Abstract
Recent developments in bone tissue engineering have paved the way for more efficient and cost-effective strategies. Additionally, utilization of autologous sources has been considered very desirable and is increasingly growing. Recently, activated platelet rich plasma (PRP) has been widely used in the field of bone tissue engineering, since it harbours a huge number of growth factors that can enhance osteogenesis and bone regeneration. In the present study, the osteogenic effects of PRP coated nanofibrous PES/PVA scaffolds on adipose-derived mesenchymal stem cells have been investigated. Common osteogenic markers were assayed by real time PCR. Alkaline phosphate activity, calcium deposition and Alizarin red staining assays were performed as well. The results revealed that the highest osteogenic differentiation occurred when cells were cultured on PRP coated PES/PVA scaffolds. Interestingly, direct application of PRP to culture media had no additive effects on osteogenesis of cells cultured on PRP coated PES/PVA scaffolds or those receiving typical osteogenic factors. The highest osteogenic effects were achieved by the simplest and most cost-effective method, i.e. merely by using PRP coated scaffolds. PRP coated PES/PVA scaffolds can maximally induce osteogenesis with no need for extrinsic factors. The major contribution of this paper to the current researches on bone regeneration is to suggest an easy, cost-effective approach to enhance osteogenesis via PRP coated scaffolds, with no additional external growth factors.
Keywords: Bone tissue engineering, Adipose-derived mesenchymal stem cell, PRP, Nanofibrous scaffold
Introduction
Large bone defect regeneration after tumor isolation, trauma and infection remains a challenging issue for orthopedic surgeons. Autologous bone implantation is the first choice for surgeons; and bone tissue engineering (BTE) is a promising approach that can help large bone defect regeneration. Stem cells are an incredible choice in BTE due to their capability of self-renewal and multi potency. The clinical application of embryonic stem cell is limited due to difficulties in controlling their differentiation, which can lead to Teratoma formation (Liao and Chen 2014). Mesenchymal stem cells (MSCs) from different origins in combination with distinct scaffold, materials or growth factors can be utilized for clinical conditions (Yamaguchi 2014). Mesenchymal stem cells are ideal cells since they can be harvested from multiple sources like adipose tissue, bone marrow, peripheral blood, placenta, pericyte, synovial membrane and umbilical cord (Ahmadyan and Kabiri 2017). In addition to immunomodulatory and differentiation potential of MSCs, they can express important cytokines including Epidermal Growth Factors (EGF), Vascular Endothelial Growth Factor (VEGF), Transforming Growth Factor (TGFβ) and bioactive molecules which are essential in tissue repair (Freitag et al. 2016). Adipose-derived mesenchymal stem cells (ADSCs) can differentiate into multiple mesodermal-derived tissues such as bone, cartilage and adipose. In this study, we used liposuction procedure, which is an easy method with fewer side effects (Liao and Chen 2014). ADSCs are immunosuppressive with the same surface marker and they are available in large amounts (Morcos et al. 2015). ADSCs have been clinically used in bone repair and implanted in a maxillary flap with beta- tetracalcium phosphate and bone morphogenic protein 2. In addition, ASCs have been used in fistular repair from Crohn’s disease (Kokai et al. 2014).
As appears in the literature, Growth factors are essential for tissue regeneration; and there are various methods for scaffold-based growth factor delivery. Some common methods for bone tissue engineering include making bioactive scaffolds, surface modification, biomineralization, nanoparticle encapsulation, and physical entrapment (Nyberg et al. 2015). However, single use of growth factors such as bone morphogenic proteins for bone tissue engineering has always been associated with some limitations such as high costs, necessity of high concentration, increased risk of toxicity, etc. Thus, it is imperative to find a source containing a mixture of more effective growth factors for bone regeneration (Oryan et al. 2016). Cellular changes that occur during tissue damage are regulated by platelets and growth factors (Zhang et al. 2013). PRP was first introduced as a great number of platelet which exist in a small volume of plasma and contain numerous growth factors (Fernandes and Yang 2016). PRP is acquired from peripheral blood, which could be obtained from xenogeneic, allogenic or autologous sources (Oryan et al. 2015). PRP has antibacterial effects in vitro, reduces the risk of inflammation, it is easy to work with and as an autologous agent the risk of immunogenic reactions and disease transmission is lower. Platelets consist of more than 300 biologically active molecules, which are secreted upon activation process (Fernandes and Yang 2016). There are many examples of growth factors which exist in PRP. Namely, transforming growth factor β (TGF-β), which in turn triggers osteoprogenitor cells to proliferate, insulin-like growth factor (IGF-I), which develops the late stage differentiation and osteoblast activity, and platelet-derived growth factor (PDGF). This also acts as a mitogen for connective tissue (Oryan et al. 2015). PRP has been used in a liquid or gel type alone or in combination with Alginate Hydrogels, collagen sponges and electrospun fibers (Kang et al. 2013). The addition of PRP to in vitro cultures leads to cell matrix production, boosting cell proliferation and promoting cell differentiation (Ramezanifard et al. 2017). The clinical application of PRP alone has been investigated in various medical fields for instance, orthopedics, sport medicine, plastic surgery, dermatology, oral implantology and dentistry (Anitua and Tejero 2013). Lately the application of MSCs and Platelet rich plasma (PRP) for bone defects has been improved (Tajima et al. 2015). Dental osteointegration, bone regeneration, periodontal defects, spinal fusions, distraction osteogenesis and fractures have been treated with the help of PRP in combination with MSCs (Fernandes and Yang 2016). Electrospinning of PRP alone or in combination with biodegradable polymers has been also evaluated (Díaz-gómez et al. 2015).
