Abstract
Long QT syndrome (LQTS) is a hereditary ion channelopathy resulting in prolonged cardiac repolarization and abnormal prolongation of the QT interval on the electrocardiogram (ECG). The patients are likely to develop ventricular arrhythmias and sudden cardiac death. Molecular biology and basic electrophysiology studies revealed an approach to the management of patients with LQTS, which includes genotype-based risk stratification. A 16-year-old-woman with QT prolongation on ECG had frequent syncopal episodes and an attack of ventricular tachycardia followed by ventricular fibrillation. The SCN5A mutation (intravene sequence 4-1 c/t) in addition to the KCNH2 mutation (Arg56Gln) was identified. Her mother and older sister were also diagnosed as having LQTS, but had only a single mutation (KCNH2). Her older sister had an episode of syncope, but her mother did not. Genetic analysis sometimes reveals 2 or more mutations in LQTS patients with clinical phenotypes of the Romano–Ward syndrome. Compound mutations in different LQTS-related genes are likely to modify clinical characteristics. In addition, comprehensive screening of LQTS-related genes might be needed when facing family members with different clinical manifestations.
<Learning objective: Molecular biology and basic electrophysiology studies revealed an approach to the management of patients with LQTS, which includes genotype-based risk stratification. We described a case of LQTS having compound mutations of KCNH2 and SCN5A who had frequent syncopal episodes and an attack of ventricular fibrillation. The mutations of 2 different genes were associated with a severe phenotype of LQTS. Comprehensive screening of LQTS-related genes might be needed for estimating the severity of LQTS.>
Keywords: Long QT syndrome, Compound mutations, KCNH2, SCN5A
Introduction
The long QT syndrome (LQTS) is a genetic cardiac channelopathy in which most affected individuals have delayed ventricular repolarization manifested with prolongation of the corrected QT (QTc) interval on the electrocardiogram (ECG) 1, 2, 3. Clinically, this condition can lead to ventricular arrhythmias with syncope and sudden death. LQTS is caused by disturbed function of ion channel subunits or proteins. Ion channels have specific ion selectivity and allow the passage of charged ions across the cell membrane [3]. Expression of abnormal sodium, calcium, or potassium channels results in aberrant ionic fluxes that can delay ventricular repolarization, manifesting as a prolonged QT intervals [2].
Congenital LQTS is identified as the Romano–Ward syndrome (prolonged QT interval, autosomal dominant transmission) and the much rarer Jervell and Lange–Nielsen syndrome (prolonged QT interval with deafness, autosomal recessive transmission) syndromes [1]. To date, 15 subtypes, including 13 of the Romano–Ward syndrome and 2 of the Jervell and Lange–Nielsen syndrome, caused by 13 genes with >1200 mutations have been identified [3]. Mutations in KCNQ1, KCNH2, and SCN5A, underlying the LQTS type 1, 2, and 3, respectively, account for 90–95% among the LQTS cases [1]. Gene-specific clinical features have been well recognized in LQTS. In particular, these 3 genetic variants have different features, risks, and responses to therapy. Patients with LQT1 have more sensitivity to sympathetic stimulation than those with LQT2 or LQT3 [4]. The trigger of Torsade de Pointes (TdP) for patients having LQT1, LQT2, and LQT3 is exercise, startle status, and sleep/rest, respectively [4]. A beta-blocker is the first choice of pharmacological therapy in LQT1 and LQT2 patients. A class IB sodium channel blocker is effective in the LQT3 syndrome [4].
Some patients with clinical phenotypes of the Romano–Ward syndrome have multiple mutations in LQTS-related genes. Herein, we report a case of LQTS with 2 mutations, i.e. KCNH2 and SCN5A.
