Abstract
Medulloblastoma (MB) is the most common malignant childhood brain tumor. MB is currently classified into four molecular subgroups (Wnt, Shh, Group 3, and Group 4). The wingless (Wnt) pathway is responsible for embryonic development and is deregulated in MB. We analyzed the activation of the Wnt pathway in MB cell lines and its correlation with the Shh pathway, with emphasis on the importance of cellular characterization. Transient β-catenin transfection led to an increase in the β-catenin gene and protein expression in MB cell lines. Wnt pathway activation resulted in a reduced number of colonies in all cell lines studied and a significant increase in the G2/M cell cycle phase only in ONS-76 cells. Regarding the Shh pathway, transfection caused a reduced expression of the PTCH1 and SMO genes only in the UW473 cells. Further studies are needed to understand the mechanism underlying the molecular events associated with the effects of Wnt activation in MB.
Keywords: Medulloblatoma, Wnt pathway, β-Catenin
Introduction
Medulloblastoma (MB) is the most common malignant childhood brain tumor. Current treatment includes resection of the tumor, followed by local and cranial-spinal radiation and/or chemotherapy (Marino 2005; Packer 2011). Initiation and progression of MB is associated with molecular deregulation in several signaling pathways, especially those related to embryonic development. This alteration has led to the classification of MB according to the activated molecular pathway (Klaus and Birchmeier 2008; Komiya and Habas 2008; Fuentes et al. 2015). Thus, MB has been classified into four molecular subgroups: Wnt, Shh, Group 3, and Group 4 (Northcott et al. 2011; Taylor et al. 2012). However, MB subgroups show extensive heterogeneity, and recently genome-wide DNA methylation and gene expression analysis have permitted the identification of 12 different subgroups (Cavalli et al. 2017). Among them, the Wnt subgroup is the rarest type and has the best prognosis (Northcott et al. 2011; Taylor et al. 2012).
The wingless (Wnt) pathway is activated by the binding of a Wnt-protein ligand to a Frizzled receptor (Fz) and their coreceptors (LRP5/6), forming the Wnt-Fz-LRP5/6 complex. This complex recruits the cytoplasmic phosphor protein Dishevelled (DVL), which inhibits GSK3-β enzyme function (Polakis 2000; Komiya and Habas 2008; Guessous et al. 2008; Davidson and Niehrs 2010; Kim et al. 2011). These events inhibit the β-catenin degradation complex, reducing its phosphorylation level and consequently leading to an increase in its cytoplasmic levels. When present at higher concentrations in the cytoplasm, the protein is translocated into the cell nucleus and can bind to TCF-LEF1 transcription factors (Taipale and Beachy 2001; MacDonald et al. 2009; GE and Wang 2010), activating the expression of Wnt target genes such as c-Myc and cyclin D1, involved in cell proliferation, migration and survival (Dale 1998; Marino 2005; Dutta-Simmons 2009; Kim et al. 2011).
Somatic mutations in the β-catenin gene (CTNNB1) have been observed in sporadic MB (Marino 2005; Guessous et al. 2008; Roussel and Hatten 2011). These mutations cause deregulation of the Wnt pathway by blocking β-catenin degradation. As a consequence, β-catenin levels increase in an uncontrolled manner, leading to the development of a transformed phenotype (Gilbertson and Ellison 2008; Rossi et al. 2008; Goschzik et al. 2015).
Recent studies have shown that β-catenin protein mutations are associated with positive nucleus immunophenotyping, with significant improvements in patient survival. These findings suggest that nuclear β-catenin accumulation may be a marker of favorable results in MB. Thus, Wnt activation may represent an indicator of good prognosis (Ellison et al. 2005, Salaroli et al. 2015). However, despite this evidence, the importance of Wnt pathway activation in MB initiation remains to be determined. Also, since many patients still have long-term side effects related to therapy (Guessous et al. 2008; Northcott et al. 2012; Goschzik et al. 2015), these studies could bring new insights into MB treatment.
We report here that the MB cell lines UW402, UW473 and ONS-76 do not show mutations in genes of the Wnt pathway. Activation of the Wnt pathway by transient expression of ß-catenin induced the expression of its targets genes and reduced the number of MB cell colonies. Moreover, Wnt activation affected Shh signaling in UW473 cells. Taken together, these findings show the importance of cellular characterization for the study of the effects of Wnt signaling on MB cells.
Materials and methods
Patients and samples
Six non-neoplastic cerebellum samples obtained at autopsy from patients aged 0–13 years (3 boys and 3 girls) whose deaths were due to non-cancer related causes were used as control tissues. All samples were microdissected and obtained from the University Hospital, Ribeirão Preto Medical School, University of São Paulo. The local Ethics Committee approved the study and the patients or persons responsible for them gave written informed consent to participate (protocol numbers 9374/2003, 10633/2012).
Cell culture conditions
The pediatric medulloblastoma cell lines UW402 and UW473 (kindly provided by Dr. Michael Bobola), classified as group 3 or 4 (Castro-Gamero et al. 2013) and ONS-76 (subtypes belonging to the MB-SHH subgroup (Triscott et al. 2013; Ivanov et al. 2016) (purchased from ATCC and Banco de Células do Rio de Janeiro, respectively) were cultured as previously described (Andrade et al. 2017). All cell lines were authenticated using STR profiling.