Incorporation of electrospun nanofiber plates is common in tissue engineering and can provide a suitable surface area for cell cultivation (Kabiri et al. 2015). High porosity and interconnected porous scaffold as well as large surface and enhanced cell growth makes nanofibers applicable for tissue regeneration (Hanaee et al. 2012). Electrospinning has shown a great potential of fabricating scaffolds with specific thickness, density and mesh size. Nanofibrous scaffold has the ability to improve cell adhesion and growth rate (Ahvaz et al. 2013). A wide range of polymers with superb biocompatibility can be constructed into nanofibers which structurally imitate the native extra cellular matrix (ECM) and they can be bio modified. Polyethersulfone (PES) is an example of a synthetic polymer which has been widely utilized for biomedical applications (Babaeijandaghi et al. 2010) and it is an aromatic polymer. It has proper mechanical strength and excellent thermal and oxidative resistance (Modesti and Boaretti 2014). PES nanofibers have been qualified for expansion and proliferation of wide range of human cell types from different tissue origins (Unger et al. 2005). It has been proven that PES nanofibers play many positive roles in in vitro osteogenesis (Ardeshirylajimi et al. 2013, 2015; Amiri et al. 2016; Pournaqi et al. 2016).
Polyvinyl alcohol (PVA) is a hydrophilic, biocompatible, nontoxic and physiologically inert polymer (Maheshwari 2013). It has many polar alcohol groups, which give rise to hydrophilicity bonds, leading to dissolution in water. Furthermore, it is an inexpensive polymer with extensive biomedical applications (Hsieh and Liau 2013) making it more desirable to be utilized.
PVA has an anabolic effect on bone formation and has been used in osteogenic studies (Song et al. 2012; Haddadi-asl 2010; Qi et al. 2013; Gomide et al. 2012).
The objective of this study was to investigate the osteogenic effects of PRP coated PES/PVA scaffolds. We hypothesized that both PRP coated scaffolds and media supplementation with PRP can lead to appreciable osteogenic differentiation of mesenchymal stem cells. In the present study, we have prepared co-electrospun PES/PVA scaffold covalently coated with PRP. Also, we have compared the effect of soluble PRP in culture medium to that stabilized on the scaffold surface in order to assess the osteogenic effects of PRP in different forms.
Materials and methods
Cell isolation and characterization
Abdominal adipose tissue was obtained from a 30-years old female by liposuction surgery after obtaining the patient’s written consent according to the ethics of Tehran University of Medical Sciences. The tissue was completely washed with PBS containing Penicillin/Streptomycin, and then incubated with collagenase type I (Sigma) for 40 min (5% CO2 at 37 °C). After neutralization and RBC lysis cells were sedimented. Culture medium contained 10% FBS, Penicillin (100U/ml)/Streptomycin (100 µg/mU) and 1% fungizone.
To characterize the stemness of the isolated cells, osteogenic differentiation (DMEM, 10% FBS, 10−7 M Dexamethasone, 10 mM β-glycerol phosphate, 50 µg/ml ascorbic acid biphosphate) and adipogenic (0.5 mM IBMX, 10−7 M dexamethasone, 66 nM insulin and 0.2 mM indomethacin) differentiation were conducted for 14 days. Surface marker proteins were also analysed using fluorescent-conjugated antibodies (CD90, 45, 34, 44).
Cells were prepared for flow cytometry analyses in accordance with Kabiri et al. (2015) and analysed on a FACS Calibur cytometer (Becton–Dickinson, San Jose, CA) with Win MDI 2.8 software.
PRP preparation
Samples of human blood were obtained (from 4 females between 30 and 40). 10 ml of blood was harvested from each donor. These samples were centrifuged at 2000 rpm, 15 min. Plasma and buffy coat layers were collected and after the next centrifugation (4000 rpm, 18 min) half of the plasma was removed. Next, platelets were activated by CaCl2 (10%) and kept at 4 °C overnight. Finally, after filtration, PRP was ready to use (Pakfar et al. 2016).
Scaffold construction and characterization
Electrospinning method was used for scaffold construction. 3.16 g of PES polymer (442.52 g/mol, BASF, Germany) was dissolved in 10 ml of DMF (Dimethylformamide) for 3 h and 0.8 g of PVA (44.00 g/mol, MERCK)was dissolved in 10 ml of hot water, then the PES solution was divided into 2 syringes. Electrospinning setting for PES was as follows: nozzle angle at 35 degree, flow rate 0.2 ml/l, high voltage 20 kv and for PVA solution was nozzle angle at 35 degree, flow rate 0.5 ml/h and high voltage 24 kv. Finally, the scaffold was placed and wrapped on the rotating collector.
Plasma surface modification
In order to boost hydrophilicity of PES/PVA scaffold, Plasma treatment was performed through DIENER electronics with settings at 40 kHz frequency, 30 W power, and 0.4 mbar gas pressures (oxygen).
PRP coated scaffold
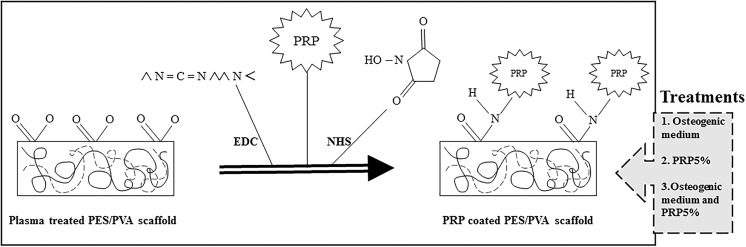
The size of scaffolds varied from 1 to 3 cm diameter circle. EDC (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide) and NHS (N-hydroxysulfosuccinimide) were used as cross linkers in order to coat PRP on the PES/PVA scaffold (Fig. 1). EDC and NHS were both dissolved in phosphate buffered saline (PBS 6 mg/ml). Next, the scaffold was coated with NHS/EDC solution and kept overnight at 4 °C. After a day, the cross linker solution was replaced with activated PRP and kept again overnight at 4 °C.
Fig. 1.