Case report
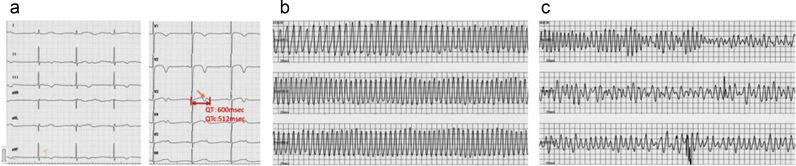
A 16-year-old female was admitted to our hospital due to frequent and severe syncopal episodes. When she was 10 years’ old, she fainted and QTc prolongation was detected on the ECG. At that time, she was diagnosed as having LQT2 syndrome on the basis of typical abnormal findings on the ECG and detection of the KCNH2 mutation (Arg56Gln). At the age of 15 years, she started to have syncopal episodes with spontaneous resolution (within 1 min). The events were provoked at the time of waking up by her alarm clock, during urination, or during conversations with her friends. The ECG showed prolonged QTc intervals (512 ms) and low-amplitude T waves with notches, which are typical for LQT2 (Fig. 1a) [3].
Fig. 1.
Electrocardiographic (ECG) findings of the patient's clinical course. (a) 12-lead ECG at rest showing a prolonged QTc interval of 512 ms and low-amplitude T waves with notches. (b) Torsades de pointes (TdP) ventricular tachycardia during syncope. (c) TdP deteriorated into ventricular fibrillation.
On admission, she had syncope with TdP ventricular tachycardia (VT) which reverted to sinus rhythm within 1 min. Although propranolol had been administered immediately, another TdP occurred and deteriorated into ventricular fibrillation (VF) (Fig. 1b and c). VF reverted to sinus rhythm within a few minutes by cardiopulmonary resuscitation. After administration of bisoprolol, verapamil, magnesium sulfate, and potassium, VT disappeared. In addition to bisoprolol and verapamil, an implantable cardioverter defibrillator (ICD) was implanted for atrial pacing and prevention of sudden death 3, 4.
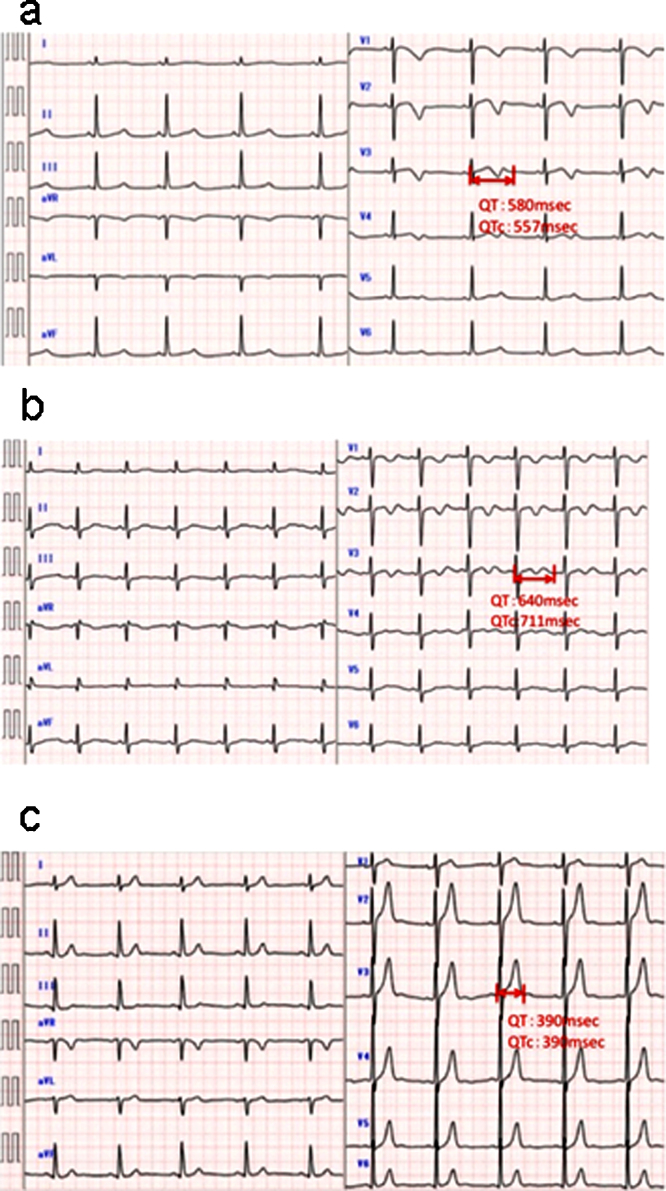
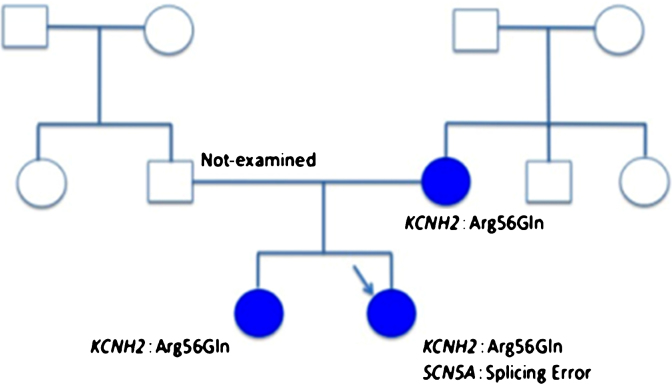
We also examined her family in detail. Her mother and her older sister had QTc prolongations, although her father had normal QTc interval (Fig. 2a–c). Her older sister had an episode of syncope at the age of 18 years, but her father and mother did not. We analyzed her family by genetic screening for gene mutations of LQTS, including KCNQ1 (LQT1), KCNH2 (LQT2), and SCN5A (LQT3), after informed consent was given. A heterozygous mutation of KCNH2 (Arg56Gln) was found in her mother and older sister. Surprisingly, the SCN5A mutation (intravene sequence (IVS) 4-1 c/t) in addition to the KCNH2 mutation (Arg56Gln) was identified in the patient (Fig. 3).