DNA extraction and Polymerase Chain Reaction (PCR)
The DNA of pediatric MB cell lines was extracted with the DNeasy Blood and Tissue kit (Qiagen) according to the manufacturer’s instructions, in order to determine mutations in the β-catenin and APC genes. Amplification of exon 3 fragments of the CTNNB1 gene (227 bp) (5′-GCTGATTTGATGGAGTTGGA-3′ (sense) and 5′-GCTACTTGTTCTTGAGTGAA-3′ (antisense)) was performed (Leal et al. 2011). Two different fragments of exon 15 of the APC gene [251pb (5′-AAGTGGTCAGCCTCAAAAGG-3′ (sense) and 5′-CTTCGCTCACAGGATCTTCAGC-3′ (antisense); and 271pb (5′-AGAATCAGCCAGGCACAAAG-3′ (sense) and 5′-GCTTGGTGGCATGGTTTGT-3′ (antisense)] were also amplified (Bougatef et al. 2008). Fragments amplified by conventional PCR were sequenced by an automated method according to manufacturer’s instructions.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the cell lines using the Trizol® reagent (Gibco BRL, Life Technologies®, Carlsbad, CA, USA). Complementary DNA (cDNA) was obtained with the High Capacity® kit (Applied Biosystems ®, Foster City, CA, USA) according to the manufacturer’s instructions. cDNA levels of genes CTNNB1 (β-catenin) (Hs00170025), c-Myc (Hs00153408_m1) and CCND1 (Hs0076555_m1) were measured using TaqMan® probes (Applied Biosystems) in the QuantStudio 12 k Flex system (Applied Biosystems). The relative expression was calculated using the 2−ΔΔCT method (Livak and Schmittgen 2001) with two internal controls, GUS (Glucuronidase) (4326320E) and HPRT (hypoxanthine guanine phosphoribosyltransferase) (4326321E). The expression levels in untreated cells or a non-neoplastic cerebellum were used as calibrators.
Lipofectamine transfection assay
Cells lines were cultured in 6-well plates at 1 × 105 cells/well and maintained in culture for 24 h. Cells were transfected with DNA of the plasmid of interest (pcDNA3-S33Y—Plasmid # 19286) (Addgene, Cambridge, MA, USA) (1 μg), a plasmid with the CTNNB1 gene (β-Catenin) with S33Y mutation (serine for tyrosine change in codon 33, the binding site for GSK3β), and the DNA of the empty vector (control) (1436 pcDNA3 Flag HA—Plasmid # 10792) (Addgene), a plasmid without the gene of interest. Plasmids were diluted in Opti-MEM using lipofectamine 2000 (Invitrogen Co., Carlsbad, CA, USA) according to manufacturer’s instructions. After 5 h, the DNA-lipofectmine mixture was replaced with appropriate medium for each cell line and the cells were cultured as previously described.
Colony-forming cell assay
MB cell lines were removed from the transfection assay and plated (5000 cells/well) with drug-free complete medium (3 ml) at 37 °C in an incubator with a 5% CO2 humidified atmosphere. Cells were treated with G418 (300 µg/ml) (Geneticin, Sigma-Aldrich Co., St. Louis, MO, USA) for 48 h and the culture medium was then replaced with a drug-free medium for an additional incubation of 10–15 days. Colonies with more than 50 cells were scored. Assays were performed in triplicate.
Western blotting
Cell lines were transfected and equal amounts of protein (20–60 μg) were size-fractionated by SDS-PAGE as described previously (Andrade et al. 2017). Proteins were immunoblotted with β-catenin antibodies (sc-7963) and with the endogenous controls anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (sc-47724) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Histone3 (#4499) (Cell Signaling Technology, Danvers, MA, USA), all diluted according to manufacturer’s instructions.
Cell cycle assay
Cells were seeded in 6-well plates at 1 × 105 cells/well and maintained in culture for 24 h. Cells were then transfected with the plasmids of interest. After 48 h, transfected cells were trypsinized and stained with propidium iodide (1 mg/mL). The cell cycle was analyzed with a Guava personal flow cytometry system (GUAVA Instruments, Hayward, CA, USA) according to the protocol provided by the manufacturer. The percentage of cells in the G0/G1, S and G2/M phases was scored using the GUAVA Cytosoft 4.2.1 version Software.
Statistical analysis
Functional assays and gene expression data were analyzed by one-way or two-way ANOVA followed by Bonferroni’s test, as appropriate, with the level of significance set at P < 0.05. Data analysis was carried out using the Geneious 8.0, ImageJ, GraphPad Prism 4.0 and SPSS 17.0 statistical software packages.
Results and discussion
Studies addressing molecular developmental pathways in MB such as Wnt still require the development of specific models, since many target genes are unique for each MB subgroup (Northcott et al. 2012). Among the major models currently available are primary cultures and established cell lines (Ivanov et al. 2016). Cell lines have the advantage of lower variability when compared to the primary culture, representing an important tool for the study of several diseases such as cancer. Nevertheless, this model needs to be used carefully. Cell lines must be well characterized before being used in several studies such as those based on specific molecular pathways. Thus, despite the broad characterization of the tumor biology of MB acquired from these methodologies, a method showing that cell lines could be useful to predict the patient’s response to treatment remains undetermined (Ivanov et al. 2016).