A schematic diagram of PRP coating on plasma treated PES/PVA scaffold
Tensile test
The mechanical properties of PES/PVA and PES/PVA grafted scaffold were measured using a SANTAM tensile tester (Iran). Scaffolds were cut into 3 pieces (1 × 4 cm) and were stuck on 4 × 4 squares. Thickness of scaffold was measured by a thickness gauge tool. Tensile machine was set at a gauge length of 20 mm with strain rate of 2 mm/min, and load cell of 100 N.
Scanning electron microscopy (SEM)
The morphology of the scaffolds was analyzed by scanning electron microscope (Philips). Seeded cell scaffolds were fixed with 2.5% glutaraldehyde solution for 3 h at 4 °C then dehydrated with ethanol gradient series up to 100% and dried afterwards at room temperature.
Protein release test
This test was conducted to estimate the residue of grafted PRP on scaffold in fluidal condition. One cm PRP grafted scaffolds were put in Phosphate-buffered saline. The PBS was replaced and collected for 1 week. Subsequently, they were checked for protein presence using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific).
Transform infrared spectroscopy (FTIR)
FTIR technique was conducted to investigate PRP absorbance on PES/PVA scaffold. To do so, samples were prepared in accordance with Dodel et al. (2016) and were analysed using ATR-Fourier transform infrared spectroscopy (ATRFTIR; Bruker, Germany). The process was equipped with a DTGS detector and ATR diamond crystal.
Viability of cells on PES/PVA scaffold
To evaluate the viability of cells, MTT test was performed on days 7, 14 and 21. Four thousand cells were seeded on 1 cm scaffolds. Samples were incubated (5% CO2 at 37 °C) with MTT solution for 2 h. After that they were vortexed with DMSO for 1 min. Finally, the optical density was detected and recorded by microplate reader (BioTek instruments, USA).
Cell seeding and differentiation
The scaffold sheet was sterilized by UV light (20 min each side) then cells (passage 3) were seeded on 3 × 104 cells/cm2 in 24 well culture plates (orange science Belgium) in a same culture medium: (DMEM, 10% FBS, and 1% penicillin/streptomycin). They allowed to attach and proliferate for 2 days to reach 60–80% confluency. After that plates were divided with 3 different culture medium. (1) DMEM, 10% FBS, and 1% penicillin/streptomycin. (2) PRP 5%, DMEM, 1% penicillin/streptomycin. (3) osteogenic culture medium (DMEM, FBS 10%, 1% penicillin/streptomycin, 50 μg/ml ascorbate acid, 10 mM β-glycerol phosphate, and 10−7 M dexamethasone). Studied groups and their treatments are listed in Table 1.
Table 1.
Study groups
| S | PES/PVA scaffold |
| SP | PRP coated PES/PVA scaffold |
| SP + PRP 5% | PRP coated scaffold treated with mixture of culture medium + liquid PRP |
| S + PRP 5% | scaffold treated with mixture of culture medium + liquid PRP |
| SP + DIF | PRP coated scaffold treated with osteogenic culture medium |
| S + DIF | scaffold treated with osteogenic culture medium |
Total RNA isolation and real time PCR
Total RNA was extracted from all samples with Trizol reagent (CinnaGen) and the cDNA synthesis (fermentas) was carried out. The quantitative polymerase chain reaction was performed using Platinum SYBR Green kit (Invitrogen).
Primers used are presented in Table 2. Changes in gene expression and their analysis were completed by a RT PCR analyzer (Corbett) and Rotor Gene software. Gene expression was normalized to the β2m housekeeping gene. The reaction mixture without cDNA was used as a negative control. To check the contamination and primer dimer formation, melt curve analysis was performed at the end of the reaction.
Table 2.
Primers used for real time PCR
| Gene | Upstream | Downstream |
|---|---|---|
| RUNX2 | GCC TTC AAG GTG GTA GCC C | CGT TAC CCG CCA TGA CAG TA |
| ALP | CCA CCT GCC TTA CTA ACT C | AGA CAC CCA TCC CAT CTC |
| Col I | TGG AGC AAG ACG CGA GAG | CAC CAG CAT CAC CCT TAG C |
| β2m | ATG CCT GCC GTG TGA AC | ATC TTC AAA CCT CCA TGA TG |
Calcium deposition
In order to estimate the amount of deposited calcium, calcium deposition test was performed on days 7 and 14. Scaffolds were washed with warm PBS, homogenized in 0.6 N HCL (Merck) and centrifuged at 1200 rpm for 5 min. The released calcium was determined using calcium assay kit (Pars Azmoon). The optical densities of the samples were measured using a microplate reader (BioTek instruments, USA). Finally, the data was normalized against total protein for all groups.
ALP activity
Alkaline phosphatase (ALP) activity was investigated on days 7 and 14. samples were washed with PBS and homogenized with 1 ml of Ripa buffer (Tris–HCL, Nacl, EDTA, EGTA, Triton X100, Glycero, SDS, H2O) and then sonicated (BANDELIN, Germany, maximum power, 2 min on ice). All samples were incubated with an alkaline buffer solution (Pars Azmoon) for 30 min (5% CO2, 37 °C). The Optical densities of the samples were evaluated by microplate reader (BioTek instruments, USA). Finally, the data was normalized against total protein for each group under study.
Histochemical analysis
In order to estimate the proliferation and differentiation of cells on scaffolds, Alizarin Red and H&E staining procedures were executed. Mesenchymal stem cells were seeded on 0.5 cm scaffolds following the same protocol for 14 days. After the 14th day, all cells were fixed by paraformaldehyde then sections were made and H&E stained at Jamali cytopathology Laboratory (Iran). Alizarin red staining was performed in order to evaluate calcium deposition during osteogenic differentiation.
