The use of the optoelectronic properties of noble metal nanoparticles has given a new dimension to the realm of cancer diagnosis and therapy (theranostics). The multifunctional catalytic properties and surface plasmon resonance of noble metal nanoparticles (NPs) along with their facile surface chemistry gives them an edge in the field of biology, including for immunotherapeutic applications. In a previous study, we reported the anticancer efficacy of silver NPs coated with murine serum albumin against fibrosarcoma in an in vivo mouse model, highlighting their role in eliciting oxystress and immune interference. In addition to addressing the bioavailability and toxicity parameters, our study reported that there was a significant reduction in the size, as well as a delay in the incidence of tumors with AgNP-MSA treatment. Transmission electron micrographs revealed AgNP-MSA uptake by sentinel immune cells associated with the tumor along with the downregulation of pro-tumorigenic inflammatory cytokines, such as TNF-α, IL-6 and IL-1β.1
Subsequently, we explored the mechanism of action of noble metal NPs, such as silver and gold on tumor-associated immune cells. Circulating monocytes and tissue resident macrophages constitute key components of the immune response to tumor cells. Macrophage plasticity functions along a M1–M2 axis.2 Classical IFN-γ-dependent activation recruits the M1 subset of macrophages that are cytotoxic and immunoregulatory in nature, whereas others exhibiting the M2 phenotype appear to participate in neoplastic transformation at inflammatory niches, where they promote tumor sustenance by inducing tumor cell proliferation, angiogenesis, and metastasis. As the latter remains associated with the tumor microenvironment, establishing a cardinal link between inflammation and carcinogenesis, they are coined tumor-associated macrophages (TAMs) and are characterized by anti-inflammatory and immunosuppressive actions.
In this study, a two-stage carcinogenesis model was used, in which a single subcutaneous dose (25 mg kg−1 b.w.) of the chemical carcinogen 3-methylcholanthrene (MCA) at the right flank was administered, followed by multiple doses of the tumor promoter phorbol myristate-13-acetate (PMA) (4.26 nM) administered on alternate days at the opposite flank. Splenic macrophages (SMs) were isolated following the method described previously,3 and TAMs were isolated from tumor-bearing mice4 and confirmed with the macrophage-specific marker CD68. Our first experiment was to elucidate the detailed mechanism of the functional repolarization of TAMs from the M2 to M1 phenotype by the noble metal NPs (hereafter, NPs) AuNP-MSA and AgNP-MSA. Because the spleen functions as a reservoir of immune cells,5 NP-induced oxidative stress was evaluated in SMs from untreated control mice and SMs from tumor-bearing mice (TSMs), as the negative control, along with the TAMs from tumor-bearing mice. It was evident that these NPs were not only immunoreactive but also triggered oxidant-sensitive signaling events in the tumor microenvironment, inducing oxidative stress. For this, non-toxic, sublethal doses of AuNP-MSA (2 mg/kg b.w.) and AgNP-MSA (4 mg/kg b.w.)3, 6 were selected after determining their dose-dependent biodistribution, accumulation, metabolism, and clearance. These doses transcribe to a particle number of 3.72 × 1015 (per dose) and 1.07 × 1015 (per dose) daily.3
Oxidative stress in the tumor microenvironment was measured as superoxide production with the nitroblue tetrazolium (NBT) reduction assay. Protein carbonylation and reactive oxygen species (ROS) estimation were evaluated using DCFH-DA. Increased O2− levels in TAMs and TSMs after NP treatment were attributed to NADPH oxidase activation or an oxidative burst that was inhibited in the tumor microenvironment to compensate for the augmented energy consumption, followed by H2O2 production, HIF-1 accumulation, and angiogenesis (Warburg phenomenon).7 Under hypoxic conditions existing in the tumor microenvironment, NADPH oxidase-driven ROS generation in TAMs caused HIF-1 destabilization by NO donors involving the enzyme prolyl hydroxylase. Moreover, both protein carbonylation and ROS levels as measured by DCFH-DA were increased in the NP-treated TAMs, likely due to disruption of their mitochondrial ultrastructure.8 Alternatively, these particles could intrinsically induce a Fenton-type reaction9 to produce ROS. Furthermore, the NPs were also reported to produce a nitrosative stress in TAMs. TAMs promote tumors by suppressing their immunoregulatory activity, as evidenced from the depleted levels of nitric oxide (NO) release. NP treatment restores the RNS levels in TAMs, reverting them to their normal phenotype. NO itself has a short half-life and is stabilized by forming adducts with the thiol groups (RNSOs) of glutathione, forming S-nitrosoglutathione. The NPs may account for the increased NO levels in TAMs because they catalyze NO production from endogenous RNSOs via gold-thiolate and silver thiolate formation.10
