The gastrointestinal tract contains one of the most abundant populations of macrophages in the body.1 These cells play a critical role in the maintenance of gut homeostasis and defend the integrity of epithelial crypts and the mucosal membrane. Chronic disruption of gut homeostasis can cause sustained inflammation mediated by intestinal macrophages. Since inflammation plays a key role in ulcerative colitis (UC) and other forms of inflammatory bowel disease (IBD),2–4 an improved understanding of how inflammation is regulated in the gut is essential for the development of effective therapies for such diseases.
Intestinal homeostasis is maintained by a population of resident macrophages that is sustained through recruitment of circulating monocytes (precursors of macrophages) as well as migration of macrophages from the lymphatic system to target mucosal tissues. These processes are driven by the concerted action of an array of chemokines and chemokine receptors.5, 6 In addition, the diverse microorganisms that densely populate the intestine continuously attract macrophages. Macrophages may encounter commensal microorganisms in the draining lymph nodes or during occasional compromise of the gut epithelial barrier.7, 8 Macrophages have diverse and plastic phenotypes and undergo a series of changes during their response, which is spatially and temporally regulated by multiple and often self-limiting signals. Several intermediate phenotypes between the so-called classically activated M1 (pro-inflammatory) and alternatively activated M2 (anti-inflammatory) phenotypes have been identified.9, 10 A surprising characteristic of intestinal macrophages is their ability to maintain a noninflammatory state. As shown by many studies, this noninflammatory state is maintained through downregulation of diverse molecules involved in the innate immune response of intestinal macrophages.11 However, whether macrophages resolve intestinal inflammation by triggering a single mechanism that targets a cohort of inflammatory genes, or whether this is achieved through the concerted action of several mechanisms was previously unknown.
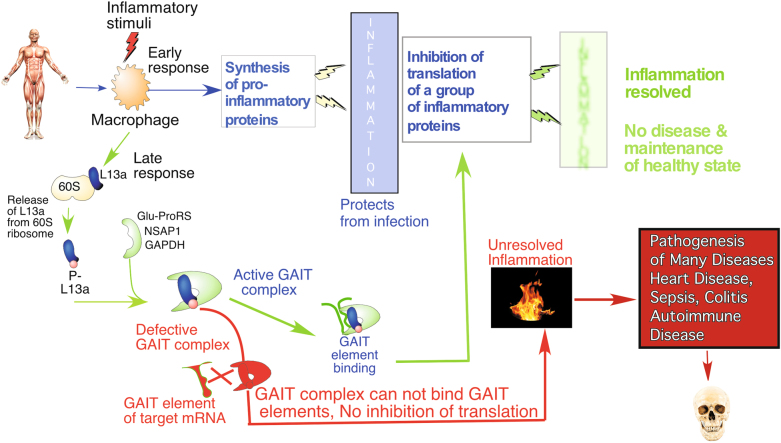
Our work published in Cellular & Molecular Immunology in 2016 identified a single physiological defense mechanism against inflammation that is active in intestinal macrophages.12 Using a murine model of dextran sodium sulfate (DSS)-induced experimental colitis, we discovered a ribosomal protein L13a-dependent mechanism by which translation of inflammatory factors is suppressed in intestinal macrophages. This work built upon our previous discovery of a unique translation control mechanism mediated by a GAIT (interferon (IFN)-gamma-activated-inhibitor of translation) sequence element present in inflammation-associated mRNAs, such as IFN-γ-induced human ceruloplasmin (Cp) mRNA.13 As a part of the multiprotein RNA-binding GAIT complex, in macrophages, L13a directly blocks translation of a group of mRNAs encoding chemokine and chemokine receptors.14 This silencing relies on binding of the L13a-containing GAIT complex to the GAIT element present in the 3′ untranslated region (3′UTR) of its target mRNAs.15 The physiological significance of these observations was tested in our laboratory by creating mice with abrogated expression of L13a specifically in cells of myeloid origin, such as macrophages. Using the inflammation model of lipopolysaccharide (LPS)-induced endotoxemia16 and high-fat diet-induced atherosclerosis,17 we found that these macrophage-specific L13a-KO mice had more severe disease compared to control mice. Together, these studies suggest that defects in the L13a-dependent GAIT-mediated translational silencing pathway can contribute into the pathogenesis of many human diseases caused by chronic or overactive inflammation. The model shown in Fig. 1 depicts the role of this pathway in the pathogenesis of inflammatory diseases.
