Abstract
This work presents a pipette tip gap closure migration assay prototype tool (semi-adherent relative upsurge—s-ARU—method) to study cell migration or wound healing in semi-adherent cell lines, such as lymph node carcinoma of the prostate (LNCaP). Basically, it consists of a 6-well cover plate modification, where pipette tips with the filter are shortened and fixed vertically to the inner surface of the cover plate, with their heights adjusted to touch the bottom of the well center. This provides a barrier for the inoculated cells to grow on, creating a cell-free gap. Such a uniform gap formed can be used to study migration assay for both adherent as well as semi-adherent cells. After performing time studies, effective measurement of gap area can be carried out conveniently through image analysis software. Here, the prototype was tested for LNCaP cells, treated with testosterone and flutamide as well as with bacteriophages T4 and M13. A scratch assay using PC3 adherent cells was also performed for comparison. It was observed that s-ARU method is suitable for studying LNCaP cells migration assay, as observed from our results with testosterone, flutamide, and bacteriophages (T4 and M13). Our method is a low-cost handmade prototype, which can be an alternative to the other migration assay protocol(s) for both adherent and semi-adherent cell cultures in oncological research along with other biological research applications.
Keywords: LNCaP, Migration assay, Semi-adherent cell, Wound healing, Gap closure assay
Introduction
Cellular migration is an essential event for normal and abnormal physiological process, such as embryo development, angiogenesis, immune response, wound healing and cancer invasion and metastasis (Albini 1998; Fidler 2002; Wolf et al. 2003; Steeg 2006; Wyckoff et al. 2006; Hulkower and Herber 2011; Justus et al. 2014). Considering the importance of cell migration leading to metastasis in cancer, for example, understanding cancer cell behavior in vitro is an extremely useful tool from clinical research to drug discovery (Gupta and Massague 2006; Hulkower and Herber 2011; Kramer et al. 2013). Monolayer wound healing assays have been extensively used for studying changes in cytoskeleton rearrangement, cell-substrate adhesions and degradation, intracellular signaling pathways, nuclear reorganization, and gene expression (Savino et al. 2004; Belo et al. 2016). Also, integrins being the major family of migration promoting receptors (Ridley et al. 2003), their binding to ECM components regulates many signalling proteins involved in cell migration and invasion (Hood and Cheresh 2002). The migration assay is efficient for studying the roles of Ras superfamily of GTPases (Rho protein—Rho GTPases) in cell growth, migration, differentiation and in invasive behavior of carcinoma cells (Keely et al. 1997; Shaw et al. 1997; Allen et al. 1998; Itoh et al. 1999; Nobes and Hall 1999; Roberts et al. 1999; Banyard et al. 2000; DeVries et al. 1999; Burridge and Wennerberg, 2004). It is also reported that migration depends on activation of the ERK pathway and is independent of Ras activation (Anand-Apte et al. 1997; Heldin and Westermark 1999).
Due to its importance, many techniques have been developed for studying cellular migration and various companies have developed commercial kits or inserts for cell migration and invasion assays. The scratch assay or wound healing assay is the most commonly used non-commercial assays for various adherent cell lines, whereas Boyden chamber assay™ or Matrigel™ assay are used for studying the migration and invasion in adherent, semi-adherent and non-adherent cell lines (Kramer et al. 2013). Moreover, many commercially kits or inserts are available to ease such studies, including, Cell Comb™ Scratch Assay (Merck Kenilworth, NJ USA,), ORIS™ & ORIS™ Pro Cell Migration & Invasion Assays (Platypus Technologies, Madison, WI, USA), Radius™ Cell Migration Assays (Cell Biolabs, Inc., San Diego, CA, USA), CytoSelect™ Wound Healing Assay (Cell Biolabs, Inc., San Diego, CA, USA), Cell migration Biogel™ (Enzo Life Sciences, Inc., NY, USA), Millicell® µ-Migration Assay Kit (Merck KGaA, Darmstadt, Germany), QCM™ 24-well colorimetric cell migration assay (Millipore-Merck KGaA, Darmstadt, Germany), and IncuCyte® Scratch Wound Assay (Essen BioScience, Inc., Ann Arbor, MI, USA). All these available commercial products are useful and are being used for studying cell migration and invasion assays. However, there are very few cost-effective options available for studying the migration assays (wound healing assays), especially for semi-adherent (like LNCaP) and non-adherent cell lines.
