied in cell therapies as well. MSCs are gaining interest due to their role in tissue damage repair (DiMarino et al. 2013), and for the treatment of patients with autoimmune diseases (Maria et al. 2017). The complex paracrine signaling network is one of their main therapeutic mechanisms. Namely, as secretors of hematopoietic cytokines, MSCs could be infused to promote hematopoiesis after myeloablative therapy (Koç et al. 2000). Whereas, they can also function as negative effectors and inhibit proliferation and cytotoxic action of immune cells, which could be exploited to prevent or mitigate complication of graft-versus-host disease (GVHD) following allogeneic HSC transplantations. The majority of early phase clinical trials showed efficacy in the treatment of acute and chronic GVHD with MSCs (Kebriaei et al. 2009; Prasad et al. 2010), additionally there are some reports regarding the prevention of GVHD after MSCs cotransplantation with HSCs (Ball et al. 2007; Wu et al. 2013).
Although MSCs have been successfully isolated from almost all types of tissues, bone marrow is usually used as cell source for cell expansion in the most common clinical applications like GVHD treatment or replacement of bone tissue. For stem cell therapies, identification and quantification of the stem cell population is crucial. The identification of HSCs is a relatively simple and frequently used method (Keeney et al. 1998), while the determination of MSC concentration before cell expansion is still a challenge. This heterogeneus population is defined as MSCs after reaching minimal criteria—being able to adhere to plastic, must be able to differentiate into trilineage cell types adipocytes, chondroblasts and osteoblasts, and cells are positive for surface markers: CD73+, CD90+, CD105+, and negative for CD45−, CD34−, CD14−, CD11−, CD79−, CD19− and HLA-DR (Dominici et al. 2006). However, due to MSC rarity in tissue and the fact that surface marker expression profiles could differ between expanded cells and those in a tissue sample, there is no standardized enumeration method for MSCs before cell culturing. Since an additional marker, antigen CD271, has been described and applied as a selective phenotypic marker for MSC identification (Quirici et al. 2002; Bühring et al. 2007; Kuçi et al. 2010), we wanted to identify MSCs as CD45−/CD73+/CD90+/CD105+ cells and in parallel as CD45−/CD271+ cells (Table 1) and compare the concentration of both populations with the functional colony-forming unit fibroblast (CFU-F) count. Although the CFU-F assay is the most reliable indicator of a MSC count, it is time consuming and inappropriate method for the routine use. As HSCs and MSCs are situated in the same niche microenvironment of bone marrow (Boulais and Frenette 2015), we wanted to find out if MSC amount could be evaluated on the basis of HSC concentration.
Table 1.
Hematopoietic and mesenchymal stem cell expression markers used in the study
| Other name/description | Target population | |
|---|---|---|
| CD34 | Transmembrane sialomucin | Hematopoietic stem and progenitor cells |
| CD45 | Leukocyte common antigen, a receptor type protein tyrosine phosphatase protein | Hematopoietic stem and progenitor cells |
| CD73 | Ecto-5′-nucleotidase | Mesenchymal stem cells |
| CD90 | Thy-1, glycosylphosphatidylinositol-linked protein involved in cell–cell and cell–matrix interactions | Mesenchymal stem cells |
| CD105 | Endoglin, type I membrane glycoprotein that functions as an accessory receptor for TGF-β superfamily ligands | Mesenchymal stem cells |
| CD271 | Nerve growth factor receptor (NGFR) | Mesenchymal stem cells |
Beside adequate cell enumeration method, knowledge about the changes in the MSC counts that occur with aging is important in cell therapy planning. Therefore, we wanted to define the effect of the patient’s age on the concentration of both types of the collected stem cells. Bone marrow was aspirated from one site or from three different sites and stem cells were quantitatively compared among these two different harvesting techniques.
Methods
Sample collection
Bone marrow was aspirated from 21 individuals, ten women and eleven men. For the needs of orthopaedic surgery, bone marrow was harvested from the anterior iliac crest using different aspiration techniques. In the 1st group (n = 7), the surgeon aspirated bone marrow from one harvesting site using 50 mL syringes prefilled with 4.5 mL of acid citrate dextrose solution A (ACD-A) (Fresenius Kabi, Bad Homburg, Germany) with an average total volume of 31.1 mL (range 24.5–34.5 mL). In the 2nd group (n = 13), bone marrow was aspirated from three different harvesting sites using 10 mL syringes prefilled with 1.5 mL ACD-A each. The bone marrow was aspirated to the average total volume of 32.2 mL (range 28.0–34.0 mL), with around 10 mL from each harvesting site. Only a small fraction of the collected bone marrow aspirate was analysed, the remaining sample was processed and applied back to the patient. The usage of surplus clinical samples for cell analyses was approved by the National Medical Ethics Committee number 0120-14/2016-2.