Statistical analysis
We employed student t test to compare the mean of the groups against the mean of the controlled group. Each experiment was repeated 3 times. In addition, One-way analysis of variance (ANOVA) was used to compare results. Gene expression data were analyzed by REST 2009 software. A p value of < 0.05 was considered to be statistically significant.
Results
Characterizations of the isolated ADSCs
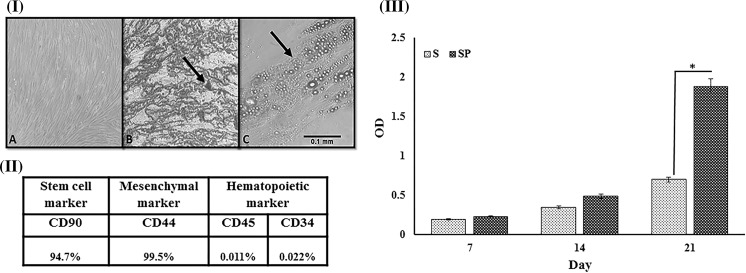
The stemness of the isolated ASCs was confirmed as the cells showed the potency to differentiate to two different lineages as presented in Fig. 2I. In addition, ASCs had positive expression for CD90 and CD44 surface markers, while being negative for hematopoietic markers CD45 and CD34 (Fig. 2II).
Fig. 2.
I. a Morphology of the isolated cells after 14 days. b Alizarin red staining visualized the presence of vesicles. c Oil red staining, brown vesicles are available all around. ×40 magnification. II Flow cytometry result of isolated adipose stem cells. III MTT assay. Cell viability in control (S), PRP coated scaffold (SP). “* p < 0.05”,**p < 0.01 and “***p < 0.001” indicate significant differences. I Scanning electron microscope analysis a PES/PVA scaffold b PRP coated PES/PVA scaffold without cells, the arrow points PRP grafted area. c PRP coated PES/PVA scaffold with seeded cells, the arrow points seeded cells. II Stability analysis of the scaffolds. PRP release during 72 h from PES/PVA scaffold measured by BCA kit. III FTIR analyses of the stability of the cross-linked PRP. PES/PVA scaffold as a control group (CNT), PRP grafted scaffold after a day from being grafted (red graph), PRP grafted scaffold after a month from being grafted in wet condition (PBS) (green graph). (Color figure online)
Construction and characterization of scaffold
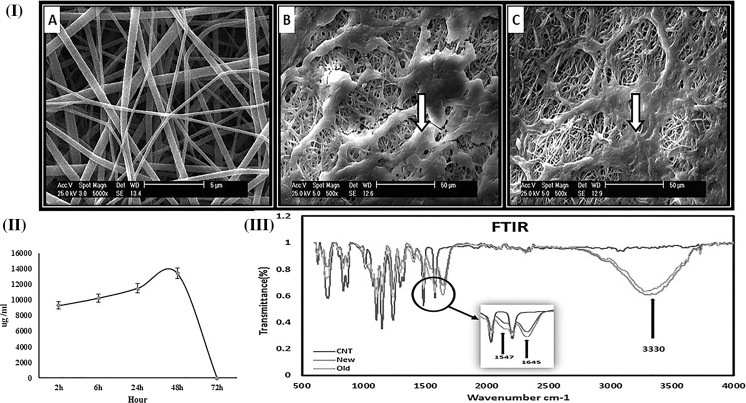
After plasma treatment of the PES/PVA scaffold, the COOH groups become present on its surface. The topography of electrospun PRP-grafted PES/PVA scaffold were demonstrated using SEM (Fig. 3I). Presence of PRP was confirmed via FTIR by monitoring the amide picks at 3330, 1645 and 1547 wavelengths (Fig. 3III).
Fig. 3.
I Scanning electron microscope analysis a PES/PVA scaffold, b PRP coated PES/PVA scaffold without cells, the arrow points PRP grafted area. c PRP coated PES/PVA scaffold with seeded cells, the arrow points seeded cells. II Stability analysis of the scaffolds. PRP release during 72 h from PES/PVA scaffold measured by BCA kit. III FTIR analyses of the stability of the cross-linked PRP. PES/PVA scaffold as a control group (CNT), PRP grafted scaffold after a day from being grafted (red graph), PRP grafted scaffold after a month from being grafted in wet condition (PBS) (green graph). (Color figure online)
Maintenance and stability of the cross linked PRP on the electrospun scaffold is presented in Fig. 3II. As it shown, PRP release reaches its maximum value after 48 h. Accordingly, after 3 days of incubation the cross-linked scaffold with culture medium, 95.57% of total protein (PRP) still remained on the scaffold.
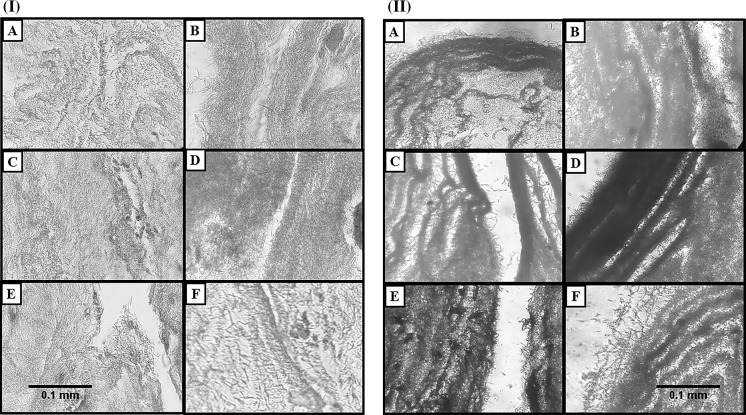
Biocompatibility of the scaffold was confirmed by MTT assay (Fig. 2III), SEM (Fig. 3I), Alizarin red staining (Fig. 4II) and H&E staining (Fig. 4I). It can be seen from Fig. 3I that PRP grafted PES/PVA scaffold is biocompatible primarily because the seeded cells proliferated after 5 days and they covered the entire surface of nanofibers.