Considering the need for an economical alternative method for studying semi-adherent cell lines as well as their importance in oncological and other biological research, we report here the developed pipette tip gap closure migration assay (s-ARU method), for studying the migration assays for the semi-adherent as well as adherent cell lines. We applied this method to our studies with LNCaP prostate cancer cell line. The LNCaP cells are androgen-dependent epithelial cells and do not produce a uniform monolayer and are slightly attached to the substrate forming clusters (http://www.lncap.com/). They are slow growing and need more incubation time to grow after sub-culturing (https://www.atcc.org/products/all/CRL-1740.aspx#characteristics). We hope that our developed low-cost handmade method will prove useful and facilitate the research activities for other adherent and semi-adherent cell lines.
Materials and methods
Cancer cell lines, bacteriophage cultures, and other prerequisites
The prostate cancer-derived LNCaP and PC-3 cell lines (androgen-independent cell) were acquired from the American Type Culture Collection (ATCC, Manassas, VA, USA). The experiments were performed according to ATCC guidelines. These cells were individually seeded at 1 × 105 cells/well in 6-well culture plates and the experiments were carried out in triplicate. In case of the PC3 cell line, cells were washed with sterile D-PBS (GIBCO/Thermo Fisher). The recommended culture medium used is Gibco™ RPMI 1640 Medium (FBS free) (GIBCO/Thermo Fisher). All procedures were performed inside a 5% CO2 incubator (Thermo Fisher, Waltham, MA, USA) under strict sterile conditions. The bacteriophage T4 and M13 were purchased from New England Biolab’s Inc (Ipswich, MA, USA). Testosterone and flutamide were purchased from MilliporeSigma (St. Louis, MO, USA).
Preparation of modified 6 well plates
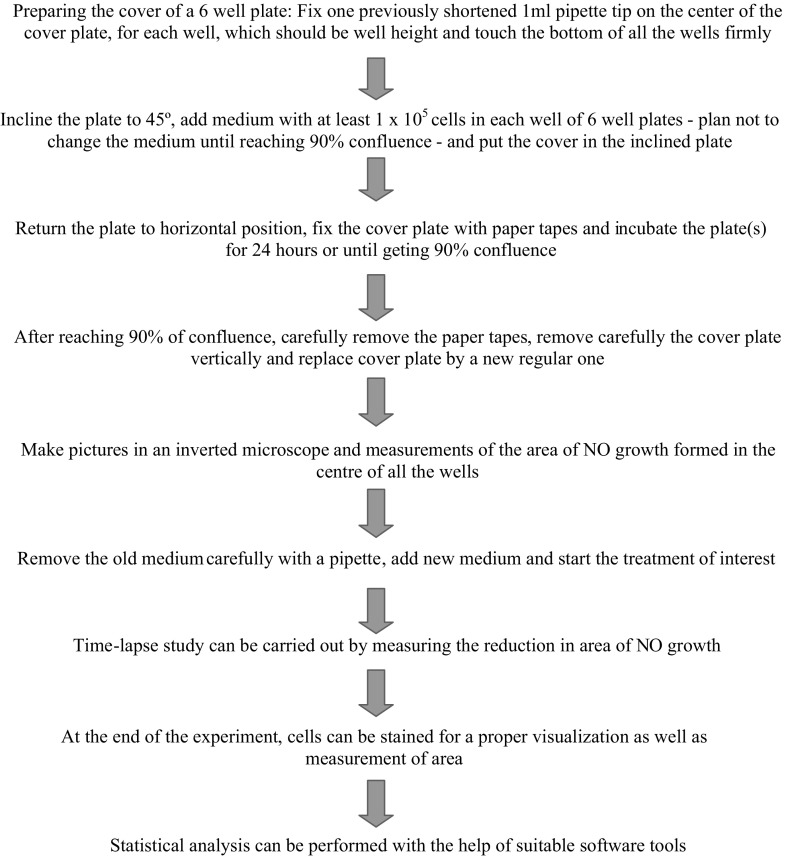
For the preparation of modified well plates, six pipette tips with filter (of 1 ml volume each) were selected and shortened equally to the height of the wells with the help of a sterile cutter (or razor blade, 70% ethanol-treated). The tips were positioned in the center of each well and a drop of silicon glue was added over the filter of the tips. The cover plate was kept over the 6 well-plate with tips positioned at the center with glue at the top of the filter. The cover plate is left to dry. It has to be made sure that all 6 pipette tips have been fixed. For 1 ml pipette tips, the filter position provides a reference for cutting tips equally and the silicon glue will adjust and correct any difference in height. The directions for plate modifications are given in Figs. 1, 2 and 3. The plates can be UV sterilized to avoid any external contamination.