Cell counting
The concentration of white blood cells (WBCs) was determined with the haematology analyzer COULTER® Ac·T diff2T (Beckman Coulter, Fullerton, CA, USA).
Flow-cytometric characterization of hematopoietic stem cells
The bone marrow aspirates (n = 21) were used to evaluate the phenotype state of cells and populations of cells. The CD34+ cells (100 µL) were analysed using standardized three-color single platform variant of the ISHAGE guidelines with the addition of the viability dye 7-aminoactinomycin D (7-AAD) (Keeney et al. 1998). Briefly, the cell samples were stained with anti-CD34 (PE), anti-CD45 (FITC) conjugated antibodies and 7-AAD, in tubes containing BD Trucount counting beads. All reagents were purchased from Becton-Dickinson (BD) (San Diego, CA, USA). After incubation (20 min at room temperature in the dark) the red blood cells were lysed with a lysing buffer (BD) for 10 min and analysed immediately using FACSCalibur™ flow cytometer and Cell Quest Pro software.
Flow-cytometric characterization of mesenchymal stem cells
Bone marrow samples (n = 21) were also analysed for mesenchymal stem cell markers using the revised ISHAGE protocol. The cell samples (200 µL) were stained with anti-CD271 (PE), anti-CD45 (FITC), and 7-AAD in BD Trucount tubes. All reagents were purchased from BD. After incubation (20 min at room temperature in the dark), the red blood cells were lysed with a lysing buffer for 10 min and analysed immediately using FACSCalibur and Cell Quest Pro software.
Bone marrow samples (n = 21) were next labeled with anti-CD45 (PE), anti-CD73 (APC), anti-CD90 (FITC), and anti-CD105 (PerCP-Cy5.5) antibodies, all from BD. Cells were incubated 20 min at room temperature in the dark, the red blood cells were lysed with a lysing buffer for 10 min, centrifuged at 400 g for 5 min, cell pellet was resuspended with DPBS (Gibco, Paisley, Scotland), and analysed immediately with BD FACSAria Cell Sorter and equivalent program software BD FACSDiva. Compensation was set using single-stained cells for each color versus unstained control. Nonspecific fluorescence was checked using isotype control. In order to determine the proper borders of fluorescent gating, fluorescence-minus-one controls were measured as well.
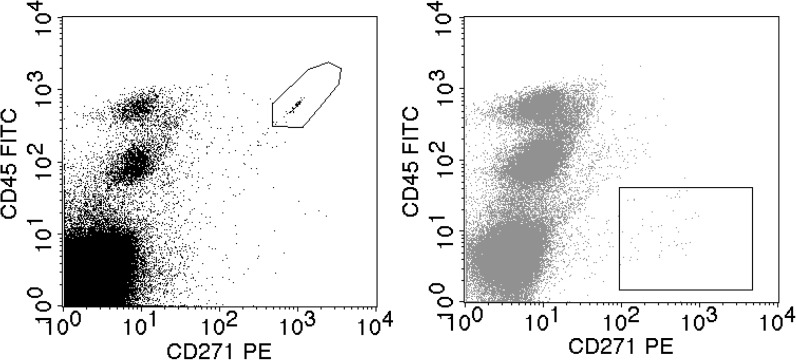
Hematopoietic and mesenchymal stem cell expression markers used in the study are listed in the Table 1. Figure 1 shows the flow cytometric analysis of viable MSCs defined as CD45-/CD271+ cell population (gate in the right dot plot), and the counting beads (gate in the left dot plot).
Fig. 1.