Fig. 4.
I Cell adhesion and growth visualization by H&E staining. a Control scaffold, b PRP coated scaffold. c Scaffold treated with osteogenic medium. d PRP coated scaffold treated with osteogenic medium. e Scaffold treated with PRP 5%. f PRP coated scaffold treated with PRP 5 %. ×40 magnification. II Alizarin red staining confirms the osteogenic differentiation of all groups. a Control scaffold, b PRP coated scaffold. c Scaffold treated with osteogenic medium. d PRP coated scaffold treated with osteogenic medium. e Scaffold treated with PRP 5%, f PRP coated scaffold treated with PRP 5%
Tensile test
As shown in Table 3, mechanical properties of PRP coated PES/PVA scaffold have decreased in comparison with the control group. Furthermore, there are significant differences in ultimate tensile strength and Young’s modulus between PRP and the control group.
Table 3.
Tensile mechanical test of PRP coated PRS/PVA scaffold and the control PES/PVA scaffold without PRP
| Group | Ultimate tensile strength (MPa) | Strain break (%) | Young’s modulus (MPa) |
|---|---|---|---|
| Control | 0.7 ± 0.09 | 28/7 ± 0.06 | 0.027 ± 0.03 |
| PRP | 0.325 ± 0.025 | 28/3 ± 0.08 | 0.056 ± 0.011 |
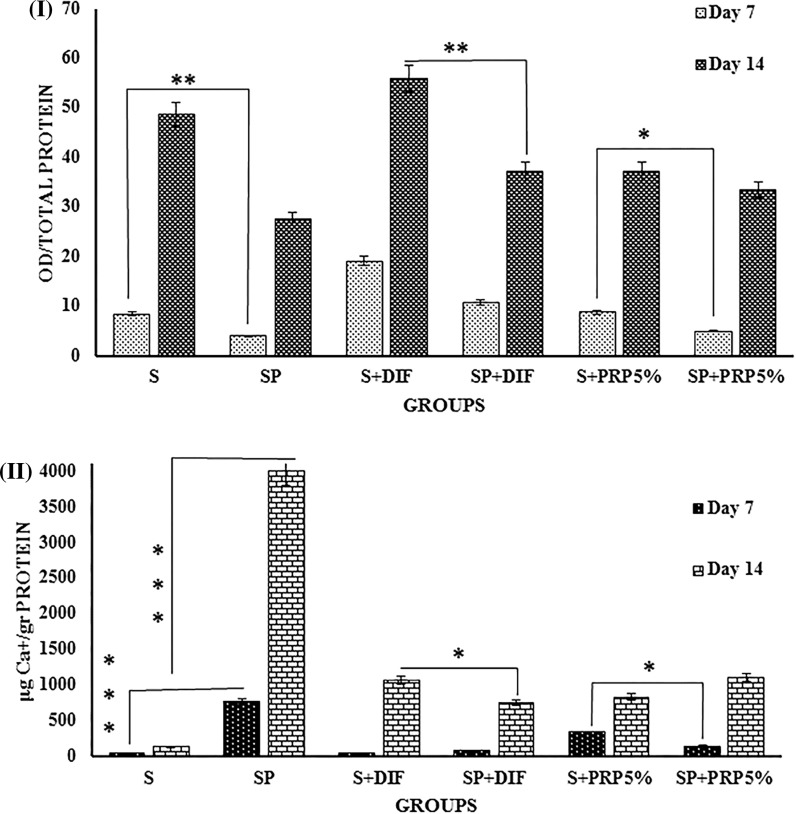
ALP activity and calcium deposition
As appears in Fig. 5I, ALP enzyme activity has shown an increasing trend in all the studied groups from days 7 to 14. The highest ALP activity was observed in scaffold group which received DIF (Osteogenic Medium) treatment. Surprisingly, the lowest response was obtained by PRP coated group. It appears that even the presence of PRP 5% in culture medium does not significantly affect the ALP enzyme activity.
Fig. 5.
I ALP activity, and II calcium content measurements. (S) Control scaffold (SP) PRP coated scaffold. (S + DIF) scaffold treated with osteogenic medium. (SP + DIF) PRP coated scaffold treated with osteogenic medium. (S + PRP 5%) scaffold treated with PRP 5% (SP + PRP 5%) PRP coated scaffold treated with PRP 5% “*p < 0.05”, **p < 0.01 and “***p < 0.001” indicate significant differences
Quantitatively, calcium was deposited in all the studied groups, and calcium deposition significantly increased from day 7 to 14 in all groups (Fig. 5II). The highest amount of deposited calcium was observed in cells seeded on PRP-coated scaffolds (Fig. 5II). In comparison with PRP coated scaffold, the combination of PRP 5% with PRP grafted scaffold lead to a decrease in the calcium deposition.
Overall, it should be noted that PRP coating on the electrospun PES/PVA scaffolds by itself significantly induced osteogenic differentiation. Therefore, this result is significant at p < 0.00 l level.
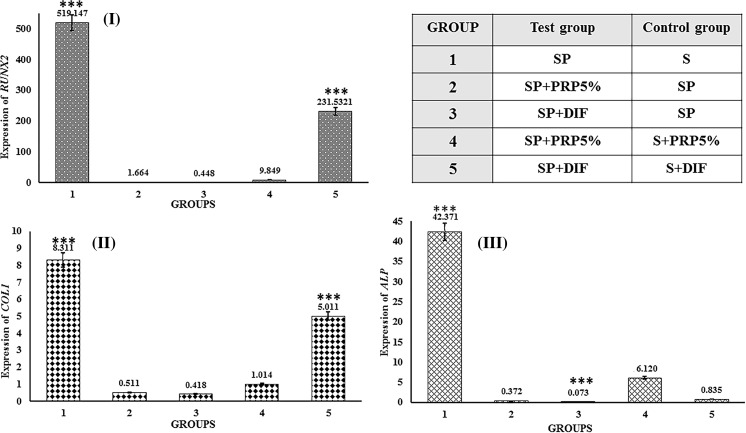
Gene expression
Figure 6 shows the expression of 3 genes on day 14. RUNX2, COL1 and ALP genes expression is essential for osteoblastic differentiation. As it appears in Fig. 6I, Runx2 was significantly up-regulated in first and fifth groups, which means that the PRP coated scaffold alone or in combination with osteogenic medium shows a higher gene expression compared to their control groups. Figure 6II demonstrates the upregulation of COL1 gene in all the groups. Interestingly, the highest amount of expression was found in first and fifth groups. Moreover, the expression of ALP gene can be seen from Fig. 6III, it shows the significant gene expression in groups one and three, which means that the expression of ALP gene of the PRP coated scaffold treated with osteogenic medium was higher than its control.