Fig. 1.
Flowchart of pipette tip gap closure migration assay (s-ARU method)
Fig. 2.

Representative image of modified-6-well culture plate prepared for s-ARU method migration assay. Shortened-1 ml pipette tips were fixed in the center of each well-cover using silicone adhesive, for creating a cell-free gap in the plate
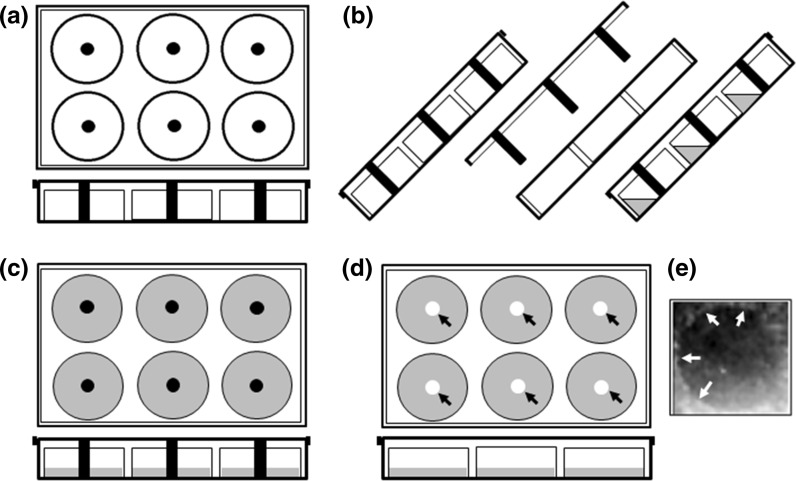
Fig. 3.
Schematic representation of modified-6-well culture plate application in the s-ARU method for migration assay using pipette tips as a barrier. a Upper and lateral view of the modified-6-well culture plate. b Lateral view of the tilted modified-6-well culture plate for application of the medium-containing cells. c Upper and lateral view of modified-6-well culture plate in horizontal position, allowing cells to attach and to reach confluence. At this moment, cover plate needs to be fixed with the help of sterile paper tape to restrict the free movement of the cover plate and preventing disturbance of the free-cell borderline. d Upper and lateral view of culture plate after cells have reached the confluence. A regular cover plate replaces the modified cover plate. The arrows point to the cell-free gap formed at the center of the wells. e Representative view of the cell-free gap formed by LNCaP cells after zone formation under normal condition. Arrows point to the borderline of the cell-free gap
Inoculation of cells to grow and to reach confluence
With the plates tilted at 45°, RPMI medium mixed with 1 × 105 LNCaP cells were inoculated in each well of the prepared plates. The modified-cover plates with fixed tips were positioned with the plates still tilted at 45°, to avoid entering cells in the cell-free gap. The plates were returned to the horizontal position and the cover plates were carefully fixed with paper tapes. The plates were gently mixed and incubated for 24 h or until getting 90% confluence at 37 °C.
Removing the modified-cover plate and measuring the cell-free area
After incubation, the plates were slowly rotated in one direction to detach loosely attached cells and then the plates were tilted to about 45° to remove the paper tapes and the modified-cover plate. The medium along with suspended detached cells were removed with the help of a pipette (do not use the suction pump as it may disturb the weakly attached monolayer due to suction).
A new medium with the supplements for treatment were added to the wells and regular cover-plates were used for further incubations.
Testing the s-ARU method assay for studying LNCaP cells migration
The s-ARU method was used to study the cell migration assay for LNCaP cells. Confluent LNCaP cells were treated for 48 h with 10 nM of testosterone, 10 μM of flutamide, 1 × 107 pfu/ml of bacteriophage T4 and M13, separately. For comparison, we also performed the scratch assay for LNCaP and PC3 cell lines. LNCaP and PC3 cells at 1 × 105 concentration were seeded in 6 well-plates. After reaching 90% confluence, scratch assay was performed for the LNCaP and PC3 cells. However, the LNCaP cells exhibited a remarkable irregular borderline after the scratch proceeding, producing significant differences in the initial gap area among the experimental groups and replicates. In this sense, it was decided not to proceed with this experiment for LNCaP cells. On the other hands, PC3 cells exhibited a uniform gap area among replicates and experimental groups and were then treated with 1 × 107 pfu/ml of bacteriophage T4 and M13, separately. After 48 h the image of the wound was digitalized and the measurements of the area were performed.