Here, we characterized the MB cell lines UW402, UW473 and ONS-76 by the sequencing of exon 3 of the CTNNB1 gene (Silva et al. 2013), and exon 15 of the APC gene (Bougatef et al. 2008). These genes encode important Wnt pathway proteins and those exons are well known as hotspots because of their high mutation frequency (Zakrzewska et al. 2004). Mutation in these genes results in higher β-catenin resistance to degradation, contributing to tumorigenesis (Bougatef et al. 2008; Bo et al. 2012). However, we did not identify any mutation in the exons described here in any cell line (data not shown). Thus, it is possible to infer that the Wnt pathway is not activated in the UW402, UW473 and ONS-76 cell lines and should be classified in another molecular MB subgroup.
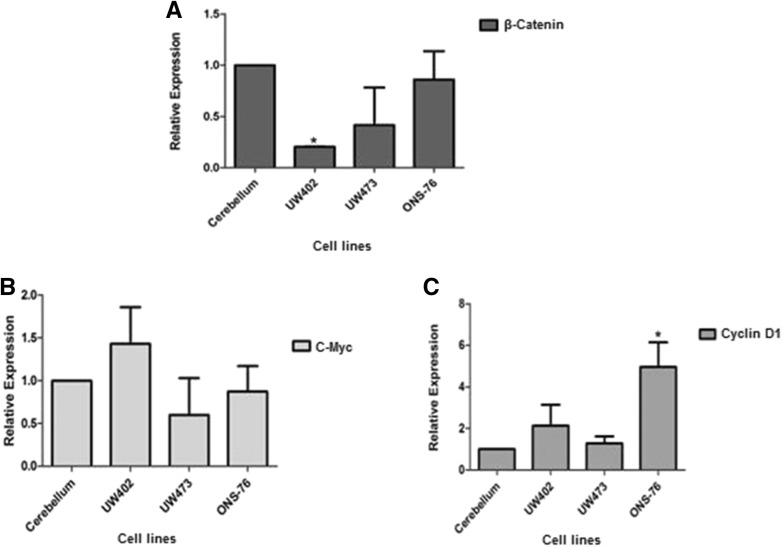
In order to confirm the sequencing results, we evaluated the gene expression of proteins involved in the Wnt pathway such as CTNNB1 (β-catenin), Cyclin D1, C-myc, in MB cell lines and compared it to their expression in non-neoplastic cerebellum (Fig. 1). We observed low expression of the CTNNB1 gene (β-catenin) in all cell lines compared to normal cerebellum, confirming the hypothesis that the Wnt pathway is not activated in any of the cell lines, in contrast to the tumors. However, the expression of the Wnt target genes Cyclin D1 and C-myc was found to be higher in the MB cell lines compared to normal cerebellum. These results may be explained by the fact that these genes might be expressed independently of β-catenin (Pogoriler et al. 2006; Diaz et al. 2015). The high β-catenin (CTNNB1) expression in non-neoplastic cerebellum was expected since this protein plays important roles in cerebellum cells such as differentiation, the formation of cellular junctions containing cadherin, and cell motility (Gilbertson and Ellison 2008; Rossi et al. 2008).
Fig. 1.
Expression of genes associated with the Wnt pathway in normal cerebellum and medulloblastoma cell lines. A β-catenin gene expression, where UW402 showed a decreased expression when compared to cerebellum (*p = 0,01); B C-Myc gene expression. C Cyclin D1 gene expression, where ONS-76 showed an increase when compared to cerebellum (*p = 0,012). All expressions are being compared to average expression in non-neoplastic cerebellum. Changes in the expression of β-catenin Cyclin D1 and c-Myc were analyzed by qRT-PCR. The reference genes GUS and HPRT were used to correct the variation in the amounts of RNA
Due to the rarity of MB type Wnt and its favorable prognosis, MB Wnt cell lines have not been immortalized and/or are not available for study (Ivanov et al. 2016). Thus, it is necessary to use techniques such as transient transfection for the activation of this pathway in available MB cell lines. Since it is not clear if the activation of the Wnt pathway may confer a less aggressive phenotype to MB, further research is needed to clarify this molecular subgroup (Salaroli et al. 2015). However, it is important to admit the limitations of this model, and the possible influence on the molecular context of other genes and pathways that are active in these cell lines.