Next, we evaluated the antioxidant defenses in TAMs in response to AuNP-MSA and AgNP-MSA. Although catalase levels remained unaltered, a significant difference in the superoxide dismutase (SOD) levels was reported. GSHs and protein-bound thiols were significantly increased in TAMs treated with NPs; however, these increases were marginal in TAMs compared to SMs and TSMs. This is because, under normal circumstances, cellular GSH and protein-bound thiols neutralize oxidative stress, but in the presence of NPs, such restoration is partial because the electron-rich SH groups that donate electrons with the help of polarizable sulfur atoms onto the NPs eventually become attached to their surfaces.
NP uptake by TAMs was determined by TEM, and NPs were found to be localized to the nucleus, ER, lysosomes, and mitochondria. Membrane integrity was uncompromised, which could be due to the redox state induced by the NPs because noble metal NPs have an intrinsic property of mimicking SOD and catalase in a pH-dependent manner. ROS and RNS play major roles in oxidant-dependent cell signaling as second messengers activating downstream signaling cascades that induce cell proliferation and the expression of target genes.
To evaluate oxidant-dependent cell signaling, the expression levels of TNF-α, IL-10, and IL-12 were assessed in TAMs. TNF-α, a key player in pro-invasive macrophage-tumor crosstalk, has a critical role in the switch of macrophages from the M1 to M2 phenotype and in the induction of pro-tumorigenic cytokine cascades, leading to inflammation and tumor promotion. TNF-α in the NF-кβ signaling cascade triggers matrix metalloproteinases (MMPs) and the epithelial mesenchymal transition (EMT) along with pro-migratory factors, such as vasoreactive epidermal growth factor (VEGF) and chemokines, such as CCL2 and CXCL12.
Expression of IL-12, a pro-inflammatory cytokine, is compromised in TAMs due to the autocrine action of IL-10 that promotes tumorigenesis. TAMs treated with noble metal AuNP-MSA and AgNP-MSA show lower expression levels of IL-10 and TNF-α, whereas IL-12, the cytokine that drives the cells toward the M1 phenotype, is augmented. This study elucidated the effect of these NPs on the redox state and cellular signaling in TAMs along with a pro-oxidative therapeutic approach in tumors.3
In an extension of the above work, we next used a novel metal nanoparticle comprising MSA-conjugated gold and manganese oxide (Au-Mn NP), in which manganese oxide was used to augment the magnetic resonance property when coupled with gold.6 Similar to AuNP-MSA and AgNP-MSA in the Au-Mn NP study, ROS, O2− and NO generation were studied along with the factors responsible for sustaining hypoxia in tumors, namely, HIF-1α, the pro-tumorigenic cytokines TNF-α and IL-10 and the immunoregulatory cytokine IL-12. The high levels of O2− and NO indicate that TAMs are maintained in a hypoxic microenvironment compared to SMs and TSMs. Unlike AuNP-MSA and AgNP-MSA, treatment with Au-Mn NP significantly decreased ROS and RNS levels in TAMs, although the cytokine profiles showed effects similar to those of AuNP-MSA and AgNP-MSA. This indicates that although the former exerted a pro-oxidative effect on the tumor microenvironment that modulated the TAMs from an M2 to M1 phenotype, Au-Mn NP caused a similar end result but by an alternative mechanism, in which ROS and RNS levels were significantly decreased, likely due to changes in tissue hypoxia and antioxidant functions, such as increased expression of SOD. For this, we studied the expression of HIF-1α in TAMs treated with Au-Mn NP. The HIF-1α subunit, under normoxic conditions, is ubiquitinated and undergoes proteosomal degradation catalyzed by O2 and iron-dependent enzymes prolyl hydroxylase (PHDs). Under hypoxic conditions, as in tumors, PHDs are inactivated, which leads to HIF-1α accumulation in TAMs. ELISA and FACS analysis of HIF-1α also showed a significant decrease in its level after Au-Mn NP treatment of TAMs, suggesting a decrease in hypoxia.