Statistical analysis
The statistical analysis was performed by using two-way ANOVA with the help of GraphPad Prism 7.04 for Windows (GraphPad Software, San Diego, CA, USA) and ImageJTM software’s (Rueden et al. 2017).
Result
The modified cover-plate with pipette tips attached was effective for creating a cell-free gap area in the center of the 6-well plate for LNCaP cells.
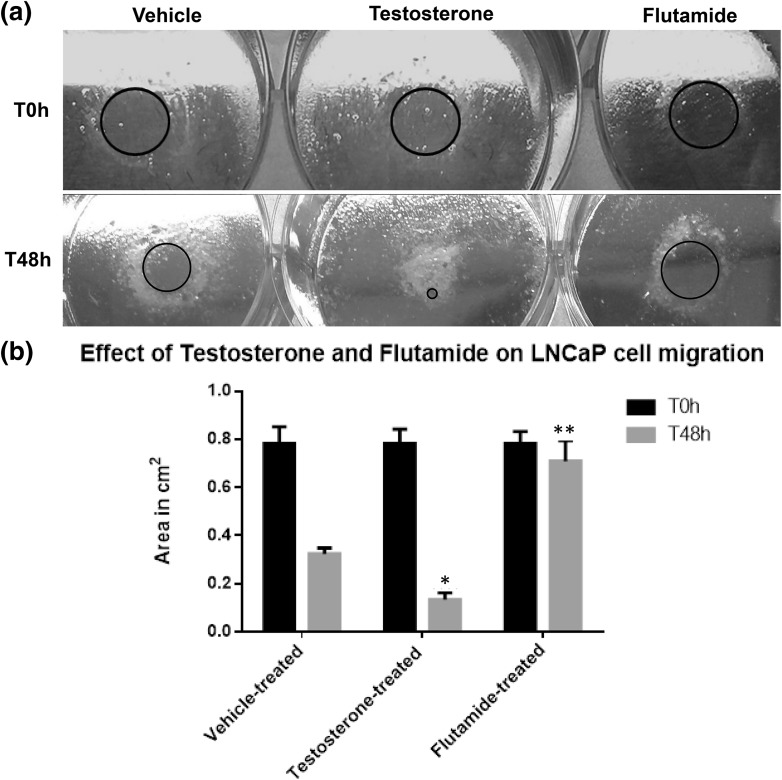
For testing and validating our s-ARU method, we evaluated the effects of testosterone and flutamide, an androgen receptor blocker, on LNCaP cells, which is an androgen-dependent prostate cancer cell line. As expected, testosterone treatment enhanced LNCaP cell growth and migration, whereas, flutamide treatment inhibited LNCaP cell growth and migration (Fig. 4).
Fig. 4.
a Representative image of LNCaP cells submitted to pipette tip assay (s-ARU method). After 24 h of plating the LNCaP cells and following removal of the pipette tip barrier, a spherical cell-free gap area could be easily identified (red circle), for all treatment, and it represents time zero hour (T0 h) or the beginning of the experiment. b Results of the measurements of the cell-free area (red circle) after 48 h (T48 h) of LNCaP cell growth and treatment with vehicle, testosterone and flutamide. The treatment with testosterone highly enhanced the growth and migration of LNCaP cell, while flutamide treatment was highly suppressive as compared with vehicle-treated cells (* means p > 0.01; ** means p > 0.001). The values are presented as the mean ± standard deviation. (Color figure online)
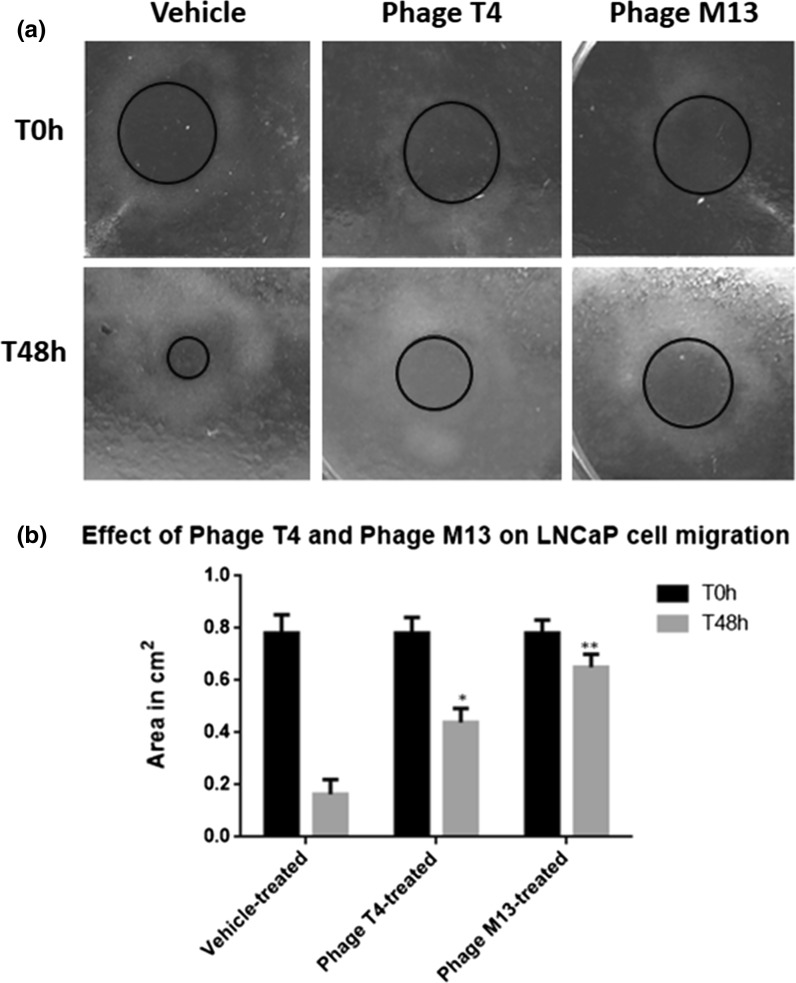