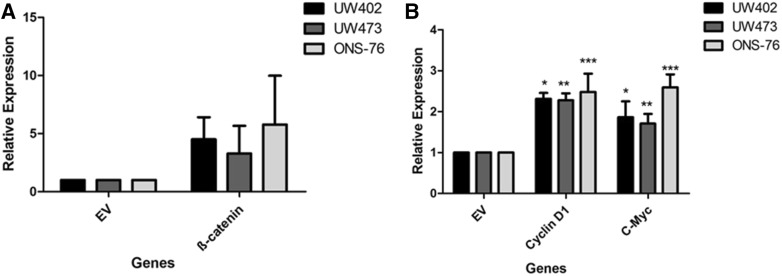
Thus, to study the effects of gene expression induction in the Wnt pathway it was necessary to induce the activation of this pathway by a transient transfection process. The specific plasmid selected was pcDNA3-S33Y, which encodes a non-degradable form of β-catenin, allowing us to evaluate biological effects under culture conditions (Kolligs et al. 1999; Salaroli et al. 2015). After transfection, the expression of β-catenin (CTNNB1) and of the Cyclin D1, C-myc genes was investigated in order to determine whether transfection had activated the Wnt pathway (Fig. 2). Cells transfected with the pcDNA3-S33Y vector showed higher expression levels of β-catenin (CTNNB1) and of its target genes Cyclin D1 and C-Myc compared to cells transfected with the empty vector. These data indicate that our transfection method was successful. Salaroli and colleagues (2015) obtained similar results and reported that transfection with the pCIneo/β-catenin S33Y vector, another plasmid that also triggers pathway activation, resulted in increased β-catenin, Cyclin D1 and C-myc expression in MB cell lines.
Fig. 2.
Effects of transfection on β-catenin, Cyclin D1 and c-Myc gene expression in medulloblastoma cell lines after 48 h. UW402, UW473 and ONS-76 cells were transfected with the vector of interest (pcDNA3-S33Y—Plasmid #19) and the empty vector (control) (1436 pcDNA3 Flag HA—Plasmid #10792) using lipofectamine. Changes in the expression in comparison with empty vector (EV) for a β-Catenin; b Cyclin D1 (*p = 0,02; **p = 0,03; ***p = 0,04) and c-Myc gene expression (*p = 0,04; **p = 0,039; ***p = 0,03) analyzed by qRT-PCR in β-catenin after transfection, all comparions are related to empty vector (EV). The reference genes GAPDH and HPRT were used to correct the variation in the amounts of RNA. The relative target gene expression was also normalized to a mean value (value = l) for control cells (empty vector). EV empty vector. *P < 0.05
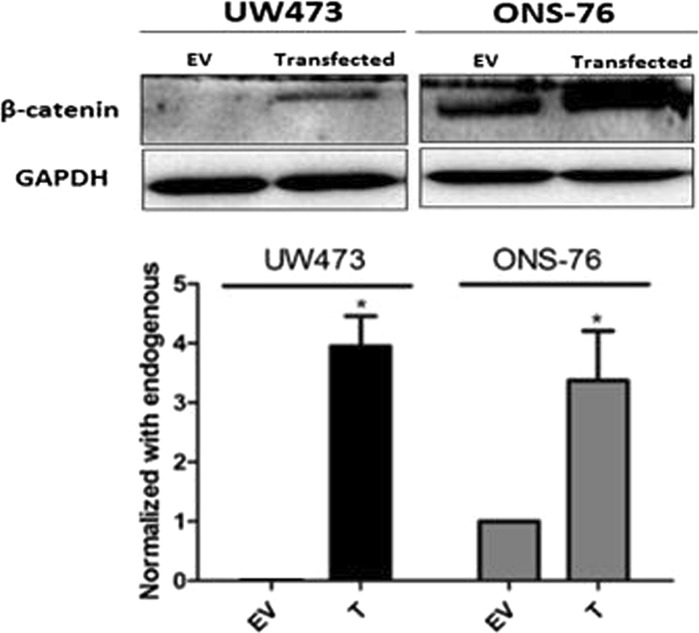
Subsequently, we investigated the effects of pcDNA3-S33Y transfection on β-catenin protein levels by western blotting (Fig. 3). Our findings showed that UW473 cells expressed higher β-catenin protein levels after transfection while ONS-76 cells, that already expressed this protein in an endogenous way, increased its expression by approximately 30% compared to control after transfection. Similar results have been reported by Salaroli et al. (2015).
Fig. 3.
