Abstract
Most bio-industrial mammalian cells are cultured in serum-free media to achieve advantages, such as batch consistency, suspended growth, and simplified purification. The successful development of a serum-free medium could contribute to a reduction in the experimental variation, enhance cell productivity, and facilitate biopharmaceuticals production using the cell culture process. Commercial serum-free media are also becoming more and more popular. However, the cell line secrets its own recombinant product and has special nutritional requirements. How can the composition of the proprietary medium be adjusted to support the specific cell’s metabolism and recombinant protein? This article uses statistical strategies to modify the commercial medium. A design of experiments is adopted to optimize the medium composition for the hybridoma cell in a serum-free condition. The supplements of peptone, ferric citrate, and trace elements were chosen to study their impact on hybridoma growth and antibody production using the response surface methodology. The stimulatory effect of the developed formulation on hybridoma growth was confirmed by the steepest ascent path. The optimal medium stimulated the hybridoma growth and antibody production in three diverse systems: a static plate, an agitated spinner flask, and a hollow fiber reactor. The cells in the developed serum-free medium had a better antibody production as compared to that in the commercial medium in the hollow fiber reactor. Our results demonstrated that the facile optimization for medium and antibody production was successfully accomplished in the hybridoma cells.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0255-z) contains supplementary material, which is available to authorized users.
Keywords: Antibody, Hybridoma, Medium optimization
Introduction
Scientists have gradually shifted from serum-containing media to serum-free media because serum ingredients have disadvantages, such as batch-to-batch variation, virus risk, and purification interference (Price 2017). Most of the mammalian cells used for the FDA-approved production of biopharmaceuticals are cultured in serum-free media since the presence of serum is a major obstacle for purification and product validation (McGillicuddy et al. 2018). However, serum-containing media are still in general use in the biological field of basic research. In addition, the question of how to develop the serum-free passage for attached cells and the weaning procedure shall be solved for the further application of serum-free media. Antibody production cells include Chinese hamster ovary cells, murine lymphoid NS0 and hybridoma cell lines, and human PER.C6. Hybridoma belongs to the hematopoietic cells, which are anchorage-independent and immortalized by the fusion of mortal splenocytes and immortal myelomas (Shukla and Thömmes 2010). Their secreted antibodies are important in the initial screening of protein drugs, future epitope determination, biomarker detection, and disease diagnosis (Corrêa et al. 2016). Concerns for animal welfare and quality assurance of the antibody drug hasten the development of serum-free media. Several commercially media are available for culturing hybridoma cells under a serum-free culture (Manna et al. 2015). One of the drawbacks of these commercial media is that their cost is several times higher than that of the basic medium. In addition, new cell lines have unique nutrient requirements for their culture environment. Specific ingredients shall be identified to optimize the proliferation and antibody production of cells (Tan et al. 2015).
A culture medium is a complex mixture of nutrients, buffers, and trace elements that maintain the physical environment for cell growth. The early basal media like minimum essential medium (MEM) had limited amino acids and glucose. Dulbecco’s modification of Eagle’s MEM (DMEM) is fortified to have fourfold the concentrations of amino acids and vitamins present in Eagle’s MEM. This medium also supplies the non-essential amino acids, glycine and serine, iron, and pyruvate (Price 2017). Blended media including DMEM/F-12 developed by Barnes and Sato and RPMI1640/DMEM/F-12 (RDF, mixing ratio 2:1:1) by Murakami have been applied to the serum-free culture of different mammalian cells (Yao and Asayama 2017). The reason for mixing two kinds of media can expand the number of nutritional constituents and the trace minerals. The modification of the medium composition is usually the first step of the cell culture process to increase antibody productivity. Finding a medium composition with the best cell growth is the main purpose of medium development. Optimization studies for medium can be carried out using multivariate methods, such as response surface methodology and the statistical design of experiments (DoEs) (Xing et al. 2011; Liu and Chang 2006). These methodologies organize statistical and mathematical tools established on the fit of a polynomial model to the data that can describe the experimental system and make statistical predictions (Yolmeh and Jafari 2017). These strategies can understand possible interactions of multiple medium compositions on the cell growth and antibody production that are not observed when changing one factor at a time (Knöspel et al. 2010). The sparsity-of-effects principle states that the effects of individual- and two-factor interactions significantly influence the experimental outcome when analyzing the results from the factorial experiments. Therefore, the statistical DoEs has been chosen by many researchers such as Dhanasekaran et al. (2013), Dong et al. (2008), and Sen and Roychoudhury (2013) to optimize the culture medium or procedure. These multivariate regression analyses significantly reduce the labor of parameter screening and process optimization.
Conventionally, the parental cells in a serum-contained medium are pre-weaned by using a commercial proprietary medium to facilitate the sequential adaption to the serum-free condition (van der Valk et al. 2010). However, these cells will be bound to this commercial medium for further production. Modification of the proprietary medium will be beneficial for the development and reduces the uncertainty of biopharmaceuticals production. The purpose of this study is to develop a serum-free medium modified from the commercial serum-free medium for a hybridoma cell line. First, several mixing-ratio media were assayed to cultivate hybridoma cells. Second, five ingredients suggested by the papers were screened to choose the most important three supplements for optimization. Third, a DoE was applied to optimize the serum-free medium for antibody production using the hybridoma cells. Accordingly, the optimal concentrations for three supplements were added to the blended media. Finally, the effects of the optimal medium on the cell growth, antibody production, and scalability were evaluated in static plate, agitation, and perfusion systems.