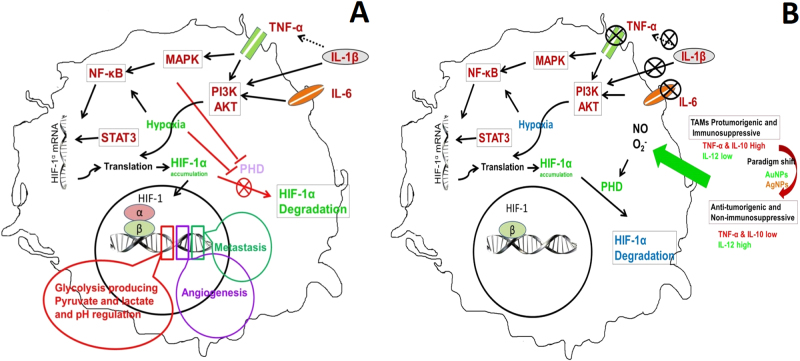
Thus, we may infer that noble metal NPs do exert anti-tumorigenic activities by shifting TAMs from the M2 to M1 phenotype as indicated by the decrease in TNF-α and IL-10 and concomitant rise in the immunoregulatory cytokine IL-12. Moreover, our study reports a decrease in the expression of HIF-1α after NP treatment that suggests a decrease in hypoxia in TAMs (Figure 1) that may account for the arrest in tumor size upon NP treatment, as we initially reported. However, the effect of noble metal NPs on oxidative and nitrosative stress has yielded contradictory findings, which require further investigation.
Fig. 1.
a HIF-1α and regulatory cytokine signaling pathways exist in the cells of the tumor microenvironment. b Likely mechanism of HIF-1α inhibition and the regulatory cytokine signaling cascade triggered by AuNPs or AgNPs
Publisher's note:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conflict of interest
The authors declare that there are no conflict of interest.
References
- 1.Chakraborty B, et al. Immunomodulatory properties of silver nanoparticles contribute to anticancer strategy for murine fibrosarcoma. Cell. Mol. Immunol. 2016;13:191–205. doi: 10.1038/cmi.2015.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hegedus C, et al. Redox control of cancer cell destruction. Redox Biol. 2018;16:59–74. doi: 10.1016/j.redox.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal R, et al. Noble metal nanoparticle-induced oxidative stress modulates tumor associated macrophages (TAMs) from an M2 to M1 phenotype: an in vitro approach. Int. Immunopharmacol. 2016;38:332–341. doi: 10.1016/j.intimp.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Wang R, et al. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J. Exp. Clin. Cancer Res. 2011;30:62–62. doi: 10.1186/1756-9966-30-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortez-Retamozo V, et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl Acad. Sci. USA. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nath A, et al. Gold-manganese oxide nanocomposite suppresses hypoxia and augments pro-inflammatory cytokines in tumor associated macrophages. Int. Immunopharmacol. 2018;57:157–164. doi: 10.1016/j.intimp.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Nagy MA. HIF-1 is the commander of gateways to cancer. J. Cancer Sci. Ther. 2011;3:35–40. [Google Scholar]
- 8.Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013;2013:15. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navalon S, de Miguel M, Martin R, Alvaro M, Garcia H. Enhancement of the catalytic activity of supported gold nanoparticles for the Fenton reaction by light. J. Am. Chem. Soc. 2011;133:2218–2226. doi: 10.1021/ja108816p. [DOI] [PubMed] [Google Scholar]
- 10.Adeyemi OS, Faniyan TO. Antioxidant status of rats administered silver nanoparticles orally. J. Taibah Univ. Med. Sci. 2014;9:182–186. [Google Scholar]