Effects of transfection on β-catenin levels. Western blotting of cell lysates from UW473 and ONS-76 cells transfected with the vector of interest (pcDNA3-S33Y—Plasmid #19) and the empty vector (control) (1436 pcDNA3 Flag HA—Plasmid #10792) using lipofectamine for 48 h at the indicated concentrations. Transient transfection induced β-catenin expression in both cell lines compared to control, and showed an increase (*p = 0,001 for UW473; and *p = 0,002 for ONS-76). However, in the ONS-76 cell line the control already expressed the protein endogenously. Expression of β-catenin was determined using the anti-β-catenin antibody. GAPDH was used as loading control. T transfected cell lines, EV empty vector. *P < 0.05
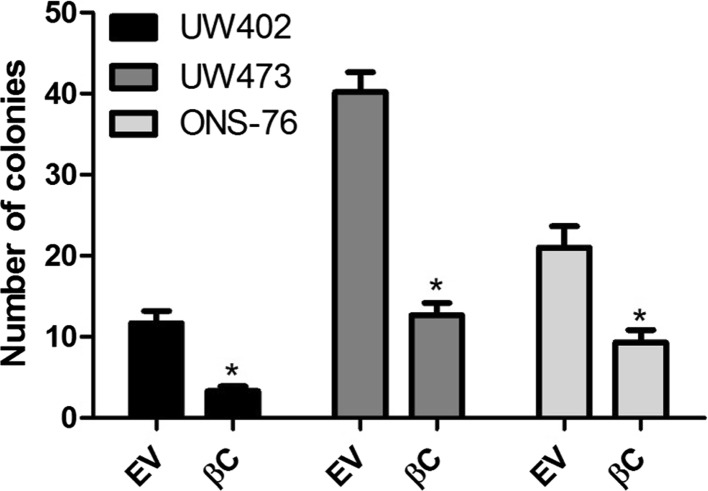
In order to analyze colony forming ability after transient transfection we performed the clonogenic assay on MB cell lines (Fig. 4). There was a reduction in colony number in cells transfected with the vector of interest (pcDNA3-S33Y) (33%, 48% and 63% for UW402, UW473 and ONS-76, respectively). In agreement with the present data, Salaroli et al. (2015) reported a reduction in the number of colonies after transfection with pCI-neo plasmid/β-catenin S33Y. These results show that the inhibition of cell proliferation after activation of Wnt in MB may be due to the cellular response to β-catenin accumulation. However, this hypothesis cannot be confirmed yet due to the absence of animal models with Wnt type MB.
Fig. 4.
Transfection with vector of interest (pcDNA-S33Y-Plasmid #19) decreased colony formation in medulloblastoma (MB) cells UW402 (*p = 0,003), UW473 (*p = 0,018); and ONS-76 (*p = 0,003) in relation to the empty vector (control) (1436 pcDNA3 Flag HA—Plasmid #10792) was performed with lipofectamine. A total of 5000 cells were plated onto 6-well plates. After 10 days, colonies greater than 50 cells were counted. Data represent the mean ± SD of three independent experiments each conducted in triplicate. βC transfected with β-catenin, EV empty vector. *P < 0.05
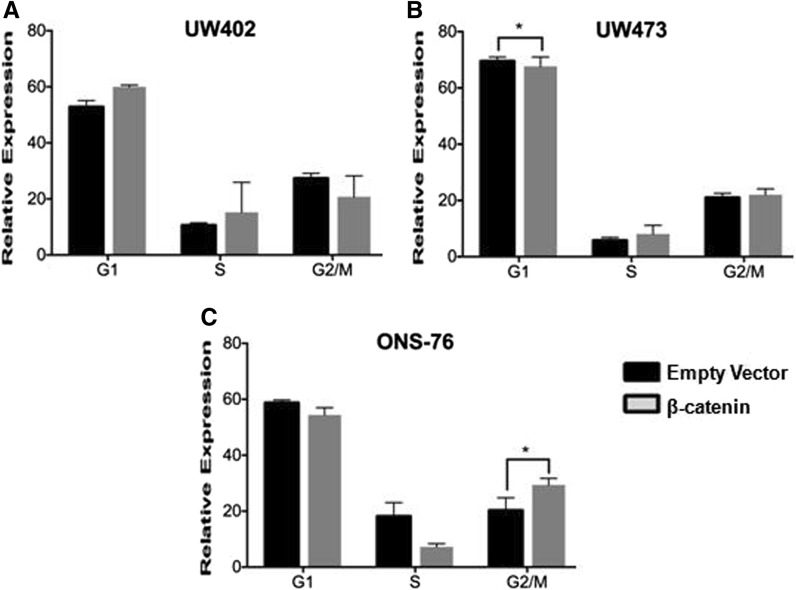
Previous studies have demonstrated that β-catenin overexpression may be linked to blockade of cell cycle progression at G2/M, which could trigger cell death (Olmeda et al. 2003; Hadjihannas et al. 2012). Thus, to determine whether pcDNA3-S33Y transfection would interfere with cycle progression, we performed a cell cycle assay with the MB cell lines (Fig. 5). We observed that overexpression of β-catenin led to a significant increase in G2/M in ONS-76 cells. However, we did not detect a significant change in cell cycle progression in the other cell lines. These results are similar to those reported in previous studies. Olmeda et al. (2003) showed that transfection with a plasmid similar to ours synchronized epithelial cells in the S-phase. High levels of β-catenin led to a maximal cell accumulation in G2/M, which triggered apoptosis. Hadjihannas et al. (2012) also showed that in colorectal cancer cells, high expression of β-catenin leads to a peak in G2/M followed by a fast decline in the G1 phase. These results could also explain the reduction of the number of colonies in the clonogenic assay, since the increase in β-catenin expression is related to the blockade of the cell cycle in G2/M, resulting in cell death (Olmeda et al. 2003).
Fig. 5.