Materials and methods
Materials
Ferric citrate, glutathione, glutamine, ascorbic acid were purchased from Sigma (St. Louis, MO, USA). SFM4MAB medium and DMEM were from Hyclone (Chicago, IL, USA). Meat peptone was from Conda (Torrejón de Ardoz, Madrid, Spain) and trace element solution (A, B) was from Corning (Tewksbury, MA, USA). Spinner flasks were from Bellco Glass (Vineland, NJ, USA) and the hollow fiber reactor was from Fibercell System (C5011, MWCO: 20kd, fiber surface area: 2100 cm2, Frederick, MD, USA). Chemically defined supplement for high cell-density hybridoma culture (CDM-HD) was also purchased from Fibercell System. DMEM supplemented with 10% CDM-HD, termed CDMHD medium, was used to compare with the optimal medium in the hollow fiber reactor using three medium circulation rates.
Cell line and cell culture
The hybridoma cell line (CRL-1754) used in this study was established by Reimer et al. (1984) and was obtained from American Type Culture Collection (Manassas, VA, USA). This cell line produces monoclonal antibody against human IgG (Fc). The cell line was weaned in the SFM4Mab medium (GE HealthCare, Logan, UT, USA) according to the company’s protocol. The cell line was maintained in T-25-flasks using a serum-free CD Hybridoma medium supplemented with 8 mM l-glutamine (Sigma) at 37 °C under a humidified atmosphere of 5% CO2 and 95% air. The mixture of SFM4Mab with DMEM (Sigma) at 1:1, 1:2, and 1:3 ratios was compared for their influence on cell proliferation and antibody production. The SFM4Mab/DMEM medium (SDM 1:2) was used for medium optimization.
Cell concentration and viability
The number of cells was determined using the Coulter Multisizer 3 (Beckman, Brea, CA, USA). For the analysis of viable cells, 1 mL of cells was harvested from the T-25-flasks (Nunc, Rochester, NY, USA) or 100 mL spinner flasks (Bellco). After mixing with Trypan blue (Sigma) for 5 min the cell viability was then determined using a hemocytometer. For DoE experiments, 2 × 105 cells in 1 mL test medium were seeded in a 48-well plate. Antibody production and cell concentration were determined after 96 h of growth. Experiments were performed in triplicate in 48-well tissue culture plates.
Experimental design and statistical analysis
Fractional factorial design and central composite design data were regressed and analyzed by running the GLM and RSREG procedures in SAS 9.4 software (Cary, NC, USA). The three-dimensional response surfaces were generated by Sigmaplot (Systat, San Jose, CA, USA) based on the second-order equation. For the factorial design, 0.5 mg/mL of ferric citrate, a trace element solution containing copper sulfate, zinc sulfate, manganese sulfate, and sodium orthosilicate, 5 mg/mL of ascorbic acid, 0.5 g/mL of peptone, and 5 mg/mL of glutathione were used as the serum supplements. According to the DoE (Table S1), the concentrated supplement (10 μL) and SDM medium was added into each well to reach a final volume of 1000 μL. (“+ 1” presents the 10 μL addition, while “− 1” presents no addition.). After 96 h of culture, the influence of the ingredient was estimated utilizing glm regression analysis. The linear-regression coefficients of the fractional factorial design can be applied in the selection of important medium components. A greater positive coefficient value indicates that the ingredient has a more stimulatory effect on cells, while a negative coefficient shows that the ingredient has an inhibitory effect on cells. Among the five ingredients, three important factors such as ferric citrate, peptone, and trace elements were chosen for further optimization based on the coefficients of the first-order linear equation. A Box–Behnken design for three levels (− 1, 0, 1) is a rotatable second-order response surface model that has been adopted for medium optimization (Souza et al. 2005; Ferreira et al. 2004). It consists of a central point and the middle points of the edges. A total of 15 experimental trials (Table S2) are needed for the three-level three-factor Box–Behnken experimental design.
Quantification of antibody using an enzyme-linked immunosorbent assay (ELISA)
The antibody secreted by hybridoma was measured using an ELISA assay. Ninety-six-well microplates coated with affinity purified antibody specific to mouse immunoglobulin G (IgG, KPL, Gaithersburg, MD, USA) and blocked with the blocking buffer (3 g of skim milk powder and 5 g of sucrose in 100 mL PBS) were used for the analysis of hybridoma antibody production. The conditioned medium after the appropriate dilution was added to each well, and the microplate was incubated for 2 h at room temperature. After washing step, 100 μL of detecting antibody-HRP conjugate (KPL) was added to each well, and then the plates were incubated for 2 h at room temperature. After the plate was washed 3 times, 100 μL of tetramethylbenzidine hydrogen peroxide solution was added to each well and was allowed to react for 20 min in the dark. The reaction was stopped by adding 2 N sulfuric acid (50 μL/well), and the absorbance was measured at 450 nm with an ELISA reader (Molecular Device, Sunnyvale, CA, USA). A serially diluted mouse IgG (SCBT, Santa Cruz, CA, USA) was prepared for the standard curve. All experiments were performed in triplicate.
Cell culture in a spinner flask and hollow fiber reactor
The cells at a final concentration of 2 × 106/mL were seeded into 50 mL of the optimal SDM medium and cultured in a 100 mL spinner flask at a 60-rpm rate. The operation of the hollow fiber reactor is based on the instruction in the Fibercell Manual. Prior to cell inoculation, the bioreactor was aseptically circulated with the phosphate-buffered saline (PBS) and DMEM. A total of 108 cells were seeded into the extra-capillary space to establish a hybridoma culture. The serum-free medium was circulated at a 50 mL/min rate between the intra-capillary space and the reservoir bottle. The cells and their antibody solution were sampled every day for analysis.
Statistical analysis
Data were reported as means ± standard deviations. Two-tailed T test in MS Excel was used for evaluating the differences between the test group and the control group. The p-value less than 0.05 was considered statistically significant: *(p < 0.05), **(p < 0.01), and ***(p < 0.001).