Flow cytometric analysis of viable bone marrow mesenchymal stem cells defined as CD45−/CD271+ cell population (gate in the right dot plot), and the counting beads (gate in the left dot plot) added to the sample. Number of CD45−/CD271+ cells/mL is directly calculated by comparing the number of target CD45−/CD271+ cells and the number of counting beads detected in the same sample
Fibroblast colony forming units assay (CFU-F)
Triplicates were seeded into 6-well culture plates at 1.25 × 105, 2.5 × 105 or 5 × 105 nucleated cells/well containing DMEM/Ham’s F12 (1:1, Gibco, Paisley, UK) supplemented with 10% FBS (Gibco), 1 ng/mL bFGF (Preprotech, London, UK), antibiotics gentamycin, penicillin, streptamycin (Gibco) and 1 IU/ml heparin (B. Braun, Melsungen, Germany). The plates were incubated at standard cell culture conditions: 37 °C, 5% CO2 with media changes (without heparin) every 2–4 days. After 14 days samples were fixed with 4% formaldehyde and stained with 0.05% crystal violet (Sigma, St. Louis, MO, USA). Colonies containing ≥ 50 fibroblastic cells were manually counted under the stereomicroscope. The concentration of the CFU-F/µL of bone marrow was calculated based on the seeding number and the initial concentration of WBC.
Statistical analysis
The data were analysed using the descriptive and inferential statistics of the tested samples. The data are expressed as mean, standard deviation (SD), minimum, maximum, number, p value and the coefficient of determination (R2). The concentration of cells was determined (1) as the ratio of the positive cells to the WBC obtained from appropriate gating of CD45 cells, detected by flow cytometry and (2) with fluorescent counting beads, which provide information on the number of cells/µL of bone marrow and was further calculated as the percent of cells/WBC (the number of WBC was obtained with hematology analyzer). The use of fluorescent counting beads provides an absolute count of the cells by measuring the ratio of beads and cells of interest. The number of cells/µL in the bone marrow sample is calculated from the flow cytometry data by the following equation:
Flow-cytometric data were analysed using the linear or exponential curve fitting shown in a graphical form with a given R2 value. A p value of less than 0.05 was considered significant.
Results
Bone marrow samples from 21 individuals undergoing orthopaedic surgery were collected and fresh samples were analysed using flow cytometry in order to determine the concentration and to assess the relationships between different stem cell populations. The viability of the cells for each bone marrow sample was monitored, and a minimal 95% value was obtained using 7-AAD nuclear dye. The mean concentration of HSC obtained identified as CD45dim/CD34+ cells was 151.2 cells/µL of bone marrow, ranging from 11.6 to 317.4 cells/µL or 0.54%, if expressed as a percentage of CD45dim/CD34+ cells/WBC, ranging from 0.05 to 0.89% (Table 2). MSCs, identified as CD45−/CD271+ cells, were counted and the mean concentration of 11.2 cells/µL of bone marrow was obtained, ranging from 4.4 to 25.2 cells/µL. As expressed by the percentage of CD45−/CD271+ cells/WBC, their mean concentration was 0.046%, ranging from 0.022–0.094%.
Table 2.
Main characteristics of the patients and results of CFU-F assay and flow cytometric analysis. The mean with the standard deviation is presented
| Number of bone marrow donors | n = 21 | |
|---|---|---|
| Average age of the bone marrow donors | 34.6 (14–59) years | |
| WBC concentration | 26.7 ± 12.2 × 103 cells/µL | |
| CFU-F concentration | 1.9 ± 1.8 CFU-F/µL | |
| Cell population of bone marrow | Cells/µLa | % of cells/WBC |
| CD45dim/CD34+ (HSC) | 151.2 ± 94.2 | 0.54 ± 0.25 |
| CD45−/CD271+ (MSC) | 11.2 ± 5.1 | 0.046 ± 0.019 |
| Cell population of bone marrow | % of cells/WBC | |
| CD45−/CD73+ | 0.081 ± 0.034 | |
| CD45−/CD90+ | 0.069 ± 0.043 | |
| CD45−/CD105+ | 0.500 ± 0.020 | |
| CD45−/CD73+/CD90+/CD105+ (MSC) | 0.018 ± 0.014 | |
aConcentration determined by fluorescent counting beads
The MSCs in the bone marrow samples were next stained with anti-CD45, anti-CD73, anti-CD90, and anti-CD105 antibodies. The mean concentrations of CD45−/CD73+ cells, CD45−/CD90+ cells, CD45−/CD105+ cells were 0.081%, 0.069%, and 0.50%, respectively, and cumulative CD45−/CD73+/CD90+/CD105+ cell concentration was 0.018%.