Fig. 1.
Silencing of a group of inflammatory proteins through a ribosomal protein L13a-dependent pathway provides an endogenous defense against many diseases
The “cytokine storm” generated by intestinal macrophages upon occasional or repeated exposure of these cells to commensal microorganisms plays an essential role in the pathogenesis of UC. Therefore, whether L13a-mediated translational silencing of a cohort of inflammatory proteins in macrophages could serve as a physiological defense mechanism against UC was investigated. To address this question, we compared the severity of disease in macrophage-specific L13a-KO mice and control mice fed DSS-containing water to induce UC. Disease severity was scored based on the clinical features of UC, including colorectal bleeding, bloody diarrhea, weight loss, shortening of the colon and death. We found that macrophage-specific deficiency of L13a significantly increased the severity of disease in this model. In the colons of KO mice, widespread and extensive damage to epithelial crypts, infiltration of macrophages and significant elevation of an array of inflammatory cytokines were observed. Moreover, consistent with the known contribution of penetration of commensal microorganisms through the damaged epithelium in UC, we detected live bacteria and significantly higher levels of endotoxin in the plasma of the KO mice than in the plasma of the controls.12
Although the above data are very intriguing, they do not directly explain the molecular basis of the increased severity of DSS-induced UC observed in macrophage-specific L13a-KO mice. To further test our hypothesis that abrogation of L13a-dependent translational silencing of GAIT target mRNAs determines disease severity, we conducted two sets of experiments. First, using luciferase reporter RNA with a natural GAIT element in its 3′-UTR and a rabbit reticulocyte lysate (RRL) in vitro translation system, we reconstituted GAIT-mediated translational silencing ex vivo by adding lysates prepared from the colons of DSS-fed macrophage-specific L13a-KO and control mice. Colon lysates from DSS-fed control mice activated translational silencing of the GAIT element-containing reporter RNA, but those from L13a-KO mice did not. Second, we tested endogenous GAIT-dependent translational silencing of three previously identified GAIT target mRNAs (CXCL13, CCL22, and CCR3) by comparing their polyribosomal association in colon lysates prepared from the DSS-fed control and L13a-KO mice. The polyribosomal abundance of all three mRNAs was substantially higher in the mice lacking expression of L13a in macrophages. Together, these results demonstrate that L13a-dependent GAIT element-mediated translational silencing serves as an endogenous defense mechanism against intestinal inflammation.12
Any disruption of the integrity of the intestinal epithelial barrier results in confrontation of intestinal macrophages with commensal bacteria. These conditions trigger an innate immune response through activation of pattern recognition receptors, such as toll-like receptors (TLRs),18 and nucleotide binding oligomerization domain (NOD)-like receptors,19 and subsequent synthesis of an array of inflammatory cytokines. Our work shows that activation of GAIT-mediated translational silencing in response to IFN-γ in the cellular model13–15 and upon LPS16 and DSS12 administration in animal models is critically important to restrain the expression of a cohort of inflammatory proteins. The presented evidence suggests that the onset of such silencing is programmed into the initial phase of the innate immune response. In addition, we identified features of GAIT elements that likely have roles in controlling the degree of silencing. We found that while no sequence conservation exists among the GAIT elements present in the mRNAs of different target chemokines and chemokine receptors, these elements share a conserved, characteristic hairpin RNA structure (as revealed by 2D-1H NMR spectroscopy of the GAIT elements of CCL22, CXCL13, and CCR4 mRNAs). Despite this similarity in secondary structure, the GAIT elements present in these mRNAs and in the Cp mRNA showed substantial differences in affinity towards the GAIT protein complex.20 These differences are consistent with more than “all-or-none” control and may allow temporal silencing of targets depending on the microenvironment of the site of inflammation, which may subsequently allow fine-tuning of the resolution phase of inflammation.