Results and discussion
Basal medium screening
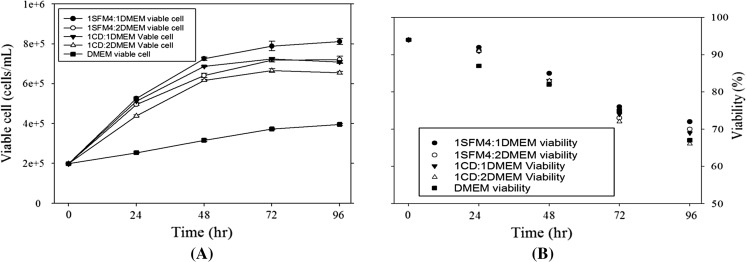
The model cell line was firstly weaned to the commercial serum-free medium. To reduce the composition complexity and develop the production medium, we tested the mixing ratios of SFM4MAB and DMEM on cell growth and antibody production. Figure 1 shows the growth of hybridoma enhanced when the proportion of SFM4MAB medium increased. The viable cell concentrations were similar in the media of the 1:1 and 1:2 mixing ratios of SFM4MAB and DMEM. The cells in the 1:3 mixed medium only reached 70% of cells in the 1:2 mixed medium. The viability of cells in the 1:1 and 1:2 mixed media were maintained above 80% during the 96-h culture. The DMEM could only support 50% of hybridoma growth compared to the cells in the 1:2 mixture of SFM4MAB and DMEM. Previously, RDF medium composed by RPMI:DMEM:F12 (2:1:1) has been applied as the basal media for hybridoma to reduce the manufacturing cost of antibody (Lee et al. 2009). The cost of antibody production using RDF can be reduced 17–50% compared with other serum-containing media (Chua et al. 1994). After considering the subsequent optimization and medium cost, the mixed SFM4MAB and DMEM in 1:2 (referred to as SDM) was used as our basal medium for the following experiments.
Fig. 1.
Effects of mixing ratio of medium on cell growth (a) and viability (b). The seeding concentration was 2 × 105 cells/mL (N = 3)
Screening of medium supplements
We used the SDM medium as the basal medium for the evaluation of the five supplements using the two-level fractional factor experiments. The results of the cell concentration and antibody production after the 72-h culture are shown in Table S1. These data were used to fit two linear polynomials for cell growth and antibody production (Table 1). The magnitude and sign of the regression constants can be used to identify the significance of the ingredients on the key outcomes of hybridoma growth. When the coefficient is relatively large, it has more significant effects on the response than a small coefficient. Furthermore, a variable with a positive fitted coefficient increases the response, and one with a negative coefficient has an inhibitory effect on the response. The stimulatory and inhibitory ingredients can be identified using the regression equations. For example, some supplements, such as ferric citrate, and trace elements, improve the cell growth at the 10% significant level (Table 1). Ferric citrate, peptone, and glutathione could enhance the antibody production significantly (p < 0.05). Our results related to the promotion of ferric citrate on mammalian cell growth are similar to the study of Coombs et al. (2015). The trace elements have been reported to enhance cell growth (Liu et al. 1986). Peptone can improve hybridoma growth and enhance the production of antibodies (Zhang et al. 1994). Protein hydrolysates (peptone) can provide small peptides that have beneficial effects on animal cell proliferation and antibody production (van der Valk et al. 2010). The low-molecular peptides, derived from peptone, in the protein-free media allow an increase of the maximal cell density (Chabanon et al. 2008). The trace elements studied herein contain CuSO4, ZnSO4, selenite, MnSO4, Na2SiO3, molybdic acid, NH4VO3, NiSO4, and SnCl2. Selenium aids in the detoxification of free radicals as a cofactor for GSH synthetase, while iron, copper, and zinc may be bound by serum protein. The stimulatory effects of trace elements like selenium and manganese on hybridoma growth in a serum-free condition have been studied (Kovár 1988). The supplement of ferric citrate can improve the growth of NS0 cells, which constitutively produce a human IgG1 antibody (Spens and Häggström 2005). Nagira et al. (1995) evaluated various iron salts and chelating agents replacing transferrin to develop a protein-free medium for a human–human hybridoma and found that ferric citrate was favorable for the production of monoclonal antibodies. The iron may be important to the control of transmembrane electron transport and intracellular DNA synthesis in in vitro culture. Alcaín et al. have reported that addition of the impermeable iron chelator bathophenanthroline disulfonate (BPS) to cultured Chinese hamster lung fibroblast (CCL 39 cells) inhibits DNA synthesis in the in vitro culture. In contrast, BPS does not inhibit cell growth stimulated by fetal calf serum. BPS treatment also inhibits transplasma membrane electron which is restored by incubation of cells with 10 μM ferric ammonium citrate (Alcaín et al. 1995). The impacts of peptone supplementation for the mammalian cell culture are summarized as follows. The specific amino acid profile of peptone can enhance the glucose utilization and reduce lactate and ammonia production (Davami et al. 2015). Therefore, ferric citrate, trace elements, and peptone are chosen for the further optimization in the basal SDM medium in the next section.
Table 1.