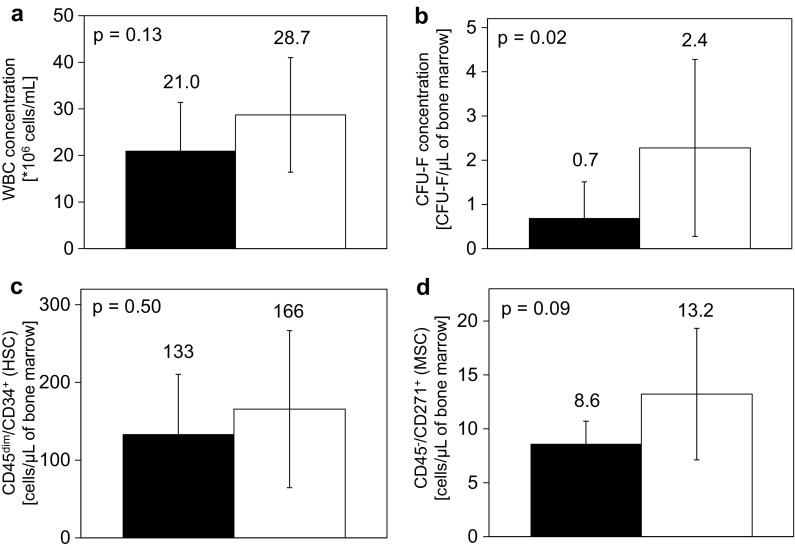
Subgrouped bone marrow samples were next compared using different kinds of harvesting techniques. The 1st group presents cells harvested by 1 × 30 mL aspiration (Fig. 2, black columns), and the 2nd group presents cells harvested by 3 × 10 mL aspiration (Fig. 2, white columns). The mean concentration of WBC, CFU-F, CD45dim/CD34+ cells and CD45−/CD271+ cells for each harvesting technique is summarized in Fig. 2.
Fig. 2.
The concentration of WBC (a), CFU-F (b), CD45dim/CD34+ cells (c) and CD45−/CD271+ cells (d) in bone marrow samples compared using two different bone marrow aspiration techniques. The 1st group aspiration technique is the collection of 30 mL of bone marrow at one harvesting site (black column); the 2nd group aspiration technique is the collection of 10 mL of bone marrow from three harvesting sites (white column), all in the anterior iliac crest. The mean with the standard deviation is presented (1st group n = 7, 2nd group n = 14)
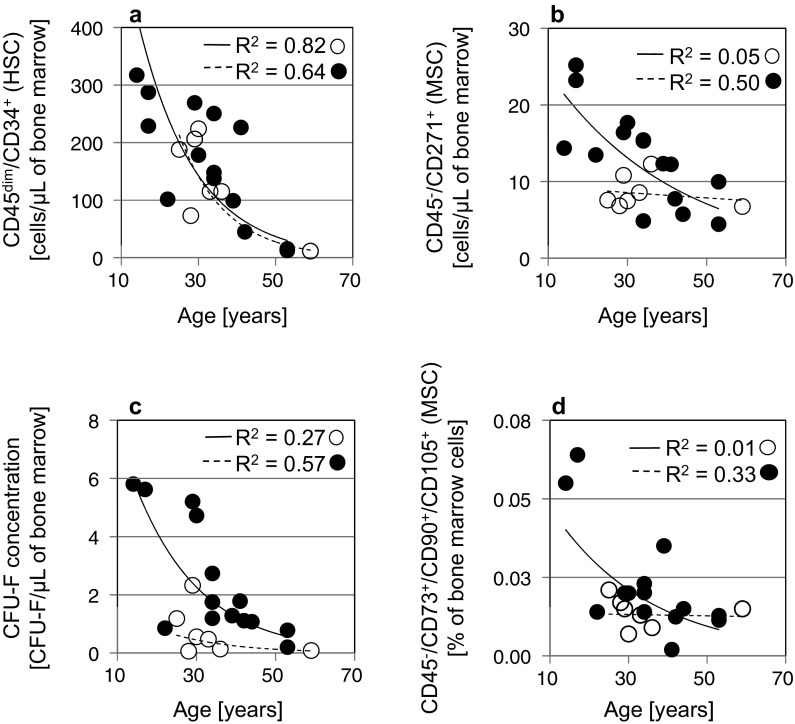
The samples were further analyzed according to the age of the bone marrow donors and a reduction in the concentration of HSC and MSC cells was observed with increasing age of the donor (Fig. 3).