We then applied our s-ARU method in another experiment. We were interested in evaluating the effects of bacteriophage T4 and M13 on prostate cancer cells migration and proliferation, as were previously reported by Dabrowska et al. (2009) for melanoma cells and by Kurzępa-Skaradzińska et al. (2013) for leukemia cells. Here we performed a gap closure assay by exposing LNCaP cell line to two bacteriophages T4 and M13 using s-ARU method. Our method showed that bacteriophage M13 has more anti-migratory effect on LNCaP cells as compared to bacteriophage T4 (Fig. 5).
Fig. 5.
Representative image of LNCaP cells cultured using pipette tips as a barrier (s-ARU method). a After the incubation of LNCaP cells with pipette tip barrier for 24 h, the cell-free gap could be easily identified (red circle), for all treatment, and it represents time zero hour (T0 h) or the beginning of the experiment. LNCaP cells were exposed for 48 h (T48 h) to bacteriophages T4 and M13 at 1 × 107 pfu/ml concentration. b Results of the measurements of the cell-free area (red circle) after 48 h (T48 h) of LNCaP cell growth and treated with bacteriophages T4 and M13. Note that both phages reduced the rate of LNCaP cell migration, in which phage M13 (** means p < 0.001) was more effective as compared with the phage T4 (* means p < 0.01). The values are presented as the mean ± standard deviation. (Color figure online)
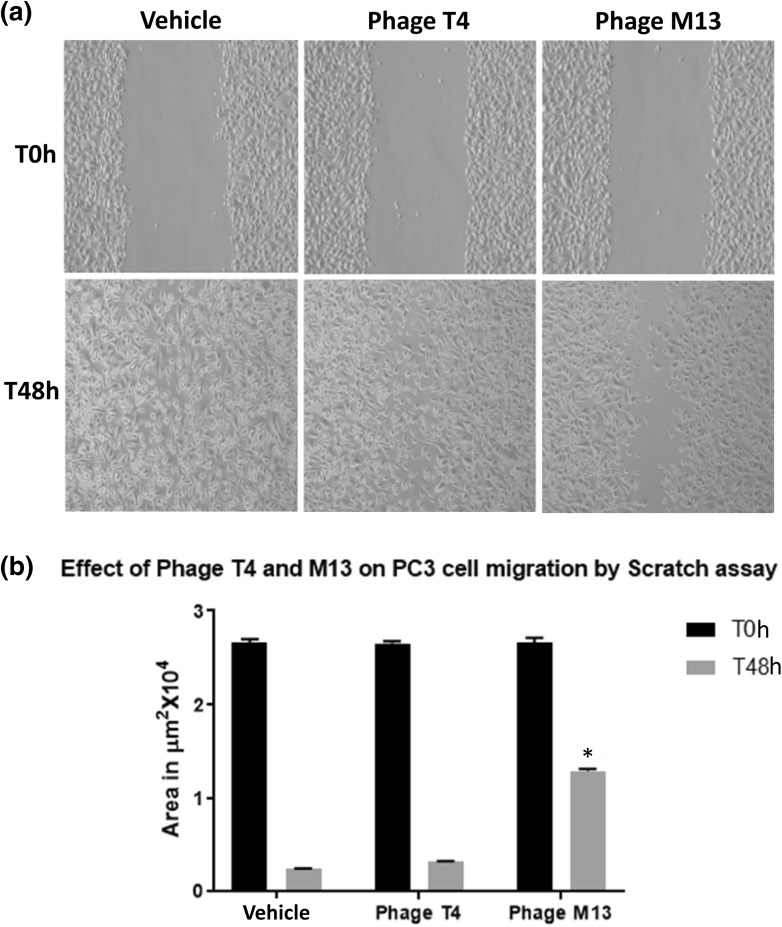
Since PC3 prostate cancer cell line is an adherent cell, for our study with bacteriophage we performed the scratch/wound healing assay. The wound healing assay performed for the PC3 cells showed that bacteriophage M13 has an anti-migratory effect on PC3 cell lines as compared to bacteriophage T4 (Fig. 6).