Regression coefficients of a fractional factorial design for cell growth and IgG production in SDM medium
| Cell concentration (105 cells/mL) | Antibody production (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | Standard error | t-value | p-value | Coefficient | Standard error | t-value | p-value | |
| Constant | 7.154 | 0.049 | 146.102 | 0.000 | 2.680 | 0.011 | 236.765 | 0.000 |
| Ferric citrate | 0.258 | 0.049 | 5.278 | 0.034* | 0.238 | 0.011 | 20.982 | 0.002* |
| Trace elements | 0.161 | 0.049 | 3.284 | 0.082* | 0.010 | 0.011 | 0.883 | 0.470 |
| Ascorbic acid | 0.131 | 0.049 | 2.686 | 0.115 | 0.045 | 0.011 | 3.976 | 0.058* |
| Peptone | 0.074 | 0.049 | 1.510 | 0.270 | 0.268 | 0.011 | 23.632 | 0.002* |
| Glutathione | 0.147 | 0.049 | 3.003 | 0.095 | 0.088 | 0.011 | 7.730 | 0.016* |
*p < 0.10
Optimization of the supplements’ concentrations for hybridoma cell growth
The effects of peptone, ferric citrate, and trace elements on cell growth and antibody production are shown in Supplementary Figs. S1–S3. Ferric citrate had a stimulatory effect on cell growth and antibody production around 3–6 μg/mL. This ferric salt would inhibit cell growth at the concentration of 12 μg/mL. The amount of antibody secreted by hybridoma is associated with its cell concentration. The effects of peptone and trace elements on hybridoma growth and antibody production were saturated at high concentrations (Figs. S1–S3). Peptone has been reported to enhance the antibody production in hybridoma cultures (Zhang et al. 1994). This one-factor-at-a-time investigation can provide important information for cell growth under a simplified medium. However, the DoE has been adapted here to optimize the multiple ingredients in the medium simultaneously and understand the interaction between two supplements in an SDM medium. The rules and design for factorial experiments and response surface methodology have been recently reviewed (Yolmeh and Jafari 2017) and applied in this study. Fractional and full factorial design data were regressed by SAS 9.4 software to obtain the first-order and second-order polynomials. The regression models can be applied in screening of the important components in the medium and the construction of the steepest ascent path. In the screening tests, the magnitude and sign of the regression constants can be used to identify the significance of the components on responses such as cell growth and antibody production. When the coefficient is relatively large, it has more significant effects on the response than a small coefficient. Furthermore, a variable with a positive fitted constant increases the response and one with a negative coefficient has inhibitory effects on the response. We can identify the stimulatory and inhibitory ingredients by the regression models. The coefficients of model can be used to construct the steepest ascent path for cell growth. The direction of the maximal increase in cell concentration is generated by the gradient of the regressed polynomial. The first-order polynomial models for hybridoma growth obtained by the glm procedure of SAS software are shown in the following equation:
The matrix of the Box-Behnken design and the comparison of data and predictions are shown in the Table S2. SAS software is applied to fit the growth data using the second-order response surface. The prediction error of the second-order polynomial was < 3%, as indicated in Table S2. The coefficients of the second-order polynomial, the p-values, and the optimal concentrations are shown in Table 2. The response surface predicted that the addition of 7.7 μg/mL of ferric citrate, 0.625 mg/mL of peptone, and 0.125% of trace elements could support maximal growth. The impacts of ferric citrate, peptone, and trace elements on cell growth are visualized in Fig. 2a, b by using the second-order polynomial. The full second-order polynomial model for hybridoma growth obtained by the rsreg procedure of SAS software is shown in the following equation:
Table 2.
Regression coefficient of the cell growth and the formulation of the optimal SDM medium predicted by DoEs
| Coefficient (cell/mL) | Standard error (cell/mL) | t-value | p-value | |
|---|---|---|---|---|
| Constant | 784,164 | 6885.41 | 113.89 | < .0001 |
| Ferric citrate | 20,147 | 5443.39 | 3.7 | 0.0076 |
| Peptone | − 121,539 | 5443.39 | − 22.33 | < .0001 |
| Trace elements | 3360.92 | 5443.39 | 0.62 | 0.5565 |
| Ferric citrate2 | − 17,302 | 7503.2 | − 2.31 | 0.0545 |
| Ferric citrate × Peptone | 1418.33 | 7698.12 | 0.18 | 0.859 |
| Peptone2 | − 82,153 | 7503.2 | − 10.95 | < .0001 |
| Ferric citrate × Trace elements | − 2042.17 | 7698.12 | − 0.27 | 0.7984 |
| Peptone × Trace elements | − 8881 | 7698.12 | − 1.15 | 0.2865 |
| Trace elements2 | − 18,126 | 7503.2 | − 2.42 | 0.0464 |
| Factor | Code | Real concentration |
|---|---|---|
| Ferric citrate | 0.54 | 7.7 μg/mL |
| Peptone | − 0.75 | 0.625 mg/mL |
| Trace elements | 0.25 | 0.125 |
| Maximal point prediction: 8.35 × 105/mL | ||
Fig. 2.