Fig. 3.
The concentration of CD45dim/CD34+ (HSC) (a), CD45−/CD271+ (MSC) cells (b), CFU-F (c), and CD45−/CD73+−/CD90+−/CD105+ (MSC) (d) in bone marrow samples correlated to the patient’s age. Empty circles present 1 × 30 mL aspiration, solid filled circles present 3 × 10 mL aspiration of bone marrow. The R2 values were obtained by exponential curve fitting for each aspiration technique separately (n = 21)
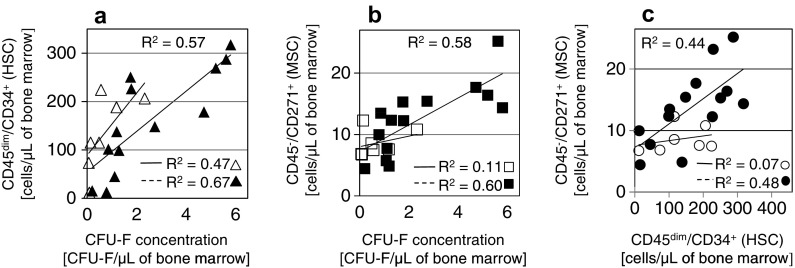
The bone marrow samples were directly applied to the plates, and colonies with more than 50 cells were counted after 14 days of culturing. The concentration of CFU-F/µL was calculated for the bone marrow aspirates. The mean concentration of CFU-F was 1.9/µL, ranging from 0.06 to 5.81/µL of bone marrow. The concentration of CFU-F compared to the concentration of CD45dim/CD34+ and CD45−/CD271+ cells obtained using flow cytometry, as well as the comparison between CD45dim/CD34+ and CD45−/CD271+ cells, displayed a positive slope of the linear regression curve (Fig. 4).
Fig. 4.
The concentration of CD45dim/CD34+ (a) and CD45−/CD271+ (b) cells in the bone marrow samples correlated to the concentration of CFU-F, and the concentration of CD45dim/CD34+ cells correlated to the concentration of CD45−/CD271+ cells (c). Empty markers present 1 × 30 mL aspiration, solid filled markers present 3 × 10 mL aspiration of bone marrow. The R2 values were obtained by linear curve fitting (n = 21)
Discussion
While cell markers CD73, CD90, CD105 are the most widely used markers for mesenchymal stem cell identification after their expansion in cell culture, alternate cell markers for their identification directly in bone marrow or other tissue samples are being investigated. For identifying MSC population in human bone marrow we chose cell marker CD271 in combination with CD45, leukocyte common antigen, which is not expressed on MSCs. The population size of CD271+ cells was compared with the population size of CD73+/CD90+/CD105+ cells in the same cell sample. The mean concentration of the mesenchymal CD45−/CD73+/CD90+/CD105+ cells in the bone marrow samples was 0.018%, which was less than the concentration of the mesenchymal CD45−/CD271+ cells, 0.046%. These data show that both MSC populations do not completely overlap, although the use of counting beads in the case of the CD45−/CD271+ cells, and not in the case of CD45−/CD73+/CD90+/CD105+ cells, could partially affect the differences. Compared to other studies, the mean percentage of our CD45−/CD271+ cells was higher than Cuthbert et al. (2012) discovered, obtaining 0.026%, and as Alvarez-Viejo et al. (2013) found, obtaining 0.0042%. Other studies determined the total number of CD271+ cells within both, CD45− and CD45+ cells, so we cannot directly compare the results. More extensive CD271+ cell population than in our samples, presenting 0.94%, was obtained by Kuçi et al. (2010), and 0.29% by Flores-Torales et al. (2010). We believe that such variability is likely due to the use of different enumeration methods or/and the analysis of relatively small number of different bone marrow donors (healthy donors, patients). At the same time it shows the complexity of the determination of this rare and heterogeneous cell population.