Fig. 6.
a Representative images of the PC3 cells migration assay by scratch assay method. After reaching confluence, a scratch was performed (T0 h) and cells were exposed to 1 × 107 pfu/ml of bacteriophages T4 and M13 per well for 48 h (T48 h). b Results of the measurements of the cell-free area after 48 h. It was observed that phage M13 was effective (* means p < 0.001) in slowing down migration rate of PC3 cells and Phage T4 showed no significant effects. The values are presented as the mean ± standard deviation
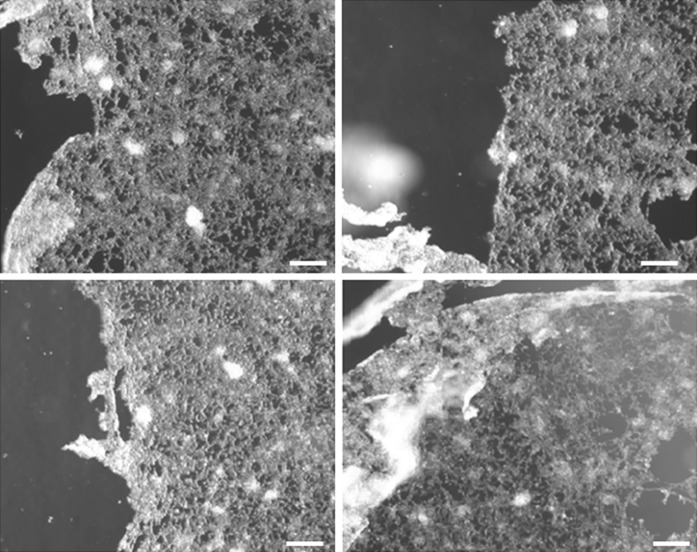
We performed a scratch assay for LNCaP cells also. As expected, LNCaP showed irregular and detached borderline and with remarkable differences among the groups and replicates, due to its semi-adherent nature (Fig. 7). This aspect did not allow comparison of the initial gap area among the groups and replicates. In this sense, our pipette tip gap closure assay (s-ARU method) seems to be an effective alternative to the scratch assay method for this kind of gap closure assay for LNCaP cell line and others semi-adherent cells.
Fig. 7.
The representative images of LNCaP cells after scratching for wound healing assay. In the scratch assay the monolayer borderline was disturbed and cells remained freely floating or loosely attached to the base of the plate, creating a non-uniform and far different gaping area among replicates and experimental groups, and hindering measurements and statistical analysis. Note typical distorted monolayer borderline (scale bar = 100 µm)
Discussion
Here, we presented a pipette tip assay (s-ARU method) to perform gap closure migration assay for semi-adherent cell lines. Our method can be applied to adherent cells lines as well, alternatively for scratching assay. However, further modifications are necessary to minimize the limitations as listed in Tables 1 and 2. Our method seems to be an effective alternative to the available cell migration assays as most of them are ineffective in carrying out such assays for the semi-adherent cell lines and are just limited to adherent cell lines (Table 3) and can be implemented for studying other semi-adherent cell lines (Kramer et al. 2013). Biotech research companies for minimizing plate construction errors and maintaining uniformity in experimental proceedings can also utilize this prototype model for developing low-cost modified cover-plate for commercial purposes.
Table 1.