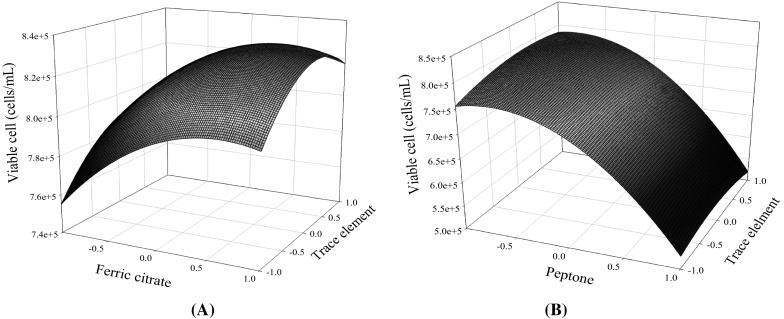
Response surfaces of ferric citrate and trace element (a) and peptone and trace elements (b) on cell growth in SDM medium
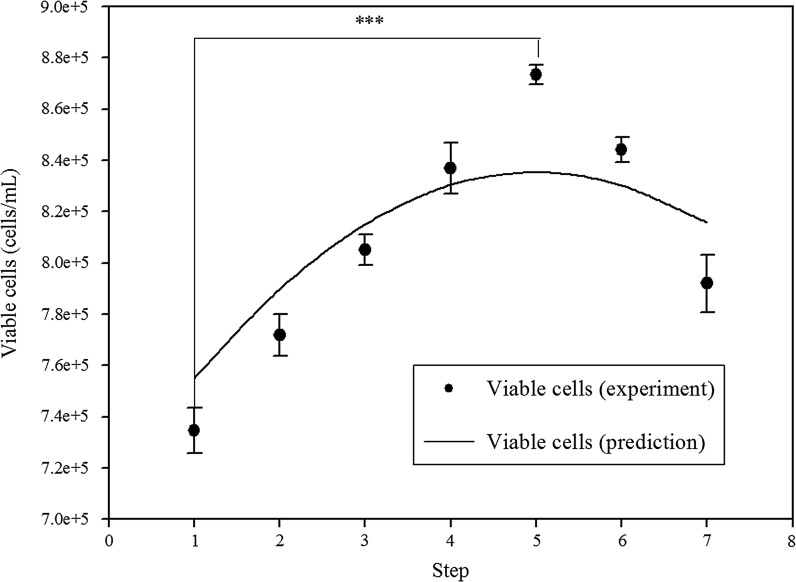
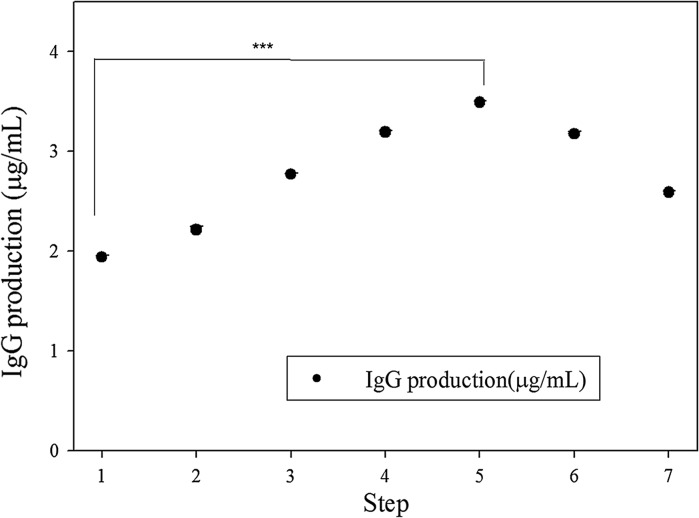
The F-value and its probability for the full quadratic equation for cell growth was 50.32 and 0.001, respectively. The determination coefficient (R2) indicated that the second-order response surface could explain 98.9% of the variability. Additionally, the optimal supplements for cell growth including 7.7 μg/mL of ferric citrate, 0.625 mg/mL of peptone, and 0.125% (v/v) of trace elements were obtained by canonical analysis in SAS software. The maximal peak of cell growth could be identified in the three-dimensional response surface plot. The steepest ascent path is used to validate the cell growth prediction in the SDM medium by the second-order polynomial. The medium composition along the ascent path and the cell growth are shown in Table S3 and Fig. 3, respectively. The maximal peak of cell growth (8.75 × 105/mL) could be observed at the fifth step, which was slightly higher than the model prediction (8.35 × 105/mL). Good agreement was shown to exist between the experimental points and the values predicted by the response model. The composition of the fifth step medium (the optimal SDM) was the same medium forecasted by the second-order polynomial, which had the maximal growth and antibody production (Figs. 3, 4).
Fig. 3.
The steepest ascent path of viable cell concentration in SDM medium. (The composition of each step is shown in Table S3, seeding cell density was 2 × 105 cells/mL, 72-h culture, 48 well; ***: p < 0.001)
Fig. 4.
The steepest ascent path of IgG production by adding peptone, ferric citrate and trace elements. (The composition of each step is shown in Table S3, seeding cells density was 2 × 105 cells/mL, 72-h culture, 48 well; ***: p < 0.001)
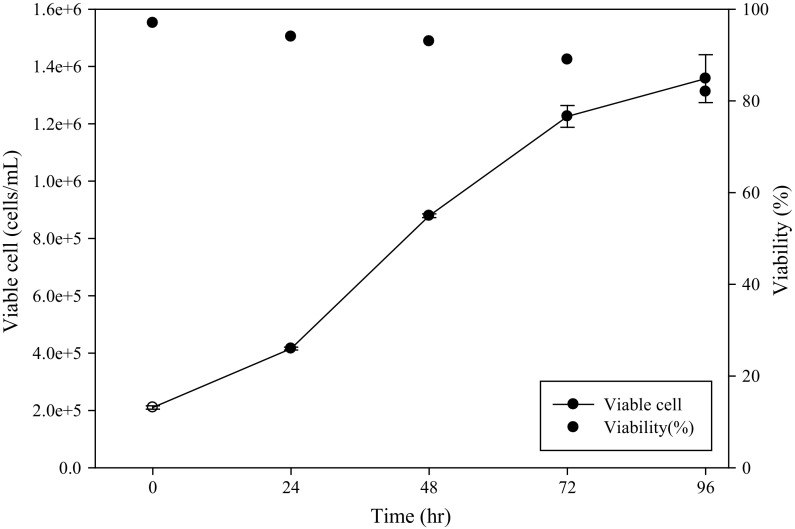
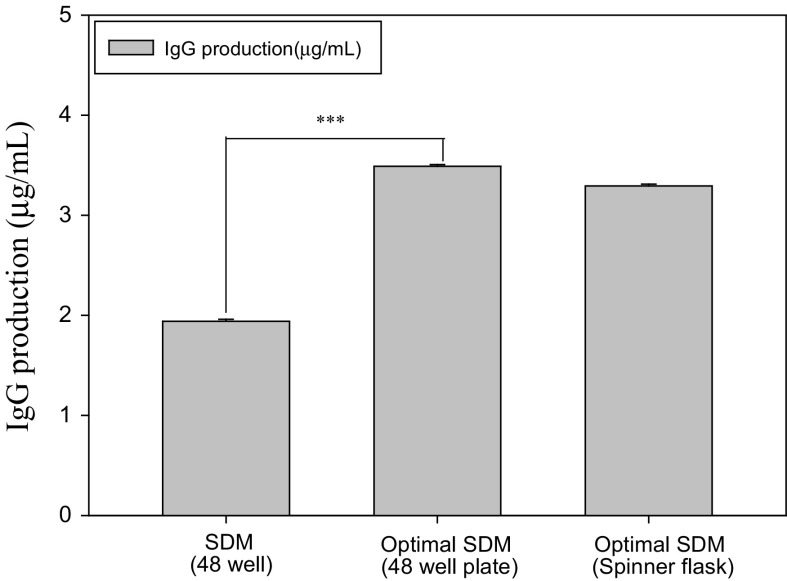
We studied the growth kinetics of the hybridoma using a spinner flask and evaluated the impact of the developed medium on mechanically agitated cells. The optimal SDM medium containing three supplements could support cell proliferation in the agitation system, as shown in Fig. 5. The antibody yields of cells in the static and agitation systems were similar when the optimal SDM medium was used (Fig. 6). However, the antibody production in the SDM medium only reached approximately 60% of that in the optimal SDM medium. This evidence suggested that the optimal SDM medium stimulated hybridoma growth and antibody production in static and agitation systems.