Fig. 6.
Gene expressions on day 14. I RUNX2 gene expression. II COL1 gene expression. III ALP gene expression. Control and test groups are defined above the figure. “*p < 0.05”, **p < 0.01 and “***p < 0.001” indicate significant differences
Discussion
Applying scaffold-based strategies brings a great many of advantages to bone tissue engineering (Liu et al. 2012). Many advances have been achieved by scaffold modification with various growth factors (Manuscript 2013). It is essential to find a way which utilizes cost-effective and simple materials for tissue engineering and introduce a functional and profitable method for growth factor utilization. According to a study, activated PRP 5% in combination with culture medium is a suitable concentration for adipose mesenchymal stem cells proliferation (Fibroblasts 2008). Moreover, our study shows that PRP improved cell proliferation. In this experiment, the osteogenic effects of activated PRP 5% and PRP-coated scaffold on mesenchymal stem cells was investigated. PRP-coated scaffold was a remarkable osteo-inductive factor in comparison with osteogenic medium containing PRP 5%, which supported osteogenic differentiation by itself.
Nanofibers formed by electrospinning method are porous, similar to extracellular matrix due to high surface area and high porosity (Song et al. 2012). They have acceptable mechanical properties and provide enough space for cell adhesion, infiltration and proliferation.
Studies have reported the mechanical and oxidative stability of PES, on the other hand there are challenges over its low biocompatibility (Pournaqi et al. 2016). In contrast, Polyvinyl alcohol (PVA) is a hydrophilic polymer which is soluble in water. PVA has excellent chemical resistance, high biocompatibility and biodegradability (Maheshwari et al. 2013).
Our investigation sought to remedy the problem of PES biocompatibility by combining PRP and PVA with PES polymers. Nanofibers can be bio-modified with biomolecules such as collagen (Sun et al. 2016), BMP (Schofer et al. 2011) and fibronectin (Gritsch et al. 2014). The functionality, availability and affordability of biomolecules are the main concerns of their utilization in tissue engineering. Furthermore, in this study, PRP-coated PES/PVA scaffolds were investigated for bone tissue engineering.
PRP contains TGF-β growth factor, which can promote osteogenic differentiation and regulate changes via SMAD pathway (Fibroblasts 2008). We confirmed the osteo-inductive properties of PRP by showing the calcification occurrence in the presence of ALP. PRP increases the ALP content due to increasing cell proliferation. It is worth noting that PRP stimulates anabolic metabolism, which can lead to increased ALP activity (Maheshwari et al. 2013). Under such circumstances, we preferred PRP bio modification of the scaffold.
There are different strategies for PRP application such as electrospinning, coating or adding supplements to the medium. However, coating of performed fibers with PRP is more beneficial; since coating with PRP enables the fibers to be prepared and become functionalized with PRP just before use. This will lead to reduced storage and stability challenges considering the protein nature and its functionality. Furthermore, dissolution of growth factors in organic solvents may alter the quality of PRP during electrospinning (Tobita et al. 2015). According to our investigation, PRP coating leads to higher differentiation rate compared to adding activated PRP 5% in culture medium.
According to tensile results, PRP-coated scaffolds had a lower Young’s modulus in comparison with control scaffolds, this demonstrated that the cross linker decreased the mechanical properties of the scaffolds. Furthermore, PRP interfered with polymer’s bond. The stability of the PRP cross-linked on the scaffolds was confirmed by FTIR analysis as the amide bonds remained on the scaffolds after a month in a wet condition. Additionally, the release of protein increased gradually about 5% for 72 h and after that almost no more protein release was detected. The gradual release (3 days) of the protein out of scaffolds ensured that cells did not encounter the high concentrations of the growth factors immediately given, which is beneficial as high concentrations of different factors can affect cells in a pathological manner with unknown possible outcomes.
Our data from MTT (Fig. 2III), SEM analyses (Fig. 3I), H&E staining (Fig. 4I) and Alizarin Red staining (Fig. 4II) supports the biocompatibility of the PRP-coated PES/PVA scaffolds as the cells were proliferated and covered the scaffold.
Another important finding about the expression of RUNX2, ALP and COL I genes was that the significant upregulation rate of PRP coated scaffold was higher than the other groups. This data supports the idea that PRP-coated scaffold by itself is the best choice for osteogenic differentiation without further chemical stimulation. Besides, PRP-coated scaffold group led to the highest calcium deposition in comparison to the other test groups.
This study reveals that PRP in all forms (PRP grafted scaffold, PRP 5% in culture medium, combination of PRP 5% and grafted scaffold) lead to osteogenic differentiation of ADSCs. Based on our results from calcium content and gene expression, PRP grafted scaffold has significantly (p < 0.001) improved osteogenic differentiation and the rate of its differentiation potential is higher than SP + PRP 5% group. Some studies show that a high concentration of PRP could suppress cell proliferation (Cho et al. 2011). PRP in grafted form could be a better choice because there is no force to refresh the PRP with culture media in every 2-3 days, therefore the primary amount of PRP is enough for osteogenic differentiation.