Cell cycle analysis. Cell cycle after transfection of cell lines UW402 (a); UW473 (b) and ONS-76 (c) with the vector of interest (pcDNA3-S33Y-β-catenin) compared to control. Overexpression of β-catenin led to a significant decrease in G1 in UW473 (p = 0,03) and an increase in G2/M in ONS-76 cells (*p = 0,02) in relation to empty vector. However, we did not detect a significant change in cell cycle progression in the other cell lines
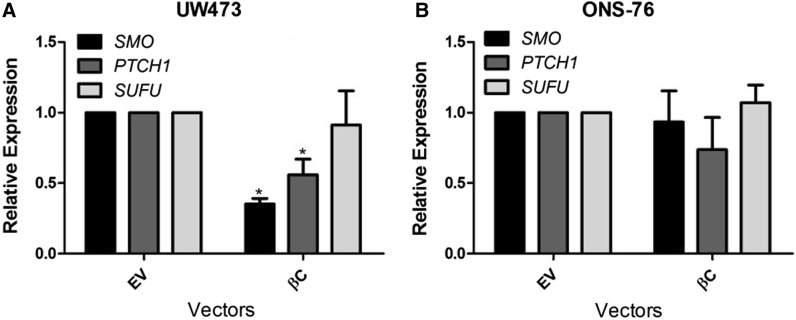
Over the last years, the initiation and progression of MB have been associated with molecular alterations including the deregulation of signaling pathways such as Sonic hedgehog (shh) and Wingless (Wnt), known for their importance during cerebellar development (Cimmino et al. 2012). Recent studies have shown that there is a cross-talk between these pathways and that they can act synergistically in the pathogenesis of MB (Baron 2003; Zinke et al. 2015). In the present study we evaluated the effect of β-catenin transfection (pcDNA3-S33Y) on the gene expression of Shh pathway members (PTCH1, SMO and SUFU) (Fig. 6). In UW473 cells, classified as group 3 or 4 (Castro-Gamero et al. 2013), transfection caused a decrease only in the expression of the PTCH1 and SMO genes. On the other hand, in ONS-76 cells, belonging to the Shh subgroup (Ivanov et al. 2016), transfection did not cause changes in the expression of any of the pathway genes. These results showed that these pathways may be acting in an antagonistic way in the UW473 line, but synergistically in the ONS-76 cell line. Different studies have shown these divergences among these pathways. Kim et al. (2010) have shown that in gastric cancer the ectopic expression of β-catenin decreases the expression of the Shh pathway genes, as shown by us in the UW473 cell line. Ma et al. (2015) have demonstrated that Shh pathway activation and Wnt pathway inactivation play an important role in cell proliferation in colon and pancreatic cancers. In addition, Zinke et al. (2015) demonstrated that in MB the stabilization of β-catenin increases its interaction with components of the Shh pathway such as Gli1, inhibiting this pathway and causing senescence of tumor cells.
Fig. 6.
Effects of transfection with the vector of interest (β-catenin) on the Shh pathway in medulloblastoma cell lines. UW473 (a) and ONS-76 (b) cells were transfected and cultured for 48 h. Changes in the expression of the SMO, PTCH1 and SUFU genes were analyzed by qRT-PCR. The reference genes GAPDH and HPRT were used to correct the variation in the amounts of RNA. The relative target gene expression was also normalized to a mean value (value = l) for the control cells (empty vector). βC transfected with β-catenin, EV empty vector. UW473 showed a decrease in SMO (*p = 0,012) and PTCH1 gene expression (p = 0,03)
Thus, our results showed that activation of the Wnt pathway through transient transfection led to an increase in β-catenin gene expression and its target genes in the three MB cell lines. In addition, transfection led to an increase in β-catenin protein expression in UW473 and ONS-76 cell lines compared to the empty vector. Wnt pathway activation resulted in the reduction of the number of colonies in the three cell lines studied and in a significant increase in the G2/M cell cycle phase only in ONS-76. Regarding the Shh pathway, transfection reduced the expression of the PTCH1 and SMO genes only in the UW473 cell line. The reasons for the distinct responses from each cell line are complex, and more studies are needed to understand the mechanism underlying the molecular events associated with these effects in MB.
Acknowledgements
We would like to thank Patrícia Vianna Bonini Palma, Camila Cristina de Oliveira Menezes Bonaldo, Daiane Fernanda dos Santos, Blood Center - USP, and Prof. Elza Tiemi Sakamoto Hojo, Departament of Genetics, Ribeirão Preto, Brazil, for their assistance with flow cytometry.