Among all procedures for determining the number of stem cells, the CFU assay is still considered as being the most reliable tool for the enumeration of proliferating cells in biological samples. Since the method is long-lasting, it is not suitable for routine use. When comparing the mesenchymal CD45−/CD73+/CD90+/CD105+ cells and the CD45−/CD271+ cells to the CFU-Fs, we found a higher correlation of the latter. The mean concentration of CFU-Fs in our study was 1.9/µL of bone marrow, which is higher than 0.612/µL measured by Hernigou et al. (2005), and 0.28/µL reported by Cuthbert et al. (2012). Nevertheless, the concentration of the CD45−/CD271+ cells obtained with flow cytometer is approximately 6.5 times higher than the concentration of MSCs measured by the CFU-F assay. This suggests that not all CD45−/CD271+ cells could form colonies, which is in agreement with the previous results (Cuthbert et al. 2012).
In bone marrow, hematopoietic stem cells share their niche environment with many other cells including MSCs. The quantification of HSCs is a routine part of the diagnostic and transplantation procedure. In contrast, the MSC enumeration before cell expansion is not evaluated in a routine practice. We analyzed the relationship among both stem cell populations in intact human bone marrow samples. The relationship between the hematopoietic CD45dim/CD34+ cell concentration and CFU-Fs (Fig. 4a) or CD45−/CD271+ cells (Fig. 4c) shows a positive linear regression. The results indicate that the standardized HSC enumeration protocol could be useful for the prediction of the MSC concentration in bone marrow. Then, the stem cell populations were quantitatively compared according to the age of the individual bone marrow donor. Many studies show that regenerative potential of HSC declines. Beside studies describing decrease in HSCs number with age (Kuranda et al. 2011; Schündeln et al. 2014; Kresnik et al. 2016), some other studies reported an increase in HSC number or in the number of HSC subpopulations with age (Kuranda et al. 2011; Pang et al. 2011). In our count, the concentration of hematopoietic CD45dim/CD34+ cells was decreasing with age (Fig. 3a).
Several research projects indicate that aging is accompanied by changes in the number and differentiation potential of MSCs (Stolzing et al. 2008; Yu et al. 2011; Maijenburg et al. 2012; Beane et al. 2014). It was shown that CD271+ MSCs are being lost with age in the dermis and epidermis (Akamatsu et al. 2016), in our study we have determined the influence of patient’s age on MSC numbers in bone marrow. From the results, a slightly negative relationship between the patient’s age and the number of collected MSCs is shown in three ways—by the decrease of CD45−/CD271+ cells (Fig. 3b), the decrease of CFU-Fs (Fig. 3c), and the decrease of CD45−CD73+/CD90+/CD105+ cells (Fig. 3d). With the knowledge on, how stem cell is number decreasing due to the age, the amount of aspirated bone marrow can be adjusted according to the age of the patient.
The initial number of stem cells also depends on the aspiration technique used to collect them from the bone marrow (McLain et al. 2005; Fennema et al. 2009). We selected two collection techniques and compared the amount of harvested cells (Fig. 2). The mean number of WBCs (Fig. 2a), CFU-Fs (Fig. 2b), hematopoietic CD45dim/CD34+ (Fig. 2c), and mesenchymal CD45−/CD271+ cells (Fig. 2d) was lower in the 1st group (1 × 30 mL aspiration) in comparison to the 2nd group (3 × 10 mL aspiration). The difference in CFU-Fs between both aspiration techniques was statistically significant and is probably due to the different dilutions of sample with peripheral blood during bone marrow taking. When bone marrow is harvested from multiple harvesting sites, as in the case of the 2nd group, the concentration of collected MSCs is higher as well as the number of CFU-Fs.
In conclusion, the enumeration of both stem cell types, MSCs and HSCs, shows a positive correlation between each other, a positive correlation with the number of grown CFU-Fs, and a negative correlation with the increasing age of bone marrow donor, regardless of the aspiration technique. This reflects in the recently evidenced thesis that both populations share the same spatial niche in the bone marrow microenvironment. Because MSC quantification is not evaluated in routine laboratory practice for various reasons, HSC concentrations could predict MSC concentration in the same bone marrow sample. CD45−/CD271+ cells show higher correlation to CFU-F numbers than CD45−/CD73+/CD90+/CD105+ cells.
Funding
The work was supported by the Slovenian Research Agency (Grant No. P3-0371).