Finally, PRP coated PES/PVA is capable of osteogenic differentiation. Additionally, it is a safe and cost-effective method without dependence on adding osteogenic markers and fresh PRP in culture medium.
Conclusion
In conclusion, PRP coated PES/PVA scaffold and PRP 5% in culture medium lead to osteogenic differentiation of ADSCs. PRP in coated form showed the highest osteogenic potential. Therefore, PRP grafted PES/PVA scaffold is an appropriate bio modified scaffold for osteogenic differentiation. the reasonable method of PRP coating scaffold shows promising application in bone tissue engineering.
Acknowledgements
This work was supported financially by Stem Cell Technology Research Center.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Nasim Hayati Roodbari, Email: Hayati@srbiau.ac.ir.
Hana Hanaee-Ahvaz, Phone: +98-21-22082120, Email: Hanaee@strc.ac.ir.
References
- Ahmadyan S, Kabiri M. Osmolyte type and the osmolarity level affect chondrogenesis of mesenchymal stem cells. Appl Biochem Biotech. 2017;185:507–523. doi: 10.1007/s12010-017-2647-5. [DOI] [PubMed] [Google Scholar]
- Ahvaz HH, Mobasheri H, Bakhshandeh B, Shakhssalim N, Naji M, Dodel M, Soleimani M. Mechanical characteristics of electrospun aligned PCL/PLLA nanofibrous scaffolds conduct cell differentiation in human bladder. Tissue Eng. 2013 doi: 10.1166/jnn.2013.7193. [DOI] [PubMed] [Google Scholar]
- Amiri B, Ghollasi M, Shahrousvand M, Kamali M, Salimi A (2016) Osteoblast differentiation of mesenchymal stem cells on modi fi ed PES-PEG electrospun fi brous composites loaded with Zn 2 SiO 4 bioceramic nanoparticles. Differentiation 1–11. 10.1016/j.diff.2016.08.001 [DOI] [PubMed]
- Anitua E, Tejero R. Platelet-rich plasma to improve the bio-functionality of biomaterials. BioDrugs. 2013 doi: 10.1007/s40259-012-0004-3. [DOI] [PubMed] [Google Scholar]
- Ardeshirylajimi A, Dinarvand P, Seyedjafari E, Langroudi L, Jamshidi Adegani F, Soleimani M. Enhanced reconstruction of rat calvarial defects achieved by plasma-treated electrospun scaffolds and induced pluripotent stem cells. Cell Tissue Res. 2013;354:849–860. doi: 10.1007/s00441-013-1693-8. [DOI] [PubMed] [Google Scholar]
- Ardeshirylajimi A, Farhadian S, Adegani FJ, Mirzaei S, Zomorrod MS, Langroudi L, Doostmohammadi A, Seyedjafari E, Soleimani M. Enhanced osteoconductivity of polyethersulphone nanofibres loaded with bioactive glass nanoparticles in in vitro and in vivo models. Cell Prolif. 2015;48:455–464. doi: 10.1111/cpr.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaeijandaghi F, Shabani I, Seyedjafari E, Naraghi ZS, Vasei M, Haddadi-Asl V, Hesari KK, Soleimani M. Accelerated epidermal regeneration and improved dermal reconstruction achieved by polyethersulfone nanofibers. Tissue Eng Part A. 2010;16:3527–3536. doi: 10.1089/ten.tea.2009.0829. [DOI] [PubMed] [Google Scholar]
- Cho HS, Song IH, Park S, Sung MC, Ahn M, Song KE. KJLM individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal. Stem Cells. 2011 doi: 10.3343/kjlm.2011.31.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-gómez L, Alvarez-lorenzo C, Concheiro A, Silva M, Sheikh FA, Cantu T, Desai R, Garcia VL (2015) NIH public access 180–188. 10.1016/j.msec.2014.03.065.Biodegradable [DOI] [PMC free article] [PubMed]
- Dodel M, Nejad NH, Bahrami SH, Soleimani M. Modifying the mechanical properties of silk nanofiber scaffold by knitted orientation for regenerative medicine applications. Cell Mol Biol. 2016;62:16–25. doi: 10.14715/cmb/2016.62.10.3. [DOI] [PubMed] [Google Scholar]
- Fernandes G, Yang S. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res. 2016 doi: 10.1038/boneres.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibroblasts HD. Proliferation-promoting effect of platelet-rich plasma on human adipose–derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008 doi: 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, Tenen A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy – a review. BMC Musculoskelet. Disord. 2016 doi: 10.1186/s12891-016-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomide VS, Zonari A, Ocarino NM, Goes AM, Pereira MM. In vitro and in vivo osteogenic potential of bioactive glass—PVA hybrid scaffolds colonized by mesenchymal stem cells. Biomed Mater. 2012 doi: 10.1088/1748-6041/7/1/015004. [DOI] [PubMed] [Google Scholar]
- Gritsch K, Salles V, Attik GN. Surface entrapment of fibronectin on electrospun PLGA scaffolds for periodontal. Tissue Eng. 2014;3:117–127. doi: 10.1089/biores.2014.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadi-asl V (2010) Prepared by electrospinning ch archive 19, pp 457–468
- Hanaee H, Masoud A, Hamid S (2012) Effective combination of hydrostatic pressure and aligned nanofibrous scaffolds on human bladder smooth muscle cells: implication for bladder tissue engineering 2281–2290. 10.1007/s10856-012-4688-1 [DOI] [PubMed]