Abbreviations
- APC
Adenomatous polyposis coli gene
- CCND1
Cyclin D1 gene
- CTNNB1
β-Catenin gene
- DAOY
Desmoplastic cerebellar medulloblastoma cell line
- DVL
Dishevelled protein
- EV
Empty vector
- Fz
Frizzled receptor
- G418
Geneticin
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- Gli1
Glioma-associated oncogene homolog 1
- GSK3-β
Glycogen synthase kinase 3 beta
- GUS
Glucuronidase
- HPRT
Hypoxanthine guanine phosphoribosyltransferase
- LRP5/6
Low-density lipoprotein-related receptors 5 and 6
- MB
Medulloblastoma
- ONS-76
Primitive neuroectodermal tumor medulloblastoma cell line
- PTCH1
Protein patched homolog 1
- Shh
Sonic hedgehog pathway
- SMO
Smoothened protein
- STR
Short tandem repeat
- SUFU
Suppressor of fused homolog
- T
Transfected cell line
- TCF-LEF1
T-cell factor/lymphoid enhancer factor 1
- UW402
Pediatric medulloblastoma cell line
- UW473
Pediatric medulloblastoma cell line
- Wnt
Wingless pathway
- βC
Transfected with β-catenin
Funding
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grant Number 2014/07124-0 and 2014/20341-0). Fundação de Apoio ao Ensino, Pesquisa e Assistência (FAEPA) do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo is also acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving patient samples were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
References
- Andrade AF, Borges KS, Suazo VK, Geron L, Corrêa CA, Castro-Gamero AM, de Vasconcelos EJ, de Oliveira RS, Neder L, Yunes JA, Dos Santos AS, Scrideli CA, Tone LG. The DNA methyltransferase inhibitor zebularine exerts antitumor effects and reveals BATF2 as a poor prognostic marker for childhood medulloblastoma. Invest New Drugs. 2017;35:26–36. doi: 10.1007/s10637-016-0401-4. [DOI] [PubMed] [Google Scholar]
- Baron M. An overview of the Notch signaling pathway. Semin Cell Dev Biol. 2003;14:113–119. doi: 10.1016/S1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- Bo N, Wang D, Wu B, Chen L, Ma R. Analysis of β-catenin expression and exon 3 mutations in pediatric sporadic aggressive fibromatosis. Pediatr Dev Pathol. 2012;15:173–178. doi: 10.2350/10-07-0866-OA.1. [DOI] [PubMed] [Google Scholar]
- Bougatef K, Ouerhani S, Moussa A, Kourda N, Coulet F, Colas C, Lahely YB, Najjar T, Ben Jilani S, Benammar-Elgaaied A, Soubrier F, Marrakchi R. Prevalence of mutations in APC, CTNNB1, and BRAF in Tunisian patients with sporadic colorectal cancer. Cancer Genet Cytogenet. 2008;187:12–18. doi: 10.1016/j.cancergencyto.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Castro-Gamero AM, Borges KS, Lira RC, Andrade AF, Fedatto PF, Cruzeiro GA, Silva RB, Fontes AM, Valera ET, Bobola M, Scrideli CA, Tone LG. Chromosomal heterogeneity and instability characterize pediatric medulloblastoma cell lines and affect neoplastic phenotype. Cytotechnology. 2013;65:871–885. doi: 10.1007/s10616-012-9529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31:e6. doi: 10.1016/j.ccell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino F, Scoppettuolo MN, Carotenuto M, De Antonellis P, Dato VD, De Vita G, Zollo M. Norcantharidin impairs meduloblastoma growth by inhibition of Wnt/β-catenin signaling. J Neurooncol. 2012;106:59–70. doi: 10.1007/s11060-011-0645-y. [DOI] [PubMed] [Google Scholar]
- Dale TC. Signal transduction by the Wnt family of ligands. Biochem J. 1998;329:209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G, Niehrs C. Emerging links between CDK cell cycle regulators and Wnt signaling. Trends Cell Biol. 2010;20:453–460. doi: 10.1016/j.tcb.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Diaz RJ, Golbourn B, Faria C, Picard D, Shih D, Raynaud D, Leadly M, MacKenzie D, Bryant M, Bebenek M, Smith CA, Taylor MD, Huang A, Rutka JT. Mechanism of action and therapeutic efficacy of Aurora kinase B inhibition in MYC overexpressing medulloblastoma. Oncotarget. 2015;6:3359–3374. doi: 10.18632/oncotarget.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta-Simmons J. Aurora kinase A is a target of Wnt/β-catenin involved in multiple myeloma disease progression. Blood. 2009;114:2699–2708. doi: 10.1182/blood-2008-12-194290. [DOI] [PubMed] [Google Scholar]
- Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, Pearson AD, Clifford SC. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom children’s cancer study group brain tumour committee. J Clin Oncol. 2005;23:7951–7957. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- Fuentes RG, Arai MA, Ishibashi M. Natural compounds with Wnt signal modulating activity. Nat Prod Rep. 2015;32:1622–1628. doi: 10.1039/C5NP00074B. [DOI] [PubMed] [Google Scholar]
- GE X, Wang X. Role of Wnt canonical pathway in hematological malignancies. J Hematol Oncol. 2010;15:33. doi: 10.1186/1756-8722-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol Mech Dis. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- Goschzik T, Zur Mühlen A, Kristiansen G, Haberler C, Stefanits H, Friedrich C, von Hoff K, Rutkowski S, Pfister SM, Pietsch T. Molecular stratification of medulloblastoma: comparison of histological and genetic methods to detect Wnt activated tumours. Neuropathol Appl Neurobiol. 2015;41:135–144. doi: 10.1111/nan.12161. [DOI] [PubMed] [Google Scholar]