The pathogenesis of IBD, UC, and Crohn’s disease is critically dependent on the concerted inputs of multiple signals from an array of inflammatory molecules. Therefore, targeting a single molecule is unlikely to achieve an effective therapeutic outcome. In contrast, our discovery of the L13a-dependent translational silencing mechanism, which impacts the synthesis of multiple inflammatory proteins, bears significant promise for the development of future clinical intervention strategies against these diseases. Our system for reconstitution of L13a-dependent translational silencing of GAIT element-containing reporter RNAs using colon lysates thus represents an invaluable model for drug discovery. This platform can be used to screen libraries of small molecules to identify compounds that promote silencing. The potential of the identified hits for human clinical trials could be further evaluated using a murine model of DSS-induced UC21 or organoid cultures from patients with UC.22
Acknowledgements
The author would like to thank Dr. Anton Komar and Dr. Aaron Severson for their valuable comments on this manuscript and Dr. Patricia Stanhope Baker for assistance with manuscript editing. Research in the author’s laboratory was funded by the National Institute of Health (NIH) Public Health Service Grant No. HL 079164. The author also received financial assistance from the Center for Gene Regulation in Health & Disease of Cleveland State University and Ohio Third Frontier Grant.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J. Exp. Med. 1985;161:475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zigmond E, Jung S. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol. 2013;34:162–168. doi: 10.1016/j.it.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 4.Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. J. Innate Immun. 2011;3:550–564. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Bogunovic M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149–154. doi: 10.1016/S0966-842X(00)88906-4. [DOI] [PubMed] [Google Scholar]
- 8.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 9.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills CD, Ley K. M1 and M2 macrophages: the chicken and the egg of immunity. J. Innate Immun. 2014;6:716–726. doi: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith PD, et al. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4:31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poddar D, Kaur R, Baldwin WM, 3rd, Mazumder B. L13a-dependent translational control in macrophages limits the pathogenesis of colitis. Cell. Mol. Immunol. 2016;13:816–827. doi: 10.1038/cmi.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazumder B, Fox PL. Delayed translational silencing of ceruloplasmin transcript in gamma interferon-activated U937 monocytic cells: role of the 3’ untranslated region. Mol. Cell. Biol. 1999;19:6898–6905. doi: 10.1128/MCB.19.10.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyas K, et al. Genome-wide polysome profiling reveals an inflammation-responsive posttranscriptional operon in gamma interferon-activated monocytes. Mol. Cell. Biol. 2009;29:458–470. doi: 10.1128/MCB.00824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapasi P, et al. L13a blocks 48S assembly: role of a general initiation factor in mRNA-specific translational control. Mol. Cell. 2007;25:113–126. doi: 10.1016/j.molcel.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poddar D, et al. An extraribosomal function of ribosomal protein L13a in macrophages resolves inflammation. J. Immunol. 2013;190:3600–3612. doi: 10.4049/jimmunol.1201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu A, et al. Ribosomal protein L13a deficiency in macrophages promotes atherosclerosis by limiting translation control-dependent retardation of inflammation. Arterioscler. Thromb. Vasc. Biol. 2014;34:533–542. doi: 10.1161/ATVBAHA.113.302573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cario E, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 19.Hisamatsu T, et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 20.Basu A, Jain N, Tolbert BS, Komar AA, Mazumder B. Conserved structures formed by heterogeneous RNA sequences drive silencing of an inflammation responsive post-transcriptional operon. Nucleic Acids Res. 2017;45:12987–13003. doi: 10.1093/nar/gkx979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okayasu I, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-H. [DOI] [PubMed] [Google Scholar]
- 22.Merker SR, Weitz J, Stange DE. Gastrointestinal organoids: how they gut it out. Dev. Biol. 2016;420:239–250. doi: 10.1016/j.ydbio.2016.08.010. [DOI] [PubMed] [Google Scholar]