References
- Akamatsu H, Hasegawa S, Yamada T, et al. Age-related decrease in CD271(+) cells in human skin. J Dermatol. 2016;43:311–313. doi: 10.1111/1346-8138.13048. [DOI] [PubMed] [Google Scholar]
- Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, et al. LNGFR (CD271) as marker to identify mesenchymal stem cells from different human sources: Umbilical cord blood, Wharton’s jelly and bone marrow. J Bone Marrow Res. 2013;1:132. [Google Scholar]
- Ball LM, Bernardo ME, Roelofs H, et al. Cotransplantation of ex vivo-expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE. 2014;9:e115963. doi: 10.1371/journal.pone.0115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125:2621–2629. doi: 10.1182/blood-2014-09-570192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- Cuthbert R, Boxall SA, Tan HB, Giannoudis PV, McGonagle D, Jones E. Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy. 2012;14:431–440. doi: 10.3109/14653249.2011.651533. [DOI] [PubMed] [Google Scholar]
- DiMarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:210. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015;125:2605–2613. doi: 10.1182/blood-2014-12-570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema EM, Renard AJ, Leusink A, van Blitterswijk CA, de Boer J. The effect of bone marrow aspiration strategy on the yield and quality of human mesenchymal stem cells. Acta Orthop. 2009;80:618–626. doi: 10.3109/17453670903278241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Torales E, Orozco-Barocio A, Gonzalez-Ramella OR, Carrasco-Yalan A, Gazarian K, Cuneo-Pareto S. The CD271 expression could be alone for establisher phenotypic marker in bone marrow derived mesenchymal stem cells. Folia Histochem Cytobiol. 2010;48:682–686. doi: 10.2478/v10042-010-0063-6. [DOI] [PubMed] [Google Scholar]
- Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transpl. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR. Single platform flow cytometric absolute CD34 + cell counts based on the ISHAGE guidelines. International Society of Hematotherapy and Graft Engineering. Cytometry. 1998;34:61–70. doi: 10.1002/(SICI)1097-0320(19980415)34:2<61::AID-CYTO1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Kresnik PK, Krasna M, Rozman P, Vrtovec B, Malicev E. Collection and immunoselection of CD34+ cells: the impact of age, sex, and diabetes in patients with chronic heart failure. Transfusion. 2016;56:1792–1800. doi: 10.1111/trf.13646. [DOI] [PubMed] [Google Scholar]
- Kuçi S, Kuçi Z, Kreyenberg H, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95:651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranda K, Vargaftig J, de la Rochere P, et al. Age-related changes in human hematopoietic stem/progenitor cells. Aging Cell. 2011;10:542–546. doi: 10.1111/j.1474-9726.2011.00675.x. [DOI] [PubMed] [Google Scholar]
- Maijenburg MW, Kleijer M, Vermeul K, et al. The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. Haematologica. 2012;97:179–183. doi: 10.3324/haematol.2011.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria AT, Maumus M, Le Quellec A, Jorgensen C, Noël D, Guilpain P. Adipose-derived mesenchymal stem cells in autoimmune disorders: state of the art and perspectives for systemic sclerosis. Clin Rev Allerg Immunol. 2017;52:234–259. doi: 10.1007/s12016-016-8552-9. [DOI] [PubMed] [Google Scholar]
- McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87:2655–2661. doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Halter J, Bucher C, et al. Hematopoietic stem cell transplantation: a review and recommendations for follow-up care for the general practitioner. Swiss Med Wkly. 2012;142:w13696. doi: 10.4414/smw.2012.13696. [DOI] [PubMed] [Google Scholar]
- Prasad VK, Lucas KG, Kleiner GI, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transpl. 2010;17(4):534–541. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/S0301-472X(02)00812-3. [DOI] [PubMed] [Google Scholar]
- Schündeln MM, Walde G, Basu O, Havers W, Kremens B. Quantification of nucleated cells, CD34-positive cells and CFU-GM colonies in single bone marrow samples and bone marrow harvests derived from healthy children. Pediatr Hematol Oncol. 2014;31:340–348. doi: 10.3109/08880018.2013.874513. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang Z, Cao Y, Xu L, Li X, Liu P, et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells with a myeloablative regimen for refractory/relapsed hematologic malignancy. Ann Hematol. 2013;92:1675–1684. doi: 10.1007/s00277-013-1831-0. [DOI] [PubMed] [Google Scholar]
- Yu JM, Wu X, Gimble J, Guan X, Freitas MA, Bunnell BA. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell. 2011;10:66–79. doi: 10.1111/j.1474-9726.2010.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]