Fig. 5.
Hybridoma cells growth in optimal SDM medium using the spinner flask (50 mL medium in 100 mL spinner flask)
Fig. 6.
Comparison of antibody production in static and suspended systems (Seeding cells density was 2 × 105 cells/mL, 72-h culture, spinner flask 100 mL; ***: p < 0.001)
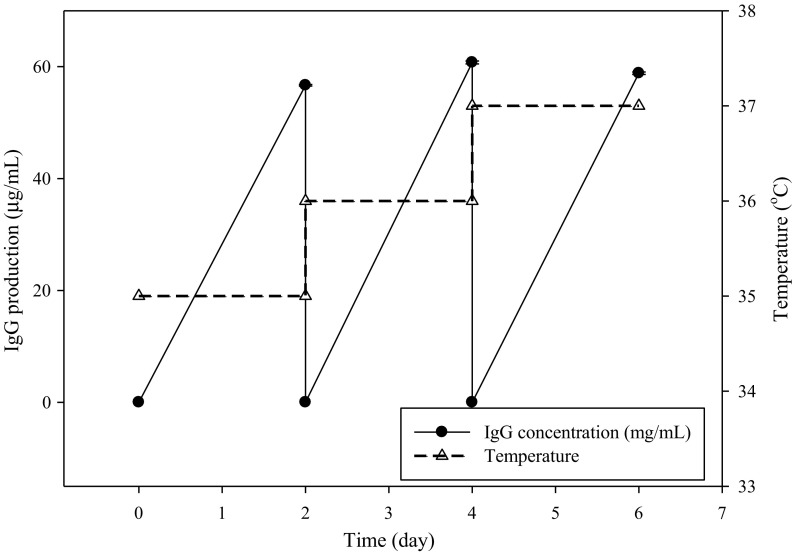
Additionally, the hollow fiber reactor can mimic a tissue-like condition with a high cell density of around 107–108/mL. The antibody production in a high-density bioreactor can maintain cell stability, enhance volumetric productivity, and reduce the production cost. However, the cell metabolic status and the medium are two critical factors for the successful development of the semi-continuous process. The optimal SDM medium was used to culture hybridoma in the hollow fiber reactor for semi-continuous IgG production. The antibody production in the high cell density culture device was similar at three culture temperatures (35, 36, and 37 °C). Cell productivity in recombinant protein can be increased by cell cycle arrest through mild hypothermia (García Münzer et al. 2015). Hypothermia can simultaneously reduce cell growth, which is regulated by the cell cycle (Coronel et al. 2016). Herein the temperature shift did not enhance the antibody production of hybridoma in the hollow fiber reactor. The optimal SDM medium could support the antibody production using the high cell-density device at a temperature of 36 ± 1 °C. These results suggested that the optimal SDM medium stimulated hybridoma growth and antibody production in the static and hollow fiber systems as indicated in Fig. 7.
Fig. 7.
Effects of the optimal SDM medium on semi-continuous IgG production (solid line) using the hollow fiber reactor at 35, 36, and 37 °C (dash line)
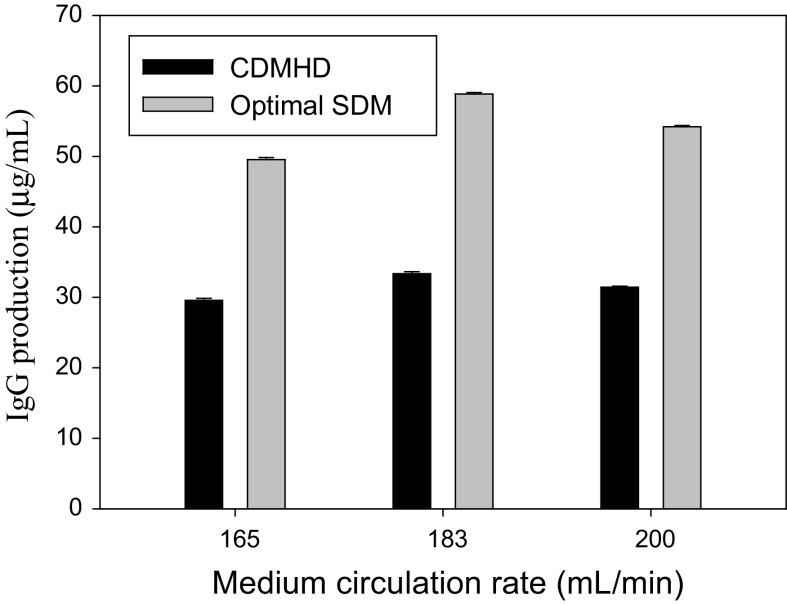
In order to prove the usefulness of our developed medium, a commercial serum-free medium, CDMHD, was used to compare with the optimal medium using three circulation rates at the temperature of 37 °C. CDM-HD is designed specifically for the culture of cells at high density by Fibercell Inc. The hybridoma cells cultivated in the optimal SDM had a 1.8-fold IgG production compared to that in the CDMHD medium at the rate of 182.5 mL/min (Fig. 8). In fact, the hybridoma cell line (CRL-1754) in the optimal SDM medium had better antibody production than those in CDMHD at all circulation rates. The medium composition dramatically affected the physiology, metabolism, growth and antibody production of the hybridoma cells. The proprietary composition of CDMHD makes it difficult to evaluate each ingredient’s impact. The hybridoma cell line (CRL-1754) in this study is a type cell line from ATCC for the academical study, which has a low IgG titer. Our data demonstrated that the facile optimization of the medium could be applied for the hybridoma cells and their antibody production by using the type cell as a model.