Fig. 2.
Familial electrocardiographic (ECG) findings. ECG of her sister, showing a prolonged QTc interval of 557 ms. ECG of her mother, showing a prolonged QTc interval of 711 ms. ECG of her father. QTc interval was not prolonged.
Fig. 3.
The pedigree of the patient. Arrow indicates a proband. Closed circles indicate QTc prolongation in electrocardiogram.
Discussion
Genetic analysis sometimes reveals 2 or more mutations in LQTS patients with clinical phenotypes of the Romano–Ward syndrome 5, 6, 7, 8. The prevalence of compound mutations is not rare, i.e. 4–11% of genotyped LQTS patients 5, 6, 7, 8. Patients with compound mutations are reported to have a more severe phenotype and younger age at onset than patients with a single mutation 5, 6, 7. Our patient had 2 mutations, i.e. in the KCNH2 (LQT2), and SCN5A (LQT3) genes. Her first event occurred at the age of 10 years and her clinical manifestation was prominent (electrical storm and VF) compared to her sister and her mother. These suggested that patients with compound mutations might occur earlier than those with a single mutation.
Regarding treatment, the first and second choice of pharmacological therapy in LQT1 and LQT2 patients was a beta-blocker and verapamil, respectively [4]. Mexiletine, a class IB sodium channel blocker, is effective in the LQT3 syndrome [4]. Bisoprolol was administered because our case had been considered to be a LQT2. This drug, however, was not effective, so verapamil was added to bisoprolol, resulting in the success of VT suppression. We did not use mexiletine, a first line of therapy in LQT3 patients, since her QTc interval was shortened by verapamil and bisoprolol. Some non-responders to beta-blockers among genetically diagnosed LQT1 patients have additional gene mutations [9]. It suggested that compound mutations in different LQTS-related genes might modify the response to treatment.