- Guessous F, Li Y, Abounader R. Signaling pathways in meduloblastoma. J Cell Physiol. 2008;217:577–583. doi: 10.1002/jcp.21542. [DOI] [PubMed] [Google Scholar]
- Hadjihannas MV, Bernkopf DB, Brückner M, Behrens J. Cell cycle control of Wnt/b-catenin signaling by conductin/axin2 through CDC20. EMBO Rep. 2012;13:347–354. doi: 10.1038/embor.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov DP, Coyle B, Walker DA, Grabowska AM. In vitro models of medulloblastoma: choosing the right tool for the job. J Biotechnol. 2016;236:10–25. doi: 10.1016/j.jbiotec.2016.07.028. [DOI] [PubMed] [Google Scholar]
- Kim JH, Shin HS, Lee SH, Lee I, Lee YS, Park JC, Kim YJ, Chung JB, Lee YC. Contrasting activity of Hedgehog and Wnt pathways according to gastric cancer cell differentiation: relevance of crosstalk mechanisms. Cancer Sci. 2010;101:328–335. doi: 10.1111/j.1349-7006.2009.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Thanendrarajan S, Schmidt-Wolf IG. Wnt/ß-Catenin: a new therapeutic approach to acute myeloid leukemia. Leuk Res Treat. 2011;2011:428960. doi: 10.4061/2011/428960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and câncer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt vias de transdução de sinal. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal LF, Mermejo LM, Ramalho LZ, Martinelli CE, Jr, Yunes JA, Seidinger AL, Mastellaro MJ, Cardinalli IA, Brandalise SR, Moreira AC, Tone LG, Scrideli CA, Castro M, Antonini SR. Wnt/β-Catenin pathway deregulation in childhood adrenocortical tumors. J Clin Endocrinol Metab. 2011;96:3106–3114. doi: 10.1210/jc.2011-0363. [DOI] [PubMed] [Google Scholar]
- Livak KL, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma J, Cheng J, Gong Y, TianL HQ. Downregulation of Wnt signaling by sonic hedgehog activation promotes repopulation of human tumor cell lines. Dis Model Mech. 2015;8:385–391. doi: 10.1242/dmm.018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S. Medulloblastoma: developmental mechanisms out of control. Trends Mol Med. 2005;11:17–22. doi: 10.1016/j.molmed.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, Pomeroy SL, Korshunov A, Lichter P, Taylor MD, Pfister SM. Medulloblastomas: the end of the beginning. Nat Rev Cancer. 2012;12:818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmeda D, Castel S, Vilaró S, Cano A. β-Catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol Biol Cell. 2003;14:2844–2860. doi: 10.1091/mbc.e03-01-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RJ. Risk stratification of medulloblastoma: a paradigm for future childhood brain tumor management strategies. Curr Neurol Neurosci Rep. 2011;11:124–126. doi: 10.1007/s11910-010-0168-5. [DOI] [PubMed] [Google Scholar]
- Pogoriler J, Millen K, Utset M, Du W. Loss of cyclin D1 impairs cerebellar development and suppresses medulloblastoma formation. Development. 2006;133:3929–3937. doi: 10.1242/dev.02556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and câncer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Rossi A, Caracciolo V, Russo G, Reiss K, Giordano A. Medulloblastoma: from molecular pathology to therapy. Clin Cancer Res. 2008;14:971–976. doi: 10.1158/1078-0432.CCR-07-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel MF, Hatten ME. Cerebellum: development and medulloblastoma. Curr Top Dev Biol. 2011;94:235–282. doi: 10.1016/B978-0-12-380916-2.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaroli R, Ronchi A, Buttarelli FR, Cortesi F, Marchese V, Della Bella E, Renna C, Baldi C, Giangaspero F, Cenacchi G. Wnt activation affects proliferation, invasiveness and radiosensitivity in medulloblastoma. J Neurooncol. 2015;121:119–127. doi: 10.1007/s11060-014-1621-0. [DOI] [PubMed] [Google Scholar]
- Silva R, Marie SK, Uno M, Matushita H, Wakamatsu A, Rosemberg S, Oba-Shinjo SM. CTNNB1, AXIN1 and APC expression analysis of different medulloblastoma variants. Clinics. 2013;68:167–172. doi: 10.6061/clinics/2013(02)OA08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Beachy PA. The Hedgehog and Wnt signaling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triscott J, Lee C, Foster C, Manoranjan B, Pambid MR, Berns R, Fotovati A, Venugopal C, O’Halloran K, Narendran A, Hawkins C, Ramaswamy V, Bouffet E, Taylor MD, Singhal A, Hukin J, Rassekh R, Yip S, Northcott P, Singh SK, Dunham C, Dunn SE. Personalizing the treatment of pediatric medulloblastoma: polo-like kinase 1 as a molecular target in high-risk children. Cancer Res. 2013;73:6734–6744. doi: 10.1158/0008-5472.CAN-12-4331. [DOI] [PubMed] [Google Scholar]
- Zakrzewska M, Rieske P, Debiec-Rychter M, Zakrzewski K, Polis L, Fiks T, Liberski PP. Molecular abnormalities in pediatric embryonal brain tumors–analysis of loss of heterozygosity on chromosomes 1, 5, 9, 10, 11, 16, 17 and 22. Clin Neuropathol. 2004;23:209–217. [PubMed] [Google Scholar]
- Zinke J, Schneider FT, Harter PN, Thom S, Ziegler N, Toftgård R, Plate KH, Liebner S. β-Catenin-Gli1 interaction regulates proliferation and tumor growth in medulloblastoma. Mol Cancer. 2015;3:17. doi: 10.1186/s12943-015-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]