- Hsieh W, Liau J. Cell culture and characterization of cross-linked poly (vinyl alcohol) -g-starch 3D scaffold for tissue engineering. Carbohydr Polym. 2013;98:574–580. doi: 10.1016/j.carbpol.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Kabiri M, Oraee-yazdani S, Dodel M, Hanaee-ahvaz H, Soudi S, Seyedjafari E, Salehi M, Soleimani M, Cell S, Cell S, Neurosurgery F, Hospital ST, Beheshti S, Cell S. Original article : Cytocompatibility of a conductive nanofibrous carbon nanotube/poly (l-lactic acid) composite. EXCLI J. 2015;14:851–860. doi: 10.17179/excli2015-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KS, Hong JM, Kang JA, Rhie J, Jeong YH, Cho D. Regulation of osteogenic differentiation of human adipose-derived stem cells by controlling electromagnetic field conditions. Exp Mol Med. 2013;45:e6–e9. doi: 10.1038/emm.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163:399–408. doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Liao H, Chen C. Osteogenic potential: comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014;6:288–295. doi: 10.4252/wjsc.v6.i3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lim J, Teoh S. Review : development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol Adv. 2012 doi: 10.1016/j.biotechadv.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Maheshwari SU. Electrospinning of polyvinylalcohol—polycaprolactone composite scaffolds for tissue engineering applications. Polym Bull. 2013 doi: 10.1007/s00289-013-1002-4. [DOI] [Google Scholar]
- Maheshwari SU, Kumar SV, Nagiah N, Uma TS. Electrospinning of polyvinylalcohol-polycaprolactone composite scaffolds for tissue engineering applications. Polym Bull. 2013;70:2995–3010. doi: 10.1007/s00289-013-1002-4. [DOI] [Google Scholar]
- Manuscript A (2013) NIH public access 30, pp 546–554. 10.1016/j.tibtech.2012.07.005.Recent
- Modesti M, Boaretti C. Electrospun polyethersulfone nanofiber membranes. Encycl Membr. 2014 doi: 10.1007/978-3-642-40872-4. [DOI] [Google Scholar]
- Morcos MW, Al-Jallad H, Hamdy R. Comprehensive review of adipose stem cells and their implication in distraction osteogenesis and bone regeneration. BioMed Res Int. 2015 doi: 10.1155/2015/842975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg E, Holmes C, Witham T, Grayson WL. Growth factor-eluting technologies for bone tissue engineering. Drug Deliv Transl Res. 2015 doi: 10.1007/s13346-015-0233-3. [DOI] [PubMed] [Google Scholar]
- Oryan A, Alidadi S, Moshiri A. Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther. 2015 doi: 10.1517/14712598.2016.1118458. [DOI] [PubMed] [Google Scholar]
- Oryan A, Alidadi S, Moshiri A. Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther. 2016 doi: 10.1517/14712598.2016.1118458. [DOI] [PubMed] [Google Scholar]
- Pakfar A, Irani S, Hanaee-ahvaz H. Expressions of pathologic markers in PRP based chondrogenic differentiation of human adipose derived stem cells. Tissue Cell. 2016 doi: 10.1016/j.tice.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Pournaqi F, Farahmand M, Ardeshirylajimi A. Increasing biocompatibility of scaffold made of polyethersulfone (PES) through combining with polyaniline (PANI) J Paramed Sci. 2016;7:1–6. [Google Scholar]
- Qi YY, Tai ZX, Sun DF, Chen JT, Ma HB, Yan XB, Liu B, Xue QJ. Fabrication and characterization of poly(vinyl alcohol)/graphene oxide nanofibrous biocomposite scaffolds. J Appl Polym Sci. 2013;127:1885–1894. doi: 10.1002/app.37924. [DOI] [Google Scholar]
- Ramezanifard R, Kabiri M, Ahvaz HH, Tech- SC. Original article: effects of platelet rich plasma and chondrocyte co-culture on MSC chondrogenesis, hypertrophy and pathological responses. EXCLI J. 2017;16:1031–1045. doi: 10.17179/excli2017-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofer MD, Roessler PP, Schaefer J, Theisen C, Schlimme S, Heverhagen JT, Voelker M, Dersch R, Agarwal S, Fuchs S, Paletta RJ. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PLoS ONE. 2011 doi: 10.1371/journal.pone.0025462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Markel DC, Wang S, Shi T, Mao G, Ren W. Electrospun polyvinyl alcohol–collagen–hydroxyapatite nanofibers: a biomimetic extracellular matrix for osteoblastic cells. Nanotechnology. 2012;23:115101. doi: 10.1088/0957-4484/23/11/115101. [DOI] [PubMed] [Google Scholar]
- Sun H, Zhang Y, Dou L, Song X, Gu X, Fu C. Nanofiber design for human stem cell culture. Rev Adv Mater Sci. 2016;44:160–167. [Google Scholar]
- Tajima S, Tobita M, Orbay H, Hyakusoku H, Mizuno H. Direct and indirect effects of a combination of adipose-derivedstem cells and platelet-rich plasma on bone regeneration. Tissue Eng Part A. 2015;21(5–6):895–905. doi: 10.1089/ten.TEA.2014.0336. [DOI] [PubMed] [Google Scholar]
- Tobita M, Tajima S, Mizuno H. Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: stem cell transplantation methods that enhance stemness. Stem Cell Res Ther. 2015;6:215. doi: 10.1186/s13287-015-0217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RE, Peters K, Huang Q, Funk A, Paul D, Kirkpatrick CJ. Vascularization and gene regulation of human endothelial cells growing on porous polyethersulfone (PES) hollow fiber membranes. Biomaterials. 2005;26:3461–3469. doi: 10.1016/j.biomaterials.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Yamaguchi DT. “Ins” and “Outs” of mesenchymal stem cell osteogenesis in regenerative medicine. World J Stem Cells. 2014;6:94–110. doi: 10.4252/wjsc.v6.i2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wu Y, Qian S, Teng C, Chen S, Li H. Research progress in the mechanism of effect of PRP in bone deficiency healing. Sci World J. 2013 doi: 10.1155/2013/134582. [DOI] [PMC free article] [PubMed] [Google Scholar]