The KCNH2 mutation, Arg56Gln, which was found in our case, had previously been reported [10]. Mutations in KCNH2 which encodes the rapidly activating delayed rectifier K + current (IKr) channel, cause loss of the channel function, thus resulting in reduced outward current during the repolarization phase of the cardiac action potential and causing prolongation of the QTc interval 2, 3. On the other hand, the splicing error of SCN5A mutation, IVS4-1 c/t, has not been reported, so we could not exactly understand the function of this mutation. This mutation, however, is speculated to induce a splicing error, resulting in a truncated protein that might lead to gain of function of SCN5A increasing the sodium current (INa) leading to prolongation of the cardiac action potential 4, 7.
The mutations of 2 different genes in our patient were associated with a severe phenotype of LQTS. She was diagnosed as LQT2 with the KCNH2 mutation at the age of 10 years. At that time, the screening of other genes was canceled because detection of the KCNH2 mutation was regarded as sufficient. However, she was found to have the SCN5A mutation as well as the KCNH2 mutation. It might not be appropriate to stop the screening for several LQTS-associated genes, if one LQTS mutation has been identified, because this might result in underestimating the severity of LQTS. Comprehensive screening of LQTS-related genes might be needed when facing family members with different clinical manifestations.
References
- 1.Zhang L., Shaik Z., Dubner S., Riera A.R.P., Schapachnik E., Yann G., Kowey P. Long QT syndrome. Rev Argent Cardiol. 2010:27–46. [Author: Please confirm volume number] [Google Scholar]
- 2.Wilde A.A., Bezzina C.R. Genetics of cardiac arrhythmias. Heart. 2005;91:1352–1358. doi: 10.1136/hrt.2004.046334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu W., Horie M. Phenotypical manifestations of mutations in genes encoding subunits of cardiac potassium channels. Circ Res. 2011;109:97–109. doi: 10.1161/CIRCRESAHA.110.224600. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu W. The long QT syndrome: therapeutic implications of genetic diagnosis. Cardiovasc Res. 2005;67:347–356. doi: 10.1016/j.cardiores.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz P.J., Priori S.G., Napolitano C. How really rare are rare disease? The intriguing case of independent compound mutations in the long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1120–1121. doi: 10.1046/j.1540-8167.2003.03339.x. [DOI] [PubMed] [Google Scholar]
- 6.Ithoh H., Shimizu W., Hayashi K., Yamagata K., Sakaguchi T., Ohno S., Makiyama T., Akao M., Ai T., Noda T., Miyazaki A., Miyamoto Y., Yamagishi M., Kamakura S., Horie M. Long QT syndrome with compound mutations is associated with a more severe phenotype: a Japanese multicenter study. Heart Rhythm. 2010;7:1411–1418. doi: 10.1016/j.hrthm.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Tester D.J., Will M.L., Haglund C.M., Ackerman M.J. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–517. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Westenskow P., Splawski I., Timothy K.W., Keating M.T., Sanguinetti M.C. Compound mutations: a common cause of severe long-QT syndrome. Circulation. 2004;109:1834–1841. doi: 10.1161/01.CIR.0000125524.34234.13. [DOI] [PubMed] [Google Scholar]
- 9.Kobori A., Sarai N., Shimizu W., Nakamura Y., Murakami Y., Makiyama T. Additional gene variants reduce effectiveness of beta-blockers in the LQT1 form of long QT syndrome. J Cardiovasc Electrophysiol. 2004;15:190–199. doi: 10.1046/j.1540-8167.2004.03212.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen J., Zou A., Splawski I., Keating M.T., Sanguinetti M.C. Long QT syndrome-associated mutations in the Per-Arnt-Sim (PAS) domain of HERG potassium channels accelerate channel deactivation. J Biol Chem. 1999;274:10113–10118. doi: 10.1074/jbc.274.15.10113. [DOI] [PubMed] [Google Scholar]