Advantages and dis-advantages of pipette tip assay (s-ARU) method
| Advantages of pipette tip assay (s-ARU method) | Disadvantages of pipette tip assay (s-ARU method) |
|---|---|
| It can be used for adherent and semi-adherent cell lines | The use of pipette tips for building the modified cover plate unit is prone to human errors |
| Effective for carrying out toxicity test, drug assay and migration assay | It is time consuming (in the case of semi-adherent cells) when having multiple samples or compounds for analysis |
| It can be utilized for different microbiological and pathological applications (e.g. testing bioactive compounds against bacterial strains) | In case of semi-adherent cell lines, it is difficult to carry out time-lapse studies with the single plate and needs multiple units to be performed |
| As compared with the available methods, this method is consistent, reliable and effective | Due to improper operator handling, contamination can be a prime factor affecting final results |
| It is economical, user friendly and cost effective | Prototype is yet to be established to fulfill all the standards required for global acceptance and commercial production |
Table 2.
Troubleshooting for the pipette tip assay (s-ARU method)
| Problems | Solutions |
|---|---|
| Growth in the cell-free gap | - The pipette tip is not properly fixed in the center of the well or |
| - Pipette tip is tilted or | |
| - Pipette tip is short | |
| The cell-free area is not spherical/circular | - The cover plate is not fixed properly with paper tape or |
| - The plate was tempered or | |
| - The medium and cells have entered under the pipette tip | |
| Contamination | - The pipette tips are not sterile/non-autoclaved or |
| - The pipette tips were not processed within the hood or | |
| - The glue/fixative/adhesive used might have contaminated the medium due to over spilling or shaking | |
| The cover plate does not fix the plate properly | - The pipette tips are not homogeneously shortened or |
| - The pipette tip is not properly fixed in the center of the well | |
| Results variation | - The variation in results in mostly due to the density of the cells or |
| - Floating cells occupying the NO growth zone |
Table 3.
Comparative analysis of different migration assays with respect to pipette tip assay (s-ARU method)
| Cell migration methods/techniques/assays | Pipette tip assay (s-ARU method) | Trans-well migration assay (Boyden chamber) | Scratch Assay | Electric fence cell migration assay or ECIS | Spheroid migration assay | Capillary tube assays | Time-lapse cell tracking |
|---|---|---|---|---|---|---|---|
| Cell types supported | Adherent and semi-adherent cell cultures | Adherent, semi-adherent and non-adherent cell cultures | Adherent cell cultures | Adherent cell cultures | Adherent cell cultures | Adherent cell cultures | Adherent cell cultures |
| Measurement | Area or diameter | Number of cells | Area | Area | Migration area | Migration tube distance | Cell migration path |
| Complexity | Extremely simple | Extremely simple | Extremely simple | Simple | Simple | Simple | Simple |
| Cost | Low | High | Low | High | High | High | High |
| References | – | Kramer et al. (2013) and Poujade et al. (2007) | Kramer et al. (2013), Liang et al. (2007) and Geback et al. (2009) | Kramer et al. (2013) and Cai et al. (2000) | Kramer et al. (2013) and Knupfer et al. (2001) | Kramer et al. (2013) and Young et al. (1999) | Kramer et al. (2013) and Zantl and Horn (2011) |
Here, using the s-ARU method, we showed that bacteriophage T4 and M13 exhibited the anti-migratory effect on both PC3 and LNCaP cell lines, but bacteriophage M13 appreciably interfered in cell migration rates. The results of this study also corroborate the work of Gorski’s group, in which they have studied the effect of bacteriophages on the migration activity of melanom with the help of purified T4 and HAP1 bacteriophages (Dabrowska et al. 2009; Kurzępa-Skaradzińska et al. 2013). They have demonstrated that bacteriophages can interfere in the migration of cancer cells. It would be very interesting to explore further applications of natural and modified bacteriophages in therapeutic approaches for different types of diseases involving cell migration studies.
Our homemade pipette-tip gap closure migration assay (s-ARU method) was developed due to the difficulties associated with studying migration LNCaP cancer cell line because of its semi-adherent nature. Further improvements or progressive development involving this method are most welcome.
Acknowledgements
This article comprises part of the Ph.D. thesis of Swapnil Ganesh Sanmukh, supported by a Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Programa de Estudantes-Convênio de Pós-Graduação (PEC-PG) funding (Process Number 8963-14-2 PEC-PG 2014). SLF received a grant from the Sao Paulo Research Foundation (FAPESP) Ref. 2016/09532-3 and from CNPq Ref. 305391/2014-3. SGS would like to dedicate this work to his daughter Miss. Arundhati (ARU) Sanmukh.