Fig. 8.
Comparison of IgG production by the hybridoma cell line (CRL-1754) in the optimal SDM medium with the commercial CDMHD medium in a hollow fiber reactor at 37 °C using three medium circulation rates
Conclusion
The statistical development of serum-free media and their applications in different culture systems for hybridoma have been investigated in this study. First, the impacts of the 1:2 mixtures of SFM4MAB and DMEM on cell growth and antibody production were investigated to reduce the medium cost. To fortify the ingredients and optimize the new formulation, five supplements were evaluated to study their influences on cell growth in the SDM medium. Sequentially, the composition of three supplements (peptone, ferric citrate, and trace elements) was optimized using the response surface methodology. Taken together, the optimal SDM medium stimulated hybridoma growth and antibody production in the static culture, the agitated spinner flask, and the hollow fiber reactor. The facile medium optimization process was successfully accomplished for the model cell line. This platform has the potential to be a starting point for process improvements in animal cell cultures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We express gratitude to Ministry of Science and Technology (MOST 106-2221-E-182-050), Chang Gung University (BMRP 758) and Chang Gung Memorial Hospital (CMRPD2G0282, 2H0071) for funding and supporting this research. We would also like to thank the valuable suggestion of bioreactor operation from Frank R. H. Wang, United BioPharma Inc., Hsinchu, Taiwan.
Compliance with ethical standards
Conflicts of interest
The authors would like to declare that no conflicting financial interests exist.
References
- Alcaín FJ, Löw H, Crane FL. Iron at the cell surface controls both DNA synthesis and plasma membrane redox system. Protoplasma. 1995;184:233–237. doi: 10.1007/BF01276926. [DOI] [Google Scholar]
- Chabanon G, Alves da Costa L, Farges B, Harscoat C, Chenu S, Goergen JL, Marc A, Marc I, Chevalot I. Influence of the rapeseed protein hydrolysis process on CHO cell growth. Biores Technol. 2008;99:7143–7151. doi: 10.1016/j.biortech.2007.12.070. [DOI] [PubMed] [Google Scholar]
- Chua F, Oh SKW, Yap M, Teo WK. Enhanced IgG production in eRDF media with and without serum, A comparative study. J Immunol Methods. 1994;167:109–119. doi: 10.1016/0022-1759(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Coombs MRP, Grant T, Greenshields AL, Arsenault DJ, Holbein BE, Hoskin DW. Inhibitory effect of iron withdrawal by chelation on the growth of human and murine mammary carcinoma and fibrosarcoma cells. Exp Mol Pathol. 2015;99:262–270. doi: 10.1016/j.yexmp.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Coronel J, Klausing S, Heinrich C, Noll T, Figueredo-Cardero A, Castilho LR. Valeric acid supplementation combined to mild hypothermia increases productivity in CHO cell cultivations. Biochem Eng J. 2016;114:101–109. doi: 10.1016/j.bej.2016.06.031. [DOI] [Google Scholar]
- Corrêa AL, Senna JPM, de Sousa ÁPB. Effects of passage number on growth and productivity of hybridoma secreting MRSA anti-PBP2a monoclonal antibodies. Cytotechnology. 2016;68:419–427. doi: 10.1007/s10616-014-9794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davami F, Eghbalpour F, Nematollahi L, Barkhordari F, Mahboudi F. Effects of peptone supplementation in different culture media on growth, metabolic pathway and productivity of CHO DG44 Cells; a new insight into amino acid profiles. Iran Biomed J. 2015;19:194–205. doi: 10.7508/ibj.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran M, Indumathi S, Lissa RP, Harikrishnan R, Rajkumar JS, Sudarsanam D. A comprehensive study on optimization of proliferation and differentiation potency of bone marrow derived mesenchymal stem cells under prolonged culture condition. Cytotechnology. 2013;65:187–197. doi: 10.1007/s10616-012-9471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Mandenius CF, Lübberstedt M, Urbaniak T, Nüssler AKN, Knobeloch D, Gerlach JC, Zeilinger K. Evaluation and optimization of hepatocyte culture media factors by design of experiments (DoE) methodology. Cytotechnology. 2008;57:251–261. doi: 10.1007/s10616-008-9168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SL, Santos WND, Quintella CM, Neto BB, Bosque-Sendra JM. Doehlert matrix: a chemometric tool for analytical chemistry—review. Talanta. 2004;63:1061–1067. doi: 10.1016/j.talanta.2004.01.015. [DOI] [PubMed] [Google Scholar]
- García Münzer DG, Ivarsson M, Usaku C, Habicher T, Soos M, Morbidelli M, Pistikopoulos EN, Mantalaris A. An unstructured model of metabolic and temperature dependent cell cycle arrest in hybridoma batch and fed-batch cultures. Biochem Eng J. 2015;93:260–273. doi: 10.1016/j.bej.2014.10.013. [DOI] [Google Scholar]