Abbreviations
- s-ARU
Semi-adherent relative upsurge
- PC3
Prostate cancer cell line
- LNCaP
Lymph node carcinoma of the prostate
- ECM
Extracellular matrix
- ATCC
American Type Culture Collection
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Albini A. Tumor and endothelial cell invasion of basement membranes. The matrigel chemoinvasion assay as a tool for dissecting molecular mechanisms. Pathol Oncol Res. 1998;4:230–241. doi: 10.1007/BF02905254. [DOI] [PubMed] [Google Scholar]
- Allen WE, Zicha D, Ridley AJ, Jones GE. A role for Cdc42 in macrophage chemotaxis. J Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand-Apte B, Zetter BR, Viswanathan A, Qiu RG, Chen J, Ruggieri R, Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J Biol Chem. 1997;5:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- Banyard J, Anand-Apte B, Symons M, Zetter BR. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene. 2000;19:580–591. doi: 10.1038/sj.onc.1203338. [DOI] [PubMed] [Google Scholar]
- Belo VA, Guimarães DA, Castro MM. Matrix metalloproteinase 2 as a potential mediator of vascular smooth muscle cell migration and chronic vascular remodeling in hypertension. J Vasc Res. 2016;52:221–231. doi: 10.1159/000441621. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/S0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Cai G, Lian J, Shapiro SS, Beacham DA. Evaluation of endothelial cell migration with a novel in vitro assay system. Methods Cell Sci. 2000;22:107–114. doi: 10.1023/A:1009864613566. [DOI] [PubMed] [Google Scholar]
- Dabrowska K, Skaradziński G, Jończyk P, Kurzepa A, Wietrzyk J, Owczarek B, Zaczek M, Switała-Jeleń K, Boratyński J, Poźniak G, Maciejewska M, Górski A. The effect of bacteriophages T4 and HAP1 on in vitro melanoma migration. BMC Microbiol. 2009;9:13. doi: 10.1186/1471-2180-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries ME, Ran L, Kelvin DJ. On the edge: the physiological and pathophysiological role of chemokines during inflammatory and immunological responses. Semin Immunol. 1999;11:95–104. doi: 10.1006/smim.1999.0165. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- Geback T, Schulz MM, Koumoutsakos P, Detmar M. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques. 2009;46:265–274. doi: 10.2144/000113083. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Hulkower KI, Herber RL. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics. 2011;3:107–124. doi: 10.3390/pharmaceutics3010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat Med. 1999;5:221–225. doi: 10.1038/5587. [DOI] [PubMed] [Google Scholar]
- Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp. 2014;88:e51046. doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Racl induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- Knupfer MM, Pulzer F, Schindler I, Hernaiz Driever P, Knupfer H, Keller E. Different effects of valproic acid on proliferation and migration of malignant glioma cells in vitro. Anticancer Res. 2001;21:347–351. [PubMed] [Google Scholar]
- Kramer N, Walzl A, Unger C, Rosner M, Krupitza G, Hengstschlaeger M, Dolznig H. In vitro cell migration and invasion assays. Mutat Res. 2013;752:10–24. doi: 10.1016/j.mrrev.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Kurzępa-Skaradzińska A, Skaradziński G, Weber-Dąbrowska B, Zaczek M, Maj T, Slawek A, Switalska M, Maciejewska M, Wietrzyk J, Rymowicz W, Gorski A. Influence of bacteriophage preparations on the migration of HL-60 leukemia cells in vitro. Anticancer Res. 2013;33:1569–1574. [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, Buguin A, Silberzan P. Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci USA. 2007;104:15988–15993. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/S1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. Image J2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017;18:529–555. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W, Mendes-Da-Cruz DA, Smaniotto S, Silva-Monteiro E, Villa-Verde DM. Molecular mechanisms governing thymocyte migration: combined role of chemokines and extracellular matrix. J Leukoc Biol. 2004;75:951–961. doi: 10.1189/jlb.1003455. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/S0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;8:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor cell invasion in vivo. Curr Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Young JD, Lawrence AJ, MacLean AG, Leung BP, McInnes IB, Canas B, Pappin DJ, Stevenson RD. Thymosin beta 4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nat Med. 1999;5:1424–1427. doi: 10.1038/71002. [DOI] [PubMed] [Google Scholar]
- Zantl R, Horn E. Chemotaxis of slow migrating mammalian cells analyzed by video microscopy. Methods Mol Biol. 2011;769:191–203. doi: 10.1007/978-1-61779-207-6_13. [DOI] [PubMed] [Google Scholar]