- Knöspel F, Schindler RK, Lübberstedt M, Petzolt S, Gerlach JC, Zeilinger K. Optimization of a serum-free culture medium for mouse embryonic stem cells using design of experiments (DoE) methodology. Cytotechnology. 2010;62:557–571. doi: 10.1007/s10616-010-9307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovár J. Hybridoma cultivation in defined serum-free media: growth-supporting substances. V. Trace elements. Folia Biol. 1988;34:35–41. [PubMed] [Google Scholar]
- Lee J, Tscheliessnig A, Chen A, Lee YY, Adduci G, Choo A, Jungbauer A. Adaptation of hybridomas to protein-free media results in a simplified two-step immunoglobulin M purification process. J Chromatogr A. 2009;1216:2683–2688. doi: 10.1016/j.chroma.2008.10.067. [DOI] [PubMed] [Google Scholar]
- Liu CH, Chang TY. Rational development of serum-free medium for Chinese hamster ovary cells. Process Biochem. 2006;41:2314–2319. doi: 10.1016/j.procbio.2006.06.008. [DOI] [Google Scholar]
- Liu YF, Tang RH, Zhang QX, Shi JY, Li XM, Liu ZQ, Zhao W. Stimulation of cell growth of Tetrahymena pyriformis and Chlamydomonas reinhardtii by trace elements. Biol Trace Elem Res. 1986;9:89–99. doi: 10.1007/BF02916518. [DOI] [Google Scholar]
- Manna L, Febo TD, Armillotta G, Luciani M, Ciarelli A, Salini R, Ventura MD. Production of monoclonal antibodies in serum-free media. Monoclon Antib Immunodiagn Immunother. 2015;34:278–288. doi: 10.1089/mab.2015.0004. [DOI] [PubMed] [Google Scholar]
- McGillicuddy N, Floris P, Albrecht S, Bones J. Examining the sources of variability in cell culture media used for biopharmaceutical production. Biotechnol Lett. 2018;40:5–21. doi: 10.1007/s10529-017-2437-8. [DOI] [PubMed] [Google Scholar]
- Nagira K, Hara T, Hayashida M, Osada K, Shiga M, Sasamoto K, Kina K, Murakami H. Development of a protein-free medium with iron salts replacing transferrin for a human–human hybridoma. Biosci Biotechnol Biochem. 1995;59:743–745. doi: 10.1271/bbb.59.743. [DOI] [PubMed] [Google Scholar]
- Price PJ. Best practices for media selection for mammalian cells. Vitro Cell Dev Biol Anim. 2017;53:673–681. doi: 10.1007/s11626-017-0186-6. [DOI] [PubMed] [Google Scholar]
- Reimer CB, Phillips DJ, Aloisio CH, Moore DD, Galland GG, Wells TW, Black CM, McDougal JS. Evaluation of thirty-one mouse monoclonal antibodies to human IgG epitopes. Hybridoma. 1984;3:263–275. doi: 10.1089/hyb.1984.3.263. [DOI] [PubMed] [Google Scholar]
- Sen S, Roychoudhury PK. Development of optimal medium for production of commercially important monoclonal antibody 520C9 by hybridoma cell. Cytotechnology. 2013;65:233–252. doi: 10.1007/s10616-012-9480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AA, Thömmes J. Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol. 2010;28:253–261. doi: 10.1016/j.tibtech.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Souza AS, Santos WNLD, Ferreira SLC. Application of Box–Behnken design in the optimisation of an on-line pre-concentration system using knotted reactor for cadmium determination by flame atomic absorption spectrometry. Spectrochim Acta Part B. 2005;60:737–742. doi: 10.1016/j.sab.2005.02.007. [DOI] [Google Scholar]
- Spens E, Häggström L. Defined protein-free NS0 myeloma cell cultures: stimulation of proliferation by conditioned medium factors. Biotechnol Prog. 2005;21:87–95. doi: 10.1021/bp049822g. [DOI] [PubMed] [Google Scholar]
- Tan KY, Teo KL, Lim JFY, Chen AKL, Reuveny S, Oh SKW. Serum-free media formulations are cell line-specific and require optimization for microcarrier culture. Cytotherapy. 2015;17:1152–1165. doi: 10.1016/j.jcyt.2015.05.001. [DOI] [PubMed] [Google Scholar]
- van der Valk J, Brunner D, De Smet K, Fex Svenningsen Å, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML, Gstraunthaler G. Optimization of chemically defined cell culture media—replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro. 2010;24:1053–1063. doi: 10.1016/j.tiv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Xing Z, Kenty B, Koyrakh I, Borys M, Pan SH, Li ZJ. Optimizing amino acid composition of CHO cell culture media for a fusion protein production. Process Biochem. 2011;46:1423–1429. doi: 10.1016/j.procbio.2011.03.014. [DOI] [Google Scholar]
- Yao T, Asayama Y. Animal-cell culture media: history, characteristics, and current issues. Reprod Med Biol. 2017;16:99–117. doi: 10.1002/rmb2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolmeh M, Jafari SM. Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 2017;10:413–433. doi: 10.1007/s11947-016-1855-2. [DOI] [Google Scholar]
- Zhang Y, Zhou Y, Yu J. Effects of peptone on hybridoma growth and monoclonal antibody formation. Cytotechnology. 1994;16:147–150. doi: 10.1007/BF00749901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.